Abstract
The Drosophila immune system is able to discriminate between classes of bacteria. Detection of Gram-positive bacteria involves a complex of two pattern recognition receptors: peptidoglycan recognition protein SA (PGRP-SA) and Gram-negative binding protein 1 (GNBP1). These activate the Toll signalling pathway. To define the cell wall components sensed by the host, we used highly purified peptidoglycan fragments of two principal Gram-positive bacterial pathogens Staphylococcus aureus and Streptococcus pneumoniae. We report that in both peptidoglycans, the minimal structure needed to activate the Toll pathway is a muropeptide dimer and that the free reducing end of the N-acetyl muramic acid residues of the muropeptides is essential for activity. Monomeric muropeptides were inactive and inhibitory in combination with dimers. Finally, peptidoglycan was degraded by the haemolymph of wild-type but not GNBP1 mutant flies. We suggest a model whereby GNBP1 is involved in the hydrolysis of Gram-positive peptidoglycan producing new glycan reducing ends, which are subsequently detected by PGRP-SA.
Keywords: innate immunity, pattern recognition, Drosophila
Introduction
Gram-positive bacteria trigger some of the most powerful inflammatory responses known to medicine (Sriskandan & Cohen, 1999; Brown, 2004). The cell wall of these bacteria is a surface consisting of several layers of two insoluble, networked carbohydrates teichoic acid and peptidoglycan (PG). Heterogeneity in cell wall composition has confounded efforts to place the immune response after Gram-positive bacterial infection into the concept of pattern recognition, although recent studies have brought forward significant information by using highly purified cell wall components to dissect inflammatory pathways (reviewed by Weber et al, 2003). In this context, Gram-positive PG has attracted much attention (reviewed by Girardin & Philpott, 2004).
In the present study, we have used Drosophila as a genetically tractable model to address the question of PG recognition by the host. Insects in general and Drosophila in particular are remarkably resistant to microbial infections. Their host reactions rely solely on innate immune defences, which have been found to share considerable similarities with those of mammals, suggesting an evolutionary relationship (reviewed by Kimbrel & Beutler, 2001; Hoffmann & Reichhart, 2002). A hallmark of the Drosophila systemic response to infection is the expression of antimicrobial peptides (AMPs). Two signalling pathways control this expression. Gram-positive bacteria and fungi activate the Toll pathway, which in turn directs expression of the AMP gene drosomycin (drs). Triggering of the Imd pathway, which responds to Gram-negative infection, culminates in the expression of several AMP genes (reviewed by Hoffmann 2003; Naitza & Ligoxygakis 2004).
It has been established recently that two pattern recognition receptors (PRRs) are needed to activate the Toll pathway after Gram-positive bacterial infection (Gobert et al, 2003). Peptidoglycan recognition protein SA (PGRPsA) was the first PRR found to act upstream of Toll, detecting invading Gram-positive bacteria in the haemolymph (Werner et al, 2000; Michel et al, 2001). Subsequently, Gram-negative binding protein 1 (GNBP1) was identified as an integral component of the PGRPsA–Toll sensing system, leading to host defence triggering (Gobert et al, 2003). Both these PRRs are needed for downstream signalling, as demonstrated by experiments in which only simultaneous overexpression resulted in challenge-independent activation of AMPs (Gobert et al, 2003). A different PGRP, PGRP-LC, seems to be a PRR for the Imd pathway, which is induced after Gram-negative bacterial infection (Choe et al, 2002; Gottar et al, 2002; Ramet et al, 2002). The structure of Gram-positive PG is characterized by a Lys-type peptidic crosslinkage, whereas Bacilli and Gram-negative bacteria have a PG with a meso-diaminopimelic (DAP) residue (Schleifer & Kandler, 1972). It has been shown recently that Lys-type PG activates the Toll pathway, whereas DAP-type PG activates the Imd pathway (Leulier et al, 2003; Kaneko et al, 2004). Finally, the silkworm Bombyx mori seems to have the ability to recognize PG fragments from Gram-positive and Gram-negative bacteria (Iketani et al, 1999).
We used PG components of the two principal Gram-positive bacterial pathogens Staphylococcus aureus and Streptococcus pneumoniae, in search of the minimal Gram-positive PG fragments able to activate Drosophila host defences. We conclude that the dimeric muropeptide functions as the smallest PG fragment able to induce an immune response. Reduction of the free reducing ends of the sugar groups abolishes activity, indicating that sensing of Gram-positive PG requires an intact C-1 residue of the N-acetyl muramic acid component. The Toll pathway mediates responses triggered by these compounds, verifying once again its role in Gram-positive sepsis in Drosophila. Our genetic studies confirm recent results (Pili-Floury et al, 2004) that both PGRPsA and GNBP1 are needed for PG detection by the host. However, our analysis suggests that PGRP-SA but not GNBP1 is responsible for sensing small PG fragments. Our data indicate that cell-wall-degrading enzymatic activity is missing in the haemolymph of GNBP1 mutant flies. This activity could assist in the release of small PG components and/or formation of new reducing ends that are subsequently recognized by PGRP-SA.
Results
Polymerized muropeptides are potent activators of Toll
To examine in more detail which substructures of Gram-positive PG are recognized by the Drosophila immune system, we isolated mono- and multimeric muropeptides from the cell wall of two principal Gram-positive pathogens S. aureus and S. pneumoniae, using high-pressure liquid chromatography (HPLC). We have monitored the immune response to these infections by measuring the levels of expression of the Toll-dependent AMP gene drs 24 h after injection. This was performed by northern blots (see Methods).
The method of isolation of different muropeptides is depicted in Fig 1, and the concentrations and volumes injected for each component are described in the Methods. We chose to test these components as (i) recent studies show that a monomeric disaccharide tetrapeptide fragment of Gram-negative PG induces AMP gene activation in Drosophila (Kaneko et al, 2004), whereas similar structures induce AMP synthesis in the silkworm B. mori (Iketani et al, 1999), (ii) teichoic acids from S. aureus did not significantly induce AMP expression in flies or tumour necrosis factor release by human peripheral monocytes in culture (P.L., data not shown; Majcherczyk et al, 2003) and (iii) in the rabbit meningitis model, the monomeric muropeptides (a disaccharide of GlcNAc–MurNAc with a tripeptide chain) represent the smallest inflammatory unit of the pneumococcal cell wall (S.R.F. & A.T., unpublished results).
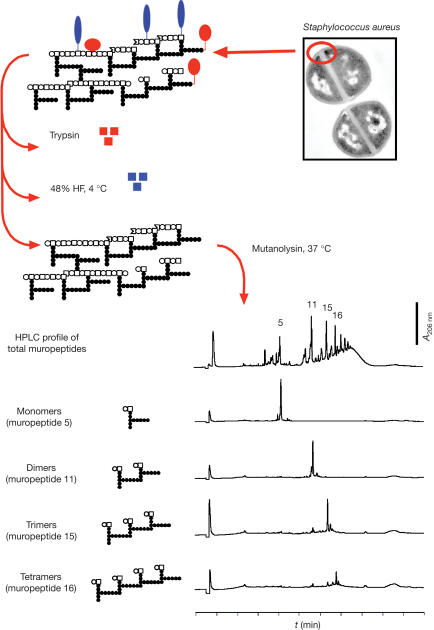
Figure 1.
Isolation of small peptidoglycan fragments from the cell wall of Staphylococcus aureus. Cell walls were purified as described in the Methods. They are represented as follows (top left corner): open circle, N-acetylglucosamine; open square, N-acetylmuramic acid; closed circles, amino-acid residues of the stem peptides; red ovals, proteins associated with the cell wall; blue ovals, teichoic acids. Cell walls were treated with trypsin to degrade associated proteins and incubated in 48% HF at 4°C to cleave the phosphodiester bonds between the repeating units of the teichoic acids and the linkage to PG. The resulting PG was digested with mutanolysin to produce the constituent muropeptides, which were then separated by HPLC in monomers (muropeptides 5), dimers (muropeptide 11), trimers (muropeptide 15), tetramers (muropeptide 16) and higher polymers (top HPLC chromatogram). A schematic representation of their chemical structure is shown along with the respective chromatograms (see Methods for details). Electron microscopy photograph was kindly provided by Dr M.G. Pinho (ITQB, Portugal).
Our results indicate that, at a concentration of 0.15 mg/ml (equivalent to 0.12 mM), monomers are inactive, but a dimeric GlcNAc–MurNAc crosslinked through its stem peptides is the minimum structure required for drs activation in both bacterial species (Fig 2; see Fig 3 for a schematic representation of the dimer). This dimeric muropeptide at a concentration of 0.15 mg/ml induces drs at levels comparable with the injection of PG at a concentration of 5 mg/ml. The tetrameric muropeptide produced from S. aureus PG showed the strongest induction (Fig 2A). Dose–response curves indicate that both the dimer and the tetramer show a response curve similar to intact PG (supplementary Fig S1 online). These response curves suggest that dimeric and tetrameric muropeptides may have higher specific inducing activities than PG. For instance, either dimeric or tetrameric muropeptides at 0.05 mg/ml were able to induce the same levels of drs as PG at 0.5 mg/ml. Dimeric muropeptides at a concentration of 0.15 mg/ml were as effective as PG at 2.5 mg/ml in drs induction (supplementary Fig S1 online). Polymerized muropeptides from S. pneumoniae were also biologically active, inducing an immune response comparable with pneumococcal PG (Fig 2B).
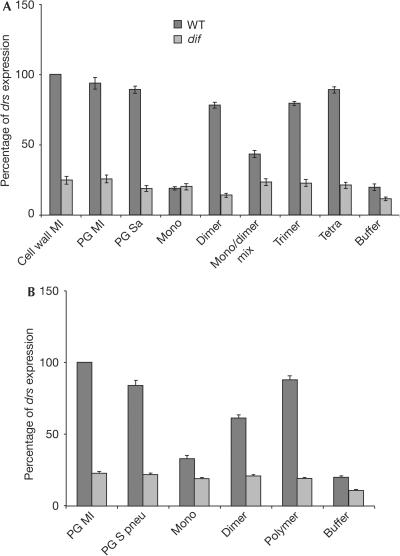
Figure 2.
Polymerized muropeptides from Gram-positive peptidoglycan activate the Toll pathway. (A) A dimeric GlcNAc–MurNAc is the minimum structure required for Staphylococcus aureus (Sa) PG fragments to induce drs expression. Polymerization augments expression levels, as seen with the injection of trimeric or tetrameric muropeptides. To compare drs expression levels induced by small PG fragments of S. aureus, we used as positive controls cell wall and PG from Micrococcus luteus (Ml), a well-documented inducer of the Toll pathway (Werner et al, 2000; Michel et al, 2001; Leulier et al, 2003). All compounds were used at a concentration of 0.15 mg/ml unless otherwise stated. Cell wall and PG were used at 5 mg/ml. (B) Purified components of the pneumonococcal cell wall were tested for their elicitor activity. PG from Streptococcus pneumoniae (S pneu) induces drs expression at levels comparable with injection of cell wall from the same bacterium. Although monomeric muropeptides do not activate drs, dimers induce a response albeit in lower levels compared with PG. Conversely, polymeric (poly) muramyl peptides show a stronger activation. These polymeric muropeptides represent fractions following the dimer in HPLC-mediated purification. All compounds were used at a concentration of 0.1 mg/ml unless otherwise stated. PG samples were used at 5 mg/ml. Values for (A,B) are mean values of five independent experiments with the relevant standard deviation.
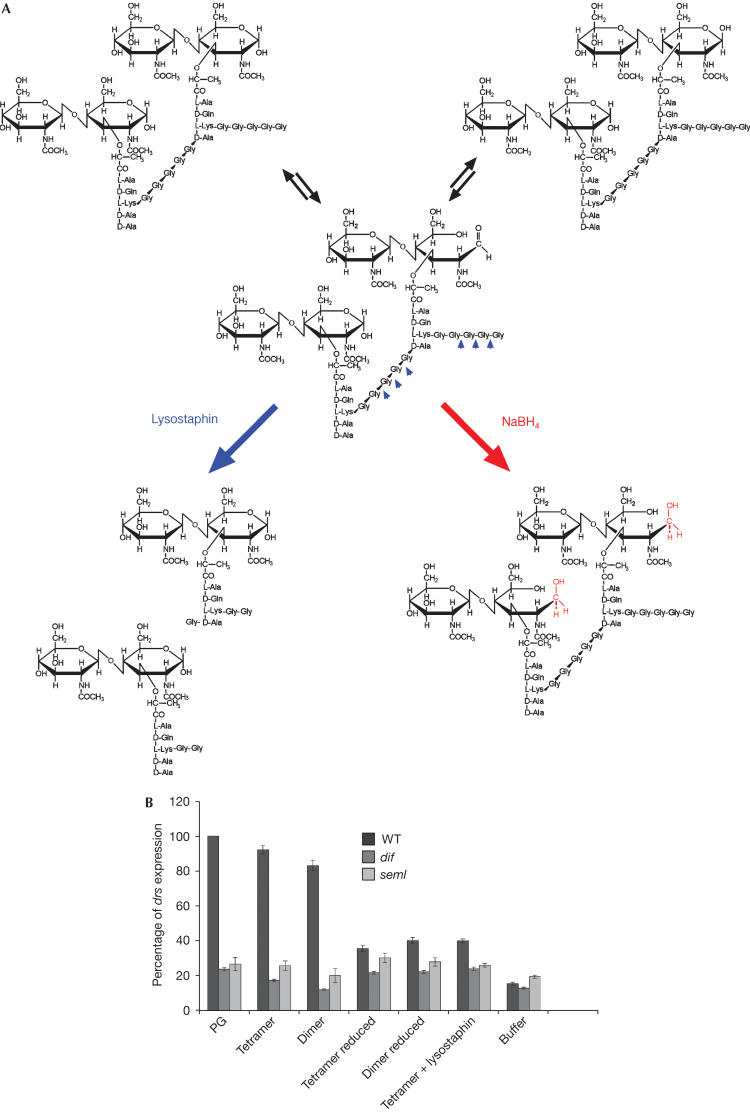
Figure 3.
Reduction of the free end in the sugar chain renders muropeptides inactive. (A) Dimeric muropeptide of Staphylococcus aureus. Muropeptides are present as anomers representing three states of MurNAc. The aldehyde group of the C1 of the muramic acid residue is converted to the respective alditol after reduction, resulting in a molecule unable to circularize. Conversely, as lysostaphin converts dimers to monomers (B), these changes abolish elicitor activity as seen with the tetrameric and dimeric muropeptides of S. aureus. No induction of drs was observed in dif1 and PGRPseml mutants.
The nuclear factor-κB transcription factor Dif has been shown to be the mediator of Toll-dependent immune responses in adult flies (Rutschmann et al, 2000a). Induction of drs by polymerized muropeptides from both pathogens was dependent on the Toll pathway, as it was compromised in dif mutant flies (Fig 2). As expected, expression of drs was not dependent on the Imd pathway, as induction was at wild-type levels in flies carrying a mutation for kenny (Rutschmann et al, 2000b), which encodes the IKK-γ subunit of the Drosophila IKK complex (data not shown).
Interestingly, we observed that when we digested staphylococcal and micrococcal PG with mutanolysin, converting it in a mixture of monomeric and polymeric muropeptides, and injected it into flies without any further purification step, drs was not induced (data not shown). This is in accordance with the results of a recent study (Leulier et al, 2003). However, we noted that purified dimeric muropeptides from S. aureus (at a concentration of 0.15 mg/ml, previously shown to induce drs expression) in the presence of monomers (0.34 mg/ml) at a ratio of 1:4.5 molecules could no longer activate the immune response (Fig 2A). This result suggests that monomers may act as competitors for recognition and/or dilute the elicitor effect of dimers.
Digestion of tetrameric muropeptides with lysostaphin (an endopeptidase that specifically cleaves the pentaglycine bridges converting them to monomers; Fig 3A) destroyed the ability of the sample to induce drs. This shows that the induction activity of the tetramer depends on its polymerization (Fig 3B).
Reduction of the muramic acid abolishes infectivity
Current protocols of muropeptide analysis include PG treatment with mutanolysin and subsequent reduction of the resulting soluble fragments before their isolation by HPLC. In contrast, we avoided the reduction step, as it produces muropeptides that are not representative of the physiological condition (Beranova-Giorgianni et al, 1998). In keeping with this idea, we tested whether reduced PG fragments would retain inflammatory activity in adult flies. Fig 3A depicts the change in the cyclic structure of the MurNAc following reduction by NaBH4 in alkaline pH. We noticed a loss of elicitor activity in the reduced muropeptides, as shown in Fig 3B. This result indicates that sensing of Gram-positive PG requires the intact C-1 reducing ends of muramic acid residues, which have an important role in recognition by the host.
Different roles for PGRP-SA and GNBP1
We wanted to establish whether PGRP-SA and/or GNBP1 are involved in muropeptide recognition. As shown in Figs 3B, 4A, flies mutant for PGRPsAseml were defective in drs induction after injection of various muropeptides from S. aureus. This indicates that PGRP-SA participates in the sensing of these PG fragments. In contrast, although in GNBP1 mutants, the levels of drs were consistently lower than in the wild-type flies, this reduction was much less severe than what was observed for PGRP-SA, and the drs activity observed (five independent experiments) was no less than 70% of the wild-type levels (Fig 4A). This reduction may result from the fact that although PGRPsA is the principal sensing factor for muropeptides, it has a reduced capacity for signalling with the absence of GNBP1, in keeping with the data from Gobert et al (see Discussion). As expected, levels of drs in PGRP-LC mutants after injection of various muropeptide species were comparable with wild-type flies, confirming the specificity of this PRR for Gram-negative bacteria (data not shown).
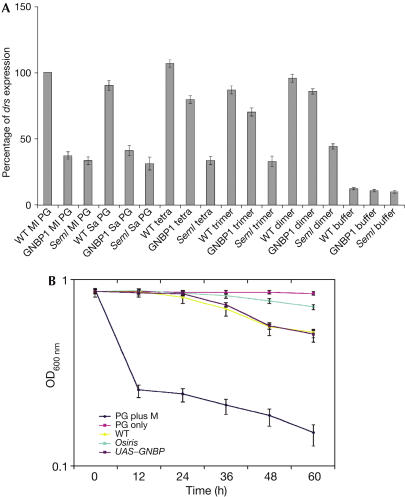
Figure 4.
PGRPsA but not GNBP1 mediates recognition of muropeptides. (A) Both GNBP1osiris and PGRP-SAseml mutants fail to respond to PG from Micrococcus luteus (Ml PG) or Staphylococcus aureus (Sa PG). Nevertheless, only flies deficient for PGRP-SA are unsuccessful in responding to purified muropeptides. (B) When Ml PG is mixed with haemolymph of wild-type flies at 37°C, hydrolysis occurs (WT). On the contrary, mixing with haemolymph from GNBP1osiris mutant flies does not result in PG hydrolysis (osiris). Bringing back GNBP1 (through a UAS–GNBP1 construct driven by yolk GAL4) in an osi background restores activity. PG incubated with mutanolysin (M) was used as a control for PG hydrolysis. Values shown are mean values from three independent experiments with the relevant standard deviation.
The above results suggest a difference between the recognition properties of the two Gram-positive bacterial PRRs, pointing towards an intriguing possibility. It has been proposed recently that GNBP1 and PGRP-SA form a protein complex that senses the presence of Gram-positive bacterial cell wall, although the precise molecular patterns recognized have not been identified experimentally (Gobert et al, 2003).
Our results indicate that GNBP1 and PGRP-SA sense PG but only the latter is required to respond to small PG fragments. Given the homology of GNBP1 with glucan recognition proteins, we wondered whether this protein could be hydrolysing PG, creating free reducing ends, which are then recognized by PGRP-SA. To test this, we isolated haemolymph from wild-type and GNBP1 mutant adults. Following mixture of these extracts with PG preparations of the Gram-positive bacterium Micrococcus luteus (at a concentration of 5 mg/ml and an optical density (OD)600 nm of 0.85), OD changes were measured during the course of 40 h (Fig 4B). We observed that in haemolymph from wild-type flies, the rate of degradation was four times faster than for GNBP1 mutants. Samples incubated with haemolymph from wild-type flies had their OD reduced from 0.85 to 0.50. Conversely, incubation with haemolymph from GNBP1 mutant flies resulted in an OD of 0.75. Flies expressing GNBP1 through a UAS construct (using the yolk GAL4 driver) in a GNBP1 mutant background showed wild-type levels of PG degradation (Fig 4B). These results indicate that the activity detected is associated with the addition of GNBP1. Similar results were obtained with PG from S. aureus (data not shown). These data illustrate the differences observed between wild-type and GNBP1 mutant haemolymph extracts, confirming the hypothesis that there is hydrolysis of Gram-positive PG associated directly or indirectly with GNBP1, which is severely reduced in the GNBP1 mutants.
Discussion
We have used highly purified PG fragments from two principal Gram-positive bacterial pathogens, in an attempt to identify the compounds that are recognized by the Drosophila immune system during Gram-positive sepsis. Our results indicate that two GlcNAc–MurNac units crosslinked through their peptide chains from the minimum pattern of S. aureus and S. pneumoniae PG sensed by the Toll pathway. The reducing end of the sugar moiety is necessary for the elicitor activity, as reduction of the terminal MurNAc in the dimeric and tetrameric muropeptides abolished elicitor activity. This result suggests a role for the free reducing end of the sugar chain in recognition, as in the silkworm and mammalian Nod2 systems (Iketani et al, 1999; Girardin et al, 2003).
Of note is our observation that digested PG from M. luteus and S. aureus did not induce drs expression in our experiments, in agreement with Leulier et al (2003; for M. luteus). Analysis of the PG composition from M. luteus showed that there were no trimeric or higher polymerized muropeptides, and most of the detectable muropeptides were monomers (S.R.F., unpublished results). Moreover, when challenged with dimeric muropeptides in the presence of monomers, the flies were not able to induce the triggering of the innate immune response. This suggests that the presence of monomeric muropeptides apart from not inducing the Drosophila host defence seems to prevent the trigger induced by polymeric muropeptides. The mechanism of such an effect is being addressed at present. Nevertheless, our study, work on the sensing of Gram-negative bacterial PG in the fruitfly (Kaneko et al, 2004; Stenbak et al, 2004), the silkworm (Iketani et al, 1999) and studies on the intracellular recognition of Gram-positive PG by mammalian Nod2 (Girardin et al, 2003) point to a minimal PG motif that is recognized by each system. In our hands, both S. aureus and S. pneumoniae oligomeric muropeptides showed powerful drs-inducing activity, in spite of the differences in the chemical composition of the stem peptide portions of these molecules. The possible role of the chemistry of the peptide components in the inflammatory activity of muropeptides remains to be determined.
From the perspective of recognition, our results indicate that both GNBP1 and PGRP-SA are needed for PG detection but that sensing of small PG fragments is mediated by PGRP-SA, as immune induction is severely reduced in PGRP-SAseml. After injection of muropeptides in GNBP1 mutants, drs levels of expression were moderately decreased, suggesting a role for this molecule in downstream signalling. GNBP1 is part of the GNBP/β(1,3)-glucan recognition family of proteins, which comprises members from several insects (Zhang et al, 2003). These proteins contain an amino-terminal glucan-binding domain and a carboxy-terminal domain similar to β(1,3)- and β(1,4)-glucanase from bacteria and a β(1,3)-glucanase from the sea urchin Strongylocentrotus purpuratus (Yahata et al, 1990; Bachman & McClay, 1996). Although crucial residues in the active sites of bacterial glucanases have been replaced with other amino acids in GNBP1 (Zhang et al, 2003; our unpublished observations), our data show that there is PG degrading activity in the haemolymph of wild-type flies, which is lost in the GNBP1 mutants. This suggests that GNBP1 could have enzymatic activity or be involved in the hydrolysis of β(1,4)-glucosaminoglycans, such as PG.
Speculation
We propose that GNBP1 and PGRP-SA cooperate in sensing Gram-positive bacterial PG. The former breaks down PG into smaller fragments freeing the reducing ends in the bacterial glycan chains, which are important in recognition by the latter. In this scenario, PG hydrolysis by GNBP1 would resemble endo-muramidase-like activity resulting in muramic acid residues with free reducing ends. Full signalling requires both molecules and this would correlate with recent findings, which indicate the formation of a complex between the two proteins and the need of both for challenge-independent expression of drs (Gobert et al, 2003). Binding and single-molecule live imaging studies in progress (S.R.F. & P.L., unpublished) will shed more light on the precise interactions between these receptors and PG.
Methods
Bacterial strains and conditions, cell wall preparation, PG degradation assays, HPLC, PG purification and enzymatic digestion are described in supplementary information online.
Drosophila strains and infections. We have used cn bw and w− isogenized flies as wild-type controls, as most of the mutant strains infected were isolated on these backgrounds. No significant differences were observed between them (data not shown). The results shown are based on w− isogenized flies as a control. Other fly strains used were dif1 (Rutschmann et al, 2000a), PGRPsAseml (Michel et al, 2001), UAS–GNBP1 and GNBP1osiris (Gobert et al, 2003), pgrp-lc (Gottar et al, 2002) and key (Rutschmann et al, 2000b). All mutant fly stocks were isogenized and maintained in non-crowded bottles on standard medium at 25°C.
Infections were performed by injecting approximately 20 nl of bacterial cell wall, PG (at 5 mg/ml) or unreduced and reduced muramyl peptides (at 0.15 mg/ml for S. aureus and 0.1 mg/ml for S. pneumoniae, except otherwise stated) into the anepisternum of adult flies (aged 3–4 days). This is particularly relevant, as wounding itself provokes a short-lived induction of AMPs. All injections were performed with thin glass capillaries mounted on a Nanoject II microinjector (Drummond Scientific, Broomall, PA, USA). Our negative control was PBS injection. Our positive control was cell wall or PG from the Gram-positive bacterium M. luteus, a microorganism widely used for Gram-positive infections in Drosophila. In all cases, flies were incubated for 24 h before RNA extraction. Northern blots were performed as described previously (Rutschmann et al, 2000a). After exposure, signals were measured using a Fuji-film FLA-3000 phosphorimager and normalized against the signal of the loading control, which was a probe for the expression of ribosomal protein 49 (rp49). We performed five independent experiments for the genotypes and treatments presented. Each time, the wild-type control was set as 100% induction and all levels of induction were measured against this value.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400371s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank J.-M. Reichhart and D. Ferrandon for fly stocks, J. Hodgkin and M. Gravato-Nobre for critical reading of the manuscript, D. Sherratt for the use of the Fuji-film FLA-3000 phosphorimager and Herminia de Lencastre for the use of the Shimadzu HPLC system. S.R.F. was supported initially by an EMBO Long Term Postdoctoral Fellowship and subsequently by a fellowship from the Fundação para a Ciência e Tecnologia, MCT, Portugal. This work was supported by a Career Establishment Grant from the Medical Research Council UK (to P.L.) and by funds from the Irene Diamond Foundation (to A.T.).
References
- Bachman ES, McClay DR (1996) Molecular cloning of the first metazoan β-1,3 glucanase from eggs of the sea urchin Strongylocentrotus purpuratus. Proc Natl Acad Sci USA 93: 6808–6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranova-Giorgianni S, Desiderio DM, Pabst MJ (1998) Structures of biologically active muropeptides from peptidoglycan of Streptococcus sanguis. J Mass Spectrom 33: 1182–1191 [DOI] [PubMed] [Google Scholar]
- Brown EJ (2004) The molecular basis of streptococcal toxic syndrome. N Engl J Med 350: 2093–2094 [DOI] [PubMed] [Google Scholar]
- Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV (2002) Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial responses in Drosophila. Science 296: 359–362 [DOI] [PubMed] [Google Scholar]
- Girardin SE, Philpott DJ (2004) The role of peptidoglycan recognition in innate immunity. Eur J Immunol 34: 1777–1782 [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott D, Sansonetti PJ (2003) Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 5509–5512 [DOI] [PubMed] [Google Scholar]
- Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, Hoffmann JA, Ferrandon D (2003) Dual activation of the Drosophila Toll pathway by two pattern recognition receptors. Science 302: 2126–2130 [DOI] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J (2002) The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416: 640–644 [DOI] [PubMed] [Google Scholar]
- Hoffmann JA (2003) The immune response of Drosophila. Nature 426: 33–38 [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart J-M (2002) Drosophila innate immunity: an evolutionary perspective. Nat Immunol 3: 121–126 [DOI] [PubMed] [Google Scholar]
- Iketani M, Nishinura H, Akayama K, Yamano Y, Morishima I (1999) Minimum structure of peptidoglycan required for induction of antibacterial protein synthesis in the silkworm Bombyx mori. Insect Biochem Mol Biol 29: 19–24 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N (2004) Monomeric and polymeric Gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 20: 637–639 [DOI] [PubMed] [Google Scholar]
- Kimbrel AD, Beutler B (2001) The evolution and genetics of innate immunity. Nat Rev Genet 2: 1–13 [DOI] [PubMed] [Google Scholar]
- Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B (2003) The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol 4: 478–484 [DOI] [PubMed] [Google Scholar]
- Majcherczyk PA, Rubli E, Heumann D, Glauser MP, Moreillon P (2003) Teichoic acids are not required for Streptococcus pneumoniae and Staphylococcus aureus cell walls to trigger the release of tumor necrosis factor by peripheral blood monocytes. Infect Immun 71: 3707–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T, Reichhart J-M, Hoffmann JA, Royet J (2001) Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414: 756–759 [DOI] [PubMed] [Google Scholar]
- Naitza S, Ligoxygakis P (2004) Drosophila antimicrobial defences: the story so far. Mol Immunol 40: 887–896 [DOI] [PubMed] [Google Scholar]
- Pili-Floury S, Leulier F, Takahashi K, Saigo K, Samain E, Ueda R, Lemaitre B (2004) In vivo RNA interference analysis reveals an unexpected role for GNBP1 in the defence against Gram-positive bacterial infection in Drosophila adults. J Biol Chem 279: 12848–12853 [DOI] [PubMed] [Google Scholar]
- Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA (2002) Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416: 644–648 [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Hetru C, Reichhart J-M, Hoffmann JA, Ferrandon D (2000a) The Rel protein DIF mediates the antifungal but not the antibacterial host defence in Drosophila. Immunity 12: 569–580 [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Zhou R, Silverman N, Hoffmann JA, Ferrandon D (2000b) Role of Drosophila IKKγ in a Toll-independent antibacterial immune response. Nat Immunol 1: 342–347 [DOI] [PubMed] [Google Scholar]
- Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36: 407–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriskandan S, Cohen J (1999) Gram-positive sepsis: mechanisms and differences from Gram-negative sepsis. Infect Dis Clin N Am 13: 397–412 [DOI] [PubMed] [Google Scholar]
- Stenbak CR et al. (2004) Peptidoglycan molecular requirements allowing detection by the Drosophila immune deficiency pathway. J Immunol 173: 7339–7348 [DOI] [PubMed] [Google Scholar]
- Weber RW, Moreillon P, Tuomanen E (2003) Innate sensors of Gram-positive bacteria. Curr Opin Immunol 15: 408–415 [DOI] [PubMed] [Google Scholar]
- Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D (2000) A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA 97: 13772–13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Watanabe T, Nakamura Y, Yamamoto Y, Kamimiya S, Tanaka H (1990) Structure of the gene encoding β-1,3-glucanase A1 of Bacillus circulans WL-12. Gene 86: 113–117 [DOI] [PubMed] [Google Scholar]
- Zhang R, Cho HY, Kim HS, Ma YG, Osaki T, Kawabata S, Sönderhäll K, Lee BL (2003) Characterisation and properties of a 1,3-β-D-glucan pattern recognition protein of Tenebrio molitor larvae that is specifically degraded by serine protease during prophenoloxidase cascade. J Biol Chem 278: 42072–42079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information