Abstract
Recruitment of β-arrestin (β-arr) to agonist-stimulated G-protein-coupled receptors (GPCRs) has a crucial role in controlling signalling efficacy and selectivity. When translocated to the receptor, β-arr is believed to undergo important conformational rearrangement necessary for its downstream actions. To probe these changes in living cells, we constructed an intramolecular bioluminescence resonance energy transfer (BRET)-based biosensor, in which β-arr is sandwiched between the Renilla luciferase (Luc) and the yellow fluorescent protein (YFP). We show that the intramolecular BRET between Luc and YFP was significantly increased following GPCR activation, suggesting a conformational rearrangement bringing the amino terminus and carboxyl terminus of β-arr in closer proximity. Kinetic analysis showed that this conformational change follows the initial β-arr/receptor engagement. In addition to providing new insights into the agonist-induced conformational rearrangements of β-arr in living cells, the double-brilliance β-arr offers a universal biosensor for GPCR activation, allowing the study of native receptors in large-scale screening analysis.
Keywords: GPCR, BRET, biosensor, arrestin
Introduction
G-protein-coupled receptors (GPCRs) relay the information provided by numerous hormones and neurotransmitters into intracellular signalling pathways, primarily through their coupling to heterotrimeric G proteins. Agonist stimulation of GPCRs also initiates their feedback desensitization, mostly mediated by GPCR kinases (GRKs) and β-arrestin (β-arr) proteins. Through their binding to GRK-phosphorylated receptors, β-arrs prevent further coupling to G proteins and promote GPCR endocytosis, thus leading to decreased signalling efficacy. In addition to their role in receptor desensitization, β-arrs can act as scaffolds, linking GPCRs to mitogen-activated protein kinase signalling pathways (Luttrell & Lefkowitz, 2002). When considering their interaction with β-arrs, GPCRs can be divided into two classes: class A receptors interact only transiently with β-arr and undergo efficient recycling when released from β-arr, whereas class B receptors stably associate with β-arr as a result of higher affinity, thus leading to the accumulation of intracellular receptor/β-arr complexes that prevent receptor recycling (Oakley et al, 2001).
Solved crystal structures (Hirsch et al, 1999; Han et al, 2001), mutagenesis (Vishnivetskiy et al, 2002) and limited tryptic proteolysis studies (Gurevich & Benovic, 1993; Xiao et al, 2004) suggest that a conformational rearrangement of the β-arr molecule accompanies its transition to the high-affinity receptor-binding state. It has been proposed that known intramolecular interactions between the amino- and carboxy-terminal domains in the inactive state are lost in the active β-arr, suggesting that the domains move relative to each other on activation. In this process, the C-tail seems to be released, thus exposing its clathrin- and adaptin 2 (AP2)-binding sites and promoting interactions with the internalization machinery (Lin et al, 1999, 2002; Gurevich & Gurevich, 2003).
To assess whether such agonist-promoted conformational changes of β-arr occur in living cells and to obtain further information on the relative positions of the C- and N-terminal domains during the activation process, we used an intramolecular bioluminescence resonance energy transfer (BRET)-based biosensor consisting of a β-arr molecule sandwiched between the bioluminescent donor Renilla luciferase (Luc) and the yellow fluorescent protein (YFP). Using this double-brilliance β-arr biosensor (Luc–β-arr–YFP), we show that β-arr undergoes important conformational rearrangement after agonist stimulation, where the N terminus and C terminus are brought in closer proximity. Comparison of the kinetics of β-arr recruitment to the receptors and its conformational change indicates that the latter follows the initial recruitment of β-arr to agonist-activated receptors. In addition to providing new insights into the structural rearrangements following β-arr activation in living cells, our study validates the use of double-brilliance β-arr as a general biosensor of GPCR activity.
Results and Discussion
Double-brilliance β-arr
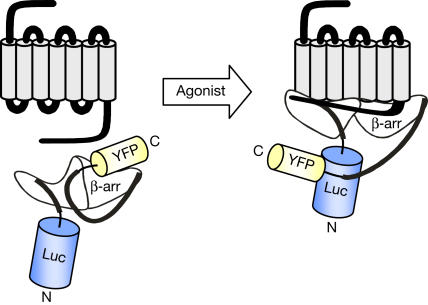
Inspired by previous reports of intramolecular fluorescence resonance energy transfer (FRET)-based biosensors (Zhang et al, 2002), showing that resonance energy transfer (RET) is sensitive to changes in the relative positions of the donor and acceptor molecules, we assessed whether conformational changes of β-arr could be monitored using an intramolecular BRET approach. We engineered a double-brilliance β-arr, in which we fused Luc to the N terminus of β-arr2 and YFP to its C terminus, yielding Luc–β-arr–YFP (Fig 1).
Figure 1.
Double-brilliance β-arr. Schematic diagram illustrating how agonist-promoted conformational rearrangement of β-arr can be measured as changes in BRET using double-brilliance β-arr. Luc and YFP are represented by cylinders proportional to their sizes, but their real orientation is unknown.
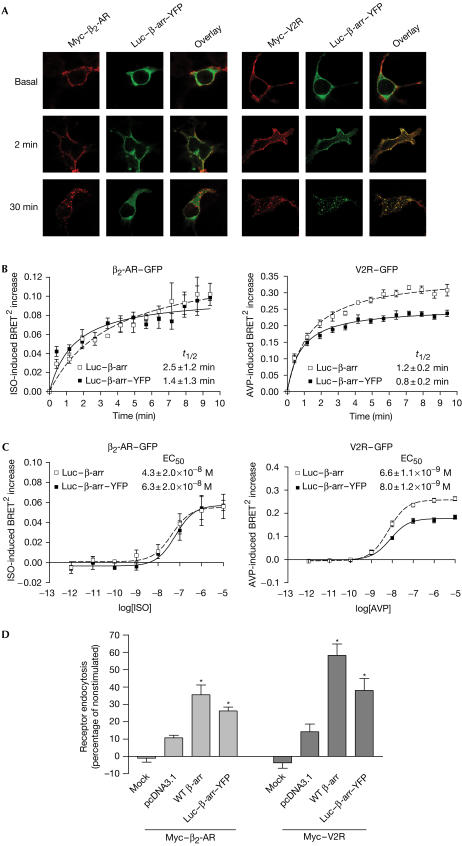
To test the functionality of Luc–β-arr–YFP, we first assessed its ability to be recruited to agonist-stimulated class A β2-adrenergic receptor (β2-AR) and class B V2 vasopressin receptor (V2R) by fluorescence microscopy. As shown in Fig 2A, agonist stimulation led to rapid translocation of Luc–β-arr–YFP to the plasma membrane, colocalizing with Myc-tagged β2-AR and V2R (Myc–β2-AR; Myc–V2R). The patterns of Luc–β-arr–YFP interaction were consistent with those observed for class A (transient β-arr interaction) and B (stable β-arr association) receptors in similar experiments using a β-arr–green fluorescent protein (GFP) conjugate (Oakley et al, 2000). Indeed, whereas Luc–β-arr–YFP was recruited to both β2-AR and V2R after 2 min of stimulation, it returned to the cytoplasm after 30 min in Myc–β2-AR-expressing cells but remained colocalized with Myc–V2R in endocytic vesicles. To quantitatively assess the recruitment of Luc–β-arr–YFP to agonist-activated GPCRs, we used an intermolecular BRET2 assay that takes advantage of the different spectral properties of Luc substrates that allow energy transfer to different fluorescent acceptors (Milligan, 2004). Luc–β-arr–YFP was transiently coexpressed with the receptors, and the agonist-induced BRET2 between Luc–β-arr–YFP and either β2-AR–GFP or V2R–GFP was measured in the presence of DeepBlueC™ coelenterazine, allowing transfer of energy to GFP. As shown in Fig 2, agonist stimulation promoted a time-dependent (Fig 2B) and dose-dependent (Fig 2C) increase in BRET2, reflecting the recruitment of Luc–β-arr–YFP to the receptors. Similar kinetics and EC50 were obtained for the recruitment of both Luc–β-arr–GFP and Luc–β-arr, indicating that double-brilliance β-arr is as efficiently recruited to the receptors as the singly conjugated construct. It should be noted that, although the maximum agonist-promoted BRET increase observed with the class A β2-AR is less than that observed with class B V2R, the stability of the signals was similar, indicating that the signal observed with β2-AR reflects a steady state corresponding to constant association and dissociation of β-arr from the activated receptors.
Figure 2.
Functionality of double-brilliance β-arr. HEK293 (A–C) or COS (D) cells were transiently transfected with the indicated plasmids. (A) Cells incubated or not in the presence of saturating concentrations of specific agonists (β2-AR, 10 μM isoproterenol (ISO); V2R, 1 μM arginine vasopressin (AVP)). Localization of Luc–β-arr–YFP and Myc-tagged receptors was analysed by confocal fluorescence microscopy. (B) Agonist-induced recruitment of β-arr measured using BRET2. t1/2=half-time of maximal β-arr recruitment. (C) Dose-dependent recruitment of β-arr to the receptors measured in BRET2 following 2 min stimulation with the agonist. EC50=concentration of agonist producing half-maximal β-arr recruitment. (D) Cells treated or not for 15 min with the specific agonists at 37°C and cellsurface receptor levels measured by enzyme-linked immunosorbent assay (ELISA). Receptor endocytosis is defined as the loss of cell-surface immunoreactivity and is expressed as a percentage of total immunoreactivity measured under basal conditions. Expression levels of β-arr were controlled using western blot (data not shown). Data are the mean±s.e.m. of at least three independent experiments. *P<0.05 between treatment and each individual control condition. Mock, nontransfected cells.
To assess the biological activity of Luc–β-arr–YFP, we tested its capacity to promote receptor endocytosis in COS cells, which express low endogenous levels of β-arr. As shown in Fig 2D, agonist-promoted β2-AR and V2R endocytosis was considerably increased when overexpressing Luc–β-arr–YFP. Even though this increase in receptor endocytosis was not as pronounced as that obtained by the overexpression of wild-type (WT) β-arr, it suggests that Luc–β-arr–YFP retains significant biological activity.
Agonist-induced conformational changes of β-arr
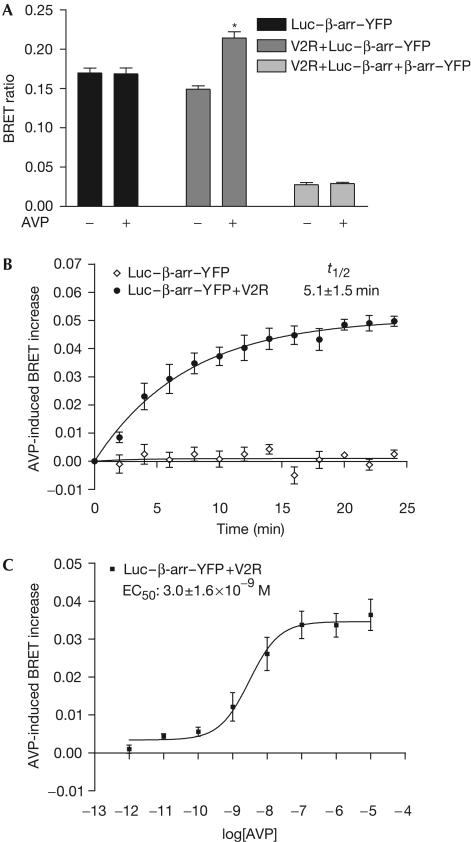
To assess whether Luc–β-arr–YFP could be used to monitor the conformational rearrangement of β-arr, the construct was expressed with or without V2R, and BRET was measured in the presence of coelenterazine h, allowing transfer of energy to YFP. As shown in Fig 3A, an important basal BRET signal could be measured in cells transfected with Luc–β-arr–YFP, reflecting the proximity of the energy donor and acceptor in the construct. Arginine vasopressin (AVP) stimulation of cells coexpressing V2R led to a significant increase in BRET, suggesting movement of Luc and YFP relative to each other. To rule out the possibility that this increased signal results from intermolecular BRET between individual Luc–β-arr–YFP molecules brought together through oligomerization (Hirsch et al, 1999) or clustering at the plasma membrane, we tested for the occurrence of BRET in cells transiently expressing Luc–β-arr and β-arr–YFP. In transfection conditions leading to equivalent fluorescence and luminescence levels as those obtained in Luc–β-arr–YFP-expressing cells, coexpression of Luc–β-arr and β-arr–YFP led to the detection of only a marginal basal BRET that could not be modulated by V2R stimulation (Fig 3A). This observation therefore demonstrates that the AVP-induced increase in BRET signal observed in cells transfected with Luc–β-arr–YFP results from a change in intramolecular BRET. As variations in RET can reflect changes in both the distance and orientation between the energy donor and acceptor molecules (Andrews & Demidov, 1999), the observed agonist-promoted increase in the Luc–β-arr–YFP intramolecular BRET could indicate that the N terminus and C terminus are either brought closer or are in a more permissive BRET orientation following activation.
Figure 3.
AVP-induced conformational change of β-arr monitored by intramolecular BRET. HEK293 cells were transfected with the indicated plasmids and BRET was measured at 25°C in the presence of coelenterazine h. (A) Specificity of agonist-induced β-arr intramolecular BRET. (B) Real-time BRET measurements of the agonist-induced β-arr conformational change. t1/2=half-time of maximal conformational change of β-arr. (C) Dose-dependent agonist-promoted increase of β-arr intramolecular BRET. Cells were stimulated with increasing concentrations of AVP for 4 min. EC50=concentration of AVP producing half-maximal conformational change of β-arr. Data are the mean±s.e.m. of at least three independent experiments. *P<0.01 between treated and control condition.
To further characterize the agonist-induced change in the conformation of β-arr, the kinetics and dose dependency of AVP-mediated BRET increase were assessed. Real-time BRET measurements show a time-dependent AVP-induced conformational change of β-arr, with half-time of maximal BRET increase (t1/2) of 5.1±1.5 min (Fig 3B). This kinetics is significantly slower (P<0.02) than that of the AVP-induced recruitment of β-arr (t1/2=0.8±0.2 min; Fig 2B, right panel), suggesting that the conformational change observed in Luc–β-arr–YFP occurs after its initial recruitment to the activated V2R. This difference in kinetics cannot result from inter-experimental variations because similar results were obtained when the two events were measured in the same cell population expressing V2R–GFP and Luc–β-arr–YFP (data not shown). Despite the difference in kinetics, the efficacy of AVP to induce a conformational change in Luc–β-arr–YFP (Fig 3C) was similar to that observed for β-arr recruitment (Fig 2C, right panel), indicating that these two events are directly linked and reflect the binding affinity of V2R for AVP (KD∼1 × 10−9 M).
The observed kinetic lag between β-arr recruitment and its conformational change could be consistent with the proposal that inactive β-arr is first recruited to the activated GPCR where its interaction with the GRK-phosphorylated residues subsequently induces the release of its C-tail (Gurevich & Gurevich, 2003). Alternatively, such a lag could indicate that the intramolecular BRET changes observed with Luc–β-arr–YFP result from the subsequent recruitment of β-arr-interacting proteins (e.g. clathrin and AP2 or signalling proteins such as csrc, Raf1, ERK1/2, ASK1 and JNK3) to the receptor-bound β-arr (Lefkowitz & Whalen, 2004) rather than from the conformational change induced by receptor binding. To distinguish between these two possibilities, we tested the agonist-promoted conformational changes of a β-arr(R169E) mutant shown to bind to GPCRs in a phosphorylation-independent manner, probably as a result of a constitutively open conformation (Kovoor et al, 1999). As shown in Fig 4, both the basal and AVPstimulated BRET signals observed for the Luc–β-arr–YFP phosphate-insensitive mutant (Luc–β-arr(R169E)–YFP) were similar to those observed with WT Luc–β-arr–YFP, ruling out the hypothesis that the observed conformational rearrangement of β-arr results from binding to phosphorylated residues on V2R. It follows that the agonist-promoted BRET increase in Luc–β-arr–YFP probably represents conformational changes promoted by the binding of β-arr-interacting proteins that follows activation.
Figure 4.
Agonist-promoted conformational change of β-arr is independent of receptor phosphorylation. HEK293 cells were transfected with V2R and either Luc–β-arr–YFP or Luc–β-arr(R169E)–YFP. Cells were stimulated or not for 10 min in the presence of 1 μM AVP. Inset: AVP-induced BRET increase. Data are the mean±s.e.m. of three independent experiments. *P<0.02 between treatment and each individual control condition.
A general biosensor to monitor GPCR activity
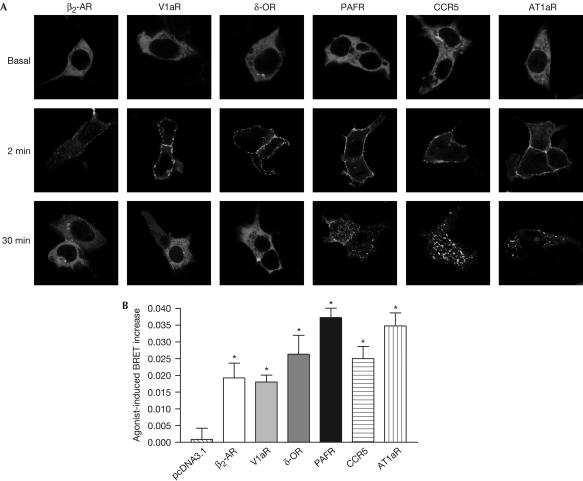
To assess whether Luc–β-arr–YFP could be used as a general GPCR activity sensor, we tested whether its agonist-induced conformational change could be promoted by other receptors, particularly those of class A, which are believed to interact only transiently with β-arr. Recruitment of Luc–β-arr–YFP and agonist-promoted intramolecular BRET were assessed in cells coexpressing different receptors of class A (β2-AR, V1 vasopressin receptor (V1aR), δ-opioid receptor (δ-OR)) and class B (platelet-activating factor receptor (PAFR), CC chemokine receptor type 5 (CCR5), angiotensin receptor type 1a (AT1aR)). As shown in Fig 5A, agonist stimulation efficiently induced the recruitment of Luc–β-arr–YFP to the plasma membrane, with the expected interaction patterns for all class A (transient) and class B (stable) receptors. In all cases, activation of Luc–β-arr–YFP mediated by class A and B receptors was accompanied by a significant increase in BRET (Fig 5B). Interestingly, although the kinetics and stability of the BRET increase were found to be similar for receptors of class A and B (data not shown), we observed a tendency of class A receptors to induce smaller BRET increases. As previously noted when comparing the BRET-detected recruitment of β-arr to class A β2-AR and class B V2R (Fig 2B), this probably indicates that the BRET assays provide a steadystate signal reflecting continuous rounds of association–dissociation cycles. In any case, these results suggest that Luc–β-arr–YFP can be used as a general biosensor to monitor GPCR activity. When compared with the intermolecular BRET-based β-arr recruitment assays (Angers et al, 2000; Bertrand et al, 2002), double-brilliance β-arr avoids the difficulty of expressing the appropriate ratio of energy donor and acceptor constructs and allows the study of unmodified GPCRs.
Figure 5.
Double-brilliance β-arr monitors GPCR activation. HEK293 cells were transfected with Luc–β-arr–YFP and either pcDNA3.1 or plasmids encoding the indicated receptors. (A) Agonist-induced translocation of Luc–β-arr–YFP measured following treatment with 1 μM of the specific agonists (β2-AR, ISO; V1aR, AVP; δ-OR, SNC80; PAFR, PAF; CCR5, hRANTES; AT1aR, angiotensin II). (B) Agonist-induced conformational change of Luc–β-arr–YFP measured following 10 min stimulation with the specific agonists mentioned in (A). Data are the mean±s.e.m. of three independent experiments. *P<0.05 between treatment and each individual control condition.
In summary, we report for the first time, to our knowledge, the real-time monitoring of agonist-promoted conformational changes of β-arr in living cells using a double-brilliance β-arr intramolecular BRET-based biosensor. This conformational rearrangement of the β-arr molecule reflects its transition from an inactive state to a biologically active state that follows its initial recruitment to activated GPCRs and involves the relative movement of the C-tail of β-arr towards its N terminus (Fig 1). As most GPCRs recruit β-arr in an agonist-dependent fashion, double-brilliance β-arr could represent a general tool to probe receptor activity that could be used advantageously in largescale screening campaigns aimed at identifying GPCR ligands. In conclusion, double-brilliance β-arr represents the first intramolecular BRET-based biosensor that allows the monitoring of protein conformational changes. This should lead the way to the development of similar tools to study other proteins believed to undergo significant conformational rearrangement linked to their function.
Methods
Expression vectors. Plasmids encoding Flag–AT1aR, CCR5 (Pleskoff et al, 1997) and Myc–PAFR (Marrache et al, 2002) were generously provided by S. Meloche, N. Heveker and S. Chemtob, respectively (Université de Montréal, Québec, Canada), and WT β-arr2 was a generous gift from S. Marullo (Institut Cochin, Paris). Myc–V2R and HA–V1aR (Terrillon et al, 2003), Myc–β2-AR (Hebert et al, 1996), Myc–δ-OR (Petaja-Repo et al, 2002), V2R–GFP (Charest & Bouvier, 2003), β2-AR–GFP (Mercier et al, 2002), β-arr2–YFP (Angers et al, 2000) and Luc–β-arr2 (Perroy et al, 2003) have been described previously. Luc–β-arr–YFP was generated by subcloning the coding sequence of enhanced YFP in-frame at the C terminus of β-arr2 in pcDNA3.1–Luc–β-arr2, yielding Luc–β-arr–YFP with flexible spacers of 23 aa between Luc and β-arr, and 10 aa between β-arr and YFP. Mutation of arginine 169 into glutamate in Luc–β-arr(R169E)–YFP was generated by PCR site-directed mutagenesis using Luc–β-arr–YFP.
Cell culture. Human embryonic kidney 293 (HEK293) cells and simian kidney fibroblast (COS) cells were maintained as described previously (Charest & Bouvier, 2003). Cells were transfected with the indicated plasmids using the calcium phosphate precipitation method (Sambrook et al, 1989) or the FuGENE 6 transfection reagent (Roche Applied Science, Laval, Canada) according to the manufacturer's protocol. The experiments were performed 48 h after transfection.
Fluorescence microscopy. To detect Myc–β2-AR and Myc–V2R, cells were incubated with anti-Myc 9E10 monoclonal antibody (ascite fluid from our core facility) for 1 h at 4°C and then treated with the appropriate agonist (Sigma, Oakville, Canada) for 2 or 30 min at 37°C. Cells were then fixed and permeabilized before adding Texas-red-conjugated secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The samples were analysed by confocal laser-scanning microscopy using a Leica TCS SP1. Measurements were as follows: YFP (green), λex=488 nm, λem=540/25 nm; Texas red (red), λex=568 nm, λem=610/30 nm.
BRET assays. Assessment of β-arr recruitment in BRET was performed as described previously (Charest & Bouvier, 2003). Briefly, cells were distributed in 96-well microplates (Corning, Corning, USA) and incubated with or without agonist for the indicated time at 25°C. The appropriate Luc substrate was added to a final concentration of 5 μM, either simultaneously with the agonist (time course) or following agonist treatment (single measurement or dose dependency), and readings were collected using a Multilabel Reader Mithras LB 940 (Berthold Technologies, Bad Wildbad, Germany). To detect BRET between Luc and YFP, coelenterazine h (Molecular Probes, Burlington, Canada) was used as substrate and light emission was detected at 460–500 nm (Luc) and 510–550 nm (YFP), whereas for BRET2 detection (Luc and GFP), DeepBlueC™ coelenterazine (Perkin-Elmer, Wellesley, MA, USA) and filters at 330–470 nm (Luc) and 495–535 nm (GFP2) were used. The BRET signal was determined by calculating the ratio of the light emitted by the fluorescent acceptor and the light emitted by Luc. The values were corrected by subtracting the background BRET signals detected when Luc–β-arr was expressed alone. Expression levels of the different receptors transfected were verified by enzyme-linked immunosorbent assay (ELISA) (Charest & Bouvier, 2003).
Receptor endocytosis assay. Receptor endocytosis was measured by ELISA as described previously (Charest & Bouvier, 2003).
Acknowledgments
We thank Dr M. Lagacé for critical reading of the manuscript. This work was supported by grants from the Canadian Institute for Health Research and the Quebec Heart and Stroke Foundation (HSF). P.G.C. was supported by doctoral fellowships from the HSF of Canada and the Fonds de Recherche en Santé du Québec. M.B. holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology.
References
- Andrews DL, Demidov AA (1999) Resonance Energy Transfer. Chichester, UK: Wiley [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M (2000) Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc Natl Acad Sci USA 97: 3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Parent S, Caron M, Legault M, Joly E, Angers S, Bouvier M, Brown M, Houle B, Menard L (2002) The BRET2/arrestin assay in stable recombinant cells: a platform to screen for compounds that interact with G protein-coupled receptors (GPCRs). J Receptor Signal Transduction Res 22: 533–541 [DOI] [PubMed] [Google Scholar]
- Charest PG, Bouvier M (2003) Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances β-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem 278: 41541–41551 [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL (1993) Visual arrestin interaction with rhodopsin. Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem 268: 11628–11638 [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2003) The new face of active receptor bound arrestin attracts new partners. Structure (Camb) 11: 1037–1042 [DOI] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C (2001) Crystal structure of β-arrestin at 1.9 Å: possible mechanism of receptor binding and membrane translocation. Structure (Camb) 9: 869–880 [DOI] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M (1996) A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem 271: 16384–16392 [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Schubert C, Gurevich VV, Sigler PB (1999) The 2.8 Å crystal structure of visual arrestin: a model for arrestin's regulation. Cell 97: 257–269 [DOI] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV (1999) Targeted construction of phosphorylation-independent β-arrestin mutants with constitutive activity in cells. J Biol Chem 274: 6831–6834 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ (2004) β-Arrestins: traffic cops of cell signaling. Curr Opin Cell Biol 16: 162–168 [DOI] [PubMed] [Google Scholar]
- Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ (1999) Feedback regulation of β-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem 274: 15971–15974 [DOI] [PubMed] [Google Scholar]
- Lin FT, Chen W, Shenoy S, Cong M, Exum ST, Lefkowitz RJ (2002) Phosphorylation of β-arrestin2 regulates its function in internalization of β(2)-adrenergic receptors. Biochemistry 41: 10692–10699 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ (2002) The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115: 455–465 [DOI] [PubMed] [Google Scholar]
- Marrache AM et al. (2002) Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol 169: 6474–6481 [DOI] [PubMed] [Google Scholar]
- Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M (2002) Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem 277: 44925–44931 [DOI] [PubMed] [Google Scholar]
- Milligan G (2004) Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur J Pharm Sci 21: 397–405 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS (2000) Differential affinities of visual arrestin, β-arrestin1, and β-arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275: 17201–17210 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG (2001) Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor–β-arrestin complexes after receptor endocytosis. J Biol Chem 276: 19452–19460 [DOI] [PubMed] [Google Scholar]
- Perroy J, Adam L, Qanbar R, Chenier S, Bouvier M (2003) Phosphorylation-independent desensitization of GABA(B) receptor by GRK4. EMBO J 22: 3816–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M (2002) Ligands act as pharmacological chaperones and increase the efficiency of δ-opioid receptor maturation. EMBO J 21: 1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M (1997) Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science 276: 1874–1878 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, Bouvier M (2003) Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol 17: 677–691 [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Hirsch JA, Velez MG, Gurevich YV, Gurevich VV (2002) Transition of arrestin into the active receptor-binding state requires an extended interdomain hinge. J Biol Chem 277: 43961–43967 [DOI] [PubMed] [Google Scholar]
- Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ (2004) Activation dependent conformational changes in β-arrestin 2. J Biol Chem 279: 55744–55753 [DOI] [PubMed] [Google Scholar]
- Zhang J, Campbell RE, Ting AY, Tsien RY (2002) Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol 3: 906–918 [DOI] [PubMed] [Google Scholar]