Abstract
BAX and BAK operate at both the mitochondria and endoplasmic reticulum (ER) to regulate the intrinsic apoptotic pathway. An unresolved issue is whether any caspases can be activated in response to intrinsic apoptotic signals in the absence of BAX and BAK. Following organelle-specific intrinsic stress signals, including DNA damage and ER stress, we detected no activation of CARD-containing caspases (initiator CASP)-1, -2, -9, -11 and -12 in Bax−/−Bak−/− doubly deficient (DKO) cells. BCL-2 overexpression in these DKO cells provided no further protection to their already strong protection from DNA damage and ER stress. Moreover, there was no activation of effector CASP-3 and -7 in DKO cells, consistent with the lack of initiator caspase activity and disfavouring a BAX, BAK-independent intrinsic apoptotic pathway to activate initiator caspases. Thus, BAX and BAK confer an essential gateway for the activation of caspases in the intrinsic apoptotic pathway.
Keywords: BCL-2, BAK, apoptosis, BAX, mitochondrial pathway
Introduction
The BCL-2 family proteins constitute critical control points in the intrinsic apoptotic pathway, and are subdivided into three groups: (1) ‘multidomain' antiapoptotic proteins (e.g. BCL-2), which share homology in four conserved regions termed BCL-2 homology (BH1–4) domains, (2) ‘multidomain' proapoptotic proteins (BAX or BAK) and (3) ‘BH3-only' proteins (e.g. BID or BIM; Danial & Korsmeyer, 2004). In healthy cells, inactive BAX is located in the cytosol or is loosely attached to membranes, but in response to stress signals, BAX is inserted into the mitochondria as a homo-oligomerized multimer, resulting in downstream mitochondrial dysfunction (Danial & Korsmeyer, 2004). Before death signals, an interaction of resident voltage-dependent anion channel protein 2 (VDAC2) with the inactive conformer of BAK keeps this potentially lethal molecule in check at the mitochondrion, and after stress signals, VDAC2 is displaced and BAK undergoes homo-oligomerization, with subsequent cytochrome c (Cyt c) release (Danial & Korsmeyer, 2004).
The intrinsic apoptotic pathway makes use of the release of Cyt c from the mitochondria to activate caspases (Wang, 2001). In vitro studies have shown that Cyt c together with apoptotic protease-activating factor-1 (APAF-1) generates an apoptosome structure that activates the initiator caspase (CASP)-9, which in turn activates downstream effectors CASP-3 and -7 (Wang, 2001). Organelle sites including endoplasmic reticulum (ER), nucleus and mitochondria participate in apoptosis (Ferri & Kroemer, 2001), and most organellespecific death responses ultimately trigger caspase activation. CASP-12 is activated specifically in response to ER stress (Nakagawa et al, 2000), whereas CASP-2 is activated in response to stress induced by DNA-damaging agents (Lassus et al, 2002). However, it remains uncertain whether distinct organellespecific caspase activation pathways specifically mediate activation of initiator caspases including CASP-2 or CASP-12. Alternatively, CASP-2 or CASP-12 activation might represent amplification loops residing downstream of common sensors and/or effectors of cellular damage. For example, cells doubly deficient for BAX and BAK are resistant to both DNA-damage-induced and ER-stress-induced apoptosis (Danial & Korsmeyer, 2004).
A crucial issue remaining to be resolved is whether caspases can function upstream as well as downstream of BAX and BAK in the intrinsic apoptotic pathway. This could be addressed by examining whether caspases are activated in the absence of BAX and BAK after organelle-specific death signals. To address these issues, we examined loss-of-function models to assess the relationship between BAX and BAK and caspase activation. Murine embryonic fibroblasts (MEFs) deficient for APAF-1 and CASP-9, and doubly deficient for BAX and BAK (DKO) showed significant protection from apoptosis during the critical 24 h period after exposure to genotoxic damage. We noted that activation of CASP-1, -2, -3, -7, -9, -11 and -12 was not detected in the absence of BAX and BAK, indicating that this critical gateway operates upstream of all these caspases. Moreover, antiapoptotic BCL-2 provided no further protection to DKO cells, suggesting that BCL-2 does not sequester substantial adaptor molecules that would activate apoptosis in the absence of BAX and BAK. We conclude that the BAX and BAK gateway is essential for the activation of multiple initiator as well as effector caspases.
Results and Discussion
BAX and BAK are required for CASP-2 activation
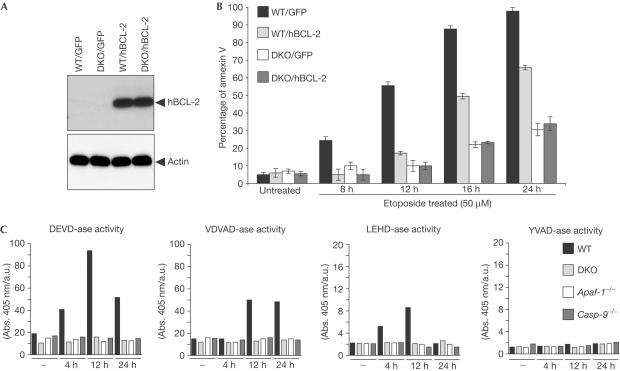
BAX and BAK seem to constitute an essential apoptotic gateway after DNA damage and can operate at the mitochondria and the ER (Danial & Korsmeyer, 2004). We investigated whether BCL-2 could provide a further level of protection from DNA-damage-induced apoptosis in the absence of BAX and BAK by expressing BCL-2 in DKO cells. We cloned (human) hBCL-2 complementary DNA in the retroviral vector pLZR-IRES/GFP to generate the construct (pLZR-hBCL-2/IRES/GFP). DKO MEFs immortalized by simian virus 40 (SV40) were transduced by retroviral infection using either pLZR-IRES/GFP or pLZR-hBCL-2/IRES/GFP to generate DKO/GFP+ and DKO/hBCL-2 cells. As a control, we transduced wild-type (WT) SV40-MEFs to produce WT/GFP+ and WT/hBCL-2 cells. GFP+ cells were monitored and sorted (see Methods), and western blots confirmed the expression of hBCL-2 in WT/hBCL-2 and DKO/hBCL-2 cells (Fig 1A). Annexin V staining over a time course after etoposide treatment confirmed the resistance of DKO/GFP+ cells, compared with WT/GFP+ cells (Fig 1B; Wei et al, 2001). Although BCL-2 overexpression delayed apoptosis in WT cells (Fig 1B), it did not confer further protection to DKO cells (Fig 1B).
Figure 1.
BCL-2 overexpression does not confer protection to DKO cells after etoposide-induced apoptosis. (A) WT and DKO SV40-MEFs were transduced using pLZR-IRES/GFP and pLZR-hBCL-2/IRES/GFP. GFP+ cells were analysed for hBCL-2 expression. (B) WT/GFP+, WT/hBCL-2, DKO/GFP+ and DKO/hBCL-2 cells were cultured either in medium alone or with etoposide (50 μM). Staining for annexin V was performed according to the manufacturer's protocol. Apoptosis was quantified by FACS analysis, followed by analysis using FlowJo software (mean±s.e.; n=4). (C) To assay caspase activity, WT, DKO, Casp-9−/− and Apaf-1−/− SV40-MEFs were either untreated or treated with etoposide (5 μM), and then cells were collected 4, 12 and 24 h later and lysates prepared as described in the Methods. Data are from a minimum of four experiments.
CASP-2 can trigger BAX translocation to mitochondria after etoposide treatment, suggesting that CASP-2 would function as an apical caspase upstream of the BAX and BAK gateway (Lassus et al, 2002). To explore whether caspases are activated in the absence of BAX and BAK, we analysed caspase activity after DNA damage in DKO SV40-MEFs and compared them with Casp-9−/−, Apaf-1−/− and WT SV40-MEFs. Typically, effectors CASP-3 and -7 can cleave the sequence DEVD (Garcia-Calvo et al, 1999), whereas initiator caspases prefer other sequences, such as LEHD (CASP-9), VDVAD (CASP-2) and YVAD (CASP-1 and -11; Garcia-Calvo et al, 1999). We prepared lysates from the cells at serial time points and assessed caspase activity. Etoposide treatment induced DEVD-ase activity in WT but not in Casp-9−/−, Apaf-1−/− or DKO cells (Fig 1C). Similar data were obtained when examining activities for the VDVAD and LEHD substrates (Fig 1C). In contrast, analysis of YVAD-ase activity indicated no evidence for CASP-1 and -11 activation after etoposide treatment (Fig 1C). We did not detect expression of CASP-11 in SV40-MEFs (Fig 2A), eliminating the involvement of CASP-11 as an apical caspase in these cells. As a positive control, we stimulated WT and DKO cells with lipopolysaccharide, which upregulates CASP-11 protein levels (Kobori et al, 2004). Furthermore, we analysed CASP-1 in a time-course analysis after etoposide treatment (Fig 2B), and detected no CASP-1 cleavage, thus confirming the results obtained from the YVAD-ase activity assay.
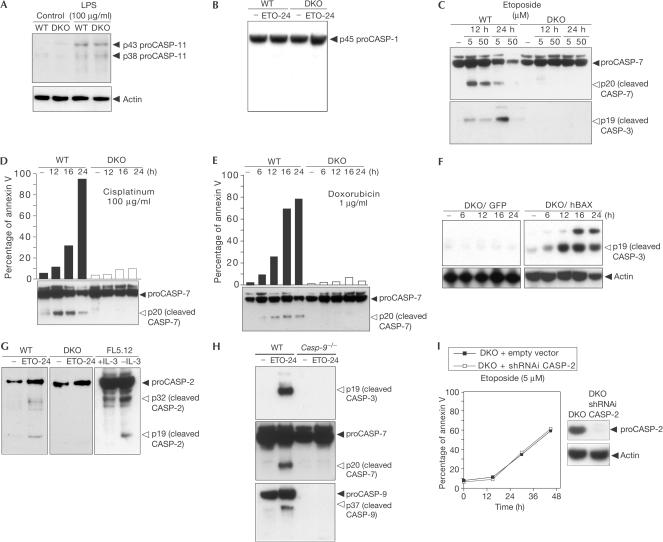
Figure 2.
CASP-2 activation is downstream of multidomain BAX and BAK after DNA damage. (A) WT and DKO SV40-MEFs were either untreated or treated with lipopolysaccharide (LPS) for 12 h (100 μg/ml), and then cells were collected and analysed for CASP-11 expression using an anti-CASP-11 antibody. (B) WT and DKO MEFs were cultured either in medium alone or with etoposide (ETO, 5 μM, 24 h). Cells were collected and analysed for CASP-1 expression using an anti-CASP-1 antibody. (C) WT and DKO SV40-MEFs were untreated or treated with etoposide (5–50 μg/ml) for the indicated times, and then analysed for CASP-3 and -7 cleavage. (D,E) WT and DKO SV40-MEFs were untreated or treated with cisplatinum (100 μg/ml) or doxorubicin (1 μg/ml) for the indicated times, and then analysed for annexin V staining and CASP-7 cleavage. (F) DKO/GFP and DKO/BAX cells were untreated or treated with etoposide (5 μg/ml), and then analysed for CASP-3 cleavage. (G) WT and DKO SV40-MEFs were untreated or treated with etoposide (5 μg/ml, 24 h), and then analysed for CASP-2 cleavage. FL5.12 cells were IL-3starved for 24 h and analysed for CASP-2 cleavage. (H) WT and Casp-9−/− SV40-MEFs were either untreated or treated with etoposide (5 μM, 24 h), and then analysed for CASP-3, -7 and -9 cleavage. (I) DKO SV40-MEFs were retrovirally infected with empty pSUPERretro or pSUPER/CASP-2 vectors to generate DKO/empty vector and DKO/shRNAi CASP-2 cells. Cells were treated with etoposide and analysed for annexin V and CASP-2 expression.
We then analysed CASP-3 and -7 cleavage by immunoblots of WT and DKO cells treated with etoposide, resulting in CASP-3 and -7 activation in WT but not in DKO cells (Fig 2C). These data confirm, in this system, that effector caspases are not activated in the absence of BAX and BAK. To validate that effector caspases are indeed activated downstream of BAX and BAK after DNA damage, we evaluated CASP-7 cleavage in response to cisplatinum and doxorubicin treatments (Fig 2D,E), and the results concurred with those obtained from etoposide treatment.
To exclude clonal selection of a highly resistant DKO cell line during the SV40 immortalization process, we overexpressed BAX in DKO cells to rescue the phenotype observed in DKO cells. To this end, we cloned BAX cDNA into pLZR-IRES/GFP to generate the construct pLZR-hBAX-IRES/GFP. DKO cells were transduced using either pLZR-IRES/GFP or pLZR-hBAX-IRES/GFP to generate DKO/GFP and DKO/BAX cells. Analysis of CASP-3 cleavage over a time-course analysis after etoposide treatment confirmed the lack of CASP-3 activation in DKO/GFP cells (Fig 2F). In contrast, overexpression of BAX in DKO cells restored caspase activation (Fig 2F).
Next, we assessed the processing of CASP-2 after etoposide treatment. CASP-2 was cleaved into p32 and p19 fragments in WT but not in DKO cells (Fig 2G). As a positive control, we examined CASP-2 cleavage in FL5.12 cells after IL-3 deprivation, noting cleaved p32 and p19 fragments. Thus, our results argue that the activation of CASP-2 is downstream of BAX and BAK. Moreover, the activation of effector CASP-3 and -7 did not occur in Casp-9−/− SV40-MEFs after etoposide treatment (Fig 2H). This supports the activation of effector caspases requiring the initiator CASP-9.
To eliminate any involvement of CASP-2 activation bypassing the BAX and BAK gateway, we designed several small hairpin RNA interference (shRNAi) CASP-2 sequences to knock down CASP-2 in DKO cells (Fig 2I). Apoptosis analysis after etoposide treatment in DKO/shRNAi CASP-2 cells showed no further protection to the already strong resistance of DKO cells (Fig 2I). Thus, the sum of our results led us to argue that CASP-2 does not bypass the BAX and BAK gateway, and it is not responsible for the residual cell death induced after etoposide treatment in DKO cells.
BAX and BAK control CASP-12 activation
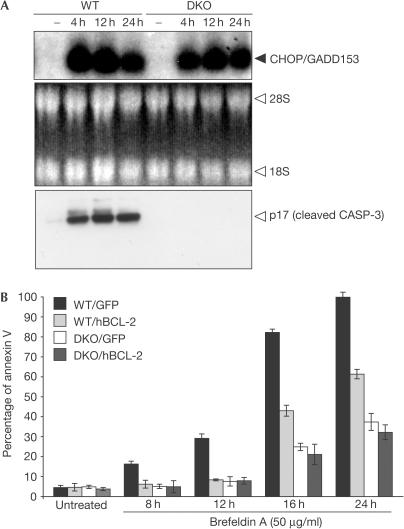
Eukaryotic cells respond to the accumulation of unfolded proteins in the ER by signalling an adaptive response termed the unfolded protein response (UPR; Reimold et al, 2000). Chronic UPR stimulation after treatment with brefeldin A (an ER–Golgi transport inhibitor) triggers apoptosis. Following UPR, the transcription factor CHOP/GADD153 is upregulated and considered a hallmark of ER stress (Harding et al, 2000). Notably, northern blot analysis showed CHOP/GADD153 upregulation in both WT and DKO cells after ER stress triggered by brefeldin A treatment (Fig 3A), indicating that an appropriate response to UPR is still initiated in the absence of BAX and BAK.
Figure 3.
CHOP/GADD153 upregulation in both WT and DKO cells after ER stress. (A) WT and DKO SV40-MEFs were treated with brefeldin A (50 μg/ml) at different time points. Cells were collected and total RNA was extracted from both cell types, and then northern blots were probed with murine C/EBP-homologous protein/growth arrest and DNA-damage inducible protein (CHOP/GADD153) cDNA. CASP-3 cleavage was analysed at the same time points. (B) WT/GFP+, WT/hBCL-2, DKO/GFP+ and DKO/hBCL-2 cells were cultured and then treated with brefeldin A (50 μg/ml). Apoptosis was quantified by annexin V staining (mean±s.e.; n=5).
To assess the effects of BCL-2 overexpression after UPR, we treated WT/GFP+, WT/hBCL-2, DKO/GFP+ and DKO/hBCL-2 cells with brefeldin A. DKO cells were protected from UPR-induced apoptosis in comparison with WT cells (Fig 3B). Expression of BCL-2 protected WT cells, but conveyed no further benefit to DKO cells after UPR (Fig 3B). This argues that the antiapoptotic function of BCL-2 occurs at the same step or upstream of the BAX and BAK gateway after ER stress.
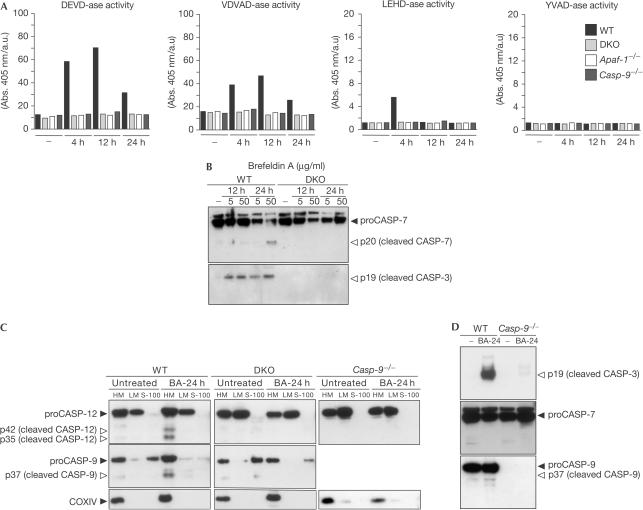
To assess the requirement of caspases during ER-stress-induced apoptosis, we first compared caspase activity in DKO, Casp-9−/−, Apaf-1−/− and WT SV40-MEFs after brefeldin A treatment. DEVD-ase, VDVAD-ase and LEHD-ase activities were detected in WT but not in Casp-9−/−, Apaf-1−/− or DKO cells (Fig 4A). Further analysis of effector CASP-3 and -7 showed processing of CASP-3 and -7 in WT but not in DKO cells after brefeldin A treatment (Fig 4B). Thus, UPR-induced apoptosis makes use of the mitochondrial pathway including BAX, BAK and CASP-9 with no evidence of initiator caspases bypassing the BAX and BAK gateway to trigger effector caspases.
Figure 4.
CASP-12 activation is downstream of the mitochondrial pathway after ER stress. (A) WT, DKO, Casp-9−/− and Apaf-1−/− SV40-MEFs were either untreated or treated with brefeldin A (5 μg/ml), and then cells were collected at 4, 12 and 24 h and lysates prepared as described in the Methods. Data are from a minimum of four experiments. (B) WT and DKO SV40-MEFs were untreated or treated with brefeldin A (5–50 μg/ml) for the indicated times, and then analysed for CASP-3 and -7 cleavage. (C) WT, Casp-9−/− and DKO SV40-MEFs were either cultured with medium alone or with brefeldin A (5 μg/ml), and subcellular fractionation was performed as described in the Methods. (D) WT and Casp-9−/− SV40-MEFs were either cultured with medium alone or with brefeldin A (5 μg/ml), and then analysed for CASP-3, -7 and -9 cleavage.
To investigate whether the activation of CASP-12 makes use of BAX and BAK, we assessed the processing of CASP-12 in WT versus DKO cells after brefeldin A treatment. We performed subcellular fractionation to obtain cytosolic fractions (S-100), mitochondrion-enriched heavy membrane fractions (HM) and ER-enriched light membrane fractions (LM) from either untreated or brefeldin-A-treated cells. This ER-stress-induced stimulus resulted in the cleavage of CASP-12 into p42 and p35 fragments in WT cells but not DKO cells (Fig 4C). Taken together, these results place CASP-12 activation downstream of BAX and BAK, rather than CASP-12 operating as an apical caspase upstream of the BAX and BAK gateway. Analysis of CASP-12 cleavage was also impaired in Casp-9−/− SV40-MEFs after brefeldin A treatment (Fig 4C), indicating that CASP-12 activation is downstream of initiator CASP-9. Our results are in agreement with those recently published (Zong et al, 2003).
We also evaluated the involvement of initiator CASP-9 after brefeldin-A-induced ER stress. The cleaved p37 fragment of CASP-9 was detected in WT but not in DKO cells (Fig 4C). Notably, there was no CASP-3 cleavage in CASP-9-deficient cells, placing CASP-3 activation downstream of CASP-9 for ERstress-induced apoptosis (Fig 4D). No detectable CASP-7 cleavage was noted after brefeldin-A-induced apoptosis (Fig 4D).
Marsden et al (2002) detected marginal CASP-7 cleavage in Casp-9−/− and Apaf-1−/− thymocytes on γ-irradiation, and speculated that CASP-7 activation was due to a putative alternative intrinsic apoptotic pathway consisting of initiators CASP-1, -2, -11 or -12 that bypasses the apoptosome. It has been shown that certain cell types undergo activation of both intrinsic and extrinsic apoptotic pathways after DNA damage (Scoltock & Cidlowski, 2004), suggesting that the residual CASP-7 processing detected in Casp-9−/− and Apaf-1−/− thymocytes might be attributed to the activation of extrinsic apoptotic signals. Specifically, γ-irradiation triggers apoptosis in thymocytes dependent on p53 transcription factors (Lowe et al, 1993). Many p53 targets have been found so far, including components of the intrinsic pathway such as NOXA or PUMA (Danial & Korsmeyer, 2004), as well as components of the extrinsic apoptotic pathway such as FAS-L/FAS (Kobayashi et al, 1998), TRAIL/DR5 (Wu et al, 1997) or CASP-10 (Rikhof et al, 2003). We have used WT, DKO, Casp-9−/− and Apaf-1−/− MEFs immortalized by SV40, which inhibits the transcriptional activity of p53 and therefore blocks all the possible downstream targets (Farmer et al, 1992). Our analysis of caspase activity after DNA damage or ER stress treatments demonstrates no discernible caspase activity in DKO, Casp-9−/− and Apaf-1−/− SV40-MEFs, disfavouring the existence of a putative apoptosome-independent intrinsic cell death pathway to activate initiators CASP-1, -2, -11 and -12.
It has been shown that ectopic expression of human BCL-2 rescues some of the developmental cell deaths in Caenorhabditis elegans, suggesting that ‘multidomain' BCL-2 performs at least some of the same antiapoptotic functions as its worm homologue CED-9 (Vaux et al, 1992). In this model, CED-9 interacts with CED-4 (the APAF-1 homologue) and prevents activation of the worm caspase CED-3 (Hengartner, 1997). However, in mammalian cells, APAF-1 does not interact with BCL-2 (Moriishi et al, 1999). Even if another CED-4-like molecule were to exist and be sequestered, the data here indicate that BAX and BAK would still be required.
Methods
Cell culture. MEFs were derived from day 13.5 embryos obtained from C57BL/6 WT, Apaf-1−/− (Yoshida et al, 1998), Casp-9−/− (Kuida et al, 1998) and doubly deficient Bax−/−, Bak−/− (Wei et al, 2001) mice.
Immunoblot analysis. Western blot analysis was performed as described previously (Ruiz-Vela et al, 2001). Antibodies used for immunoblot analysis included the following: anti-human BCL-2 (clone 6C8, Pharmingen, San Diego, CA, USA), anti-actin (clone AC-15, Sigma-Aldrich, St Louis, MO, USA), anti-CASP-2 (clone 10C6, Chemicon, Temecula, CA, USA), anti-CASP-3 (Cell Signaling, Beverly, MA, USA), anti-CASP-7 (Cell Signaling), anti-CASP-9 (Cell Signaling), anti-CASP-1 (clone C4851, Sigma-Aldrich), anti-CASP-12 (clone 14F7, Sigma-Aldrich), anti-CASP-11 (clone 17D9, Sigma-Aldrich) and anti-COXIV (Molecular Probes, Eugene, OR, USA). As secondary antibodies, we used horseradish peroxidase-linked anti-rabbit (DAKO), anti-rat (Jackson ImmunoResearch, West Grove, PA, USA), anti-hamster (Jackson ImmunoResearch) or anti-mouse (DAKO, Glostrup, Denmark).
Subcellular fractionation. Cells were collected, washed in PBS and then resuspended in isotonic buffer A (250 mM sucrose, 20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulphonyl fluoride, 2 mg/ml aprotinin, 2 mg/ml leupeptin and 1 mg/ml pepstatin) on ice for 20 min. After incubation, cells were disrupted by Dounce homogenization, followed by 30 strokes through a 30-gauge needle. Cell lysates were centrifuged at 800g for 10 min at 4°C, and the supernatant obtained was further centrifuged at 8,000g for 20 min at 4°C. The resulting pellet HM was collected, and the supernatant was further centrifuged at 100,000g for 1 h at 4°C. The resulting pellet constituted LM, whereas the supernatant was saved as S-100. Subsequently, HM and LM fractions were re-lysed in RIPA buffer for western blot analysis.
Cell viability assay. Apoptosis was evaluated by annexin-V-Cy3 (BioVision, Mountain View, CA, USA) staining according to the manufacturer's protocols. Apoptosis was quantified by FACS analysis, followed by analysis using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Caspase assays. Cells were collected, washed in PBS and then lysed for 30 min at 4°C in buffer B (100 mM HEPES, pH 7.2, 10% sucrose, 0.1% CHAPS and 10 mM dithiothreitol), followed by 15 strokes through a 30-gauge needle. After centrifugation at 16,000g for 30 min, the resulting supernatants were adjusted to 1 μg/μl with buffer B. Caspase activity was measured using 50 μg protein. Samples were incubated at 37°C for 30 min with 80 μM of different caspase substrates (DEVD-pNA, LEHD-pNA, VDVAD-pNA and YVAD-pNA) in 100 μl of buffer B (final volume; Garcia-Calvo et al, 1999). Substrate hydrolysis was measured at 405 nm using an ELISA microplate reader (Bio-Rad, Hercules, CA, USA).
Retroviral-mediated gene transfer. Retroviral gene transfer was performed as described previously (Ruiz-Vela et al, 2001), and GFP+ cells were monitored and sorted by flow cytometric analysis using a FACScalibur (Becton Dickinson).
Small hairpin RNA interference. shRNA was produced using pSUPER-retro vector (Oligoengine). Oligonucleotides for murine Casp-2 messenger RNA included TGTGGAACTCCTCAACCTG, GCAGCTCCGCCTATCCACA and TGTTCTTCATC CAAGCATG.
Acknowledgments
We thank all members of the lab for helpful discussions and Dr D. Ron for the gift of murine CHOP cDNA. A.R.-V. is supported by a postdoctoral fellowship from the European Molecular Biology Organization (EMBO), and J.T.O. is a Damon Runyon Cancer Research Foundation fellow.
References
- Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C (1992) Wild-type p53 activates transcription in vitro. Nature 358: 83–86 [DOI] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G (2001) Organellespecific initiation of cell death pathways. Nat Cell Biol 3: E255–E263 [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Rasper DM, Vaillancourt JP, Zamboni R, Nicholson DW, Thornberry NA (1999) Purification and catalytic properties of human caspase family members. Cell Death Differ 6: 362–369 [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Hengartner MO (1997) Apoptosis. CED-4 is a stranger no more. Nature 388: 714–715 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ruan S, Jabbur JR, Consoli U, Clodi K, Shiku H, Owenschaub LB, Andreeff M, Reed JC, Zhang W (1998) Differential p53 phosphorylation and activation of apoptosis-promoting genes Bax and Fas/APO-1 by irradiation and ara-C treatment. Cell Death Differ 5: 584–591 [DOI] [PubMed] [Google Scholar]
- Kobori M et al. (2004) Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ 11: 123–130 [DOI] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94: 325–337 [DOI] [PubMed] [Google Scholar]
- Lassus P, Opitz-Araya X, Lazebnik Y (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297: 1352–1354 [DOI] [PubMed] [Google Scholar]
- Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849 [DOI] [PubMed] [Google Scholar]
- Marsde VS et al. (2002) Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419: 634–637 [DOI] [PubMed] [Google Scholar]
- Moriishi K, Huang DC, Cory S, Adams JM (1999) Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc Natl Acad Sci USA 96: 9683–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulumspecific apoptosis and cytotoxicity by amyloid-β. Nature 403: 98–103 [DOI] [PubMed] [Google Scholar]
- Reimold AM et al. (2000) An essential role in liver development for transcription factor XBP-1. Genes Dev 14: 152–157 [PMC free article] [PubMed] [Google Scholar]
- Rikhof B, Corn PG, El-Deiry WS (2003) Caspase 10 levels are increased following DNA damage in a p53-dependent manner. Cancer Biol Ther 2: 707–712 [PubMed] [Google Scholar]
- Ruiz-Vela A, Serrano F, Gonzalez MA, Abad JL, Bernad A, Maki M, Martinez AC (2001) Transplanted long-term cultured pre-BI cells expressing calpastatin are resistant to B cell receptor-induced apoptosis. J Exp Med 194: 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoltock AB, Cidlowski JA (2004) Activation of intrinsic and extrinsic pathways in apoptotic signaling during UV-C-induced death of Jurkat cells: the role of caspase inhibition. Exp Cell Res 297: 212–223 [DOI] [PubMed] [Google Scholar]
- Vaux DL, Weissman IL, Kim SK (1992) Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science 258: 1955–1957 [DOI] [PubMed] [Google Scholar]
- Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15: 2922–2933 [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GS et al. (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 17: 141–143 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW (1998) Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94: 739–750 [DOI] [PubMed] [Google Scholar]
- Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162: 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]