Abstract
Ligands for nuclear receptors facilitate the exchange of co-repressors for coactivators, leading to chromatin modifications that favour the activation of gene transcription. Here, we show that the repressed state of an endogenous retinoic acid-regulated gene is quickly re-established after ligand removal. As expected, repression is characterized by recruitment of N-CoR/SMRT–HDAC3 (histone deacetylase 3) co-repressor complexes, leading to local histone hypoacetylation. The achievement of the repressed state involves the ordered deacetylation of lysines in H4 tails. This order is determined by the inherent substrate specificity of HDAC3, and unexpectedly predicts the binding preference of N-CoR/SMRT for submaximally acetylated H4 tails. The match between the specificity of acetyl-histone deacetylation by HDAC3 and the histone-binding preference of N-CoR/SMRT allows the co-repressor complex to stabilize and propagate repression of nuclear hormone receptor gene targets.
Introduction
Nuclear receptors (NRs) comprise a large family of transcription factors that have crucial roles in all aspects of growth, development and metabolism (Mangelsdorf et al, 1995). Transcriptional activities of NRs are regulated by small lipophilic ligands, including hormones, vitamin derivatives, intermediary metabolites and xenobiotics (Chawla et al, 2001). In the absence of ligands, many NRs repress transcription of target genes to which they are bound (Hu & Lazar, 2000). The repression function of NRs has been implicated in several important and medically relevant aspects of biology, including (i) the severity of thyroid-hormone resistance due to thyroid-hormone receptor (TR) gene mutations (Tagami & Jameson, 1997), (ii) the efficacy and side effects of selective oestrogen-receptor modulators as therapy for breast cancer (Shang & Brown, 2002), and (iii) the pathophysiology of acute promyelocytic leukaemia (APL), which is due invariably to chromosomal translocations involving retinoic-acid receptors (RARs; Melnick & Licht, 1999). The unliganded RAR is a particularly potent transcriptional repressor, and relief of repression by all-trans retinoic acid (ATRA) is the cornerstone of therapy for APL (Minucci & Pelicci, 1999).
Repression is mediated by recruitment of co-repressors N-CoR (Horner et al, 1995) or SMRT (Chen & Evans, 1995; referred to as N-CoR/SMRT when discussing common features) to unliganded NRs bound to target genes. The carboxyl terminus of N-CoR/SMRT contains CoRNR motifs that specifically recognize and bind to unliganded NRs (Hu & Lazar, 1999; Nagy et al, 1999; Perissi et al, 1999). The amino terminus contains several protein–protein interaction domains that mediate the formation of a core complex containing WD-40 proteins TBL1/TBLR-1, GPS2 and histone deacetylase 3 (HDAC3; Guenther et al, 2000; Li et al, 2000; Zhang et al, 2002). HDACs have a well-characterized role in transcriptional repression—the deacetylation of lysine residues in the N-terminal tails of histone proteins (Grozinger & Schreiber, 2002). This has at least two repressive functions: the increased positive charge of lysine favours a closed nucleosomal structure (Kouzarides, 2000), and the loss of acetylation reduces the affinity of coactivators containing bromodomains (Zeng & Zhou, 2002).
In addition to serving as platform proteins delivering the co-repressor complex to the genes bound by unliganded NR, N-CoR/SMRT actively contribute to the repression process. In particular, the enzymatic activity of HDAC3 requires interaction with a region of N-CoR/SMRT referred to as the deacetylase-activating domain (DAD; Guenther et al, 2001; Zhang et al, 2002). The DAD contains a SANT motif, referred to as SANT1, in addition to an N-terminal extension, which is also crucial for DAD activity. N-CoR/SMRT also contain a second SANT motif, referred to as SANT2, which is located approximately 120 amino acids downstream, and does not have DAD function but is an important component of a histone-interaction domain (HID; Yu et al, 2003). Histone binding is a property shared by several other SANT-containing proteins, including Ada2 (Boyer et al, 2002) and ISWI (Grune et al, 2003). The histone-binding domain of N-CoR/SMRT enhances the enzymatic activity of HDAC3 (Yu et al, 2003).
Although most studies of NR function have emphasized the switch from repression to activation, co-repressors may be equally or more important in reversing the activated state and creating a repressed chromatin environment. To address this, we studied the switch from activation to repression of an endogenous ATRA-regulated gene. We determined that ATRA induction of the RARγ2 gene is rapidly reversible after ligand removal. N-CoR/SMRT and HDAC3 were recruited to the gene in minutes after ligand removal, and this was associated with a reduction in histone acetylation. We showed that the pattern of histone H4 deacetylation was non-random, with H4 lysine 5 (H4 K5) being most affected. This specificity for H4 K5 was recapitulated in studies of the substrate specificity of the HDAC3/SMRT enzyme in vitro, and knockdown of HDAC3 markedly affected K5 acetylation in living cells. Finally, histone binding by N-CoR/SMRT was destabilized by H4 K5 acetylation. Together, the data show that the substrate specificity of the HDAC3/co-repressor complex serves to stabilize co-repressor binding and propagate the repressed state.
Results
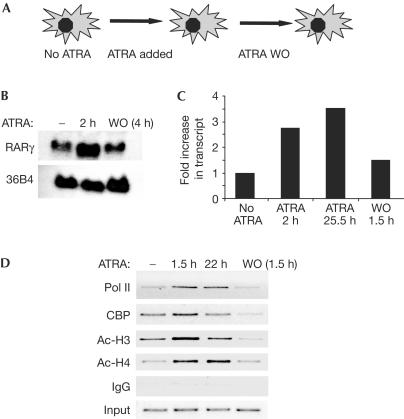
We investigated the reversal of ATRA-dependent gene activation and the re-establishment of the repressed state (Fig 1A). In agreement with earlier reports (Kamei et al, 1993), the RARγ2 gene was markedly upregulated in response to ATRA treatment of 3T3-L1 fibroblasts (Fig 1B). Intron-containing transcripts were upregulated by ATRA, which was consistent with transcriptional activation of the gene (Fig 1C). Notably, the level of the ATRA-responsive RARγ2 messenger RNA returned to baseline in 4 h after ATRA withdrawal (Fig 1B), and the level of new transcripts was near baseline 1.5 h after ATRA removal from the cell medium (Fig 1C). Chromatin immunoprecipitation (ChIP) analysis of the RARγ2 promoter showed increases in local histone acetylation as well as maximal recruitment of the coactivator/histone acetyltransferase CREB-Binding Protein (CBP) and RNA polymerase II (Pol II) in 1.5 h after ATRA treatment (Fig 1D), which was consistent with the rapid increase in transcription. Moreover, in 1.5 h after ATRA removal, these changes had reverted to near-baseline levels (Fig 1D).
Figure 1.
Rapid reversal of gene activation after ATRA withdrawal. (A) Schematic of ATRA addition and washout (WO) protocol. (B) ATRA induction of RARγ gene expression in 3T3-L1 fibroblasts is rapidly reversed after WO. Northern analysis of RARγ gene expression at indicated times is shown. 36B4 is shown as a loading control. (C) ATRA induction of RARγ primary transcripts is rapidly reversed after WO. Analysis of heterogenous nuclear RNA using intronic primers for quantitative PCR is shown. (D) ATRA-induced histone hyperacetylation and recruitment of CBP and Pol II to the RARγ2 gene promoter are rapidly reversed after ATRA WO.
The rapid reversal of histone acetylation suggested that the removal of ATRA actively promoted histone deacetylation. The mechanism of this effect was further investigated by studying histone modification as well as N-CoR/SMRT–HDAC3 recruitment at early time points after ATRA removal. As expected, ATRA led to a reduced association of N-CoR/SMRT and HDAC3 with the target gene (Fig 2A). N-CoR/SMRT and HDAC3 then returned to the promoter at the shortest time point that was feasibly tested, that is, 1.5 min after ATRA removal (Fig 2A). The return of N-CoR/SMRT–HDAC3 corresponded to a rapid reversal of histone acetylation (Fig 2B) as well as the dismissal of RNA Pol II (Fig 2C) from the promoter. The histone deacetylation was examined in more detail by performing ChIP analysis with antibodies specific for each of the four acetylated lysines in the H4 tail. Surprisingly, the rate and extent of histone deacetylation were not uniform. Rather, H4 K5 was deacetylated first, followed by K8, K12 and K16 (Fig 2D). By contrast, individual lysine residues in the H3 tail were deacetylated at similar rates at these early time points (data not shown). Similar patterns of H4 tail deacetylation after ligand removal were observed for CRBPI (Fig 2E), another direct target gene of RA (Smith et al, 1991). Hence, we focused on understanding the mechanism of differential deacetylation of H4 tail lysines.
Figure 2.
Co-repressor recruitment and non-random histone deacetylation occur rapidly after ATRA removal. (A) ChIP for N-CoR/SMRT and HDAC3 at the RARγ2 gene before, during and after withdrawal of ATRA. Although N-CoR/SMRT recruitment seems reduced 5 min after ligand removal in this experiment, this was not consistently observed. (B) Acetylation of histone H4 decreases rapidly after ATRA washout (WO). (C) Association of Pol II decreases rapidly after ATRA WO. (D) Time course of specific H4 lysine deacetylation after ATRA WO. *P<0.05 versus ATRA, **P<0.01 versus ATRA; ‡range of duplicate samples. (E) Same as (D), for CRBPI gene.
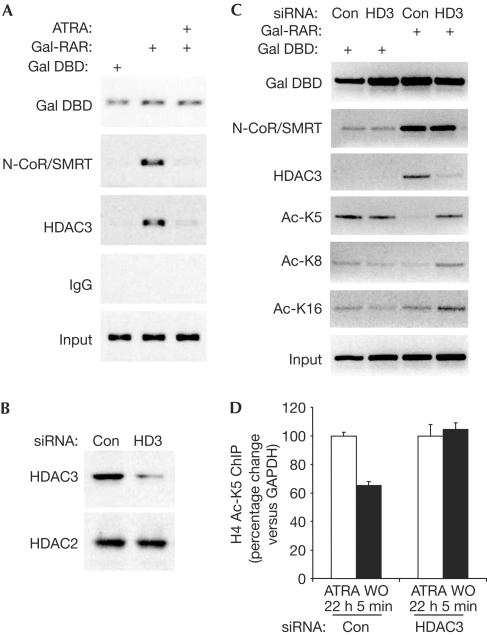
We next used small interfering RNA (siRNA) to determine whether HDAC3 was responsible for differential deacetylation of H4 tail lysines in living cells. These experiments were performed in 293T cells, in which we previously determined that Gal4-TR represses transcription of a (UASx5)-SV40 luciferase reporter gene by a mechanism that involves the N-CoR/SMRT–HDAC3 complex; this mechanism pertains when the reporter is transiently transfected or stably integrated into the genome (Ishizuka & Lazar, 2003). Gal-RAR also is a potent repressor in this system (data not shown), recruiting HDAC3 and N-CoR/SMRT to the promoter region of the reporter gene in a manner that is reversible by ATRA (Fig 3A). HDAC3 knockdown was highly successful in 293T cells (Fig 3B), and thus led to a marked reduction in HDAC3 recruitment by RAR, although recruitment of N-CoR/SMRT remained intact (Fig 3C). Using control siRNA, Gal-RAR led to histone deacetylation that was marked for H4 K5, and was also observed for K8, with little deacetylation of K12 (not shown) and K16 observed under these conditions (Fig 3C). This acetylation pattern was completely reversed when HDAC3 levels were depleted (Fig 3C). Moreover, on the endogenous RARγ2 gene in NIH 3T3 fibroblasts, HDAC3 knockdown prevented the reduction in H4 K5 acetylation normally observed 5 min after washout of ATRA (Fig 3D). Together, these data show that HDAC3 itself is crucial for the preferential deacetylation of H4 K5.
Figure 3.
Specific increase in acetylation of histone H4 lysine 5 at an RAR-regulated gene after HDAC3 knockdown. (A) Gal4-RAR recruits N-CoR/SMRT and HDAC3 to Gal4 UAS luciferase reporter in 293T cells. ChIP analysis of endogenous N-CoR/SMRT and HDAC3, and Gal4-RAR on transiently transfected reporter is shown. (B) siRNA knockdown of HDAC3. Nonspecific control (Con) doublestranded RNA is used as a negative control, and HDAC2 is shown as a control for the specific effect of the siRNA. (C) HDAC3 knockdown differentially increases H4 lysine acetylation at the Gal-RAR-bound reporter gene. (D) Effect of HDAC3-specific siRNA or control siRNA on H4 K5 acetylation at the endogenous RARγ2 gene in NIH 3T3 fibroblasts. Mean and range of duplicate samples are shown. WO, washout.
Having shown the requirement of HDAC3 for lysine-specific deacetylation in the context of an active promoter in living cells, we next tested whether the preferential deacetylation of H4 K5 was an inherent substrate specificity of the HDAC3 enzyme in vitro. Recombinant SMRT/HDAC3 complex was purified on beads and incubated with hyperacetylated histones isolated from butyrate-treated HeLa cells (Fig 4A). Hyperacetylation of K5, K8 and K12 was confirmed by immunoblotting with acetylationspecific H4 antibodies (Fig 4B). Although the total amount of histone H4 protein remained constant during the incubation, deacetylation of K5 was detected in 5 min (Fig 4C). By contrast, K8, K12 and K16 deacetylation became increasingly more modest and delayed (Fig 4C). Thus, the inherent substrate specificity of HDAC3 underlies the pattern of histone deacetylation observed on an endogenous ATRA-responsive gene after ligand removal.
Figure 4.
HDAC3 preferentially deacetylates histone H4 lysine 5. (A) Schematic of the assay to determine lysine specificity of histone deacetylation by HDAC3. O/N, overnight. (B) Assessment of H4 lysine hyperacetylation in the substrate. (C) HDAC3 specificity for lysine deacetylation in histone H4. Immunoblots for total H4 and acetylated K5, K8, K12 and K16 are shown at various time points.
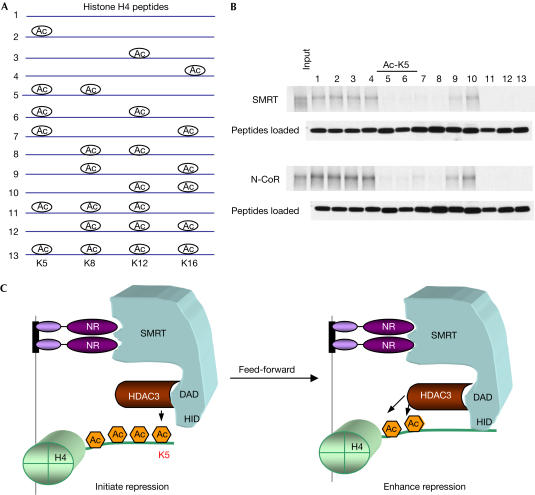
Next, we examined the binding of N-CoR and SMRT to a series of mono-, di- and tri-acetylated H4 tail peptides, in addition to the unacetylated and tetra-acetylated tails (Fig 5A). As expected from earlier studies (Yu et al, 2003), N-CoR and SMRT bound to unacetylated histone H4 tails, but had much lower affinity for tetra-acetylated H4 tails (Fig 5B). N-CoR/SMRT also bound uniformly well to mono-acetylated tails and poorly to tri-acetylated tails (Fig 5B). We observed that binding to di-acetylated tails was highly specific. Acetylation of K5 prevented binding when coupled with acetylation of any other lysine (Fig 5B). At the other extreme, K16 acetylation had no effect on binding unless it was in the context of acetylated K5. Thus, there is a code for binding of N-CoR/SMRT to acetylated K4 tails, with the important determination being deacetylation of K5 matching the substrate specificity of HDAC3.
Figure 5.
Acetylation of histone H4 lysine 5 preferentially inhibits N-CoR/SMRT binding. (A) Schematic of peptides used for binding assay. (B) Binding of peptides to N-CoR and SMRT. Equal amounts of peptides were presented for binding as shown. (C) Feed-forward model of how the match between HDAC3 substrate specificity and N-CoR/SMRT histone-binding preference serves to stabilize the repression complex. NR recruitment of HDAC3 by means of N-CoR/SMRT favours deacetylation of H4 K5, leading to increased chromatin association of N-CoR/SMRT, and thereby enhancing deacetylation and maintaining the repressed state.
Discussion
We have shown that the repressed state of a ligand-responsive gene, characterized by recruitment of N-CoR/SMRT–HDAC3 and by histone hypoacetylation, is quickly attained after ligand removal. The deacetylation after ligand removal requires the presence of HDAC3. Achievement of the repressed state involves the ordered deacetylation of lysines in H4 tails. This order is determined by the substrate specificity of HDAC3, which matches the binding preference of N-CoR/SMRT for submaximally acetylated H4 tails. Histone binding by N-CoR/SMRT requires a SANT motif, similar to that found in other histone-binding proteins including ISWI and Ada2 (Boyer et al, 2002; Grune et al, 2003). It will be of interest to determine whether other SANT motifs discriminate between differently modified histone tails, as we have shown here for N-CoR/SMRT.
Our findings have important implications for transcriptional regulation. To the best of our knowledge, HDAC3 is the first example of a mammalian HDAC with substrate specificity. Yeast HDACs Clr3 and Sir2 selectively deacetylate histone tails, but the specificity is different from that of HDAC3 (Imai et al, 2000; Bjerling et al, 2002; Suka et al, 2002). Thus, it is possible that other mammalian HDACs will prove to have specificity that may be unique. Progress in this area is inhibited by the observation that several other HDACs do not have enzymatic activity when purified to homogeneity (Guenther et al, 2001; Li et al, 2004). Immunoprecipitates from cells do have activity, but often contain several HDACs (Fischle et al, 2002; Li et al, 2004). The ability to activate HDAC3 by N-CoR/SMRT in the absence of other HDACs has made the present studies feasible.
The matching of HDAC3 H4 lysine substrate preference with the H4 binding specificity of N-CoR/SMRT confirms and extends the model that co-repressor histone binding serves the feed-forward function of stabilizing and enhancing the function of the repression complex (Yu et al, 2003; Fig 5C). The specificity for H4 K5 is thematically consistent with the tenet that specific enzymatic histone modifications regulate the binding of transcriptional regulators to nucleosomes (Strahl & Allis, 2000). Thus, HDAC3 marks its presence on the H4 tail, promoting SMRT/N-CoR binding and function. Another member of the SMRT/N-CoR complex—the WD-40 repeat protein TBL1—has been shown to bind to histones, also preferring unacetylated to fully acetylated histone tails (Li et al, 2002). It will be of interest to determine whether TBL1 shows a similar or overlapping binding code, which could, in principle, either enhance or restrict its association with nucleosomes that have been modified by HDAC3 in the context of the entire co-repressor complex. Moreover, as other HDACs exist in multiprotein complexes, their individual substrate preferences may act analogously to modulate the function of other members of the complex.
Methods
Mammalian cell culture and transfection. 3T3-L1 fibroblasts were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, USA) supplemented with 10% fetal bovine serum (Sigma, St Louis, MO, USA). Confluent cells were washed with phosphate-buffered saline (PBS) and maintained in DMEM supplemented with 10% charcoal/dextran-stripped fetal bovine serum for 24 h. After 24 h, cells were collected (no ATRA) or treated with 1 μM ATRA (Sigma) for different durations. For the ligand washout experiments, cells were washed twice with PBS maintained at 37°C and then incubated with DMEM supplemented with 10% charcoal/dextran-stripped serum for different durations. SMART pool siRNAs (Dharmacon, Lafayette, CO, USA) were validated (data not shown) and used for mouse HDAC3 knockdown in NIH 3T3 fibroblasts. 293T cells were maintained and transfected as described previously (Yu et al, 2003), and HDAC3 was knocked down as described previously (Ishizuka & Lazar, 2003).
Analysis of RNA and protein. Total RNA from treated cells was collected using the RNeasy midi kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. A 20 μg portion of RNA was analysed for murine RARγ1/2 and 36B4 expression by northern analysis. Heterogenous nuclear RNA was assayed as described previously (Elferink & Reiners, 1996), using reverse transcription primers specific for exon 6 of murine RARγ (5′-GGAATGTCACAGTGCGTGG-3′). Complementary DNA was then analysed by quantitative PCR using primer 5′-CACAGCCTGCCAGTCTACAATG-3′ in exon ID and 5′-CCTGGTCCCAAACTTTACACAAG-3′ in intron 6. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as a control. Immunoblotting with chemiluminescent detection was performed as described previously (Ishizuka & Lazar, 2003) using rabbit polyclonal antibodies that recognized HDAC2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), HDAC3 (Abcam, Cambridge, MA, USA), Flag–HRP (horseradish peroxidase; Sigma), histone H4 (Upstate, Waltham, MA, USA) and streptavidin–HRP (Upstate), in addition to antibodies used in the ChIP assay described below.
Chromatin immunoprecipitation. ChIP assays in 3T3-L1 and 293T cells were performed as described previously (Hartman et al, 2002; Ishizuka & Lazar, 2003). Samples were immunoprecipitated with rabbit IgG or polyclonal antibodies that recognized CBP(A22), HDAC3, Gal4 DBD and RAR(454) (Santa Cruz Biotechnology); acetylated histone H3, acetylated histone H4, acetylated histone H4 K8 and K12 (Upstate); acetylated histone H4 K5 and K16 (Abcam); Pol II (Covance, Berkeley, CA, USA); and N-CoR/SMRT (PA1-842, Affinity BioReagents, Golden, CO, USA). Note that the N-CoR/SMRT antibody is an antibody to SMRT, but we have found it to recognize and immunoprecipitate both N-CoR and SMRT (T.I. and M.A.L., data not shown). Samples were analysed by conventional PCR using primers spanning the RA-responsive region in the RARγ2 promoter region (5′-GCACCAAGATGAGCAGAGC-3′ and 5′-TGTCGAGAGAGCTTGATTGC-3′). Samples were also analysed by real-time PCR using primers specific for RARγ2 (5′-CCTTCACTCCTTCCCCCAAT3′ and 5′-CGACAAGCTGGAGGTCTGAAC-3′), CRBPI (5′-CAGACACTGATTGTCATCTCTT-3′ and 5′-GCGTGTTCCGTGGCTTCT-3′) and GAPDH as a control (5′-AAGACACCAGTAGACTCCACG-3′ and 5′-CAAATTCAACGGCACAGTCAA-3′). Primers used for UASx5sV40 luciferase reporter were 5′-TGTATCTTATGGTACTGTAACTG-3′ and 5′-CTTTATGTTTTTGGCGTCTTCCA-3′.
HDAC3 assay. HDAC assays were performed as described previously (Yu et al, 2003) with the following modifications. HDAC3–Flag and Gal4sMRT(1–763) polypeptides were translated individually using the TNT T7 Quick coupled transcription–translation kit (Promega, Madison, WI, USA), and 10 μl of each of the polypeptides was mixed and added to 100 μl of buffer D-150 (150 mM KCl, 20 mM HEPES pH 7.9, 0.25 mM EDTA, 10% glycerol and 0.1% Tween 20). Complexes were purified by incubation with anti-Gal4 agarose beads (Santa Cruz) in the presence of protease inhibitors (Roche, Basel, Switzerland) for 16–18 h at 4°C. Beads were washed three times in buffer D-150 and then incubated with 10 μg of hyperacetylated HeLa histones (Upstate) in a total volume of 200 μl of buffer D-150 at 37°C for different durations. To stop each reaction, 40 μl of SDS protein loading buffer was added to the reaction. A 25 μl aliquot of each reaction was subjected to SDS–polyacrylamide gel electrophoresis.
Histone-binding assay. Biotinylated histone H4 tail peptides (SGRGKGGKGLGKGGAKRH), which were differentially acetylated, were synthesized commercially (SynPep, Dublin, CA, USA). Binding of in vitro-translated 35S-methionine-labelled full-length SMRT–Flag and N-CoR–Flag was measured as described previously (Yu et al, 2003). A short biotinylated peptide CHKtide (KKKVSRSGLYRSPSMPENLNRPR; Upstate), which contains three unmodified lysines, was used as a control.
Acknowledgments
We thank M. Guenther for helpful discussions. This work was supported by grants from NIDDK including the Nuclear Receptor Signaling Atlas (www.nursa.org).
References
- Bjerling P, Silverstein RA, Thon G, Caudy A, Grewal S, Ekwall K (2002) Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol Cell Biol 22: 2170–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Langer MR, Crowley KA, Tan S, Denu JM, Peterson CL (2002) Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol Cell 10: 935–942 [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294: 1866–1870 [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM (1995) A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377: 454–457 [DOI] [PubMed] [Google Scholar]
- Elferink CJ, Reiners JJ Jr (1996) Quantitative RT–PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477 [DOI] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E (2002) Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 9: 45–57 [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Schreiber SL (2002) Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol 9: 3–16 [DOI] [PubMed] [Google Scholar]
- Grune T, Brzeski J, Eberharter A, Clapier CR, Corona DF, Becker PB, Muller CW (2003) Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol Cell 12: 449–460 [DOI] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R (2000) A core SMRT corepressor complex containing HDAC3 and a WD40 repeat protein linked to deafness. Genes Dev 14: 1048–1057 [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Barak O, Lazar MA (2001) The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21: 6091–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman HB, Hu X, Tyler KX, Dalal CK, Lazar MA (2002) Mechanisms regulating adipocyte expression of resistin. J Biol Chem 277: 19754–19761 [DOI] [PubMed] [Google Scholar]
- Horner MA, Chen T, Thummel CS (1995) Ecdysteroid regulation and DNA binding properties of Drosophila nuclear hormone receptor superfamily members. Dev Biol 168: 490–502 [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA (1999) The CoRNR motif controls the recruitment of corepressors to nuclear hormone receptors. Nature 402: 93–96 [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA (2000) Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 11: 6–10 [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Lazar MA (2003) The N-CoR/HDAC3 deacetylase complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23: 5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Kawada T, Kazuki R, Sugimoto E (1993) Retinoic acid receptor gamma 2 gene expression is up-regulated by retinoic acid in 3T3-L1 preadipocytes. Biochem J 293: 807–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T (2000) Acetylation: a regulatory modification to rival phosphorylation? EMBO J 19: 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J (2000) Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19: 4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin Q, Wang W, Wade P, Wong J (2002) Specific targeting and constitutive association of histone deacetylase complexes during transcriptional repression. Genes Dev 16: 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Staver MJ, Curtin ML, Holms JH, Frey RR, Edalji R, Smith R, Michaelides MR, Davidsen SK, Glaser KB (2004) Expression and functional characterization of recombinant human HDAC1 and HDAC3. Life Sci 74: 2693–2705 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83: 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick A, Licht JD (1999) Deconstructing a disease: RARa, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 93: 3167–3215 [PubMed] [Google Scholar]
- Minucci S, Pelicci PG (1999) Retinoid receptors in health and disease: co-regulators and the chromatin connection. Semin Cell Dev Biol 10: 215–225 [DOI] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Love JD, Li C, Banayo E, Gooch JT, Krishna V, Chatterjee K, Evans RM, Schwabe JW (1999) Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev 13: 3209–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG (1999) Molecular determinants of nuclear receptor–corepressor interaction. Genes Dev 13: 3198–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Brown M (2002) Molecular determinants for the tissue specificity of SERMs. Science 295: 2465–2468 [DOI] [PubMed] [Google Scholar]
- Smith WC, Nakshatri H, Leroy P, Rees J, Chambon P (1991) A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J 10: 2223–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403: 41–45 [DOI] [PubMed] [Google Scholar]
- Suka N, Luo K, Grunstein M (2002) Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet 32: 378–383 [DOI] [PubMed] [Google Scholar]
- Tagami T, Jameson JL (1997) Nuclear corepressors enhance the dominant negative activity of mutant receptors that cause resistance to thyroid hormone. Endocrinology 139: 640–650 [DOI] [PubMed] [Google Scholar]
- Yu J, Li Y, Ishizuka T, Guenther MG, Lazar MA (2003) A SANT motif in the SMRT corepressor interprets the histone code and promotes histone deacetylation. EMBO J 22: 3403–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Zhou MM (2002) Bromodomain: an acetyl-lysine binding domain. FEBS Lett 513: 124–128 [DOI] [PubMed] [Google Scholar]
- Zhang J, Kalkum M, Chait BT, Roeder RG (2002) The N-CoR–HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell 9: 611–623 [DOI] [PubMed] [Google Scholar]