Summary
Stabilized lipid rafts in membrane transport
Keywords: lipid sorting, membrane curvature, membrane fission, membrane tubules, phase separation
The enrichment of sphingolipids and cholesterol in the mammalian plasma membrane, and unsaturated phosphatidylcholine (PC) in the endoplasmic reticulum, requires lipid sorting in the Golgi apparatus. Most sphingolipids are synthesized in the Golgi lumen (Holthuis & Levine, 2005). Therefore, unsaturated PC must be segregated from sphingolipids and cholesterol, and selectively included in retrograde carrier vesicles (van Meer, 1989). Lipid-phase separations into a fluid liquid-crystalline phase and a solid-gel phase were characterized in the 1970s, but mammalian membranes are always fluid: membranes similar to the endoplasmic reticulum contain unsaturated lipids, whereas the saturated lipids in plasma membranes are 'fluidized' by cholesterol. In the 1980s, PC–cholesterol mixtures were found to display fluid–fluid immiscibility, which yielded a liquid-disordered (ld) phase and a less-fluid liquid-ordered (lo) phase. Since then, ternary phase diagrams have shown that sphingomyelin (SM)/cholesterol/PC mixtures, which mimic the non-cytosolic leaflet of Golgi and plasma membranes, segregate into ld and lo phases, with the latter being enriched in SM and cholesterol (Simons & Vaz, 2004). Although this could explain lipid segregation, lipid sorting requires the selective incorporation of one of the two phases into a transport carrier with a specific destination. Interestingly, in model membranes, the phases bud away from each other (Baumgart et al, 2003). However, when no external force is applied, they do so on a micrometer scale compared with the typical 40–70 nm size of cellular transport vesicles. A new study has shown that the induction of a curvature that is typical of cellular systems can, by itself, concentrate the more fluid lipid phase into a highly curved membrane (Roux et al, 2005).
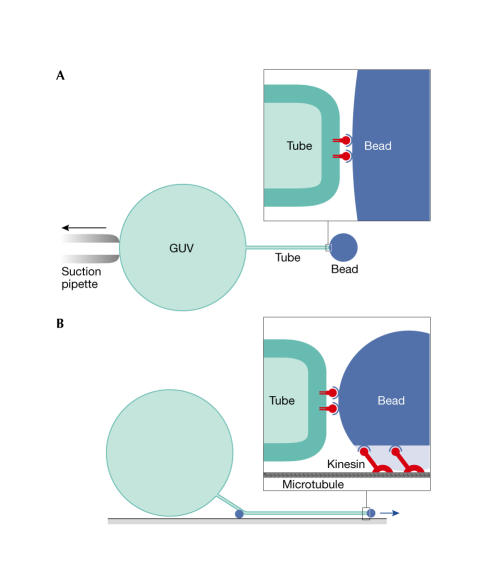
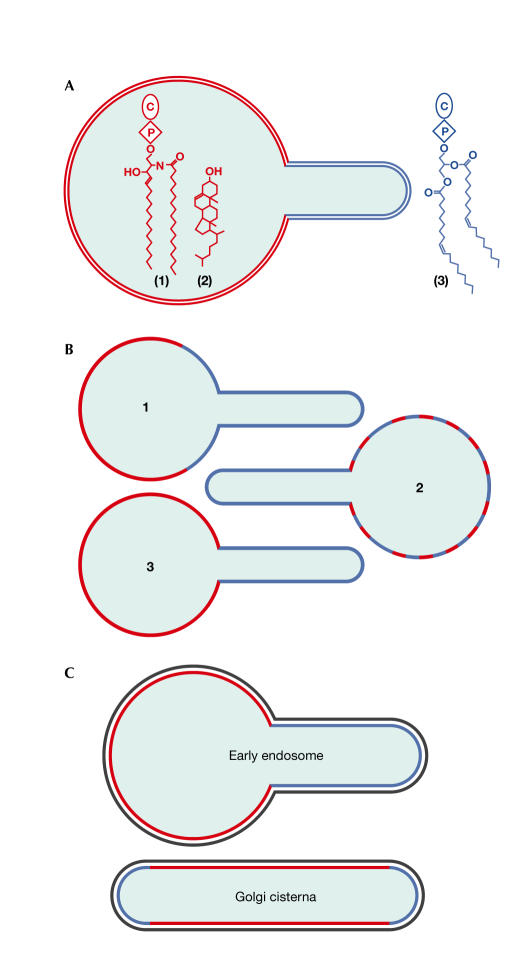
Roux and colleagues (2005) initially made giant unilamellar vesicles (GUVs) that were composed of the ternary mixture, brain SM/ cholesterol/dioleoyl PC, and used two different fluorescent-molecule markers of the ld phase (Bodipy-PC) and the lo phase (Cy3-cholera toxin/GM1 ganglioside) to construct a simple phase diagram. The authors then attached beads to the GUVs, and drew tubules from them using an optical trap and a suction pipette (Fig 1A), which allowed the measurement of the bending rigidity of the different lipid phases. Finding a greater than twofold reduced rigidity for the ld phase, Roux and colleagues predicted its preferential presence in the tubules. To study this further, they used a different tubulation system (Fig 1B). A biotinylated lipid without discernible phase preference in the GUV was attached to kinesin molecular motors, which were subsequently allowed to run along a microtubule by ATP addition. Using egg PC GUVs, this system was previously reported to generate tubules with a diameter of 40 nm, which is in the physiological range of cellular tubules and transport vesicles. As expected, tubules that were pulled from biphasic liposomes always connected to the ld phase (Fig 2A,B). This implies that the generation of tubules or buds from an organellar membrane that contains preexisting domains will generally load the resulting tubule or transport vesicle with the ld phase. Next, a tubule was pulled from a liposome that contained an equimolar mixture of SM/cholesterol/PC, which was shown by microscopy to form a single phase. Remarkably, tubulation induced a visible lipid heterogeneity, which enriched the ld lipid in the tubule. Phase properties of the tubes were used as evidence that the fluorescence result reflected a macroscopic phase separation. As the authors did not apply a high-resolution technique, such as fluorescence resonance-energy transfer or fluorescence lifetime-imaging microscopy, it remains unclear whether segregation was induced by the application of high curvature or involved a coalescence of preexisting nanoscopic domains (Fig 2B); the latter would be consistent with the idea that lipid rafts in biomembranes are small (below the 100–200 nm resolution of the light microscope) and transient unless they are stabilized (Kusumi et al 2004).
Figure 1.
Pulling tubes from giant unilamellar vesicles (GUVs). (A) A 1-μm streptavidin bead is fixed in an optical trap. The GUV is attached by biotin–lipids and is slowly pulled by moving the suction pipette, which results in tubulation. This set-up allowed Roux and colleagues (2005) to measure the pulling force and the bending rigidities of the two phases. (B) Biotin–kinesin on a 100-nm streptavidin bead is bound to microtubules on a support. GUVs bind the beads via biotin–lipids and settle down on the support. The addition of ATP results in movement and force generation by the kinesin, which draws the bead away from the GUV and results in tubule formation. This method has been further optimized by coupling biotin–kinesin to biotin–lipid through free streptavidin (Koster et al, 2003; Leduc et al, 2004).
Figure 2.
Lipid selection by tubes. (A) Tubules drawn from GUVs consisting of (1) sphingomyelin (SM), (2) cholesterol and (3) phosphatidylcholine (PC) contained the liquid-disordered (ld) phase (enriched in PC). (B) (1) Tubules connected to a preexisting ld phase. Either the tubule formed at the site of the ld phase, or ld phase lipids rapidly displaced the liquid-ordered (lo) phase during budding. (2) In equimolar GUVs, preexisting small transient rafts might have coalesced into a stabilized ld phase in the tubule. (3) Alternatively, the bud might have induced a phase separation while curving. (C) Lipid segregation has been observed in tubulated early endosomes and must occur in the Golgi apparatus. The rim of Golgi cisternae is curved like a tube and might stabilize lipid segregation even before tubulation or vesicle budding. The organization of the cytosolic surface (black) is unknown.
The discovery that the induction of a physiological curvature can assemble ld phase lipids into a tubule is of great interest. This mechanism might selectively drive ld lipids into the sorting tubules of early endosomes (Fig 2C; Mukherjee & Maxfield, 2000; Sharma et al, 2003), into the many types of tubule and tubularsaccular carriers that arise from the Golgi apparatus (Mogelsvang et al, 2004), and maybe even into budding transport vesicles. After laterally segregating the lipids, this is the first requirement for lipid sorting. One of the next questions that will need to be addressed is how the budding tubule or vesicle incorporates the proteins that direct it to its destination. This will require a molecular level understanding of the relevant lipid–protein interactions. Do complex biological lipid mixtures behave like those in the Roux study? How many types of lipid environment coexist in biomembranes in which several kinds of sorting are required (van Meer, 1989)? Considering the lipid organization on the cytosolic surface, is the enrichment of phosphatidylserine on the plasma membrane generated by its enhancement in lipid domains on the cytosolic side of carriers that are budded from the Golgi apparatus? Finally, what process drives tubule fission in vivo? Fission can be executed by budding machineries, such as COP coats. However, it sometimes requires specific proteins, such as dynamin, or the local conversion of lipid molecules to change their molecular shape. In the Roux study, fission could be achieved by inducing a lipid-phase separation in the tubule through a change in cholesterol levels. This is an elegant example of how important principles can be uncovered by physical studies on simple systems. However, as always, the challenge is to find out whether and how cells use these principles for their vital functions.
References
- Baumgart T, Hess ST, Webb WW (2003) Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 425: 821–824 [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Levine TP (2005) Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6: 209–220 [DOI] [PubMed] [Google Scholar]
- Koster G, VanDuijn M, Hofs B, Dogterom M (2003) Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc Natl Acad Sci USA 100: 15583–15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Koyama-Honda I, Suzuki K (2004) Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steadystate rafts. Traffic 5: 213–230 [DOI] [PubMed] [Google Scholar]
- Leduc C, Campas O, Zeldovich KB, Roux A, Jolimaitre P, Bourel-Bonnet L, Goud B, Joanny JF, Bassereau P, Prost J (2004) Cooperative extraction of membrane nanotubes by molecular motors. Proc Natl Acad Sci USA 101: 17096–17101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE (2004) Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic 5: 338–345 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR (2000) Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic 1: 203–211 [DOI] [PubMed] [Google Scholar]
- Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B (2005) Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J advance online publication, 10.1038/sj.emboj.7600631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DK, Choudhury A, Singh RD, Wheatley CL, Marks DL, Pagano RE (2003) Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem 278: 7564–7572 [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL (2004) Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 33: 269–295 [DOI] [PubMed] [Google Scholar]
- van Meer G (1989) Lipid traffic in animal cells. Annu Rev Cell Biol 5: 247–275 [DOI] [PubMed] [Google Scholar]