Abstract
Anaplerotic enzyme reactions are those which replenish tricarboxylic acid intermediates that are withdrawn for the synthesis of biomass. In this study, we examined recombinant protein production in Escherichia coli containing activity in an additional anaplerotic enzyme, pyruvate carboxylase. In batch fermentations, the presence of pyruvate carboxylase resulted in 68% greater production of the model protein, β-galactosidase, 41% greater cell yield, and 57% lower acetate concentration. We discuss why these results indicate that acetate concentration does not limit cell growth and protein synthesis, as predicted by other researchers, and suggest instead that the rate of acetate formation represents an inefficient consumption of glucose carbon, which is reduced by the presence of pyruvate carboxylase.
Escherichia coli is widely used for recombinant protein production, largely because it is well-characterized, fast and inexpensive to grow, and relatively easy to alter genetically (17, 18). During the biochemical synthesis of proteins, production of nonessential metabolites can waste carbon and energy that might otherwise be directed toward the protein product. A prominent example of an apparently nonessential metabolite that accumulates during aerobic growth of E. coli on glucose is acetate. Enzymatically synthesized from acetyl coenzyme A (acetyl-CoA) in two steps-phosphotransacetylase (pta gene) converts acetyl-CoA to the intermediate acetyl phosphate, which is then converted to acetate with the generation of ATP by acetate kinase (ack)-acetate is also widely considered inhibitory to growth and protein production. Studies indicate that the concentration at which acetate significantly reduces cell growth rate lies in the range of 3 to 5 g/liter (3, 9, 26, 31). Although the detailed mechanism remains unknown, this by-product is generally thought to accumulate in E. coli fermentations as a result of the tricarboxylic acid (TCA) cycle not keeping pace with glycolysis (1, 24, 28, 36). In other words, acetate accumulates as a result of insufficient oxaloacetate being present in the first step of the TCA cycle, the conversion of oxaloacetate and acetyl-CoA to citrate via citrate synthase.
Approaches for increasing cell density or protein yield in E. coli often focus on the reduction of acetate formation, and a variety of methods have been studied. The production of acetate can be blocked altogether, for example, by mutations in the pta and/or ack genes (10, 12, 30, 35). Alternatively, acetate accumulation can be reduced by redirecting this biochemical or its precursors to other benign biochemicals. For example, pyruvate can be converted to acetoin by acetolactate synthase (3, 4). Other methods include the following: altering glucose uptake (9), using carbon sources other than glucose (5), controlling feeding rates to better synchronize the TCA cycle and glycolysis (15, 26-29, 31, 36-38), or supplementing amino acids to reduce the demand for biosynthetic precursors (11, 34). Unfortunately, many of these strategies reduce the glucose uptake rate, which can simultaneously reduce the rate of protein production.
As noted above, recombinant protein production is believed to diminish flow in the TCA cycle through the withdrawal of the intermediates that serve as precursor biochemicals. Indeed, 10 amino acids are biochemically derived from TCA cycle intermediates: aspartate, asparagine, methionine, threonine, isoleucine, and lysine are derived from oxaloacetate, while glutamate, arginine, proline, and glutamine are derived from α-ketoglutarate. The additional metabolic burden resulting from recombinant protein production would likely further diminish the availability of oxaloacetate, which could lead to additional acetate formation from acetyl-CoA. If withdrawal of TCA cycle intermediates limits cell growth and protein production and consequently increases acetate accumulation, then providing cells with improved metabolic means to replenish these TCA cycle intermediates should represent an approach to increase protein production.
Anaplerotic biochemical pathways are the enzymatic reactions that replenish TCA cycle intermediates. In E. coli, the principal anaplerotic pathway during growth on glucose is the formation of oxaloacetate from phosphoenolpyruvate (PEP) by PEP carboxylase, and this single conduit must supply carbon for the 10 amino acids and other cellular building blocks derived from TCA cycle intermediates. A previous study showed that overexpression of PEP carboxylase in E. coli resulted in 17% higher specific growth rate and 44% lower specific acetate production compared to those of isogenic controls (16). Since PEP is required for the PEP phosphotransferase system, the initial step in glucose consumption, overexpressing PEP carboxylase unfortunately also diminishes glucose uptake. In aerobically grown E. coli, Chao and Liao (8) showed a 30% decrease in the glucose uptake rate as a result of overexpression of PEP carboxylase. Similarly, Gokarn et al. (21) found a 14% reduction in the anaerobic glucose consumption rate in a strain overexpressing PEP carboxylase.
Another anaplerotic pathway present in some prokaryotes but not in E. coli is the biotin-dependent enzyme pyruvate carboxylase, which converts pyruvate directly to oxaloacetate (6). Previous studies on the expression of Rhizobium etli pyruvate carboxylase in E. coli focused on anaerobic growth and fermentation (20, 21). A detailed analysis (21) of E. coli strains which either lacked or overproduced several levels of PEP carboxylase and pyruvate carboxylase demonstrated that the specific rate of glucose consumption was 34% greater in E. coli ppc mutants with low pyruvate carboxylase activity (0.03 U/mg) than in E. coli (without pyc) with heightened PEP carboxylase activity (1.25 U/mg). Another possible benefit of pyruvate carboxylase in recombinant protein production is that it redirects carbon from pyruvate, the immediate metabolic precursor of acetyl-CoA, and thus could potentially reduce acetate formation.
To improve our understanding of recombinant protein synthesis in E. coli, we analyzed the production of a model recombinant protein, β-galactosidase, in response to the additional anaplerotic pathway afforded by pyruvate carboxylase.
MATERIALS AND METHODS
Strains and plasmids.
E. coli MG1655 (CGSC6300, wild type λ−) was the host strain used in this study (23). Table 1 shows the plasmids used in this study. β-Galactosidase encoded by the lacZ gene (19) was expressed via the plasmid pACYC184-lacZ, while pyruvate carboxylase encoded by the pyc gene from R. etli (13) was expressed via the plasmid pTrc99A-pyc (22). Because both the pTrc99A-pyc and pACYC184-lacZ expression plasmids utilize the lacPO, isopropyl-β-thiogalactopyranoside (IPTG) was added to cultures of E. coli MG1655/pACYC184-lacZ/pTrc99A and MG1655/pACYC184-lacZ/pTrc99A-pyc to induce protein production.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristics | Reference |
|---|---|---|
| pTrc99A | bla lacIqtrc ColE1 | 2 |
| pTrc99A-pyc | bla lacIqtrc ColE1 R. etli pyc | 22 |
| pTrc99A-lacZ | bla lacIqtrc ColE1 E. coli lacZ | This study |
| pACYC184 | tet cat P15A | 7 |
| pACYC184-lacZ | tet cat P15A trc E. coli lacZ | This study |
| pTer7 | bla ColE1 E. coli wild-type lacZ coding region | R. Young, Texas A&M University |
Initially, the lacZ gene was cloned into the pTrc99A expression vector. To construct pTrc99A-lacZ, primers 5′ TAT CAT GGA TCC AGG AAA CAG CTA TGA CCA TGA TTA CGG ATT CAC TG 3′ and 5′ TAC ATA CTC GAG CAG GAA AGC TTG GCC TGC CCG GTT ATT ATT ATT TT 3′ were used to PCR amplify a 3,138-bp fragment from the pTer7 plasmid (restriction enzyme sites are indicated with a double underline, while the regions of homology to lacZ are indicated with a single underline). The resulting fragment was gel isolated, digested with BamHI and HindIII, and then ligated into the pTrc99A vector which had been digested with the same two restriction enzymes. To construct pACYC184-lacZ, a 3,504-bp fragment from pTrc99A-lacZ that contained the lacZ gene and the trc promoter was ligated to a 3,178-bp fragment from pACYC184 that contained the cat gene and the origin of replication (ori). The 3,504-bp lacZ fragment was prepared by digesting pTrc99A-lacZ with NarI and HindII and then filling in with Klenow, while the 3,178-bp fragment containing cat and ori was prepared by digesting pACYC184 with HincII. A clone was then selected which transcribed lacZ in the clockwise direction with respect to pACYC184.
Media and growth conditions.
Several colonies were used to inoculate 1 ml of Luria-Bertani (LB) broth with 100 mg of ampicillin per liter to keep selective pressure on the pTrc99A plasmids and with 20 mg of chloramphenicol per liter to keep selective pressure on the pACYC184-lacZ plasmid. Such inocula were incubated with agitation at 37°C for approximately 6 h before transferring the contents to 100 ml of preculture media in 500-ml shaking flasks. Preculture media modified from Horn et al. (25) contained the following (per liter): Na2HPO4 · 2H2O, 6.42 g; KH2PO4, 3.00 g; NH4Cl, 1.00 g; NaCl, 0.50 g; citric acid, 2.0 g; Fe2(SO4)3, 50 mg; H3BO3, 3.0 mg; MnCl2 · 4H2O, 15 mg; disodium EDTA · 2H2O, 9.6 mg; CuCl2 · 2H2O, 1.5 mg; Na2MoO4 · 2H2O, 2.5 mg; CoCl2 · 6H2O, 2.5 mg; ZnCl2 · 2H2O, 5.0 mg; glucose, 20 g; MgSO4 · 7H2O, 0.6 g; CaCl2 · 2H2O, 70 mg; ampicillin, 100 mg; and chloramphenicol, 20 mg. Precultures were grown at 250 rpm and 37°C to an optical density of about 1.5 before transferring the contents to a fermentor.
All batch fermentations of 1.5 liters were conducted in bench-top fermentors (Bioflow III; New Brunswick Scientific, Co., Edison, N.J.) operated at 1,000 rpm and 37°C and with a flow rate of sterile air at 1.20 liters/min. These conditions ensured that the dissolved oxygen concentration was greater than 50% of saturation for the duration of the fermentations. The fermentation media contained (per liter): KH2PO4, 6.00 g; (NH4)2HPO4, 8.00 g; citric acid, 2.1 g; Fe2(SO4)3, 62.5 mg; H3BO3, 3.8 mg; MnCl2 · 4H2O, 18.8 mg; disodium EDTA · 2H2O, 12 mg; CuCl2 · 2H2O, 1.9 mg; Na2MoO4 · 2H2O, 3.1 mg; CoCl2 · 6H2O, 3.1 mg; Zn(CH3COO)2 · 2H2O, 10 mg; glucose, 25 g; MgSO4 · 7H2O, 1.5 g; CaCl2 · 2H2O, 70 mg; biotin, 1 mg; thiamine-HCl, 1 mg; ampicillin, 100 mg; and chloramphenicol, 20 mg. The fermentations were controlled at pH 6.5 to 6.7 with 10% NaOH and 10% H2SO4, and cultures were induced with 1.0 mM IPTG when the optical density was approximately 1.5. Fermentations were completed in triplicate, and statistical analyses were completed using Student's t test, with a P of <0.10 considered the criterion for statistical significance.
Analytical methods.
During fermentation, samples were withdrawn and stored at −20°C for subsequent analysis. Cell growth was monitored by measuring optical density at 550 nm (OD550) (DU-650 spectrophotometer; Beckman Instruments, San Jose, Calif.), and this measurement was correlated with dry cell mass concentration. Glucose and acetate were analyzed by high-pressure liquid chromatography as previously described (14) using a Coregel 64-H ion-exclusion column (Interactive Chromatography, San Jose, Calif.). Carbon dioxide and oxygen were measured continuously in the fermentation off-gas (Ultramat 23 gas analyzer; Siemens, Munich, Germany).
Enzyme assays.
Aliquots (1.5 ml) of the samples were thawed and centrifuged (6,000 × g for 20 min). The cells were washed and resuspended in 1.0 M Tris buffer (pH 8.0), ruptured with a French pressure cell (850 lb/in2), and centrifuged (25,000 × g for 20 min at 4°C). The cell extract was analyzed for pyruvate carboxylase by the method of Payne and Morris (33). One unit of pyruvate carboxylase activity converts 1 μmol of pyruvate per min to oxaloacetate at 30°C and pH 8. For β-galactosidase activity, aliquots (1.5 ml) were thawed and diluted to an OD550 of 0.1 with LB broth. Diluted samples were analyzed for β-galactosidase activity by the protocol of Pardee et al. (32). One unit of β-galactosidase activity produced 1 nmol of o-nitrophenol per min at 30°C and pH 7. For cellular protein content, samples were thawed and centrifuged (6,000 × g for 20 min at 4°C). Samples were disrupted with Bper II Bacterial Protein Extraction Reagent (Pierce, Rockville, Ill.), and the total cellular protein content was determined using a BCA Protein Assay Kit (Pierce).
RESULTS
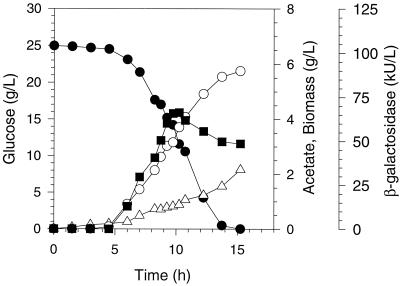
We compared the production of recombinant β-galactosidase and acetate during aerobic batch fermentations of E. coli MG1655 with and without pyruvate carboxylase activity. Figure 1 shows typical fermentation results for E. coli MG1655/pACYC184-lacZ/pTrc99A, while Fig. 2 shows fermentation results for MG1655/pACYC184-lacZ/pTrc99A-pyc. In the absence of pyruvate carboxylase for E. coli MG1655/pACYC184-lacZ/pTrc99A, 25 g of glucose per liter was consumed within 14 to 15 h, yielding a cell mass concentration of about 6 g/liter. During these fermentations, acetate accumulated to about 2 to 3 g/liter, with the volumetric rate of acetate formation visibly increasing during the last 3 to 4 h of the fermentations. β-Galactosidase concentration (i.e., volumetric activity) increased in concert with cell mass during the first 9 to 11 h of the fermentations. However, the concentration of β-galactosidase consistently remained constant or decreased slightly during the last 3 to 4 h of the fermentations, even though more than 10 g of glucose per liter remained in the media. It was noteworthy that the time interval during which protein production ceased corresponded with an increased rate of acetate formation.
FIG. 1.
Concentrations of glucose (•), cell dry mass (○), acetate (▵), and β-galactosidase (▪) from the aerobic fermentation of E. coli MG1655/pACYC184-lacZ/pTrc99A.
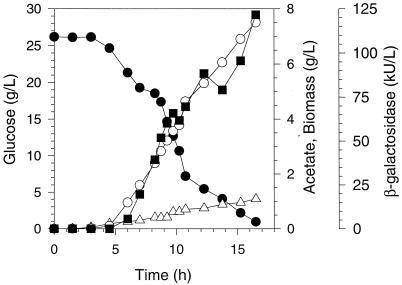
FIG. 2.
Concentrations of glucose (•), cell dry mass (○), acetate (▵), and β-galactosidase (▪) from the aerobic fermentation of E. coli MG1655/pACYC184-lacZ/pTrc99A-pyc.
In the presence of pyruvate carboxylase for E. coli MG1655/pACYC184-lacZ/pTrc99A-pyc (Fig. 2), the 25 g of glucose per liter was consumed in 16 to 19 h, yielding a cell mass concentration of about 8 g/liter. During fermentation, acetate accumulated to about 1.0 to 1.5 g/liter. Moreover, the volumetric rate of acetate production was approximately constant during these fermentations. β-Galactosidase concentration increased in concert with cell mass; therefore, the ultimate protein concentration was substantially greater than that observed in the fermentations for E. coli MG1655/pACYC184-lacZ/pTrc99A lacking pyruvate carboxylase. Interestingly, the rate of glucose consumption was similar during the first 10 to 11 h of fermentations using E. coliMG1655/pACYC184-lacZ/pTrc99A and MG1655/pACYC184-lacZ/pTrc99A-pyc. However, during the final 4 to 5 h, the rate of glucose consumption consistently slowed in E. coli MG1655/pACYC184-lacZ/pTrc99A-pyc fermentations. The specific activity of pyruvate carboxylase increased during the three fermentations from about 0.15 U/mg at 6 h to 0.4 U/mg at the time glucose was depleted.
Table 2 summarizes the key statistical comparisons of fermentations using E. coli MG1655/pACYC184-lacZ/pTrc99A and MG1655/pACYC184-lacZ/pTrc99A-pyc. We observed 68% greater β-galactosidase activity, 41% greater cell yield, and 57% less acetate concentration in recombinant E. coli fermentations containing pyruvate carboxylase activity. The results of these batch fermentations suggested that differences between E. coli with and without pyruvate carboxylase activity occurred in the rate parameters during the mid-log phase and late log phase of the fermentations. We therefore calculated several specific rate parameters for each of these two phases (Table 3). For all samples, mid-log phase was regarded as the time interval when the dry cell mass increased from 2.0 to 3.0 g/liter (commonly about 7 to 9 h). The late log phase was regarded as the time interval when the glucose concentration decreased from 10 g/liter to about 1 g/liter (approximately 12 to 14 h for E. coli MG1655/pACYC184-lacZ/pTrc99A and 15 to 16 h for MG1655/pACYC184-lacZ/pTrc99A-pyc). For each calculation, three to five samples were used. There was no significant difference between E. coli MG1655/pACYC184-lacZ/pTrc99A and MG1655/pACYC184-lacZ/pTrc99A-pyc in any of the parameters during the mid-log phase. However, during the late-log phase fermentations with E. coli MG1655/pACYC184-lacZ/pTrc99A-pyc, we observed 37% lower specific glucose consumption and 48% lower specific acetate formation than those during the analogous phase with MG1655/pACYC184-lacZ/pTrc99A. Moreover, in the late log phase, E. coli MG1655/pACYC184-lacZ/pTrc99A-pyc remained a significant producer of β-galactosidase with a specific production rate of 2.1 kilounits/g/h in contrast to MG1655/pACYC184-lacZ/pTrc99A. Although not significant at the 90% level, the respiratory quotient was generally greater for E. coli MG1655/pACYC184-lacZ/pTrc99A-pyc regardless of the growth phase, a result which is surprising because due to the presence of pyruvate carboxylase, these organisms have an additional means of carbon dioxide consumption.
TABLE 2.
Comparison of mean cell yields and maximum product concentrations in E. coli MG1655/pACYC184-lacZ with and without pyruvate carboxylase activity (expressed by plasmid pTrc99A-pyc)a
| Plasmid | Parameterb
|
||
|---|---|---|---|
| Maximum β-galactosidase activity (kilounits/liter) | Maximum acetate concn (g/liter) | Mean cell yield (g/g) | |
| pTrc99A | 66 A | 2.8 A | 0.22 A |
| pTrc99A-pyc | 111 B | 1.2 B | 0.31 B |
Three fermentations were conducted.
Values in a column followed by different letters were significantly different at the 90% confidence level.
TABLE 3.
Comparison of mean specific production and consumption rates in mid-log and late log phases in E. coli MG1655/pACYC184-lacZ with and without pyruvate carboxylase activity (expressed by plasmid pTrc99A-pyc)
| Phasea and plasmid | Parameterb
|
||||||
|---|---|---|---|---|---|---|---|
| μ (h−1) | qglucose (mmol/g/h) | qacetate (mmol/g/h) | qCO2 (mmol/g/h) | qoxygen (mmol/g/h) | RQ | qβ-Gal (kilounits/g/h) | |
| Mid-log phase | |||||||
| pTrc99A | 0.27 A | 5.8 A | 0.58 A | 7.9 A | 10.2 A | 0.78 A | 5.5 A |
| pTrc99A-pyc | 0.29 A | 5.4 A | 0.41 A | 8.0 A | 9.5 A | 0.85 A | 6.0 A |
| Late log phase | |||||||
| pTrc99A | 0.10 B | 5.1 A | 0.64 A | 4.6 B | 6.4 B | 0.73 A | −0.5 B |
| pTrc99A-pyc | 0.14 B | 2.7 B | 0.33 B | 5.4 AB | 6.5 B | 0.85 A | 2.1 C |
For all samples, mid-log phase is regarded as that time interval when the dry cell mass increases from 2.0 to 3.0 g/liter and late log phase is regarded as that time interval when the glucose concentration decreases from 10 to 0 g/liter.
μ, growth rate; qglucose, specific consumption rate; qacetate, specific production rate; qCO2, specific production rate; qoxygen, specific consumption rate; RQ, respiratory quotient; qβ-Gal, specific production rate of β-galactosidase. Values in a column followed by different letters were statistically significantly different at the 90% confidence level.
DISCUSSION
In this study, production of the recombinant protein β-galactosidase was analyzed in E. coli which had been provided an additional anaplerotic pathway via the enzyme pyruvate carboxylase. In comparison to E. coli MG1655/pACYC184-lacZ/pTrc99A without pyruvate carboxylase activity, MG1655/pACYC184-lacZ/pTrc99A-pyc containing pyruvate carboxylase activity yielded significantly more cell mass and β-galactosidase, while generating less acetate. Thus, this work has demonstrated that an additional anaplerotic pathway benefits β-galactosidase production in aerobically grown cultures of E. coli.
However, this study also suggests some subtle effects occurring in the production of β-galactosidase. Although acetate is considered inhibitory to growth and protein production, the inhibitory effects are thought to occur in the range of 3 to 5 g of acetate per liter (3, 31). We did not observe a decrease in cell growth rate as a result of the presence of acetate, and indeed, the acetate concentration was always less than 3 g/liter. Thus, acetate concentration, per se, does not appear to account for the increase in final protein concentration in the strain having pyruvate carboxylase activity.
Additional information was gleaned by considering specific production and consumption rates in the strains during the mid-log phase compared to the rates of strains in late log phase. Although no differences between the strains were observed in mid-log phase, the presence of pyruvate carboxylase greatly slowed both the specific glucose consumption rate and the specific acetate production rate in the late log phase. Together with the substantially greater cell mass yield in the strain with pyruvate carboxylase, the results suggest that pyruvate carboxylase allows the cell to use carbon more efficiently, in fact prolonging cell growth late in the growth phase. This result is consistent with approaches used by many others to slow glycolysis and in so doing generate fewer by-products (15, 26-29, 31, 36-38). In this case of using pyruvate carboxylase, the advantage appears to be that glucose is more effectively directed toward biomass and protein formation and away from acetate formation.
An important consideration is the carbon equivalence between acetate and the recombinant protein. On the basis of mass/activity of purified β-galactosidase, we calculated that there is enough carbon in 1 mg of acetate for approximately 9.7 kilounits of β-galactosidase activity. Thus, the significant difference observed in β-galactosidase activity between E. coli MG1655/pACYC184-lacZ/pTrc99A and MG1655/pACYC184-lacZ/pTrc99A-pyc fermentations can be accounted for by less than 5 mg of acetate per liter. The conclusion from this calculation is that only a small redirection of the quantity of carbon flowing to acetate to protein synthesis could substantially increase the ultimate yield of β-galactosidase.
Acknowledgments
We thank Ry Young of Texas A&M for providing the pTer7 plasmid. We also acknowledge the assistance of S. A. Lee, R. E. B. Ball, K. DeWitt, P. Reeves, L. Sanderson, and G. N. Vemuri.
This work was supported in part by funds from the University of Georgia College of Agricultural and Environmental Sciences and the Georgia Experiment Station.
REFERENCES
- 1.Åkesson, M., E. N. Karlsson, P. Hagander, J. P. Axelsson, and A. Tocaj. 1999. On-line detection of acetate formation in Escherichia coli cultures using dissolved oxygen responses to feed transients. Biotechnol. Bioeng. 65:590-598. [DOI] [PubMed] [Google Scholar]
- 2.Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. [DOI] [PubMed] [Google Scholar]
- 3.Aristidou, A. A., K. Y. San, and G. N. Bennett. 1994. Modification of central metabolic pathway in Escherichia coli to reduce acetate expression of the Bacillus subtilis acetolactate synthase gene. Biotechnol. Bioeng. 44:944-951. [DOI] [PubMed] [Google Scholar]
- 4.Aristidou, A. A., K. Y. San, and G. N. Bennett. 1995. Metabolic engineering of Escherichia coli to enhance recombinant protein production through acetate reduction. Biotechnol. Prog. 11:475-478. [DOI] [PubMed] [Google Scholar]
- 5.Aristidou, A. A., K. Y. San, and G. N. Bennett. 1999. Improvement of biomass yield and recombinant gene expression in Escherichia coli by using fructose as the primary carbon source. Biotechnol. Prog. 15:140-145. [DOI] [PubMed] [Google Scholar]
- 6.Attwood, P. V. 1995. The structure and mechanism of action of pyruvate carboxylase. Int. J. Biochem. Cell Biol. 27:231-249. [DOI] [PubMed] [Google Scholar]
- 7.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao, Y., and J. C. Liao. 1993. Alteration of growth yield by overexpression of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase in Escherichia coli. Appl. Environ. Microbiol. 59:4261-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, C. H., G. N. Bennett, and K. Y. San. 1994. Effect of modified glucose uptake using genetic engineering techniques on high-level recombinant protein production in Escherichia coli dense cultures. Biotechnol. Bioeng. 44:952-960. [DOI] [PubMed] [Google Scholar]
- 10.Dedhia, N. N., T. Hottiger, and J. E. Bailey. 1994. Overproduction of glycogen in Escherichia coli blocked in the acetate pathway improves cell growth. Biotechnol. Bioeng. 44:132-139. [DOI] [PubMed] [Google Scholar]
- 11.Delgado, J., and J. C. Liao. 1997. Inverse flux analysis for reduction of acetate excretion on Escherichia coli. Biotechnol. Prog. 13:361-367. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Ricci, J. C., L. Regan, and J. E. Bailey. 1991. Effect of the alteration of the acetic acid synthesis pathway in the fermentation pattern of Escherichia coli. Biotechnol. Bioeng. 38:1318-1324. [DOI] [PubMed] [Google Scholar]
- 13.Dunn, M. F., G. Encarnacion, M. C. Vargas, A. Daralos, H. Peralta, Y. Mora, and J. Mora. 1996. Pyruvate carboxylase from Rhizobium etli mutant characterization, nucleotide sequence, and physiological role. J. Bacteriol. 178:5960-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiteman, M. A., and M. J. Chastain. 1997. Optimization of the ion-exchange analysis of organic acids from fermentation. Anal. Chem. Acta 338:69-75. [Google Scholar]
- 15.El-Mansi, E. M. T., and W. H. Holms. 1989. Control of carbon flux to acetate excretion during growth of Escherichia coli in batch and continuous cultures. J. Gen. Microbiol. 135:2875-2883. [DOI] [PubMed] [Google Scholar]
- 16.Farmer, W., and J. C. Liao. 1997. Reduction of aerobic acetate production by Escherichia coli. Appl. Environ. Microbiol. 63:3205-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari, F. A., and J. Cappello. 1997. Biosynthesis of protein polymers, p. 37. In K. McGrath and D. Kaplan (ed.), Protein-based materials. Birkhauser, Boston, Mass.
- 18.Fothergill-Gilmore, L. A. 1993. Recombinant protein technology, p. 467. In F. Franks (ed.), Protein biotechnology. Humana Press, Totawa, N.J.
- 19.Fowler, A. V., and I. Zabin. 1978. Amino acid sequence of β-galactosidase: peptide ordering procedures and complete sequence. J. Biol. Chem. 253:5521-5525. [PubMed] [Google Scholar]
- 20.Gokarn, R. R., M. A. Eiteman, and E. Altman. 1998. Expression of pyruvate carboxylase enhances succinate production in Escherichia coli without affecting glucose uptake. Biotechnol. Lett. 20:795-798. [Google Scholar]
- 21.Gokarn, R. R., M. A. Eiteman, and E. Altman. 2000. Metabolic analysis of Escherichia coli in the presence and absence of carboxylating enzymes phosphoenolpyruvate carboxylase and pyruvate carboxylase. Appl. Environ. Microbiol. 66:1844-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gokarn, R. R., J. D. Evans, J. R. Walker, S. A. Martin, M. A. Eiteman, and E. Altman. 2001. The physiological effects and metabolic alterations caused by the expression of Rhizobium etli pyruvate carboxylase in Escherichia coli. Appl. Microbiol. Biotechnol. 56:188-195. [DOI] [PubMed] [Google Scholar]
- 23.Guyer, M. S., R. R. Reed, J. A. Stietz, and K. B. Low. 1980. Identification of a sex-factor-affinity site in E. coli as γδ. Cold Spring Harbor Symp. Quant. Biol. 45:135-140. [DOI] [PubMed] [Google Scholar]
- 24.Han, K., H. C. Lim, and J. Hong. 1992. Acetate formation in Escherichia coli fermentation. Biotechnol. Bioeng. 39:663-671. [DOI] [PubMed] [Google Scholar]
- 25.Horn, U., W. Strittmatter, A. Krebber, U. Knupfer, M. Kujau, R. Wenderoth, K. Muller, S. Matzku, A. Pluckthun, and D. Riesenberg. 1996. High volumetric yields of functional dimeric miniantibodies in Escherichia coli, using an optimized expression vector and high-cell-density fermentation under non-limited growth conditions. Appl. Microbiol. Biotechnol. 46:524-532. [DOI] [PubMed] [Google Scholar]
- 26.Jensen, E. B., and S. Carlsen. 1990. Production of recombinant human growth hormone in Escherichia coli: expression if different precursors and physiological effects of glucose, acetate, and salts. Biotechnol. Bioeng. 36:1-11. [DOI] [PubMed] [Google Scholar]
- 27.Kleman, G. L., and W. R. Strohl. 1994. Acetate metabolism in Escherichia coli in high-cell-density fermentation. Appl. Environ. Microbiol. 60:3952-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konstantinov, K., M. Kishimoto, T. Seki, and T. Yoshida. 1990. A balanced DO-stat and its application to the control of acetic acid excretion by recombinant Escherichia coli. Biotechnol. Bioeng. 36:750-758. [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. Y. 1996. High density culture of Escherichia coli. Trends Biotechnol. 14:98-105. [DOI] [PubMed] [Google Scholar]
- 30.LeVine, S. M., F. Ardeshir, and G. Ferro-Luzzi Ames. 1980. Isolation and characterization of acetate kinase and phosphotransacetylase mutants of Escherichia coli and Salmonella typhimurium. J. Bacteriol. 143:1081-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luli, G. W., and W. R. Strohl. 1990. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 56:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardee, A. B., F. Jacob, and J. Monod. 1959. The genetic control and cytoplasmic expression of “inductibility” in the synthesis of β-galactosidase by E. coli. J. Mol. Biol. 1:165-178. [Google Scholar]
- 33.Payne, J., and J. G. Morris. 1969. Pyruvate carboxylase in Rhodopseudomonas spheroides. J. Gen. Microbiol. 59:97-101. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez, D. M., and W. E. Bentley. 1993. Enhancement of recombinant protein synthesis via coordinated amino acid addition. Biotechnol. Bioeng. 41:557-565. [DOI] [PubMed] [Google Scholar]
- 35.San, K. Y., G. N. Bennett, A. A. Aristidou, and C. H. Chou. 1994. Strategies in high-level expression of recombinant protein in Escherichia coli. Ann. N. Y. Acad. Sci. 614:257-267. [DOI] [PubMed] [Google Scholar]
- 36.Shiloach, J., J. Kaufman, A. S. Guillard, and R. Fass. 1996. Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli BL21 (λDE3) and Escherichia coli JM109. Biotechnol. Bioeng. 49:421-428. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu, N., S. Fukozono, Y. Harada, K. Fujimori, K. Gotoh, and Y. Yamazaki. 1991. Mass production of human epidermal growth factor using fed-batch cultures of recombinant Escherichia coli. Biotechnol. Bioeng. 38:37-42. [DOI] [PubMed] [Google Scholar]
- 38.Yee, L., and H. W. Blanch. 1992. Recombinant protein expression in high cell density fed-batch cultures of Escherichia coli. Bio/Technology 10:1550-1556. [DOI] [PubMed] [Google Scholar]