Abstract
Vibrio cholerae can shift to a “rugose” phenotype, thereby producing copious exopolysaccharide (EPS), which promotes its environmental survival and persistence. We report conditions that promote high-frequency rugose EPS production (HFRP), whereby cells switch at high frequency (up to 80%) to rugose EPS production. HFRP appeared to be more common in clinical strains, as HFRP was found in 6 of 19 clinical strains (32%) (including classical, El Tor, and non-O1 strains) but in only 1 of 16 environmental strains (6%). Differences were found between strains in rugose colony morphology, conditions promoting HFRP, the frequency of rugose-to-smooth (R-S) cell reversion, and biofilm formation. We propose that rugose EPS and HFRP provide an evolutionary and adaptive advantage to specific epidemic V. cholerae strains for increased persistence in the environment.
Cholera, caused by Vibrio cholerae, is a severe, life-threatening diarrheal disease which continues to be a scourge throughout much of the world; there have been seven global pandemics recorded since 1817 (15). The explosive epidemic nature and severity of the disease have prompted its listing as a high-priority organism in biodefense research. Epidemic V. cholerae strains can persist for many years in the marine environment while regularly causing significant cholera epidemics (3, 5, 6, 9, 16, 20, 21); however, the specific mechanism underlying the adaptation to long-term persistence, particularly that of epidemic strains, has remained a mystery. Several studies have proposed that the persistence of V. cholerae in the environment is due to a viable but nonculturable state (VBNC) in response to starvation and cold (4, 18, 19, 27); however, it is not known whether the VBNC phenotype promotes persistence in regions where cholera is endemic and under conditions other than starvation and cold. Alternatively, “rugose” exopolysaccharide (rEPS) production might promote the survival and persistence of V. cholerae in the aquatic environment.
A rugose colony phenotype was first reported and described in both sixth (classical biotype) and seventh (El Tor biotype) pandemic strains in the late 1930s by Bruce White (25, 26) and is characterized by wrinkled colonies expressing copious amounts of EPS. Since then, studies have shown that smooth V. cholerae cells under nutrient-limiting conditions can spontaneously shift at a low frequency to express rEPS and exhibit wrinkled (rugose) colonies (13, 14, 22, 25, 26, 29) that are virulent in rabbit ileal loop models and in human volunteers (14, 17). Although a gene cluster (vps) for the biosynthesis of rEPS and a gene (vpsR) that regulates the expression of several vps biosynthetic genes have been identified (2, 28, 29), the physiology, genetics, and importance of rEPS expression are not well understood and studies on the rugose phenotype have been hampered by the low frequency of switching to the rugose phenotype when available conditions are used. Previously, Morris et al. (14) reported the isolation of only spontaneous rugose variants from a variety of V. cholerae strains after 3 to 4 days of incubation in standard alkaline peptone water (APW) and plating on L agar at 37°C. Wai et al. (22) then reported that V. cholerae El Tor O1 strain TSI-4 was able to spontaneously shift to a rugose colony morphology under starvation conditions. These investigators isolated a few spontaneous rugose variants after smooth colonies were starved in M9 salts for 2 months at 16°C and then plated on L agar at 37°C. Yildiz and Schoolnik (29) showed that, under the nutrient-limiting (carbon-limiting) conditions they tested (30°C incubation in M9 minimal media supplemented with 0.02% glucose), smooth El Tor strains were able to shift to the rugose colonial variant after 20 days of culture but at a frequency of only ∼1%.
In pursuit of our interests in the factors and processes involved in the emergence, pathogenesis, and persistence of epidemic V. cholerae, we studied the prevalence, physiology, and role of rEPS production in various V. cholerae strains which either have been described previously (10, 11) or were obtained from A. Huq (University of Maryland) and L. Campos (FIOCRUZ, Rio de Janeiro, Brazil). The strains included 19 clinical isolates (O1 and non-O1 strains) and 16 environmental isolates (O1 and non-O1 strains) collected over many years and from several continents.
We discovered that a nutrient-poor medium, a modified form of APW, which we have termed APW#3 (1% proteose peptone #3 [Difco], 1% NaCl, pH 8.5), resulted in a high frequency (up to 80%) of N16961 smooth cells shifting to the rugose phenotype. A single smooth colony was inoculated into 3 ml of APW#3 in glass test tubes or 45 ml of APW#3 in 250-ml flasks and incubated statically for 24 to 72 h at 30°C or 37°C. Sterile glass beads (4 mm in diameter) were then added, and the cultures were vortexed to disrupt any aggregates of rugose cells. Following incubation, we observed only a minor decrease in the pH of the culture medium (pH ∼8.3). Appropriate dilutions of each culture were plated on Luria-Bertani (LB) agar (Difco), and colonies were counted by standard plate counting to determine the total number of CFU per milliliter and the frequency of rugose cells. On the basis of at least eleven independent experiments, we found that, after 24 to 48 h of growth at 37°C (in glass tubes), approximately 60% of the smooth cells had switched to the rugose phenotype (Table 1 and Fig. 1). Accordingly, we called this phenotype high-frequency rugose EPS production (HFRP). Time course studies analyzing samples every other hour showed that HFRP was not detected in N16961 until at least 14 h of incubation, at which time we observed a sudden increase in the percentage of rugose variants (0 to 50%). After this initial HFRP spike the percentage of rugose cells increased in the culture, resulting in approximately 60 to 70% rugose cells at 24 h. We found that HFRP was independent of LuxS, RpoS, and ToxT (data not shown). The mechanism of HFRP is presently being studied and might be regulated by phase variation. Phase variation can occur via DNA inversion, DNA recombination, and slipped-strand mispairing and is known to be involved in controlling the expression of several surface structures of gram-negative bacteria, including fimbriae, flagella, outer membrane proteins, lipopolysaccharide, and capsular polysaccharide (8, 12).
TABLE 1.
Frequency of rugose EPS production by V. cholerae strainsa
| Strainb | Serogroup/biotype | Origin | Sourcec (year) | % Rugose coloniesd,e,f
|
|||
|---|---|---|---|---|---|---|---|
| Flask
|
Tube
|
||||||
| 30°C | 37°C | 30°C | 37°C | ||||
| N16961 | O1/E1 Tor | Bangladesh | C (1971) | 31 (1.5) | 47 (0.9) | 70 (0.7) | 74 (1.9) |
| C6709 | O1/E1Tor | Peru | C (1991) | 1 | 23 | 15 | 70 |
| NCTC 6585 | O1/classical | India | C (1943) | 43 (1.4) | 44 (0.2) | 0 | 0 |
| AMS20A73 | O1/classical | China | C (1945) | 3 | 4 | 0 | 0 |
| Aldova | O37 | Czechoslovakia | C (1965) | 0 | 1 | 72 (0.2) | 41 (2.8) |
| 1803 | Non-O1 | Brazil | C (1992) | 0 | 0 | 16 | 87 |
| 1837 | O139 | Bangladesh | C (1992) | 0 | 0.2 | 0 | 0 to 2 |
| P44 | Non-O1 | Peru | E (2000) | 12 | 0 | 0 | 2 |
| 1085-93 | O37 | Germany | E (1993) | 0 | 0 | 0.1 | 0 |
| 141-94 | O70 | Germany | E (1994) | 0 | 0 | 0.3 | 0 |
| 928-93 | O6 | Argentina | E (1993) | 0 | 0.2 | 0.4 | 0 |
A smooth colony was inoculated into glass tubes containing 3 ml of APW#3 or into flasks containing 45 ml of APW#3, and the cultures were incubated statically for 48 and 72 h at 30°C and 37°C, respectively.
Listed are only strains showing HFRP or spontaneous rugose colonies under each condition.
C, clinical; E, environmental.
Values represent the average percentages of cells shifting to a rugose colony morphology.
Readings were taken at 72 h for all strains except N16961, for which the reading was taken at 48 h.
Values in parentheses represent standard errors and are shown only for specific strains showing high levels of HFRP.
FIG. 1.
Colony morphology of smooth and rugose variants of V. cholerae. (A) Colonies at 24 h. (B) Colonies at 72 h.
In order to determine whether HFRP was found in other V. cholerae strains, we tested various clinical and environmental isolates. Consistent with previous studies (14), we found a low frequency (<0.5%) shift to the rugose phenotype in several strains. However, we were surprised to find that 6 of 19 clinical isolates (32%) and only 1 of 16 nonpathogenic strains (6%) could shift to HFRP under the conditions tested (t test; P < 0.05) (Table 1). Of all strains tested, N16961 consistently had the highest HFRP in flasks (24 to 38%, 30°C; 42 to 51%, 37°C) and tubes (68 to 74%, 30°C; 60 to 80%, 37°C) (Table 1). While the results revealed that not all epidemic strains showed HFRP, indicating that HFRP is not essential for persistence, our results do suggest that rEPS has a role in V. cholerae and that HFRP might be more common in clinical strains and might promote persistence of these specific strains. The association of HFRP with clinical epidemic strains is being studied further. Just as many studies rely on in vitro data (in some cases obtained from a single strain) to draw conclusions on the relevance of a phenotype in vivo or in the natural environment, we propose that HFRP has an important role in nature. Interestingly, it is known that persistent bacterial diseases such as cystic fibrosis are caused by only a subpopulation of the Pseudomonas aeruginosa species in which specific cells have the ability to express copious amounts of EPS and form biofilms in the lung (7). In the case of V. cholerae, rEPS and HFRP production may provide some evolutionary or adaptive advantage to that subpopulation of cells in a particular environment.
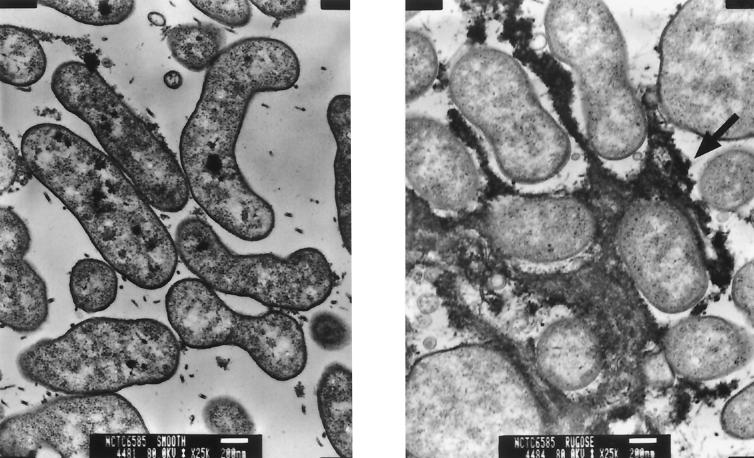
Interestingly, NCTC 6585, a sixth pandemic (classical biotype) strain, consistently showed HFRP in flasks (33 to 48%, 30°C; 44 to 45%, 37°C). In addition, the sixth pandemic strain AMS20A73 also showed HFRP, albeit at a lower frequency (3 to 4%), with HFRP being defined as a >3% shift to the rugose phenotype. A previous study showed that homologous sequences were found in sixth pandemic (classical biotype) strains; however, these investigators were unable to demonstrate rEPS expression in sixth pandemic strains (29). To demonstrate that the rugose classical biotype strain NCTC 6585 overexpressed rEPS, we performed transmission electron microscopy (TEM) on ruthenium red-stained thin sections. For TEM, 2-day-old smooth and rugose colonies on LB agar were removed as 0.5-cm2 blocks and then fixed and stained in a solution of 2% glutaraldehyde-0.075% ruthenium red-50 mM lysine monohydrochloride in 0.1 M cacodylate buffer (pH 7.2) for 1 h at room temperature and then for 18 h at 4°C. Samples were washed twice in 0.1 M cacodylate buffer (pH 7.2), encased in 2% molten Noble agar, and postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.2) overnight at 4°C. Samples were then dehydrated in 30, 50, 70, and 90% ethanol for 10 min each time and twice in 100% ethanol for 15 min each time, followed by two treatments with propylene oxide for 15 min each time and then infiltration with a 1:1 solution of propylene oxide and Epon for 2 h at room temperature and then with 3:1 Epon-propylene oxide overnight. Samples were then placed in pure Epon for 1 h, embedded in Epon, and put in a 60°C oven for 2 days, followed by thin sectioning (50- to 80-nm-thick sections). Sections were stained with uranyl acetate for 20 min and then with lead citrate for 20 min. Samples were examined under a JEOL 1200 EX II transmission electron microscope at 80 kV. Consistent with previous studies that have shown that rugose El Tor cells are surrounded by EPS (22, 29), TEM of rugose NCTC 6585 confirmed the presence of EPS between cells and the absence of this material between smooth cells (Fig. 2).
FIG. 2.
The rugose variant of V. cholerae classical biotype (sixth pandemic) strain NCTC 6585 produces an extracellular glycocalyx. TEM of ruthenium red-stained thin sections of smooth and rugose cells of classical strain NCTC 6585. Micrographs show no stained matrix between smooth cells (left panel) and densely stained matrix between rugose cells (arrow, right panel).
We found that there were differences between strains in colony morphology and in the conditions that induce HFRP (Table 1 and Fig. 1). Examination of rugose classical strains NCTC 6585 and AMS20A73 at 24 to 48 h after plating revealed that they were not as pronounced in structure as N16961 or O139 rugose colonies (Fig. 1). Although it has been known for over 60 years that rugose colony morphology is the result of copious EPS production (25, 26), different colony morphologies and different conditions triggering HFRP suggest that there are differences in the regulation and genetics of rEPS production between strains.
Table 1 shows that HFRP was not restricted to clinical V. cholerae O1 strains. HFRP was consistently found in the Aldova (serogroup O37) strain after culture in tubes (71 to 72%, 30°C; 23 to 50%, 37°C). Interestingly, this strain was responsible for an epidemic in Czechoslovakia in 1965 (1) and in the Sudan in 1968 (30) but has not caused any major outbreaks since. A previous study reported rEPS production in an O139 serogroup strain (MO10) (13), and although we were unable to detect HFRP in the O139 strain (strain 1837) we did find that 0 to 2% of strain 1837 cells could shift to the rugose phenotype and that the colony morphology was similar to that of N16961 rugose colonies. We also found HFRP in strain 1803, a non-O1 clinical isolate. Interestingly, of 16 environmental strains tested, which included many different serogroups, only one strain (P44; serogroup unknown) showed HFRP (12%).
We speculated that there might be differences between strains in the stability of the rugose phenotype. A single colony, obtained by plating a fresh glycerol stock of rugose variants of N16961, NCTC 6585, and Aldova, was inoculated into tubes and examined for the frequency of switching back to the smooth phenotype after 3 days of incubation in nutrient-rich LB (Miller) broth. This medium is optimal for growth of the smooth cell phenotype but still supports the rugose variant. The results in Table 2 show a very low level of rugose-to-smooth (R-S) reversion for N16961 and NCTC 6585 (<1%). Interestingly, statistical analysis using the two-sided Wilcoxon rank sum test showed that the R-S reversion frequency was significantly higher for the Aldova strain than for N16961 and NCTC 6585 at 30°C under static conditions (P = 0.01 and P = 0.02, respectively), at 30°C with shaking (P = 0.03 and P = 0.04, respectively), at 37°C under static conditions (P = 0.002 and 0.006, respectively), and at 37°C with shaking (t test; P < 0.0001). In particular, for cells at 37°C with shaking we observed that 77.7% of the cells had undergone R-S reversion. In addition, using the two-sided Wilcoxon rank sum test, we also found a significant difference (1,000-fold) in the number of CFU per milliliter for strain NCTC 6585 compared with strain N16961 (P = 0.03) and the O37 Aldova strain (P = 0.02) at 37°C with shaking. This implies that this condition inhibits the actual viability of the rugose variant of strain NCTC 6585. These results suggest that there are differences in the stability of the rugose phenotype between strains and in the conditions or genetics that regulate the reversion back to the smooth-cell phenotype. From the R-S reversion data it is tempting to speculate that the stability and viability of the rugose variant could be associated with long-term persistence.
TABLE 2.
Rugose stability and frequency of reversion to the smooth cell phenotypea
| Strain | % of cells undergoing R-S reversionb at:
|
|||
|---|---|---|---|---|
| 30°C
|
37°C
|
|||
| Static | Shaking | Static | Shaking | |
| N16961 | 0.8 ± 0.2 (5.3 × 108) | 0.4 ± 0.1 (1.7 × 108) | 0.9 ± 0.3 (1.7 × 108) | 0 (5.8 × 107) |
| NCTC6585 | 0.9 ± 0.2 (4.1 × 108) | 0.2 ± 0.1 (4.0 × 107) | 0.5 ± 0.2 (3.9 × 108) | 0 (3.4 × 104) |
| Aldova | 4.4 ± 0.9 (4.6 × 108) | 27.5 ± 2.3 (1.5 × 108) | 27.2 ± 2.2 (1.9 × 108) | 77.7 ± 2.2 (4.1 × 107) |
A single rugose colony was inoculated into LB (Miller) broth and incubated with shaking or statically for three days at 30°C or 37°C. LB was chosen since it provides stable maintenance of rugose and smooth cells (14). Cultures were plated, and colonies were counted to determine the relative numbers of rugose and smooth colonies. Results presented are the mean values of at least four independent experiments.
The ± values represent standard error, and the values in parentheses represent total CFU per milliliter after three days of culture.
We found that subculture of rugose colonies onto thiosulfate-citrate-bile salts-sucrose (TCBS) agar resulted in “smooth” colonies. However, repeat subculturing of these colonies on LB agar produced typical rugose colonies. This result demonstrates that TCBS agar inhibits (or masks) the rugose colony morphology and suggests that studies using TCBS agar to isolate V. cholerae from the environment (or clinical sources) will not detect the rugose variant from a particular sample. On the basis of this result, we suggest that the existence of V. cholerae in the rugose phenotype in the environment might be more common than was previously thought.
Production of the rEPS has been shown to promote V. cholerae resistance to a variety of environmental stresses, such as chlorine, UV light, hydrogen peroxide, and complement (14, 17, 23, 29). In order to determine whether rEPS production by classical strain NCTC 6585 promoted resistance to environmental stresses, we compared the levels of resistance to chlorine exposure between a smooth-cell strain and a rugose variant. Chlorine resistance was assayed (four independent experiments) by using a 1:50 dilution of an overnight culture of strain NCTC 6585 smooth and rugose cells in 3 ml of fresh LB (Miller) broth. Cultures were then incubated statically at 37°C for 3 h until reaching a density of ∼2 × 108 CFU/ml; the cells were then harvested by centrifugation and suspended in phosphate-buffered saline (pH 7.2) containing 3 mg of free chlorine per liter (sodium hypochlorite; Sigma). After 5 min of exposure in 3 mg of chlorine per liter, cultures were serially diluted and plated on LB agar to determine the number of surviving bacterial cells. Consistent with the findings of rugose El Tor strains (29), we found that the rugose variant of classical strain NCTC 6585 was 10,000-fold more resistant to chlorine (5 min of exposure to 3 mg/liter) than its smooth-cell counterpart. This result demonstrates that the rEPS of classical biotype V. cholerae strains also promotes cell survival and persistence.
Previous studies have reported an association among EPS, motility, and biofilm formation. Traditional motility tests in semisolid media containing 0.3% agar after 4 h at 37°C were performed (in triplicate) to compare smooth and rugose variants of N16961. Colony counts of overnight cultures to be used in motility tests showed no difference between the smooth and rugose variants in the number of CFU per milliliter. Compared with the average motility of smooth N16961 (20 mm), we found a 2.5-fold reduction in the motility of the rugose variant (8.5 mm). Interestingly, we found that smooth and rugose cells obtained from fresh colonies were flagellated, as were rugose planktonic cells and rugose cells in a biofilm from 48-h LB broth cultures incubated statically at 37°C (data not shown). These results suggest that V. cholerae cells expressing rEPS have flagella but are less motile than smooth strains under the conditions tested. It might be that the flagellar movement of rugose cells is inhibited by copious amounts of EPS or is defective due to some other mechanism. Although motility is required for biofilm formation and motility is impaired in rugose variants, this impairment might be offset by the elevated production of the EPS and appears to result in greater biofilm-forming ability than in the smooth variant.
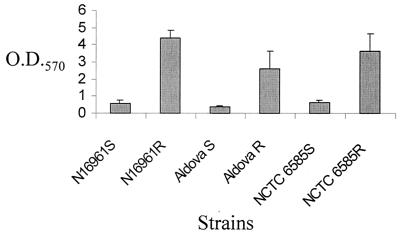
Since EPS is known to be important for biofilm formation, we investigated and compared the abilities of the rugose and smooth variants to form biofilms. While smooth strains of NCTC 6585 and N16961 incubated statically in LB at 37°C for 18 h produced no visible biofilm, rugose variants of both strains produced an obvious biofilm at the air-broth interface. We also quantitatively determined the biofilm-forming abilities of El Tor strain N16961, classical strain NCTC 6585, and the O37 Aldova strain by the method of Watnick et al. (24) with minor modifications (Fig. 3). In the tube biofilm assay (performed in triplicate), borosilicate glass tubes were used as surfaces of bacterial attachment and contained 500 μl of LB broth inoculated with a 1:100 dilution of overnight bacterial culture. The cultures were incubated statically at room temperature for 24 h, after which time the cultures were discarded and the tubes were rinsed vigorously with distilled water to remove nonadherent cells, filled with 600 μl of 0.1% crystal violet (Sigma), incubated for 30 min at room temperature, and again rinsed with water. Quantitative biofilm formation was assayed by measuring the optical density at 570 nm of the solution produced by extracting cell-associated dye with 600 μl of dimethyl sulfoxide (Sigma). The quantitative biofilm results illustrated in Fig. 3 show that the biofilm-forming abilities of the rugose variants of N16961, NCTC 6585, and Aldova strains are 7.7-, 6.6-, and 5.5-fold greater, respectively, than those of their corresponding smooth variants. These results clearly indicate that rEPS production in El Tor, classical, and non-O1 strains is associated with increased biofilm-forming ability.
FIG. 3.
Biofilm formation by smooth and rugose colony variants of V. cholerae. Glass test tubes containing 500 μl of LB broth were inoculated with a 1:100 dilution of overnight cultures of N16961, NCTC 6585, and Aldova O37 strains and incubated statically at room temperature for 24 h. Quantitative biofilm assays were performed as described previously (24).
In conclusion, in this paper we describe a novel phenotypic switch, called HFRP, in V. cholerae. Our findings link several common themes that have recently been shown to be important for long-term persistence and survival, such as EPS expression, resistance to environmental stresses, and biofilm formation. It appears that some V. cholerae strains have evolved an efficient mechanism for a high-frequency shift to rEPS expression that might provide an adaptive advantage in particular niches. Our findings are important to human public health as they might explain why some epidemic strains of V. cholerae can persist for many years in the environment and why rugose (and HFRP) V. cholerae strains would be resistant to various antibacterial treatments and might be selectively favored in certain stressful environments. On the basis of our results, we propose that rEPS expression and HFRP are important in the epidemiology and long-term persistence of V. cholerae and that similar evolutionary selection might occur in other bacterial pathogens to promote their long-term persistence, virulence, and adaptation in various niches. Understanding the mechanisms that promote the persistence of V. cholerae in the environment is a key factor in better understanding this important human pathogen and might lead to ways to predict, detect, and prevent cholera epidemics.
Acknowledgments
We thank Amanda King for technical assistance and Glenn Morris for reviewing the manuscript. We thank Karl Klose for supplying the LuxS mutant, Andrew Camilli for supplying the rpoS mutant plasmid, and Vic DiRita for supplying the toxT mutant plasmid.
This work was supported by NIH grant AI45637 (D.K.R.K.). D.K.R.K. is a recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
REFERENCES
- 1.Aldova, E., K. Laznickova, E. Stepankova, and J. Lietava. 1968. Isolation of nonagglutinable vibrio from an enteritis outbreak in Czechoslovakia. J. Infect. Dis. 118:25-31. [DOI] [PubMed] [Google Scholar]
- 2.Ali, A., J. A. Johnson, A. A. Franco, D. J. Metzger, T. D. Connell, J. G. J. Morris, and S. Sozhamannan. 2000. Mutations in the extracellular protein secretion pathway genes (eps) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect. Immun. 68:1967-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barua, D. 1972. The global epidemiology of cholera in recent years Proc. R. Soc. Med. 65:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colwell, R. R., P. R. Brayton, D. J. Grimes, D. R. Roszak, S. A. Huq, and L. M. Palmer. 1985. Viable, but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology. 3:817-820. [Google Scholar]
- 5.Felix, H. 1971. The development of the cholera epidemic in West Africa. Bull. Soc. Pathol. Exot. Filiales 64:561-582. (In French.) [PubMed] [Google Scholar]
- 6.Goodgame, R. W., and W. B. G. Greenough III. 1975. Cholera in Africa: a message for the West. Ann. Intern. Med. 82:101-106. [DOI] [PubMed] [Google Scholar]
- 7.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson, I. R., P. Owen, and J. P. Nataro. 1999. Molecular switches—the ON and OFF of bacterial phase variation. Mol. Microbiol. 33:919-932. [DOI] [PubMed] [Google Scholar]
- 9.Kamal, A. M. 1974. The seventh pandemic of cholera, p. 1-14. In D. Barua and W. Burrows (ed.), Cholera. Saunders, Philadelphia, Pa.
- 10.Karaolis, D. K. R., R. Lan, and P. R. Reeves. 1994. Molecular evolution of the seventh pandemic clone of Vibrio cholerae and its relationship to other pandemic and epidemic V. cholerae strains. J. Bacteriol. 176:6199-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaolis, D. K. R., R. Lan, and P. R. Reeves. 1995. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J. Bacteriol. 177:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krinos, C. M., M. J. Coyne, K. G. Weinacht, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414:555-558. [DOI] [PubMed] [Google Scholar]
- 13.Mizunoe, Y., S. N. Wai, A. Takade, and S. I. Yoshida. 1999. Isolation and characterization of rugose form of Vibrio cholerae O139 strain MO10. Infect. Immun. 67:958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris, J. G., Jr., M. B. Sztein, E. W. Rice, J. P. Nataro, G. A. Losonsky, P. Panigrahi, C. O. Tacket, and J. A. Johnson. 1996. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J. Infect. Dis. 174:1364-1368. [DOI] [PubMed] [Google Scholar]
- 15.Pollitzer, R. 1959. Cholera monograph series 43. World Health Organization, Geneva, Switzerland. [PubMed]
- 16.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karasawa, H. Kurazano, A. Pal, and Y. Takeda. 1993. Emergence of a novel strain of Vibrio cholerae with epidemic potential in Southern and Eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 17.Rice, E. W., C. H. Johnson, R. M. Clark, K. R. Fox, D. J. Reasoner, M. E. Dunnigan, P. Panigrah, J. A. Johnson, and J. G. J. Morris. 1993. Vibrio cholerae O1 can assume a “rugose” survival form that resists killing by chlorine, yet retains virulence. Int. J. Environ. Health Res. 3:89-98. [Google Scholar]
- 18.Roszak, D. B., D. J. Grimes, and R. R. Colwell. 1984. Viable but non-recoverable stage of Salmonella enteritidis in aquatic systems. Can. J. Microbiol. 30:334-338. [DOI] [PubMed] [Google Scholar]
- 19.Shiba, T., R. T. Hill, W. L. Straube, and R. R. Colwell. 1995. Decrease in culturability of Vibrio cholerae caused by glucose. Appl. Environ. Microbiol. 61:2583-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow, D. L., and A. A. Ries. 1992. Cholera in the Americas. Guidelines for the technician. JAMA 267:1495-1499. [PubMed] [Google Scholar]
- 21.Tauxe, R. V., and P. A. Blake. 1992. Epidemic cholera in Latin America. JAMA 267:1388-1390. [PubMed] [Google Scholar]
- 22.Wai, S. N., Y. Mizunoe, A. Takade, S. I. Kawabata, and S. I. Yoshida. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, P. B. 1940. The characteristic hapten and antigen of rugose races of cholera and El Tor vibrios. J. Pathol. Bacteriol. 50:160-164. [Google Scholar]
- 26.White, P. B. 1938. The rugose variant of vibrios. J. Pathol. Bacteriol. 46:1-6. [Google Scholar]
- 27.Xu, H.-S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of non-culturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 28.Yildiz, F. H., N. A. Dolganov, and, G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zinnaka, Y., and C. C. J. Carpenter, Jr. 1972. An enterotoxin produced by noncholera vibrios. Johns Hopkins Med. J. 131:403-411. [PubMed] [Google Scholar]