Abstract
The subcellular localization, interacting partners, and function of GS15, a Golgi SNARE, remain to be established. In our present study, it is revealed that unlike proteins (Bet1 and the KDEL receptor) cycling between the Golgi and the intermediate compartment (IC, inclusive of the ER exit sites), GS15 is not redistributed into the IC upon incubation at 15°C or when cells are treated with brefeldin A. Immuno-electron microscopy (immuno-EM) reveals that GS15 is mainly found in the medial-cisternae of the Golgi apparatus and adjacent tubulo-vesicular elements. Coimmunoprecipitation experiments suggest that GS15 exists in a distinct SNARE complex that contains SNAREs (syntaxin5, GS28, and Ykt6) that are implicated in both ER-to-Golgi and intra-Golgi transport but not with SNAREs involved exclusively in ER-to-Golgi traffic. Furthermore, components of COPI coat can be selectively coimmunoprecipitated with GS15 from Golgi extracts. Overexpression of mutant forms of GS15 affects the normal distribution of cis- and medial-Golgi proteins (GS28, syntaxin 5, and Golgi mannosidase II), whereas proteins of the trans-Golgi and TGN (Vti1-rp2/Vti1a and syntaxin 6) and Golgi matrix/scaffold (GM130 and p115) are less affected. When the level of GS15 is reduced by duplex 21-nt small interfering RNA (siRNA)-mediated knockdown approach, diverse markers of the Golgi apparatus are redistributed into small dotty and diffuse labeling, suggesting an essential role of GS15 in the Golgi apparatus.
INTRODUCTION
Two models have been proposed to account for the vectorial movement of proteins through the Golgi apparatus. The vesicle transport model suggests that forward movement of cargo proteins through the Golgi is mediated by vesicles that bud from one cisternae and fuse with the next adjacent cisternae in the Golgi stack. An important criterion of this model is that a rapid flux of protein traffic through the Golgi occurs, whereas the structural integrity and positioning of each cisternae remains static (Rothman and Wieland, 1996). The maturation model suggests that cargo proteins are transported through the Golgi via cisternal progression. In this model each cisternae is constantly being remodeled via an active retrograde vesicle transport pathway. By selectively removing proteins that function early in the Golgi while retaining those that function later it is possible that anterograde flux is mediated via a nonvesicular pathway. According to this model the major vesicle transport pathway in the Golgi is the retrograde pathway, which is likely mediated by COP1 (Glick and Malhotra, 1998; Allan and Balch, 1999). These two models are not mutually exclusive and both could operate in a concerted manner according to the physiological needs of the cell (Pelham and Rothman, 2000).
Vesicle-mediated transport, either in the anterograde or retrograde direction, involves three major steps. The first, which is executed by coat proteins, involves vesicle budding and cargo selection (Scales et al., 2000; Antonny and Schekman, 2001; Boehm and Bonifacino, 2001; Pfeffer, 2001). COPII coats mediate ER export at ER exist sites (ERES), whereas COPI coats mediate retrograde transport both between the Golgi and ER and between various Golgi cisternae. In addition, COPI coats have also been implicated in anterograde transport through the Golgi apparatus and in endocytic vesicle transport (Gruenberg, 2001; Whitney et al., 1995). The second step involves vesicle movement to and tethering with the relevant acceptor compartment. Members of the Rab GTPase family, their effector proteins, and the cytoskeletons play an important role in this step (Pfeffer, 1999; Zerial and McBride, 2001). Finally, vesicle docking and fusion with the acceptor compartment is mediated, at least in part, by N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs; Söllner et al., 1993; Jahn and Südhof, 1999; Bock et al., 2001; Chen and Scheller, 2001). Approximately 35 members of the SNARE superfamily have been identified in mammalian cells, and they are distributed in various compartments of the secretory and endocytic pathways. An interaction between SNAREs on transport vesicles (v-SNAREs) and on the target compartment (t-SNAREs) leads to the formation of a high-affinity trans-SNARE complex that underlies the final stages of vesicle docking and/or fusion. A current emerging theory is that combinatory use of different SNAREs will give rise to a wide array of different SNARE complexes, and a given SNARE could function in several distinct SNARE complexes. In this regards, it is important to define the subcellular localizations, interacting partners and potential functions of those SNAREs that are not fully studied.
The identification of SNAREs and the resulting complexes that function within the Golgi will ultimately lead to a greater understanding of intra-Golgi protein transport. We have previously identified a molecule (GS15) that is homologous to other SNAREs but that is concentrated within the Golgi apparatus (Xu et al., 1997). In the present study we show that GS15 is often found in the medial-cisternae of the Golgi apparatus and the associated structures. Biochemical studies suggest that it forms a complex with three other SNAREs (Syntaxin5, GS28, and Ykt6) to form a distinct GS15/Syn5/GS28/Ykt6 SNARE complex. An role of GS15 in the Golgi apparatus was suggested by studies employing overexpression of mutant forms of GS15 and by reducing its expression using siRNA-mediated knockdown approach.
MATERIALS AND METHODS
Antibodies
The following rabbit polyclonal antibodies were described previously: GS15 (Xu et al., 1997); Bet1 (Zhang et al., 1997); Sec22b (Zhang et al., 1999); Ykt6 (Zhang and Hong 2001); syntaxin 5, syntaxin 6, and Vti1-rp2/Vti1a (Xu et al., 1998). Antibodies against α-, β-, β′-, γ-, ε-COP were kind gifts from F. T.Wieland (University of Heidelberg, Germany). Mouse monoclonal antibodies against GS15, GM130, p115 were obtained from BD Transduction Laboratories (Franklin Lakes, NJ). mAb against mammalian KDEL receptor has been described previously (Tang et al., 1995). mAb against Golgi mannosidase II was purchased from Babco (Berkeley, CA).
DNA Constructs
The following oligonucleotides read from 5′ to 3′: 1, TTGGAATTCAGCAGATTGGACTCGAGCTCAGAGT; 2, CTTGTCGACTCA-CTTCCGGGTGTCTCGCCCAGACC; 3, ACGGAAGACGCGAACCGATACCTAGATGGCATG; 4, GTATCGGTTCGCGTCTTCCGTG-TCCCTGTCGAT; 5, GGAGTCGACTCACGTCCTTGTCCTCGA-AAAGAG.
To construct GS15ΔTM, oligonucleotides 1 and 2 were used to retrieve the cytoplasmic domain of GS15 (1–86). The EcoRI/SalI fragment was isolated and then inserted into pDmyc vector (pCI-neo vector from Promega with two myc epitopes inserted in between NheI and XhoI sites). For constructing GS15Q49A, oligonucleotides 1 and 4 were used to retrieve the N-terminal portion of GS15 (1–49). Oligonucleotide 4 contains sequence complementary to GCG (Ala) instead of CAG (Gln). Oligonucleotides 3 and 5 were used to retrieve the C-terminal fragment of GS15 (49–111). Oligonucleotide 3 harbors the mutation of GCG (Ala) instead of CAG (Gln). Both PCR products were gel purified and used as templates for the subsequent PCR reaction utilizing oligonucleotides 1 and 5 to produce a construct of GS15 (Q49A), in which the Q49 of GS15 was mutated to Ala. This cDNA was cloned into pDmyc vector and confirmed by DNA sequencing.
Cell Culture and Transfection
NRK, Vero, and CHO cells were purchased from the ATCC (Rockville, MD). Cells were cultured in minimal essential Eagle's media supplemented with 10% fetal bovine serum and antibiotic-antimycotic. The medium was changed daily. Transfection was performed using LipofectAMINE system as described by the manufacturer (Life Technologies, Rockville, MD).
Indirect Immunofluorescence Microscopy
Immunofluorescence microscopy was performed as described previously (Xu et al., 1997, 1998). For temperature treatment of cells, NRK cells were incubated at 15°C for 3 h and then fixed for immunofluorescence microscopy. For brefeldin A treatment, cells grown on coverslips were incubated in the presence of brefeldin A (10 μg/ml) for 1 h at 37°C, washed twice with PBSCM (PBS with 1 mM CaCl2, 1 mM MgCl2), and then fixed in 3% paraformaldehyde. Fixed cells were then permeabilized and incubated with antibodies against GS15 and antibodies against mannosidase II, the KDEL receptor, or Bet1 for double labeling.
Immunoprecipitation
Specific antibodies (5–10 μg) against SNARE proteins bound to protein A sepharose beads (Pharmacia, Piscataway, NJ) were incubated overnight with 500 μg of Golgi extracts in immunoprecipitation buffer (20 mM HEPES, pH 7.3, 100 mM KCl, 1 mM DTT, 5 mM EDTA, 0.2 mM ATP, and 1% Triton X-100) at 4°C. Beads were then washed twice with buffer A (identical to immunoprecipitation buffer except that it contains 0.5% Triton X-100) and three times with buffer B (identical to buffer A except that it contains 0.2% Triton X-100) before being resuspended in SDS sample buffer. Immunoprecipitated proteins and 10% of Golgi extracts used in the immunoprecipitation were separated on SDS-PAGE and transferred to a Hybond-C nitrocellulose filter before sequential incubation with the respective primary antibodies and secondary antibodies. The signals were visualized by the SuperSignal chemiluminescent kit (Pierce Chemical, Rockford, IL). For large-scale immunoprecipitation, proteins immunoprecipitated from 50 mg of Golgi membrane extract were pooled, separated on SDS-PAGE, and then stained with Coomassie blue. Protein bands were excised, trypsin-digested, and then eluted. Finally, HPLC purification of peptides and Edman microsequencing were carried out to determine the amino acid sequences.
Immuno-EM
Immuno-EM was performed essentially as described previously (Martin et al., 2000).
GS15 Knockdown by Duplex 21-nt Small Interfering RNA (siRNA)
This was performed essentially as reported previously (Elbashir et al., 2001). siRNA was purchased from Dharmacon (Lafayette, CO). The GS15-specific siRNA sequence was from position 92–112 or 148–168. Transfection of siRNA was performed as described in the manual using Oligofectamine (Life Technologies). Briefly, Hela cells were grown in 24-well plate overnight. Twelve microliters OPTIMEM 1 medium and 2 μl oligofectamine per well were premixed for 5 min. Meanwhile, 50 μl OPTIMEM 1 medium were mixed with 3 μl siRNA. These two mixtures were then combined and incubated for 20–25 min. The final concentration of siRNA was 100 nM. The entire mixture was then added to the cells and incubated for 24–48 h before being fixed for immunofluorescence analysis.
RESULTS
GS15 Does Not Recycle to Earlier Elements of the Secretory Pathway
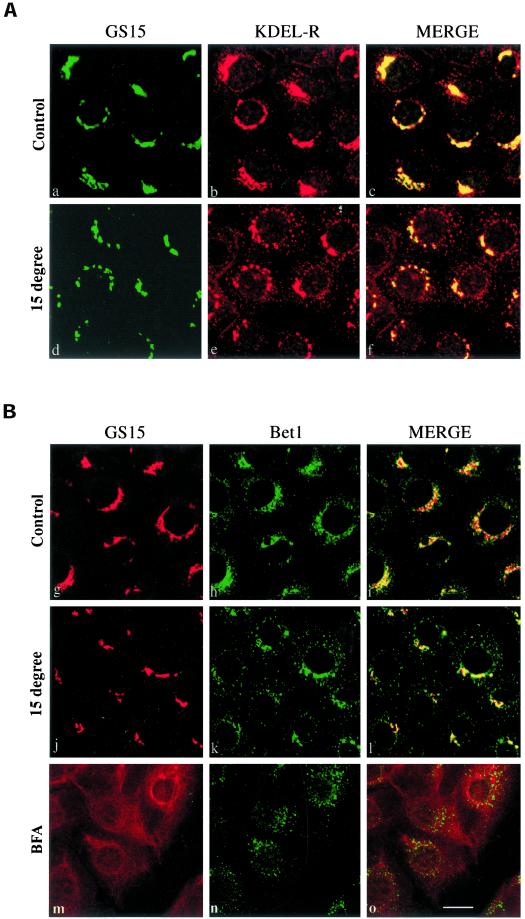
Several proteins that traffic between the ER and the Golgi apparatus constantly recycle via early elements of the secretory pathway. This can be visualized by prolonged incubation of cells at 15°C, under which proteins that normally traffic between the ER and the Golgi such as ERGIC53/p58 (Hauri et al., 2000), the KDEL receptor (Tang et al., 1993), Bet1 (Zhang et al., 1997), Sec22b/ERS-24 (Zhang et al., 1999), syntaxin 5 (Rowe et al., 1998), and some members of the p24 protein family (Fullekrug et al., 1999) accumulate in the intermediate compartment (IC; inclusive of ERES as well as post-ER transport intermediates). GS15, a Golgi SNARE, was identified based on its sequence homology with yeast and mammalian Bet1 (Xu et al., 1997). Bet1p and Bet1 are involved in ER-to-Golgi transport in yeast (Newman et al., 1990) and mammalian cells (Zhang et al., 1997), respectively. Bet1 has been shown to cycle between the Golgi and the IC (Zhang et al., 1997). In view of the sequence homology between GS15 and Bet1, it is of interest to examine whether GS15 also cycles via the IC. As shown in Figure 1A, GS15 exhibited a very compact peri-nuclear localization that corresponds to the Golgi apparatus (see below). Although there was some overlap between the KDEL receptor and GS15 in this peri-nuclear region, the KDEL receptor was also present on numerous peripheral structures characteristic of the IC (Figure 1A, a–c). Labeling of the KDEL receptor in peripheral IC structures became more prominent and more extensive when cells were incubated at 15°C (Figure 1Ae). In marked contrast, the localization of GS15 was essentially unchanged after prolonged incubation at 15°C (Figure 1A, d and f). Similar results were obtained when we compared the distribution of GS15 and Bet1 (Figure 1B). GS15 and Bet1 were both enriched in the peri-nuclear Golgi region in control cells (Figure 1B, g–i). In contrast to the compact Golgi labeling of GS15, Bet1 labeling was distributed more extensively in vesicular structures surrounding the Golgi apparatus marked by GS15 and also expanding into the cell periphery. When cells were incubated at 15°C, the peripheral labeling characteristic of the IC became more profound and extensive for Bet1 (Figure 1Bk), with concomitant reduction in the peri-Golgi labeling. GS15 labeling remained in the compact Golgi apparatus with no detectable levels in the IC (Figure 1Bj).
Figure 1.
GS15 does not recycle back to the IC. NRK cells cultured at 37°C (A: a–c; B: g–i), preincubated at 15°C for 3 h (A: d–f; B: j–l), or pretreated with brefeldin A at 10 μg/ml for 60 min (B: m–o) were fixed and processed for double-labeling to reveal the distribution of GS15 and the KDEL receptor (KDEL-R) (A) or GS15 and Bet1 (B). As shown, 15°C incubation resulted in a shift of KDEL-R and Bet1 from the Golgi to peripheral IC, while having no effects on GS15 distribution in the compact Golgi. Brefeldin A redistributed GS15 to diffuse ER-like structure, while Bet1 was redistributed into more peripheral dotted structures. Bar, 10 μM.
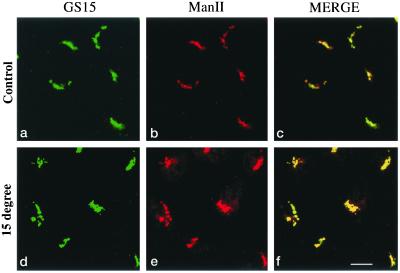
Proteins that cycle via the early secretory pathway have also been shown to be redistributed into the IC consisting mainly of ERES (Ward et al., 2001) after treatment of cells with brefeldin A, whereas nonrecycling Golgi proteins are redistributed back into the ER-like structures (Klausner et al., 1992; Tang et al., 1995). We have therefore performed double labeling of GS15 with Bet1 in brefeldin A–treated cells. As shown (Figure 1B, m–o), unlike Bet1, which was redistributed into spotty structures (Figure 1Bn), GS15 was redistributed into ER-like structures (Figure 1Bm). In addition, GS15 and ManII were colocalized in the compact Golgi apparatus in both control cells (Figure 2, a–c) as well as in cells preincubated at 15°C (Figure 2, d–f). These results suggest that like Man II, but unlike Bet1 or other cycling proteins, GS15 does not recycle back to the IC and behaves as a resident Golgi protein.
Figure 2.
GS15 behaves the same as Golgi mannosidase II (ManII). NRK cells cultured at 37°C (panels a–c) or preincubated at 15°C for 3 h (panels d–f) were fixed and processed for double-labeling to reveal the distribution of GS15 and ManII. As shown, both GS15 and ManII are distributed in compact Golgi structure at both temperatures. Bar, 10 μM.
GS15 Is Enriched in the Medial Cisternae and Associated Tubular Vesicular Elements
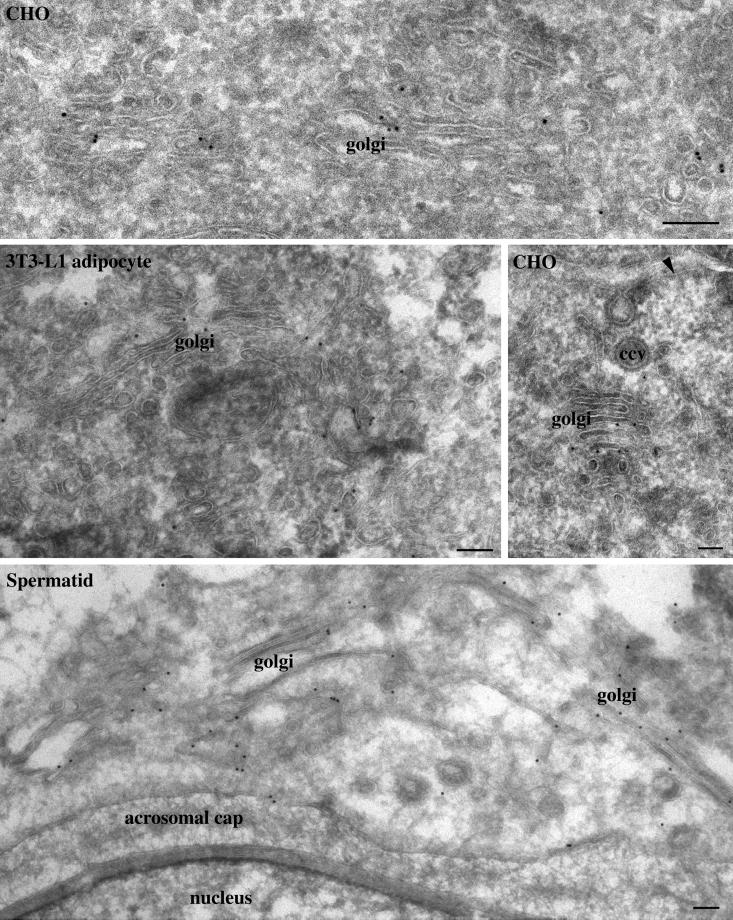
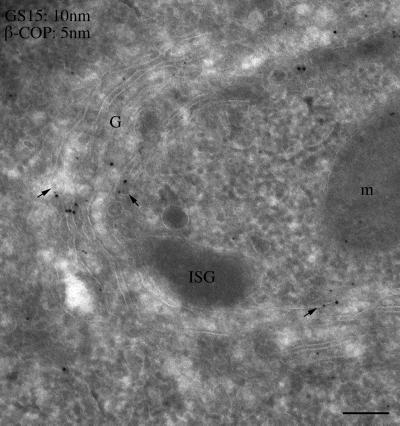
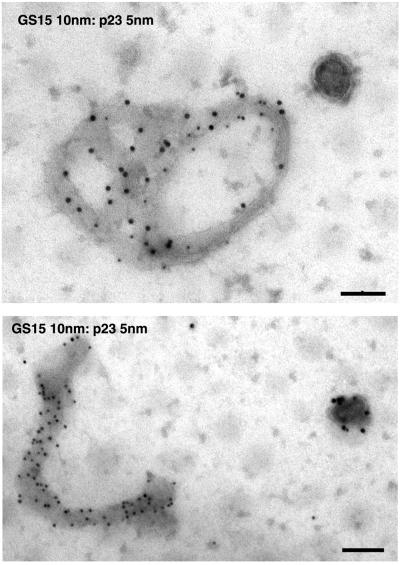
To define the Golgi structures in which GS15 is mainly localized, immunogold labeling of GS15 was performed using cryosections derived from CHO and 3T3-L1 cells as well as from testis. As shown in Figure 3, GS15 labeling was often found in the medial cisternae of the Golgi apparatus. In addition, GS15 labeling was also seen in vesicular tubular elements on the edges of the Golgi cisternae. Similar enrichment of GS15 labeling in medial cisternae and associated structures was observed in spermatid in cryosections derived from testis (Figure 3). In addition, GS15 was also mainly detected in the medial cisternae in exocrine cells in cryosections derived from pancreas (Figure 4). Importantly, some GS15 labeling could be seen in the vicinity of β-COP labeling (arrows in Figure 4, small arrows), indicating that GS15 might be incorporated into COPI-coated vesicles in the medial cisternae of the Golgi apparatus. When whole-mount of isolated Golgi apparatus was immunogold-labeled, abundant labeling for GS15 and the KDEL receptor (p23) could be observed (Figure 5). These results indicate that GS15 is likely enriched in the medial cisternae of the Golgi apparatus and its adjacent tubular and vesicular structures.
Figure 3.
GS15 is enriched in the medial cisternae and adjacent vesicular-tubular structures. Cryosections derived from indicated cells or tissues were processed for immunogold labeling at the electron microscopy level. As shown, GS15 is detected mainly on medial cisternae and its associated vesicular-tubular structures in CHO cells, 3T3-L1 adipocytes, and spermatid. Size bar, 100 nm.
Figure 4.
GS15 is detected on vesicular-tubular profiles marked by COPI coat in the medial cisternae of the Golgi. Cryosections derived from pancreas was double-labeled with antibodies against GS15 (revealed by 10 nm gold particles) and mAb against β-COP (revealed by 5-nm gold particles). As shown, GS15 and β-COP could be seen in the vicinity of the same vesicular-tubular structures (indicated by arrows). Size bar, 100 nm.
Figure 5.
GS15 is present at high concentrations on isolated Golgi cisternae. Isolated Golgi apparatus were double-labeled with antibodies against GS15 (revealed by 10-nm gold particles) and mAb against the KDEL receptor (p23) (revealed by 5-nm gold particles). The whole-mount Golgi cisternae were examined by EM. Size bar, 200 nm.
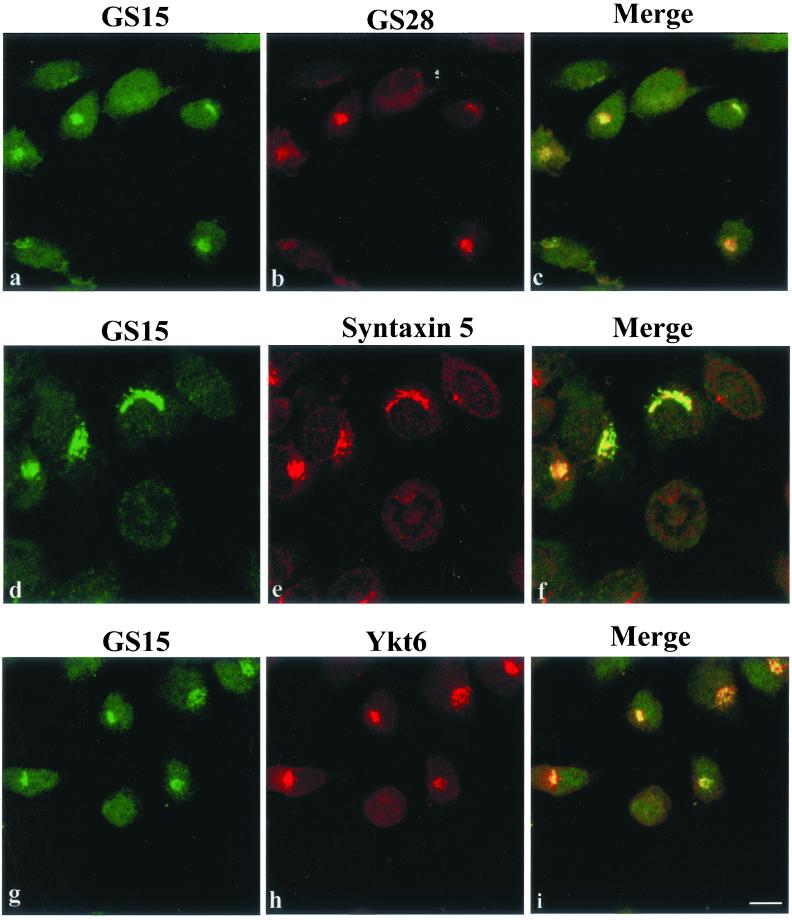
A Distinct SNARE Complex Consisting of GS15, Syntaxin 5, Ykt6, and GS28
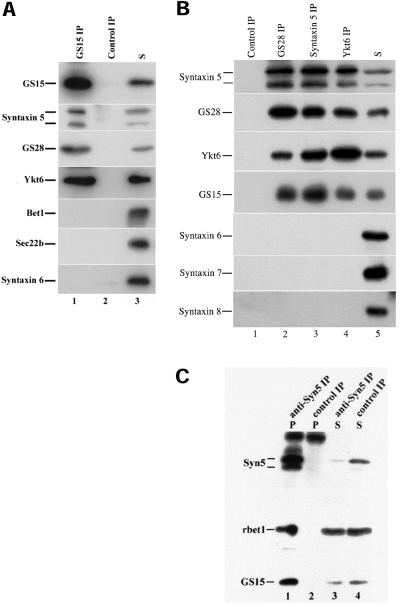
Interactions among SNAREs participate in the final stage of vesicle docking and also in fusion (Söllner et al., 1993; Jahn and Südhof, 1999; Chen and Scheller, 2001). To define the potential partners of GS15, we have performed coimmunoprecipitation experiments (Figure 6A). When the detergent extract of Golgi-enriched membranes derived from rat liver was immunoprecipitated with antibodies against GS15, the majority of GS15 was found in the immunoprecipitate (lane 1). Control antibodies did not precipitate GS15 (lane 2). Significantly, about 20% of syntaxin 5 was coimmunoprecipitated by antibodies against GS15 but not by control antibodies. Significant amounts (>10%) of GS28 and Ykt6 were also coimmunoprecipitated by antibodies against GS15 but not by control antibodies. In marked contrast, Bet1, Sec22b, or syntaxin 6 were neither coimmunoprecipitated by antibodies against GS15 nor by control antibodies. These results suggest that GS15 exists in a SNARE complex(es) with syntaxin 5, GS28, and Ykt6 but not Bet1, Sec22b, or syntaxin 6. To gain further insight into the nature of GS15-containing SNARE complex(es), detergent extracts of Golgi-enriched membranes were also immunoprecipitated with antibodies against GS28, syntaxin 5, and Ykt6. As shown in Figure 6B, syntaxin 5, GS28, Ykt6, and GS15 could be specifically immunoprecipitated. The reciprocal coimmunoprecipitation of syntaxin 5, GS28, Ykt6, and GS15 by antibodies against each of them suggest that there exists a unique SNARE complex consisting of these four SNAREs. Consistent with the fact that syntaxin 5 exists in several distinct SNARE complexes (Xu et al., 2000; Zhang and Hong 2001), antibodies against syntaxin 5 not only coimmunoprecipitated GS15 but also Bet1 (Figure 6C). Because GS15 antibodies did not coimmunoprecipitate Bet1, the identified GS15/syntaxin5/GS28/Ykt6 SNARE complex, represents a unique syntaxin 5-containing SNARE complex.
Figure 6.
Reciprocal coimmunoprecipitation of GS15, syntaxin 5, GS28 and Ykt6. A: Golgi detergent extracts were immunoprecipitated with antibodies against GS15 (lane 1) or control antibodies (lane 2). The immunoprecipitates of anti-GS15 (lane 1) or control antibodies (lane 2) and 10% of the starting material (lanes 3) were resolved by SDS-page and processed for immunoblot to detect the respective proteins as indicated on the left. As shown, syntaxin 5, GS28 and Ykt6, but not Bet1, Sec22b or syntaxin 6 were coimmunoprecipitated by anti-GS15 antibodies. B: Golgi detergent extracts were immunoprecipitated with antibodies against GS28 (lane 2), syntaxin 5 (lane 3), Ykt6 (lane 4), or control antibodies (lane 1). The immunoprecipitates, along with 10% starting material (S) were resolved by SDS-page and analyzed by immunoblot to detect the respective proteins as indicated on the left. C. Golgi detergent extracts were immunoprecipitated with antibodies against syntaxin 5 (lanes 1 and 3) or control antibodies (lanes 2 and 4). The immunoprecipitates (lanes 1 and 2) and 10% supernatants (lanes 3 and 4) as indicated were resolved by SDS-page and processed for immunoblot to detect the indicated proteins.
Coimmunoprecipitation of Components of COPI Coat with GS15
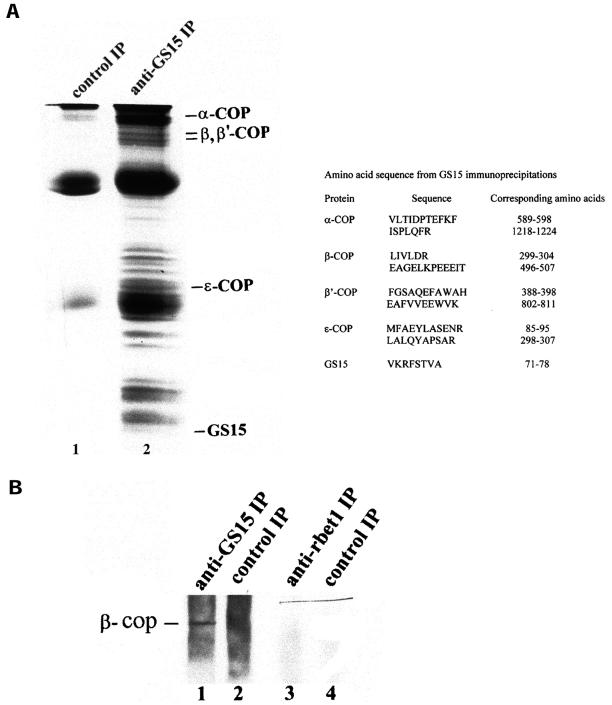
To gain additional understanding about proteins interacting with GS15, immunoprecipitation of Golgi detergent extracts with GS15 antibodies was performed in a large scale. As shown in Figure 7A, numerous proteins were coimmunoprecipitated by antibodies against GS15 (lane 2), and these proteins were absent in the control immunoprecipitate using rabbit IgG (lane 1). Abundant protein bands were excised from the gel and subjected to proteolytic digestion, and resultant peptides were microsequenced. Intriguingly, several components of coatomer (COPI coat) were revealed, including α-COP, β-COP, β′-COP, and ε-COP. Coimmunoprecipitation of COPI components with GS15 is specific because components of COPI coat were not coimmunoprecipitated by antibodies against Bet1 (Figure 7B) or control IgG (Figure 7, A and B). These results suggest that components of the COPI coat can interact with GS15 or a GS15-containing protein complex. In conjunction with the coexistence of some GS15 in the vicinity of COPI-positive vesicular tubular structures adjacent to the medial cisternae, these results imply that GS15 may be a component of COPI-mediated transport intermediates originating from the medial cisternae.
Figure 7.
Coimmunoprecipitation of components of COPI coat by anti-GS15 antibodies. A: Golgi detergent extracts were immunoprecipitated with control antibodies (lane 1) or anti-GS15 antibodies (lane 2). The immunoprecipitates were resolved by SDS-page. After staining with Coomassie-blue, the proteins bands immunoprecipitated by anti-GS15 were excised for amino acid sequence identification. The amino acid sequences of the indicated proteins were shown on the right. B. Golgi detergent extracts were immunoprecipitated with antibodies against GS15 (lanes 1), anti-Bet1 (lane 3), or control antibodies (lanes 2 and 4). The immunoprecipitates were resolved by SDS-page and processed for immunoblotting with antibodies against β-COP.
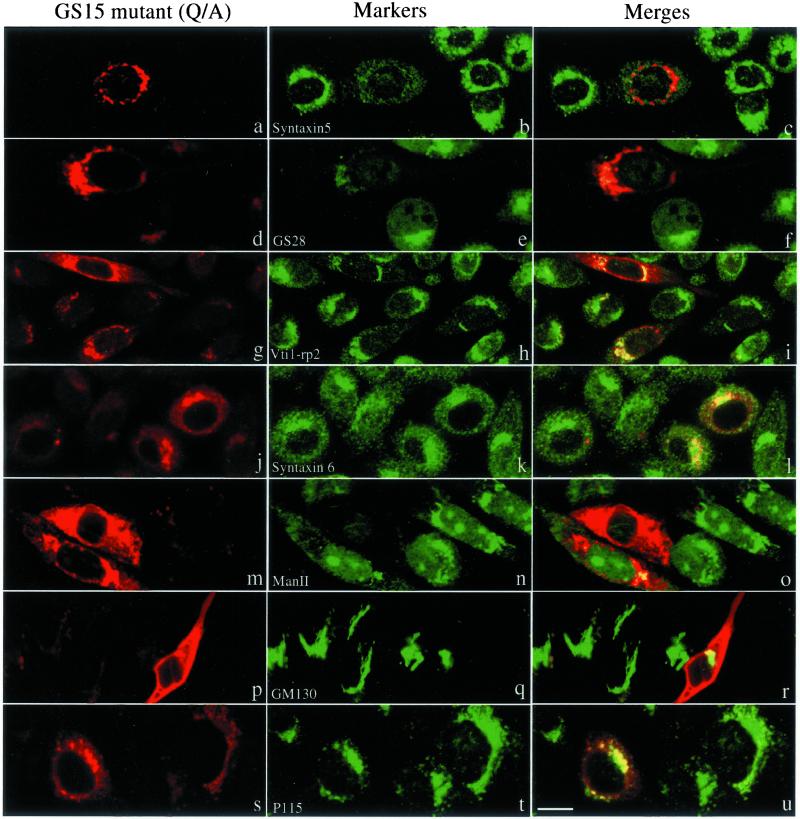
Mutant Forms of GS15 Disrupt the Localization of cis/medial-Golgi Proteins
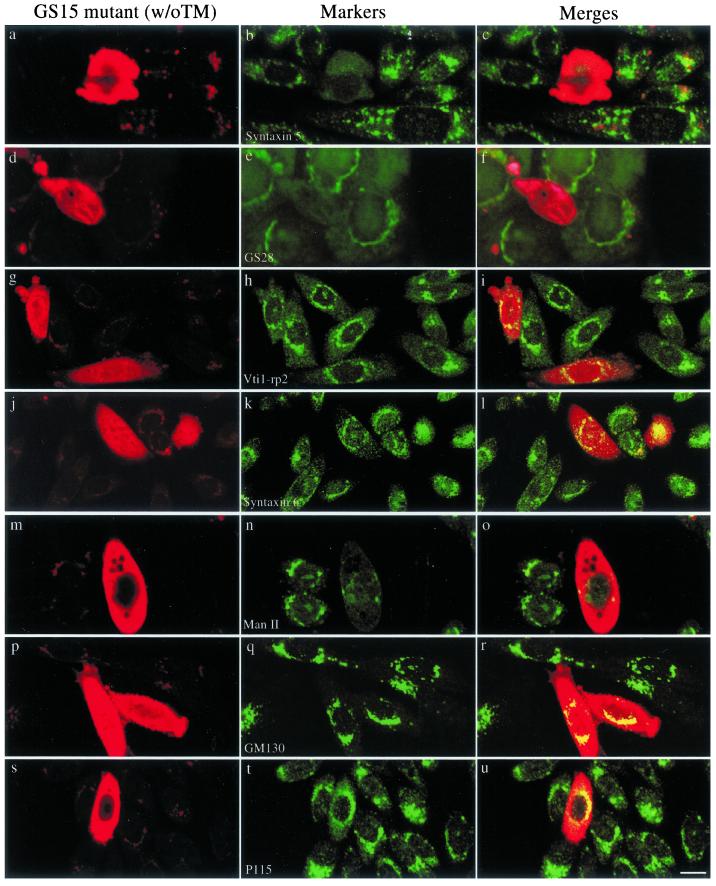
The fact that GS15 does not cycle back to the IC and that several different GS15 antibodies do not inhibit ER-to-Golgi transport of VSVG in vitro (unpublished observations) suggests that GS15 might not participate in the transport events between ER and the Golgi apparatus. To explore a potential function of GS15 within the Golgi apparatus, we have constructed two GS15 mutants. GS15ΔTM has a deletion of the C-terminal 24 residues such that the membrane anchor is deleted. The mutant protein will be expressed as cytosolic form because cytosolic SNAREs have been shown to function in a dominant-negative manner over endogenous proteins (Millar et al., 1999). GS15(Q49A) was created by substituting its conserved Gln (Q49) at the ionic zero layer of the SNARE domain by Ala because such mutations have been suggested to perturb the function of the resulting SNARE complex (Fasshauer et al., 1998; Ossig et al., 2000). On expression of GS15ΔTM (Figure 8), the compact perinuclear labeling observed for syntaxin 5 (Figure 8, a–c), GS28 (Figure 8, d–f), and Man II (Figure 8, m–o) was almost completely lost. These proteins, which are normally cis- and medial-Golgi resident proteins, were instead dispersed throughout the cytoplasm. In contrast, the localization of Vtir1-rp2/Vti1a (Figure 8, g–i) and syntaxin 6 (Figure 8, j–l), which are normally found in the trans-Golgi and trans-Golgi network, were less affected by expression of the GS15 mutant. In addition, the distribution of Golgi matrix proteins, such as GM130 (Figure 8, p–r) and p115 (Figure 8, s–u), was not significantly affected by overexpression of GS15ΔTM. Similar dispersion of syntaxin 5 (Figure 8, a–c), GS28 (Figure 8, d–f), and mannosidase II (panels Figure 8, m–o) but not Vti1-rp2/Vti1a (Figure 8, g–i), syntaxin 6 (Figure 8, j–l), GM130 (Figure 8, p–r), or p115 (Figure 8, s–u) was observed upon overexpression of GS15(Q49A) (Figure 9). These results indicate that GS15 may play a selective role in maintaining normal distribution and function of proteins of the cis- and medial-cisternae of the Golgi apparatus, and these two mutants interfered with Golgi traffic in a subtle and selective way.
Figure 8.
Expression of GS15 mutant without TM (GS15ΔTM) disrupts normal Golgi distribution of syntaxin 5, GS28, and Man II, while the Golgi distribution of Vit1-rp2/Vit1a, syntaxin 6 and Golgi matrix GM130 and p115 were less affected. NRK cells were transiently transfected with a construct for expressing GS15ΔTM. Transfected cells were fixed and processed for indirect immunofluorescence to detect the expressed mutant GS15 (left panels) and indicated Golgi proteins in central panels. The merged images are presented. Bar, 10 μM.
Figure 9.
Expression of GS15(Q49A) mutant affects normal Golgi distribution of syntaxin 5, GS28, and Man II. NRK cells were transiently transfected with a construct for expressing GS15(Q49A). Transfected cells were fixed and processed for indirect immunofluorescence to detect the expressed mutant GS15 (left panels) and indicated Golgi proteins in center panels. The merged images are presented in the right panels. Bar, 10 μM.
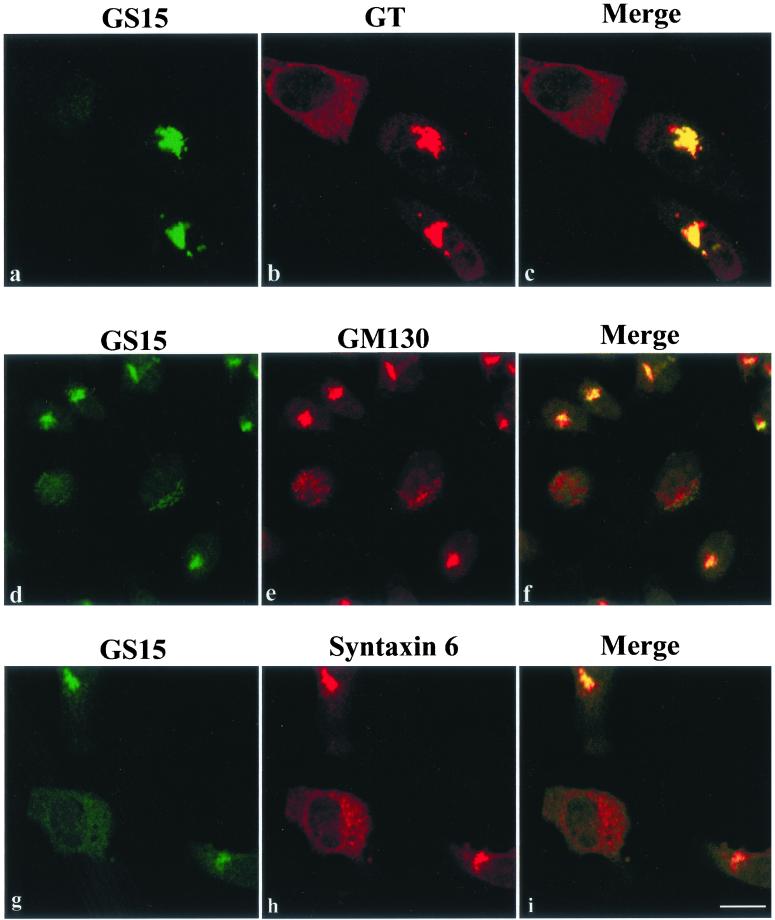
An Essential Role of GS15 in the Golgi Apparatus
To complement our results obtained by overexpressing mutant forms of GS15, we have used 21-nt duplex siRNA specific for GS15 to knockdown the cellular level of GS15 and to examine the consequence on the Golgi apparatus. As shown in Figure 10A, in cells with reduced/undetectable GS15 level, GS28 (Figure 10Ab), syntaxin 5 (Figure 10Ae), and Ykt6 (Figure 10Ah) were redistributed into small dotty and diffuse labeling. In contrast to expression of GS15 mutants, the distribution of trans-Golgi/TGN markers (GT for β1,4-galactosyltransferase and syntaxin 6; Figure 10B, b and h, respectively) was also significantly and similarly affected. Furthermore, even the Golgi matrix protein GM130 (Figure 10Be) was redistributed into small dotty structures when GS15 was knocked-down. The redistribution of markers of cis/medial-Golgi, trans-Golgi/TGN as well as Golgi matrix suggest that the entire Golgi apparatus has been disassembled when GS15 was knocked-down, establishing an essential role of GS15 in the Golgi apparatus.
Figure 10.
An essential role of GS15 in the Golgi apparatus. Hela cells were transfected with GS15-specific siRNA and then analyzed by immunofluorescence microscopy to detect GS15 and other indicated markers of the Golgi apparatus. (A) cis/medial proteins GS28, syntaxin 5, and Ykt6 were redistributed into small dotty and diffuse labeling in cells whose GS15 levels were significantly reduced by siRNA. (B) Even trans-Golgi/TGN β1,4-galactosyltransferase (GT), TGN syntaxin 6, and Golgi matrix GM130 were noticeably affected by GS15 knockdown. Bars: 10 μM.
DISCUSSION
Combined efforts using biochemical, genetic/genomic, and molecular approaches have uncovered ∼35 members of the SNARE family in mammalian cells (Jahn and Südhof, 1999; Bock et al., 2001). Several SNARE complexes have already been defined that function in various transport events. Owing to a well-established semi-intact cell system that reconstitutes ER-to-Golgi transport (Balch et al., 1994), two distinct SNARE complexes have been suggested to mediate sequential events during transport from the ER to the Golgi. The complex consisting of syntaxin5, Bet1, GS27/membrin, and Sec22b is implicated in the homotypic fusion of ER-derived COPII vesicles to form large transport intermediates (Xu et al., 2000; Zhang et al., 1997, 1999). Heterotypic fusion of these large transport intermediates with the cis-Golgi may be mediated by the complex of syntaxin5, Bet1, GS28, and Ykt6 (Zhang and Hong, 2001). The existence of this syntaxin5/Bet1/GS28/Ykt6 complex has been independently verified by a report published during the revision (Shorter et al. 2002). The other well-characterized SNARE complexes include syntaxin1/SNAP-25/VAMP2, syntaxin4/SNAP23/VAMP2, and syntaxin7/syntaxin8/Vti1b/VAMP-8, which regulate synaptic vesicle docking/fusion (Jahn and Südhof, 1999), GLUT4 translocation to the surface (Rea and James, 1997), and late endosomal fusion (Antonin et al., 2000; Wade et al. 2001), respectively. Recently, two SNARE complexes consisting of syntaxin6/syntaxin16/Vit1a in complex with VAMP4 or VAMP3/cellubrevin have been implicated in traffic from the early endosome to the TGN (Mallard et al., 2002). From these available discoveries, it seems that combinatory use of the 35 mammalian SNAREs will results a diverse array of different SNARE complexes to mediate various intracellular transport events. Despite these progresses, relatively little is known about the SNARE complexes that mediate traffic within or through the Golgi. It has been demonstrated that in addition to their well-established role in ER-to-Golgi transport, syntaxin5, GS28, and Ykt6 may also play a role in intra-Golgi transport (Nagahama et al., 1996; McNew et al., 1998; Orci et al., 2000; Dilcher et al., 2001). Other mammalian Golgi SNAREs have also been described (Xu et al., 1997, 1998), including GS15 and Vti1-rp2/Vti1a. Because Vti1a interacts with both the TGN t-SNARE syntaxin 6, as well as with the cis- and medial-Golgi t-SNARE syntaxin 5, it is likely to mediate traffic between the trans-side and the early compartment of the Golgi apparatus (Xu et al., 1998; Mallard et al., 2002). Additionally, although GS27/membrin is implicated in homotypic fusion of COPII vesicles to form transport intermediates (Xu et al., 2000), it has also been suggested to play a role in transport from the cis/medial-Golgi to the trans-Golgi/TGN (Lowe et al., 1997). GS15 is an unexplored SNARE of the Golgi and its further characterization should provide more insightful understanding about its role in trafficking events associated with the Golgi apparatus.
In the present study, we provide evidence that one of the potential roles of GS15 may be to regulate a transport step between the cis- and medial-cisternae of the Golgi apparatus. This conclusion is based upon several observations. Firstly, by incubating cells at 15°C or in the presence of brefeldin A we show that, in contrast to Bet1 and the KDEL receptor (Tang et al., 1993, 1995; Zhang et al., 1997), GS15 does not accumulate in peripheral structures characteristic of the IC (Klausner et al., 1992). Second, antibodies against GS15 that exhibited inhibitory effects on VSVG transport in intact cells did not inhibit in vitro ER-to-Golgi transport using a semi-intact cell assay (Balch et al., 1994, Zhang et al., 1997). Third, immunogold labeling of cryosections derived from several cell types or tissues indicates that GS15 is most often found in the medial cisternae of the Golgi apparatus and associated tubular-vesicular structures. Colocalization of some GS15 with a component of the COPI coat was observed in some vesicular-tubular profiles. Fourth, several components of the COPI coats could be coimmunoprecipitated by antibodies against GS15, indicating that GS15 could be a components of transport intermediates derived from COPI-mediated budding. Despite several experiments, we have not been able to demonstrate a direct interaction of COPI components with immobilized GST-GS15, suggesting that the observed coimmunoprecipitation of COPI components by anti-GS15 antibodies likely relies on other components in the GS15 complex. Furthermore, GS15, syntaxin 5, GS28, and Ykt6 appear to form a stable complex in cells, based on coimmunoprecipitation analyses. Syntaxin 5, GS28, and Ykt6 have been implicated in both ER-to-Golgi (Dascher et al., 1994; Subramaniam et al., 1996; McNew et al., 1997, 1998; Zhang and Hong, 2001) as well as intra-Golgi traffic (Nagahama et al., 1996; McNew et al., 1998; Orci et al., 2000; Dilcher et al., 2001). However, the GS15 complex is unique as compared with the ER-to-Golgi SNARE complexes or SNARE complexes functioning later in the Golgi, because we were unable to detect Bet1, Sec22b, Vti-rp2/Vti1a, or syntaxin6 in the GS15 complex (Zhang et al., 1997, 1999; Xu et al., 1998, 2000). A report published during the revision (Shorter et al., 2002) has independently shown the existence of this GS15/syntaxin5/GS28/Ykt6 complex. Finally, although sequence comparisons have failed to identify a yeast orthologue of GS15, GS15 shares similar structure with the two yeast SNAREs Bet1p and Sft1p as well as with mammalian Bet1. Because yeast Bet1p and mammalian Bet1 participate in ER-to-Golgi transport (Newman et al., 1990; Zhang et al., 1997), GS15 is more likely to be the mammalian counterpart of Sft1p. This is consistent with the subcellular localization and protein-interaction pattern observed for Sft1p (Banfield et al., 1995; Wooding and Pelham, 1998). Sft1p was proposed to function as a v-SNARE in retrograde transport from the distal cisternae to the cis-Golgi compartment based on the observations that it is localized to the medial-Golgi and interacts with the cis-Golgi t-SNARE Sed5p (the yeast counterpart of syntaxin5) and its role in maintaining an intact Golgi in yeast (Banfield et al., 1995; Wooding and Pelham, 1998). The demonstration of a distinct yeast SNARE complex consisting of Sft1, Sed5, Gos1 (a yeast counterpart of GS28; McNew et al., 1998), and Ykt6, and its implication in intra-Golgi traffic during the revision (Parlati et al., 2002) is consistent with the possibility. This Sft1/Sed5/Gos1/Ykt6 complex may well correspond to GS15/syntaxin5/GS28/Ykt6 complex reported here. Although GS15 may not be a structural homologue of Sft1p, the observations are consistent with the possibility that GS15 could be a functional homologue of Sft1p. These observations, taken together, indicate that one of the functions of GS15 may be to mediate a COPI-mediated trafficking pathway, likely from the medial- to the cis-cisternae of the Golgi apparatus.
The functional importance of GS15 in traffic through the Golgi was first investigated by monitoring the Golgi structure upon expression of mutant forms of GS15. Remarkably, overexpression of GS15ΔTM and GS15(Q49A) had more profound effects on proteins of the cis- and medial-cisternae of the Golgi apparatus. The highly compact peri-nuclear Golgi labeling pattern of GS28, syntaxin 5, and mannosidase II was disrupted in cells expressing these mutants. In contrast, the localization of proteins found in other Golgi regions including the trans cisternae, the TGN, and the Golgi matrix was less affected by the GS15 mutants. More importantly, an essential role for GS15 in maintaining an intact Golgi apparatus was revealed when GS15 expression was knocked-down by GS15-specific siRNA. More global and severe effects were observed when GS15 was knocked-down compared with overexpression of GS15 mutants in that markers of cis/medial-Golgi, trans-Golgi/TGN, and Golgi matrix were all redistributed into small dotty and diffuse labeling. This suggests that a selective involvement of GS15 in traffic in early cisternae as revealed by mutant expression has more profound effect on trafficking events later in the pathway when its function was abolished by knock-down approach.
Other studies have also been able to uncouple the relocalization of different Golgi proteins under various experimental conditions. For example, in response to brefeldin A, GM130 and p115 remain associated with Golgi-like and/or IC-like structures, whereas other proteins, such as mannosidase II and β1,4-galactotransferase, were dispersed throughout the cell presumably because of their redistribution back to the ER. Similar findings were reported in cells overexpressing a mutant Sar1 protein that blocks protein export from the ER (Seemann et al., 2000). Interestingly, two recent studies have reached somehow different conclusions regarding the fate of Golgi matrix/scaffold proteins upon treatment with brefeldin A or the presence of mutant forms of Sar1 or ARF1 (Miles et al., 2001; Ward et al., 2001). More independent studies using other types of blockers to inhibit ER-to-Golgi transport may be necessary to sort out these discrepancies. Our results suggest that the Golgi matrix/scaffold was not significantly affected by the GS15 mutants. The selective effect of GS15 mutants on cis- and medial-Golgi proteins but less on proteins of the trans-Golgi and TGN suggest that GS15 may regulate a protein trafficking loop between early elements of the Golgi stack. The molecular basis underlying this selective dispersion of cis- and medial-Golgi proteins upon overexpresion of GS15 mutants remains to be established by future experiments. However, the most likely interpretation of these experiments is that GS15 is a v-SNARE that regulates the docking and fusion of transport vesicles, derived from the medial Golgi, with the cis-Golgi via a specific interaction with the t-SNAREs GS28, syntaxin 5, and Ykt6. This speculation is consistent with recent demonstration of Sft1 functions as a v-SNARE to interact with t-SNARE consisted of Sec5/GS28/Ykt6 during the revision of our manuscript (Parlati et al., 2002). The mutant forms of GS15 presumably block the docking of these transport vesicles with the cis-Golgi, resulting in their random dispersal throughout the cytoplasm. However, these data raise a number of important questions. Most notably, why is the distribution of proteins in the trans-Golgi and/or TGN less affected by an inhibition of this early step in Golgi transport. The fact that Golgi matrix/scaffold marked by GM130 and p115 remains intact may suggest that the normal distribution of these proteins could be stabilized by the intact matrix. Another possibility is that endogenous GS15 may be still able to maintain a borderline activity to ensure that later events are less affected even in the presence of its competing mutants. This borderline activity is not sustainable when GS15 is knocked-down, resulting in more severe effects on later events. Alternatively as suggested by Balch and colleagues (Allan and Balch, 1999) it is possible that transport pathways on either side of the Golgi are intimately linked such that a reduction in flux on one side leads to an equivalent reduction in flux on the other. Our current studies not only have uncovered a unique Golgi SNARE complex likely operating in the early cisternae of the Golgi apparatus but also have provided significant implications about the organization and regulation of trafficking routes within the Golgi. In addition, an essential role for GS15 in the Golgi apparatus is established.
ACKNOWLEDGMENTS
We thank Dr. F.T. Wieland for generous gift of antibodies against COPI components. This work was support by a grant from Agency for Science, Technology, and Research (A*STAR), Singapore (to W.H.) and from the National Health and Medical Research Council of Australia (to D.E.J.). W.H. is also a faculty member of the Department of Biochemistry, National University of Singapore.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0004. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0004.
REFERENCES
- Allan BB, Balch WE. Protein sorting by directed maturation of Golgi compartments. Science. 1999;285:63–66. doi: 10.1126/science.285.5424.63. [DOI] [PubMed] [Google Scholar]
- Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure, and function. EMBO J. 2000;19:6453–6464. doi: 10.1093/emboj/19.23.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Schekman R. ER export. public transportation by the COPII coach. Curr Opin Cell Biol. 2001;13:438–443. doi: 10.1016/s0955-0674(00)00234-9. [DOI] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plunter H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Pelham HR. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. Adaptins. The final recount. Mol Biol Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Dascher C, Matteson J, Balch WE. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J Biol Chem. 1994;269:29363–29366. [PubMed] [Google Scholar]
- Dilcher M, Kohler B, von Mollard GF. Genetic interactions with the yeast Q-SNARE VTI1 reveal novel functions for the R-SNARE YKT6. J Biol Chem. 2001;276:34537–34544. doi: 10.1074/jbc.M101551200. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J, Suganuma T, Tang BL, Hong W, Storrie B, Nilsson T. Localization and recycling of gp27 (hp243): complex formation with other p24 family members. Mol Biol Cell. 1999;10:1939–1955. doi: 10.1091/mbc.10.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway. a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Hauri HP, Kappeler F, Andersson H, Appenzeller C. ERGIC-53, and traffic in the secretory pathway. J Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SL, Peter F, Subramaniam VN, Wong SH, Hong W. A SNARE involved in protein transport through the Golgi apparatus. Nature. 1997;389:881–884. doi: 10.1038/39923. [DOI] [PubMed] [Google Scholar]
- Mallard F, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes, and a Rab6 isoform. J Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Millar CA, Lyttle CT, Meerloo T, Marsh BJ, Gould GW, James DE. Effects of insulin on intracellular GLUT4 vesicles in adipocytes. evidence for a secretory mode of regulation. J Cell Sci. 2000;113:3427–3438. doi: 10.1242/jcs.113.19.3427. [DOI] [PubMed] [Google Scholar]
- McNew JA, Sogaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Sollner TH. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- McNew JA, Coe JG, Sogaard M, Zemelman BV, Wimmer C, Hong W, Sollner TH. Gos1p, a Saccharomyces cerevisiae SNARE protein involved in Golgi transport. FEBS Lett. 1998;435:89–95. doi: 10.1016/s0014-5793(98)01044-8. [DOI] [PubMed] [Google Scholar]
- Millar CA, Shewan A, Hickson GR, James DE, Gould GW. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles S, McManus H, Forsten KE, Storrie B. Evidence that the entire Golgi apparatus cycles in interphase HeLa cells. sensitivity of Golgi matrix proteins to an ER exit block. J Cell Biol. 2001;155:543–556. doi: 10.1083/jcb.200103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M, Orci L, Ravazzola M, Amherdt M, Lacomis L, Tempst P, Rothman JE, Söllner TH. A v-SNARE implicated in intra-Golgi transport. J Cell Biol. 1996;133:507–516. doi: 10.1083/jcb.133.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AP, Shim J, Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990;10:3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Volchuk A, Engel T, Gmachl M, Amherdt M, Perrelet A, Sollner TH, Rothman JE. Anterograde flow of cargo across the golgi stack potentially mediated via bidirectional. “percolating” COPI vesicles. Proc Natl Acad Sci USA. 2000;97:10400–10405. doi: 10.1073/pnas.190292497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossig R, Schmitt HD, de Groot B, Riedel D, Keranen S, Ronne H, Grubmuller H, Jahn R. Exocytosis requires asymmetry in the central layer of the SNARE complex. EMBO J. 2000;19:6000–6010. doi: 10.1093/emboj/19.22.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, Varlamov O, Paz K, McNew JA, Hurtado D, Sollner TH, Rothman JE. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc Natl Acad Sci USA. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HR, Rothman JE. The debate about transport in the Golgi—two sides of the same coin? Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Membrane transport. retromer to the rescue. Curr Biol. 2001;11:R109–R111. doi: 10.1016/s0960-9822(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Rowe T, Dascher C, Bannykh S, Plutner H, Balch WE. Role of vesicle-associated syntaxin 5 in the assembly of pre-Golgi intermediates. Science. 1998;279:696–700. doi: 10.1126/science.279.5351.696. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Gomez M, Kreis TE. Coat proteins regulating membrane traffic. Int Rev Cytol. 2000;195:67–144. doi: 10.1016/s0074-7696(08)62704-7. [DOI] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Seemann J, Jokitalo E, Pypaert M, Warren G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 2000;407:1022–1026. doi: 10.1038/35039538. [DOI] [PubMed] [Google Scholar]
- Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins, and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Peter F, Philip R, Wong SH, Hong W. GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science. 1996;272:1161–1163. doi: 10.1126/science.272.5265.1161. [DOI] [PubMed] [Google Scholar]
- Tang BL, Wong SH, Qi XL, Low SH, Hong W. Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J Cell Biol. 1993;120:325–338. doi: 10.1083/jcb.120.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Low SH, Hong W. Differential response of resident proteins and cycling proteins of the Golgi to brefeldin A. Eur J Cell Biol. 1995;68:199–205. [PubMed] [Google Scholar]
- Wade N, Bryant NJ, Connolly LM, Simpson RJ, Luzio JP, Piper RC, James DE. Syntaxin 7 complexes with mVti1b, Syntaxin 6, VAMP8, and VAMP7 in B16 melanoma cells. J Biol Chem. 2001;276:19820–19827. doi: 10.1074/jbc.M010838200. [DOI] [PubMed] [Google Scholar]
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure, and function depends on the integrity of ER export. J Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wooding S, Pelham HR. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wong SH, Zhang T, Subramaniam VN, Hong W. GS15, a 15-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) homologous to rbet1. J Biol Chem. 1997;272:20162–20166. doi: 10.1074/jbc.272.32.20162. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wong SH, Tang BL, Subramaniam VN, Zhang T, Hong W. A 29-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (Vti1-rp2) implicated in protein trafficking in the secretory pathway. J Biol Chem. 1998;273:21783–21789. doi: 10.1074/jbc.273.34.21783. [DOI] [PubMed] [Google Scholar]
- Xu D, Joglekar AP, Williams AL, Hay JC. Subunit structure of a mammalian ER/Golgi SNARE complex. J Biol Chem. 2000;275:39631–39639. doi: 10.1074/jbc.M007684200. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wong SH, Tang BL, Xu Y, Peter F, Hong W. The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus J. Cell Biol. 1997;139:1157–1168. doi: 10.1083/jcb.139.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wong SH, Tang BL, Xu Y, Hong W. Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment. Mol Biol Cell. 1999;10:435–453. doi: 10.1091/mbc.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Hong W. Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1, and participates in a late stage in endoplasmic reticulum-Golgi transport. J Biol Chem. 2001;276:27480–27487. doi: 10.1074/jbc.M102786200. [DOI] [PubMed] [Google Scholar]