Abstract
Remodeling of extracellular matrices occurs during development, wound healing, and in a variety of pathological processes including atherosclerosis, ischemic injury, and angiogenesis. Thus, identifying factors that control the balance between matrix deposition and degradation during tissue remodeling is essential for understanding mechanisms that regulate a variety of normal and pathological processes. Using fibronectin-null cells, we found that fibronectin polymerization into the extracellular matrix is required for the deposition of collagen-I and thrombospondin-1 and that the maintenance of extracellular matrix fibronectin fibrils requires the continual polymerization of a fibronectin matrix. Further, integrin ligation alone is not sufficient to maintain extracellular matrix fibronectin in the absence of fibronectin deposition. Our data also demonstrate that the retention of thrombospondin-1 and collagen I into fibrillar structures within the extracellular matrix depends on an intact fibronectin matrix. An intact fibronectin matrix is also critical for maintaining the composition of cell–matrix adhesion sites; in the absence of fibronectin and fibronectin polymerization, neither α5β1 integrin nor tensin localize to fibrillar cell–matrix adhesion sites. These data indicate that fibronectin polymerization is a critical regulator of extracellular matrix organization and stability. The ability of fibronectin polymerization to act as a switch that controls the organization and composition of the extracellular matrix and cell–matrix adhesion sites provides cells with a means of precisely controlling cell-extracellular matrix signaling events that regulate many aspects of cell behavior including cell proliferation, migration, and differentiation.
INTRODUCTION
Extracellular matrix remodeling plays an important role during development, wound healing, atherosclerosis, ischemic injury, and angiogenesis. Perturbing matrix remodeling by preventing the turnover of collagen I or by altering the levels of matrix-degrading proteases or protease inhibitors has been shown to result in fibrosis, arthritis, reduced angiogenesis, and developmental abnormalities (Liu et al., 1995; Vu et al., 1998; Holmbeck et al., 1999; Ducharme et al., 2000). In normal adult tissue, some extracellular matrix components such as elastin and fibrillar collagen have half lives of months to years (Krane, 1985; Debelle and Tamburro, 1999). Other extracellular matrix components, such as proteoglycans, thrombospondin-1 and -2, and vitronectin, can be endocytosed and degraded in the lysosomes (McKeown-Longo et al., 1984; Yanagishita and Hascall, 1984; Murphy-Ullrich and Mosher, 1987; Hausser et al., 1992; Godyna et al., 1995a; Pijuan-Thompson and Gladson, 1997; Memmo and McKeown-Longo, 1998). Extracellular matrix molecules can also be degraded extracellularly by proteases such as matrix metalloproteinases (MMPs), plasminogen activators, and plasmin (Hynes, 1990; Marchina and Barlati, 1996; Shapiro, 1998).
Recent data indicate that polymerized forms of extracellular matrix proteins have properties distinct from protomeric, nonpolymerized proteins. For example, the state of collagen polymerization has been shown to alter its growth regulatory properties (Koyama et al., 1996). Emerging evidence also indicates that the extracellular matrix form of fibronectin is functionally distinct from soluble protomeric fibronectin (Morla et al., 1994; Pasqualini et al., 1996; Mercurius and Morla, 1998). Our data indicate that fibronectin deposition into the extracellular matrix increases adhesion-dependent cell growth (Sottile et al., 1998) and cell contractility (Hocking et al., 2000). Others have shown that inhibiting fibronectin deposition or disrupting a preexisting fibronectin matrix can inhibit adhesion-dependent (Clark et al., 1997; Bourdoulous et al., 1998; Mercurius and Morla, 1998) and -independent cell growth (Wu et al., 1998).
Remodeling of the extracellular matrix by proteases promotes cell migration, a critical event in the formation of new vessels. Remodeling of the extracellular matrix could alter the cell response to extracellular matrix by production of fragments of matrix proteins with distinct properties from the native proteins. For example, MMP-2 cleavage of laminin V generates a proteolytic fragment that promotes cell migration (Giannelli et al., 1997). Extracellular matrix remodeling could also alter cellular responses to matrix through exposure of neoepitopes within matrix proteins that alter their function. For example, proteolytic cleavage of collagen IV has been shown to expose a cryptic site within the collagen triple helix that promotes angiogenesis (Xu et al., 2001).
Although much is known about the interactions between different extracellular matrix molecules, less is known about how extracellular matrix composition, organization, and stability are regulated. We developed a unique cell culture system using fibronectin-null embryo cells that enables us to study the effects of fibronectin polymerization on extracellular matrix assembly and disassembly in the absence of any cell- or serum-derived fibronectin. Our data indicate that polymerization of fibronectin into the extracellular matrix globally controls the composition and stability of the extracellular matrix and of cell–matrix adhesion sites and thus is likely to control extracellular matrix signaling cascades that regulate many aspects of cell behavior.
MATERIALS AND METHODS
Immunological Reagents and Chemicals
Polyclonal antifibronectin antibody and the mAb 9D2 (Chernousov et al., 1991; Sottile and Mosher, 1997) were generous gifts from Dr. Deane Mosher (University of Wisconsin, Madison, WI). Antibodies to mouse collagen I were purchased from Chemicon (Temecula, CA); antibodies to tensin, α5 integrin, and paxillin were from BD Biosciences (San Diego, CA); antibodies to focal adhesion kinase (FAK) were from UBI (Lake Placid, NY); antibodies to vinculin were from Sigma Chemicals (St. Louis, MO). Actinonin, amastatin, antipain, aprotinin, bestatin, chymostatin, E64, leupeptin, pepstatin, and 1,10 phenanthroline were from Sigma; illomastat was from Chemicon; jasplakinolide was from Molecular Probes (Eugene, OR).
Proteins
Human fibronectin was purified from Cohn's fractions I and 2 (a generous gift from Dr. Ken Ingham, American Red Cross, Bethesda, MD) as previously described (Miekka et al., 1982). Production and purification of recombinant rat 70- and 40-kDa fibronectin fragments and recombinant wild-type fibronectin and fibronectin lacking the RGD site (FNΔRGD) have been previously described (Sottile and Mosher, 1997; Sottile et al., 2000). The 160/180- and 120-kDa fibronectin fragments were generated as described (Sottile et al., 1998). Vitronectin (Yatohgo et al., 1988), thrombospondin-1 (Mosher et al., 1982), and a fusion protein containing GST linked to fibronectin's III-9 and III-10 modules (GST/III-9,10; Hocking et al., 1996) were prepared as described previously.
Cell Culture
Fibronectin-null cells were derived from fibronectin-null embryos and adapted to grow under serum-free conditions in defined medium (a 1:1 mixture of Cellgro (Mediatech, Herndon, VA) and Aim V (Life Technologies, Gaithersburg, MD) in the absence of serum as described (Sottile et al., 1998). Thus, the cells are cultured under conditions where no exogenous source of fibronectin or other extracellular matrix proteins is present. TJ6F normal human foreskin fibroblasts were established by Dr. Lynn Allen Hoffmann (University of Wisconsin, Madison). These cells were maintained in DMEM containing 10% FBS. Human aortic smooth muscle cells were obtained from Cell Applications (San Diego, CA), and maintained in serum containing media (Cell Applications).
Fibronectin Pulse-Chase Experiments
Fibronectin-null cells were plated onto glass coverslips precoated with 5 μg/ml vitronectin, 10 μg/ml laminin (Collaborative Research, Bedford, MA), 5 μg/ml GST/III-9,10, or onto 35-mm tissue culture dishes precoated with rat type I collagen (UBI) as described (Sottile et al., 1998). Cells were seeded in defined medium and incubated at 37°C for various lengths of time. Cells were grown to 80% confluence and were then incubated (“pulse”) overnight with 20 nM fibronectin. Cells were then either processed for immunofluorescence or were washed and then incubated (“chase”) with culture medium containing or lacking 20 nM fibronectin. In some experiments, the fibronectin used was conjugated to either Texas Red (Molecular Probes Inc., Eugene, OR) or to FITC (Cappel, West Chester, PA) as described (Sottile and Mosher, 1997). The chase medium was supplemented with various inhibitors, as described in the figure legends. Cells were fixed with paraformaldehyde, permeabilized with 0.5% Triton X-100 and then mounted in glycerol gel (Sigma). For analysis of focal contact and cell–matrix contact proteins, cells were grown to 40% confluence before addition of fibronectin. After fixing and permeabilizing, cells were incubated with antibodies to α5 integrin, paxillin, vinculin, FAK, or tensin, followed by Texas-Red– or FITC-conjugated secondary antibodies. Cells were examined using an Olympus BX60 microscope equipped with epifluorescence. For some experiments, images were obtained with an Olympus scanning confocal microscope.
Protease Inhibitor Studies
Fibronectin-null cells were grown to 80% confluence and then incubated with 20 nM FITC-conjugated fibronectin. After an overnight incubation, cells were washed and then incubated in the absence or presence of fibronectin and in the absence or presence of 0.02–0.2 mM actinonin, 10 μM amastatin, 100 μM antipain, 20–200 μg/ml aprotinin, 130–580 μM bestatin, 100 μM chymostatin, 10 μM E64, 10–20 μM illomostat, 100 μM leupeptin, 1 μM pepstatin, or 1–20 μM 1,10 phenanthroline for 16–24 h. None of the inhibitors were able to maintain the stability of the preexisting fibronectin matrix as assessed by indirect immunofluorescence microscopy. The presence of protease inhibitors had no effect on the ability of cells to assemble a fibronectin matrix when fibronectin was present in the chase media.
Iodination of Proteins and Binding Assays
Fibronectin was iodinated using the chloramine T method as described (McKeown-Longo and Mosher, 1985). Labeled proteins were separated from unincorporated iodine by gel filtration on Pharmacia PD-10 columns (Piscataway, NJ). Iodinated proteins were dialyzed against PBS at room temperature for 3 h. The specific activity of iodinated fibronectin was: 6.71 × 1010 μCi/mol. Binding assays were performed essentially as described (Sottile and Wiley, 1994). Briefly, fibronectin-null cells were seeded at 3.5 × 104 cells/well into 12-well cluster dishes in Cellgro:Aim V (1:1). Cells were allowed to grow to 80% confluence for 2 d. Cells were washed with Cellgro:Aim V and then incubated with medium containing iodinated fibronectin. After a 14-h incubation, cells were either processed as described below or were washed with Cellgro:Aim V and then incubated in culture medium containing or lacking 10–20 nM unlabeled fibronectin for 12 or 23 h. After this incubation period, cells were washed and then processed to determine the amount of matrix-associated fibronectin by extracting the cells in 1% deoxycholate as described (Sottile and Wiley, 1994). The cell extract was centrifuged at 4°C at 18,000 × g for 30 min to separate deoxycholate-insoluble (matrix-associated) from deoxycholate-soluble (cell-associated) counts. Nonspecific binding was determined by incubating cells in the presence of excess unlabeled recombinant 70-kDa protein (0.3 μM).
Map Kinase Activity
Fibronectin pulse-chase experiments were performed as described above, using 20 nM unlabeled fibronectin for the pulse and chase. In some wells, the chase medium also contained 50 μg/ml the mAb 9D2 or control IgG. Cells were lysed in lysis buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 25 mM β-glycerophosphate, 25 μg/ml leupeptin, 25 μg/ml aprotinin, 50 μg/ml soybean trypsin inhibitor, 0.5 mM sodium vanadate, 2 mM phenylmethyl sulfonyl fluoride, 1 mM hydrogen peroxide) on ice and then centrifuged at 4°C at 14,000 × g. Proteins in the supernatant were quantitated using a Pierce BCA kit (Rockford, IL). Equal amounts of proteins were analyzed under reducing conditions by SDS PAGE, transferred to nitrocellulose paper (Towbin et al., 1979), and probed with antibodies that recognize activated ERK1 (pERK1) and ERK2 (pERK2), activated p38 (pp38), and activated JNK/SAPK (New England Biolabs, Beverly, MA). To ensure equal protein loading, the blots were stripped and reprobed with polyclonal antibodies that recognize both active and inactive ERK 1 and 2 (ERK1, ERK2), p38, or JNK/SAPK.
RESULTS
Retention of Fibronectin Matrix Depends on Fibronectin Polymerization
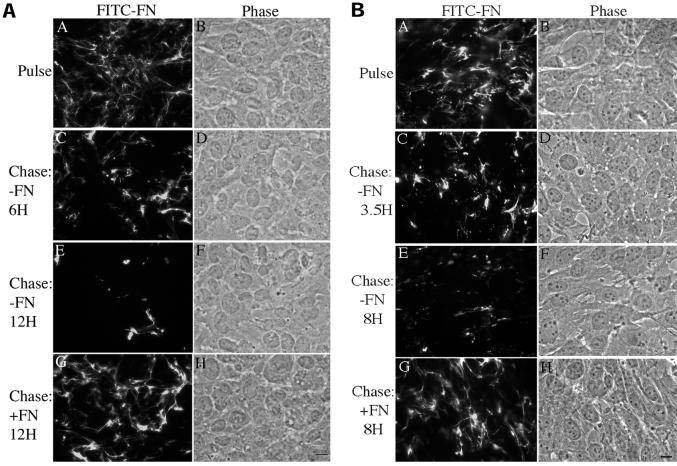
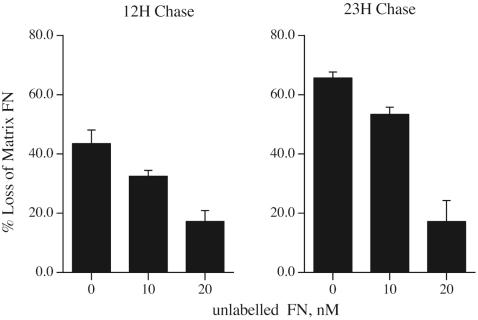
Agents that control the organization and stability of the extracellular matrix are likely to be critical in regulating the cell response to injury. We have established a cell culture system using fibronectin-null cells in order to determine the role of fibronectin and fibronectin polymerization in regulating the organization and stability of the extracellular matrix. These cells are grown in defined media that does not require serum supplementation, thus allowing us to determine the effect of exogenously added fibronectin on matrix stability in the absence of any serum- or cell-derived fibronectin. To examine the stability of fibronectin that has been deposited into the extracellular matrix, fibronectin-null cells seeded on vitronectin-coated dishes were incubated with FITC-labeled fibronectin. After the cells had elaborated an extensive fibronectin matrix (Figure 1A, panel A), the media containing the labeled fibronectin was removed and replaced with fresh media, with and without unlabeled fibronectin (“chase”). The absence of fibronectin in the chase culture medium resulted in a dramatic loss of fibronectin fibrils from the extracellular matrix (Figure 1A, panels C and E) as soon as 6 h after soluble fibronectin removal (Figure 1A, panel C). More extensive loss of fibronectin was evident 12 h after fibronectin removal (Figure 1A, panel E). In contrast, addition of unlabeled fibronectin to the chase media prevented the loss of the preestablished fibronectin matrix (Figure 1A, panel G). The cell monolayers remained intact, and the cells well spread, despite disruption of the fibronectin matrix (Figure 1A, panels B, D, F, and H). Similar results were found when cells were seeded on dishes coated with collagen (Figure 1B), laminin, or a recombinant protein containing fibronectin's cell adhesion domain, III-9,10. To quantitate the amount of fibronectin matrix that is lost upon removal of fibronectin from the cell culture medium, experiments were performed with fibronectin-null cells in which the fluorescently labeled fibronectin was replaced with 125I-fibronectin. In the absence of fibronectin in the chase media, as much as 65% of the original 125I-fibronectin matrix, was lost (Figure 2). The presence of 20 nM unlabeled fibronectin in the chase media resulted in the retention of >80% of the matrix fibronectin. These data indicate that fibronectin alters the stability of extracellular matrix fibronectin fibrils.
Figure 1.
Fibronectin matrix stability depends on the continuous presence of fibronectin. Fibronectin-null cells were grown to 80% confluence on dishes coated with vitronectin (A) or collagen (B) and then incubated with 20 nM FITC-conjugated fibronectin. After an overnight incubation, cells were either processed for immunofluorescence (Pulse, A) or were washed and then incubated with either 20 nM unlabeled fibronectin (Chase: +FN; G and H) or an equivalent volume of PBS (−FN; C–F) for the indicated times. Cells were then fixed and permeabilized. FITC-fibronectin staining and the corresponding phase pictures are shown. Bar, 10 μm.
Figure 2.
Quantitation of fibronectin matrix turnover. Fibronectin-null cells were incubated overnight with 125I-fibronectin. Cells were then either processed to determine the amount of matrix-associated fibronectin or were washed and then incubated with culture medium lacking or containing 10 or 20 nM unlabeled fibronectin for 12 or 23 h. Cells were then processed to determine the amount of matrix-associated fibronectin by extracting the cells in 1% DOC as described (Sottile and Wiley, 1994). The cell extract was centrifuged at 4°C at 18,000 × g for 30 min to separate DOC-insoluble (matrix-associated) from DOC-soluble (cell-associated) counts. The amount of matrix-associated counts before the chase was set equal to 100%. The data is presented as % loss of matrix-associated counts. Error bars represent the range of duplicate determinations.
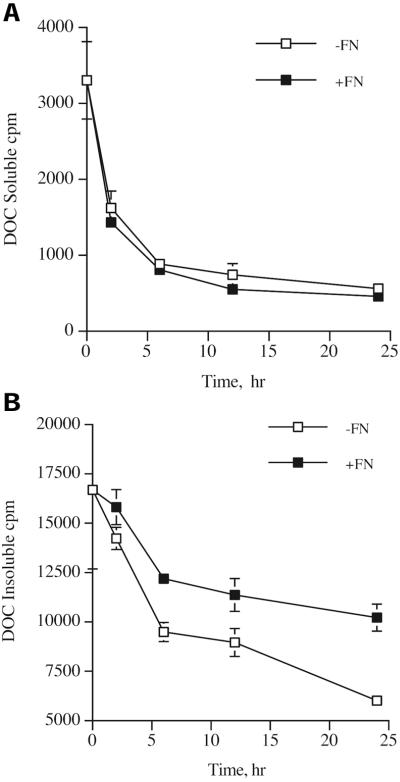
To determine the kinetics of loss of fibronectin from the cell surface, we analyzed the loss of matrix fibronectin, as well as loss of cell-associated fibronectin during fibronectin pulse-chase experiments with 125I-fibronectin. It has been previously shown that matrix fibronectin is insoluble in 1% DOC, whereas cell-associated fibronectin is soluble in 1% DOC (Choi and Hynes, 1979; McKeown-Longo and Mosher, 1983). This cell-associated fibronectin is thought to represent fibronectin that is bound to cell surface receptors, but has not yet been assembled into fibronectin fibrils. As shown in Figure 3, ∼85% of the fibronectin is incorporated into the matrix fraction at the start of the chase. Cell-associated (DOC soluble) fibronectin is rapidly lost from the cell surface during the chase (Figure 3A). The rate of loss of cell-associated fibronectin is similar when the chase is performed in the presence or absence of fibronectin (Figure 3A). In contrast, there is an enhanced rate of loss of matrix fibronectin in cells that are incubated in the absence of fibronectin in comparison with cells incubated in the presence of 10 nM fibronectin (Figure 3B). Figure 2 demonstrates that less fibronectin is lost from the matrix pool when the chase is performed with 20 nM of unlabeled fibronectin than with 10 nM fibronectin (Figures 2 and 3B). These data indicate that fibronectin is preferentially lost from the extracellular matrix when soluble fibronectin is removed from the cell culture media.
Figure 3.
Kinetics of loss of fibronectin from the cell surface. Fibronectin-null cells were incubated overnight with 125I-fibronectin. Cells were then either processed to determine the amount of matrix- or cell-associated fibronectin (t = 0) or were washed and then incubated with culture medium lacking (−FN, □) or containing (+FN, ▪) 10 nM unlabeled fibronectin for 2–24 h. Cells were then processed to determine the amount of cell-associated (A) or matrix-associated (B) fibronectin by extracting the cells in 1% DOC as described in the legend to Figure 2. Error bars represent the range of duplicate determinations.
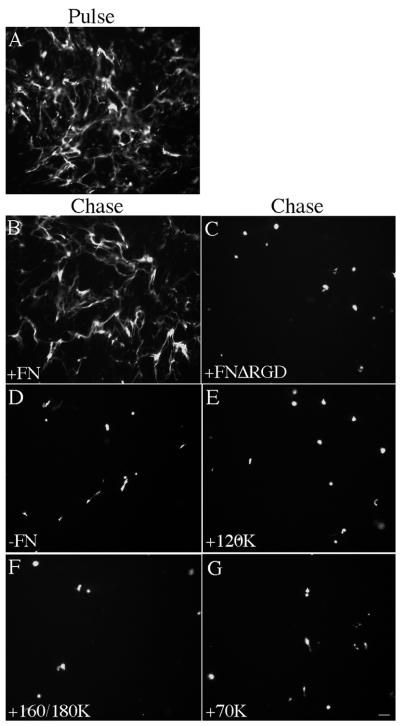
To determine whether the binding of fibronectin to integrin receptors is required to maintain extracellular matrix fibronectin fibrils, cells containing a preestablished FITC-fibronectin matrix were incubated in the presence of fibronectin lacking the integrin-binding RGD site (FnΔRGD). Addition of FnΔRGD did not prevent matrix reorganization (Figure 4C), indicating that maintenance of extracellular matrix fibronectin requires fibronectin-integrin binding. However, integrin ligation was not sufficient to maintain extracellular matrix fibronectin, because addition of integrin-binding fibronectin fragments to the chase media did not prevent the loss of fibronectin fibrils (Figure 4, E and F). These data suggest that the binding of fibronectin to integrins is necessary, but not sufficient, to maintain fibronectin matrix fibrils. The 70-kDa amino terminal fragment of fibronectin binds to the surface of adherent cells and is necessary for fibronectin matrix polymerization (McKeown-Longo and Mosher, 1985; Quade and McDonald, 1988). The 70-kDa fragment can also bind to the α5β1 integrin (Hocking et al., 1998). Therefore, we tested whether addition of the 70-kDa fragment could maintain extracellular matrix fibronectin. As shown in Figure 4G, addition of the 70-kDa fragment to the chase media did not stabilize extracellular matrix fibronectin fibrils.
Figure 4.
Fibronectin fragments do not stabilize matrix fibronectin. Fibronectin-null cells were incubated overnight with FITC-fibronectin (20 nM). Cells were then either processed for immunofluorescence (Pulse, A), or were washed and then incubated with culture medium containing 20 nM unlabeled fibronectin (Chase: +FN; B), 20 nM unlabeled FNΔRGD (C), 40 nM 120-kDa cell-binding fragment (E), 40 nM 160/180-kDa cell, heparin-binding fragment (F), 40 nM 70-kDa amino-terminal fragment (G), or an equivalent volume of PBS (−FN; D) for 24 h. Cells were then processed as described in the legend to Figure 1. FITC-fibronectin staining is shown. Bar, 20 μm.
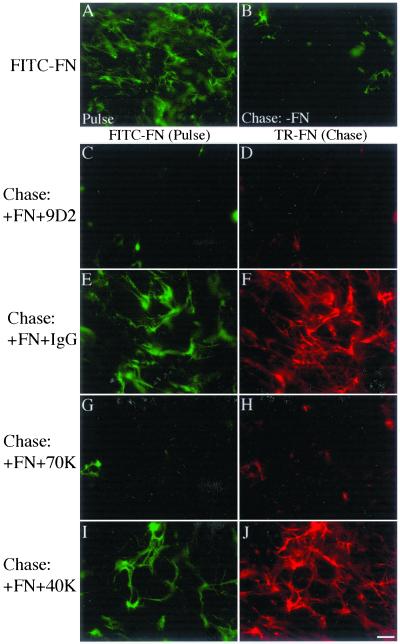
To determine whether fibronectin polymerization into the matrix is required to maintain preexisting fibronectin fibrils, we asked whether agents that inhibit fibronectin polymerization could block the retention of fibronectin fibrils. Cells were allowed to elaborate a FITC-fibronectin matrix (Figure 5A) and were then chased in the absence (Figure 5B) or presence of Texas Red (TR)-fibronectin, to allow simultaneous detection of both the preestablished (FITC) matrix and the “chase” (TR) fibronectin. The chase media also contained either the antifibronectin III-1 antibody, 9D2, or the 70-kDa amino-terminal fragment of fibronectin. The 9D2 antibody and the 70-kDa fragment inhibit fibronectin polymerization into the extracellular matrix but do not interfere with cell adhesion to fibronectin (McKeown-Longo and Mosher, 1985; Quade and McDonald, 1988; Chernousov et al., 1991 and our unpublished results). In addition, the 9D2 antibody does not block the binding of soluble fibronectin to adherent cells (Chernousov et al., 1991). Control cells were incubated with control IgG or the 40-kDa gelatin-binding fibronectin fragment. As shown in Figure 5, the Texas Red “chase” fibronectin was elaborated into an extensive fibronectin matrix in cells cultured in the presence of IgG (Figure 5F) or the 40-kDa fragment (Figure 5J). In addition, the presence of Texas Red-fibronectin in the chase media resulted in maintenance of the preexisting FITC-fibronectin matrix when the chase media also contained IgG or 40-kDa fragment (Figure 5, E and I). In contrast, when TR-fibronectin was added together with 9D2 IgG (Figure 5C) or with the 70-kDa fragment (Figure 5G), the organization of the preexisting fibrillar FITC-fibronectin network was not preserved. As shown previously, these agents also prevented the deposition of TR-fibronectin into the matrix (Figure 5, D and H). Thus, fibronectin is not able to preserve the preexisting fibronectin matrix under conditions where fibronectin polymerization is inhibited. These data indicate that the process of fibronectin polymerization regulates fibronectin matrix stability and suggest a novel mechanism whereby the extent and organization of extracellular matrix fibronectin can be controlled by the regulated polymerization of a fibronectin matrix.
Figure 5.
Fibronectin polymerization is required for stabilization of preexisting fibronectin matrix. Fibronectin-null cells were grown to 80% confluence and then incubated with 20 nM FITC-fibronectin. After a 17.5-h incubation, cells were either processed for immunofluorescence (Pulse, A) or were washed and then incubated with culture medium lacking fibronectin (Chase: −FN; B) or containing 20 nM Texas Red (TR)-fibronectin in the presence or absence of 50 μg/ml antifibronectin antibody 9D2 (C and D), 50 μg/ml control IgG (E and F), 320 nM 70-kDa amino-terminal fragment of fibronectin (G and H) or 320 nM control 40-kDa gelatin-binding fibronectin fragment (I and J) for 24 h. Cells were then processed as described in the legend to Figure 1. FITC (Pulse) and Texas Red (TR; Chase)-fibronectin staining are shown. Bar, 10 μm.
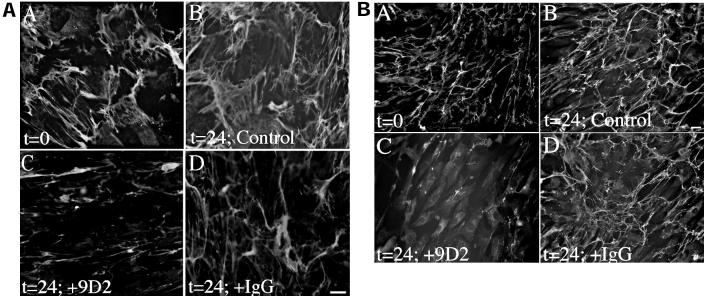
To determine whether fibronectin deposition also regulates the maintenance of extracellular matrix fibronectin in cells that produce fibronectin, we asked whether the mAb 9D2 could disrupt a fibronectin matrix produced by fibroblasts or smooth muscle cells. Fibroblasts were grown to confluence in serum-containing media. Cells were then incubated in the absence (Figure 6A, panels A and B) or presence of 9D2 (Figure 6A, panel C) or control IgG (Figure 6A, panel D) for an additional 24 h. Inhibition of fibronectin polymerization with the 9D2 antibody resulted in a dramatic rearrangement and loss of the fibronectin matrix in comparison with cells incubated in the absence of 9D2 (Figure 6A, panel B) or in the presence of control IgG (Figure 6A, panel D). Similarly, inhibition of fibronectin polymerization with the 9D2 antibody resulted in a reduction of fibronectin matrix (Figure 6B, panel C) in human aortic smooth muscle cells in comparison with cells incubated in the absence of 9D2 (Figure 6B, panel B) or in the presence of control IgG (Figure 6B, panel D). The striking reduction in the amount of fibronectin matrix after 9D2 treatment (Figure 6B, panel C) in comparison to levels present before 9D2 addition (Figure 6B, panel A) indicates that inhibiting fibronectin polymerization leads to the loss of the preexisting fibronectin matrix. Similar results were found with microvascular endothelial cells. These data indicate that the process of actively polymerizing a fibronectin matrix is a critical factor determining fibronectin matrix stability in cells that produce fibronectin.
Figure 6.
Fibronectin matrix stability in fibroblasts and smooth muscle cells. Human skin fibroblasts (A) and human aortic smooth muscle cells (B) were seeded onto coverslips in serum-containing media and grown to 90% confluence. Cells were then incubated with 50 μg/ml antifibronectin antibody 9D2 (C) or 50 μg/ml control IgG (D) for 24 h. Control cells were given an equivalent volume of PBS (B). At the time of 9D2 addition, some cells were processed for immunofluorescence (t = 0 h) to determine the amount of matrix deposited by the cells before 9D2 addition. After incubation with 9D2 or IgG, cells were permeabilized and then incubated with a polyclonal antibody to fibronectin, followed by FITC anti-rabbit IgG. Bar, 10 μm (A); bar, 20 μm (B).
Deposition and Retention of Thrombospondin-1 and Collagen I in the Matrix Depends on Fibronectin Polymerization
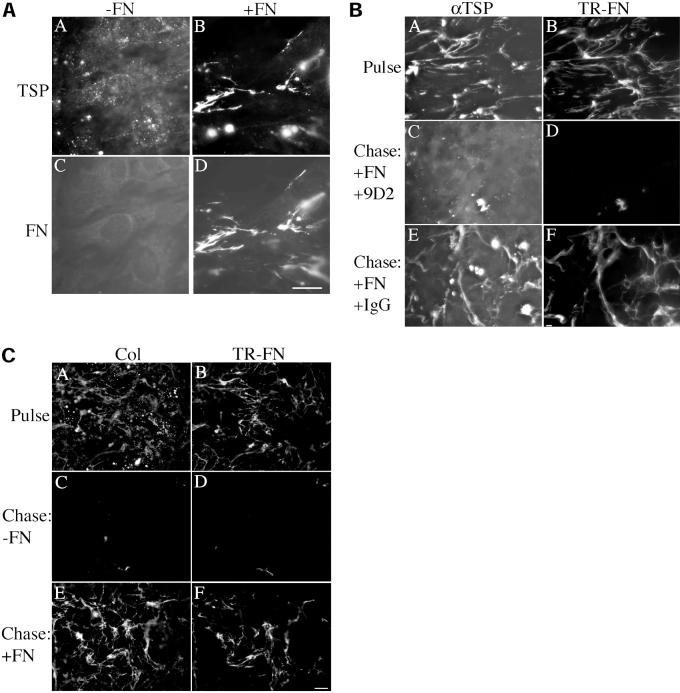
Fibronectin contains binding sites for a number of extracellular matrix molecules, including collagens, proteoglycans, fibulin, and thrombospondin (Yamada, 1989; Hynes, 1990; Chung et al., 1995; Sasaki et al., 1996) and has been shown to be required for the matrix deposition of fibulin and fibrinogen (Roman and McDonald, 1993; Sasaki et al., 1996; Pereira et al., 2002). In addition, inhibition of fibronectin matrix deposition has been correlated with a reduction in collagen type I deposition (McDonald et al., 1982). To determine whether deposition of thrombospondin-1 and collagen I in the extracellular matrix is regulated by fibronectin polymerization, we examined the localization of thrombospondin-1 in fibronectin-null cells cultured in the presence and absence of fibronectin. As shown in Figure 7A, deposition of thrombospondin-1 into fibrillar networks depended on the presence of fibronectin (Figure 7A, panel B). In the absence of fibronectin, thrombospondin-1 was not organized into fibrils but was present in a punctate staining pattern (Figure 7A, panel A). The assembly of collagen I into fibrillar networks also depends on the presence of fibronectin (our unpublished data). Both collagen I and thrombospondin 1 showed extensive colocalization with fibronectin fibrils. These data indicate that fibronectin is a critical component that regulates the assembly of other proteins into the extracellular matrix.
Figure 7.
(A) Effect of fibronectin polymerization on thrombospondin-1 deposition. Fibronectin-null cells were grown to confluence and then incubated with (+FN) or without (−FN) 20 nM fibronectin for 24 h. The culture medium was also supplemented with 15 nM thrombospondin-1. Cells were washed, fixed, and permeabilized. Cells were dual-labeled with antibodies to fibronectin (FN) and thrombospondin (TSP) followed by incubation with FITC- and Texas red-conjugated secondary antibodies. The same fields of view are shown in panels A and C; and B and D. (B) Fibronectin polymerization is required for maintenance of thrombospondin-I fibrils. Fibronectin-null cells were grown to 80% confluence and then incubated with 20 nM Texas Red-conjugated fibronectin (TR-FN) and 15 nM thrombospondin-1. After a 16-h incubation, cells were either processed for immunofluorescence (Pulse) or were washed and then incubated with culture medium containing 20 nM fibronectin, 15 nM thrombospondin, and either the antifibronectin antibody 9D2 (C and D) or mouse IgG (E and F) for 24 h. Cells were fixed and permeabilized. Thrombospondin was detected using a polyclonal antibody (αTSP) followed by a FITC-conjugated anti-rabbit IgG (A, C, and E). Corresponding fields of view are depicted in B, D, F, which show TR-fibronectin. (C) Fibronectin matrix assembly is required to maintain fibrillar collagen I. Fibronectin-null cells were grown to 80% confluence and then incubated with Texas-Red-fibronectin (20 nM). After a 16-h incubation, cells were either processed for immunofluorescence (Pulse) or were washed and then incubated with culture medium containing (E and F) or lacking (C and D) 20 nM unlabeled fibronectin for 24 h. Cells were fixed and then incubated with a polyclonal antibody to mouse collagen I (Col), followed by incubation with fluorescein-conjugated anti-rabbit IgG. A, C, and E show the FITC (collagen) staining. The same fields are depicted in B, D, and F, which show Texas Red (fibronectin) staining. Bar, 10 μm.
To determine whether fibronectin polymerization is required to maintain thrombospondin-1 and collagen-I fibrils, we asked whether thrombospondin and collagen fibrils could be maintained when cells are cultured in the presence of both fibronectin and an inhibitor of fibronectin polymerization, the mAb, 9D2. As shown in Figure 7B, thrombospondin fibrils are not maintained when fibronectin polymerization is inhibited by the 9D2 antibody (Figure 7B, panel C). In contrast, when fibronectin is coincubated with control IgG, thrombospondin fibrils are maintained (Figure 7B, panel E). Similarly, there was a striking loss of fibrillar collagen-I from the extracellular matrix (Figure 7C, panel C) that paralleled the loss of fibrillar fibronectin when fibronectin was omitted from the chase media (Figure 7C, panel D). These data indicate that the maintenance of the fibrillar organization of fibronectin, collagen I, and thrombosponin-1 requires fibronectin polymerization and support the idea that fibronectin polymerization is a critical control factor that regulates extracellular matrix composition and stability.
Analysis of MAPK During Fibronectin Matrix Turnover
Turnover of extracellular matrix fibronectin after inhibition of fibronectin deposition could result from intracellular signaling events that upregulate extracellular proteases, stimulate receptor mediated endocytosis, alter actin cytoskeletal organization, or alter the levels or cell surface localization of fibronectin-binding integrins or proteoglycans. During turnover of the fibronectin matrix, cells remain attached and spread (see Figure 1). Analysis of attached cells, as well as the few nonattached cells by vital dye staining indicate that there are no differences in cell viability between fibronectin-treated and untreated cells. Others have shown that treatment of cells with a fragment from fibronectin's III-1 module results in disruption of the fibronectin matrix and also causes upregulation of active p38 mitogen-activated protein kinase (MAPK; Bourdoulous et al., 1998). However, we did not find any correlation between the levels of active (phosphorylated) ERK1/2, p38, and JNK and the retention of fibronectin matrix (our unpublished data). Thus, the intracellular signaling pathways that are triggered after inhibition of fibronectin matrix deposition in our system differ from those triggered by addition of the fibronectin fragment III-1C (Bourdoulous et al., 1998).
Protease Inhibitors Do Not Affect Fibronectin Matrix Turnover
Loss of matrix fibronectin during fibronectin turnover could result from increased production or activation of fibronectin-degrading proteases. It should be emphasized that the loss of fibronectin matrix does not cause major changes in cell adhesion and spreading (Figure 1), making it unlikely that proteases directly act on the substrate to weaken cell adhesion and induce changes in cell shape that then lead to fibronectin loss. If proteases are involved in the reorganization and loss of fibronectin matrix that results from inhibition of fibronectin polymerization, then agents that inhibit proteolysis would be expected to prevent the loss of fibronectin matrix. We performed fibronectin pulse-chase experiments in the presence of a variety of protease inhibitors, including MMP inhibitors (1,10-phenanthroline, illomostat), aminopeptidase inhibitors (actinonin, bestatin, amastatin), serine protease inhibitors (leupeptin, aprotinin), aspartate protease inhibitor (pepstatin), and cysteine protease inhibitors (E64). None of the inhibitors tested, alone, or in combination, was able to prevent fibronectin matrix turnover. Although these data do not rule out a role for MMPs or other secreted proteases in some aspect of fibronectin matrix reorganization, they suggest that they do not play a critical role in regulating fibronectin matrix turnover.
Changes in Actin Cytoskeletal Dynamics are Critical for Fibronectin Matrix Reorganization
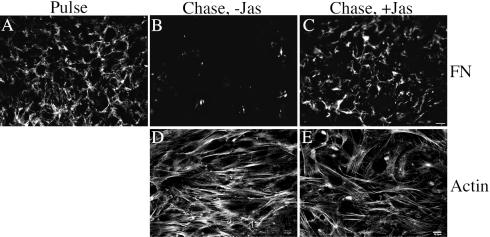
We have previously shown that fibronectin polymerization increases cell contractility and thus cytoskeletal organization, by a process that depends on Rho activity (Hocking et al., 2000). To determine whether changes in actin cytoskeletal dynamics induced by inhibition of fibronectin matrix polymerization contribute to turnover of the fibronectin matrix, we asked whether jasplakinolide could prevent fibronectin matrix turnover. Jasplakinolide has been reported to stabilize actin microfilaments and can also enhance the nucleation of actin polymerization (Bubb et al., 1994, 2000). Fibronectin-null cells containing a preestablished fibronectin matrix were incubated in the presence and absence of fibronectin and in the presence or absence of jasplakinolide. As shown in Figure 8, removal of fibronectin from the culture medium resulted in a dramatic loss of the preestablished fibronectin matrix (Figure 8B). This loss of matrix fibronectin was prevented by 150 nM jasplakinolide (Figure 8C). Addition of 100 nM jasplakinolide resulted in partial retention of fibronectin matrix, whereas 20–50 nM jasplakinolide had no effect. Actin stress fibers were present in both jasplakinolide-treated and nontreated cells (Figure 8, D and E), indicating that loss of fibronectin matrix is not correlated with a dramatic loss of organized actin fibrils. However, the ability of jasplakinolide to prevent fibronectin matrix reorganization indicates that loss of matrix fibronectin requires changes in actin cytoskeletal dynamics.
Figure 8.
Jasplakinolide stabilizes fibronectin matrix. Fibronectin-null cells were incubated overnight with 20 nM Texas Red (TR)-conjugated fibronectin. Cell were then either processed for immunofluorescence (Pulse, A) or were washed and then incubated with culture medium lacking fibronectin (Chase), in the absence (B and D) or presence (C and E) of 150 nM jasplakinolide, an actin stabilizing agent, for 24 h. Cells were fixed, permeabilized, and then incubated with FITC-phalloidin (to detect actin). TR-fibronectin (A–C) and actin (D and E) staining are shown. D and E are confocal images. Bar, 20 μm (A–C); bar, 10 μm (D and E).
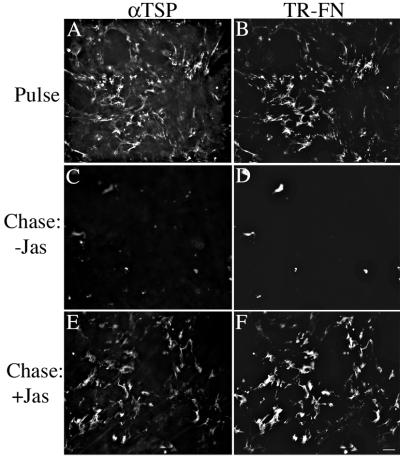
The ability of jasplakinolide to stabilize the fibronectin matrix in the absence of fibronectin polymerization allowed us to determine whether thrombospondin-1 fibrils could also be maintained under these conditions. Fibronectin-null cells with a preestablished fibronectin- and thrombospondin 1-matrix were chased in the presence of jasplakinolide and in the absence of fibronectin. As shown in Figure 9, thrombosponin fibrils were maintained when jasplakinolide-treated cells were chased in the absence of fibronectin. These data indicate that fibronectin polymerization maintains thrombospondin fibrils by preserving a fibrillar fibronectin matrix.
Figure 9.
Fibronectin fibrils are sufficient to maintain thrombospondin-1 fibrils. Fibronectin-null cells were incubated overnight with 20 nM Texas Red (TR)-conjugated fibronectin. The culture medium was also supplemented with 15 nM thrombospondin-1. Cells were then either processed for immunofluorescence (Pulse, A and B) or were washed and then incubated with culture medium lacking fibronectin (Chase), in the absence (C and D) or presence (E and F) of 150 nM jasplakinolide. Cells were labeled with antibodies to thrombospondin (TSP), followed by incubation with FITC-conjugated secondary antibodies. The same fields of view are shown in A and B; C and D; E and F. Bar, 20 μm.
Focal Contact Proteins Are Maintained during Turnover of the Fibronectin Matrix
Integrin ligation leads to clustering of integrins into focal contacts, areas of the cell that are in close apposition to the substrate (Izzard and Lochner, 1980; Chen and Singer, 1982), and that are rich in signaling and cytoskeletal proteins, including FAK, paxillin, and vinculin (Singer, 1982; Parsons et al., 2000; Turner, 2000). Clustering of integrins can also lead to the coclustering of signaling proteins (Miyamoto et al., 1995). Hence, disruption of the fibronectin matrix by inhibition of fibronectin polymerization, could be a consequence of alterations in the composition of focal contact signaling complexes. To determine whether the composition of focal contacts is altered during turnover of the fibronectin matrix, we examined the localization of vinculin, paxillin, and FAK in cells that contained, or lacked, an intact fibronectin matrix. Cells in which the fibronectin matrix was disrupted because of removal of fibronectin from the culture medium, retained prominent focal contacts that were rich in vinculin, paxillin, and FAK (our unpublished data). These proteins were also present in focal contacts of fibronectin-treated cells.
Fibronectin Matrix Stability Regulates the Composition of Cell–Matrix Fibrillar Adhesions
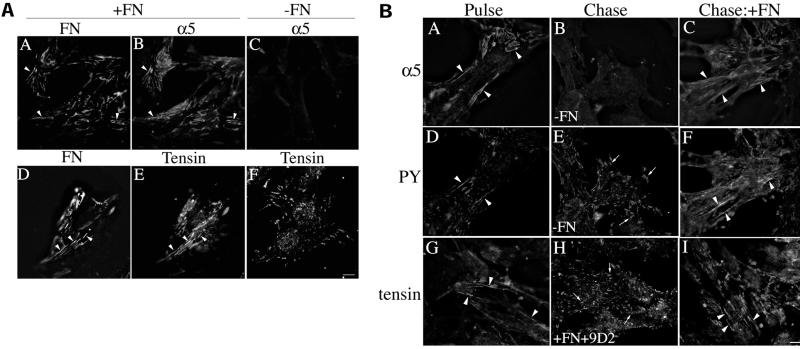
Cell–matrix fibrillar adhesions (also referred to as extracellular matrix contacts) are formed in response to integrin-extracellular matrix interactions (Chen and Singer, 1982; Singer et al., 1988). Fibrillar adhesions are dynamic structures that arise from focal contacts (Zamir et al., 2000), although their composition is distinct from focal contacts. Fibrillar adhesions have been shown to contain fibronectin, α5β1 integrin, and tensin (Katz et al., 2000; Zamir et al., 2000). Fibronectin fibrils are a prominent component of fibrillar adhesions (Singer et al., 1988; Zamir et al., 2000). To determine whether the presence of fibronectin affects the composition of fibrillar adhesions, we examined the localization of α5β1 integrin and tensin in cells cultured in the presence or absence of fibronectin. As shown in Figure 10A, α5β1 integrin localized to fibrillar adhesions in cells cultured in the presence of fibronectin; there was extensive colocalization of α5β1 with fibronectin fibrils (compare Figure 10A, panels A and B). Tensin also colocalized with fibronectin in fibrillar adhesions under these conditions (Figure 10A, panels D and E). In contrast, in the absence of fibronectin, α5β1 was not localized to any type of adhesion structure (Figure 10A, panel C), whereas tensin was found in structures resembling focal contacts (Figure 10A, panel F) but not in fibrillar adhesions. These data demonstrate that fibronectin is a critical factor that regulates the cell surface distribution of α5β1 and tensin.
Figure 10.
(A) α5β1 and tensin localize to fibrillar adhesions only in the presence of fibronectin. Fibronectin-null cells were incubated in the absence (C and F) or presence of 20 nM FITC-fibronectin (D and E) or 20 nM unlabeled fibronectin (A and B). After an overnight incubation, cells were fixed and permeabilized and then incubated with antibodies to fibronectin and α5 integrin (A–C) or antibodies to tensin (D–F). Fibronectin staining is shown in A and D; α5 integrin staining in B and C; and tensin staining in E and F. The same fields of view are shown in A and B and in D and E. Areas of colocalization of fibronectin and α5 integrin, or fibronectin and tensin are shown by the arrowheads. Bar, 10 μm. (B) Fibronectin matrix stability regulates maintenance of cell–matrix fibrillar adhesions. Fibronectin-null cells were incubated with 20 nM fibronectin. After an overnight incubation, cells were washed and then incubated in the absence (Chase; B and E) or presence of fibronectin (C, F, H, and I) for 23 h. In H, cells were coincubated with fibronectin and 9D2 IgG. Cells were fixed, permeabilized, and then coincubated with antibodies to α5 integrin (A–C) and phosphotyrosine (D–F) or antibodies to tensin (G–I). Panels A and D, B and E, and C and F are the same fields of view. Fibrillar adhesions are shown by the arrowheads and focal contacts by the arrows. Bar, 10 μm.
To determine whether turnover of the fibronectin matrix affects the composition of fibrillar adhesions, cells containing a preestablished fibronectin matrix were chased in the presence and absence of fibronectin, and the localization of α5β1 integrin, tensin, and phosphotyrosine was examined. As shown in Figure 10B, α5β1 integrin was organized into fibrillar adhesion sites (Figure 10B, panels A and C) in cells that contained an intact fibronectin matrix but was lost from these adhesions in cells in which fibronectin polymerization was blocked by removal of fibronectin from the cell culture media (Figure 10B, panel B) or by addition of the 9D2 antibody (our unpublished data). Flow cytometry data indicate that the levels of α5 integrin on the cell surface do not decrease when cells with a preformed fibronectin matrix are incubated in the absence of fibronectin (our unpublished data). Hence, the loss of α5β1 staining in fibrillar adhesions represents a reorganization of α5β1 and not a loss from the cell surface. Similarly, tensin was present in cell–matrix fibrillar adhesions in cells containing a fibronectin matrix (Figure 10B, panels G and I) but not in cells in which the fibronectin matrix was disrupted because of removal of fibronectin from the culture supernatant (our unpublished data) or to the presence of the fibronectin polymerization inhibitor, 9D2 (Figure 10B, panel H). In these cells, tensin was localized to structures resembling focal contacts (Figure 10B, panel H). Phosphotyrosine containing proteins are present in both cell–matrix fibrillar adhesions (Figure 10B, panels D and F) and focal contacts in cells that have a fibronectin matrix. Confocal image analysis indicates that the phosphotyrosine staining in fibrillar adhesions colocalizes with α5β1 integrin and tensin (our unpublished data). This phosphotyrosine staining pattern is lost from fibrillar adhesions but not from focal contacts (Figure 10B, panel E), in cells in which the fibronectin matrix is disrupted. These data indicate that disruption of fibronectin matrix fibrils leads to a loss of cell–matrix adhesion structures and that fibronectin polymerization is required to maintain fibrillar adhesions.
DISCUSSION
Assembly and maintenance of the extracellular matrix plays an important role in preserving the structural integrity of blood vessels. Mice lacking fibronectin die during embryogenesis because of impaired integrity of the vasculature (George et al., 1993, 1997). Similar defects have been found in mice lacking various integrin subunits (Bader et al., 1998; Yang et al., 1993, 1995). Collagen I, collagen III, and fibrillin also contribute to vessel wall stability, because mutations in these molecules can lead to blood vessel rupture (Pereira et al., 1997; Gustafsson and Fassler, 2000). Others have shown that cells that lack fibronectin fibrils also lack tenascin C fibrils (Chung and Erickson, 1997). In addition, fibronectin deposition regulates the deposition of fibulin (Roman and McDonald, 1993; Godyna et al., 1995b; Sasaki et al., 1996) and fibrinogen (Pereira et al., 2002) in the extracellular matrix. Our data indicate that collagen I and thrombosponin-1 are deposited into fibrillar structures in the extracellular matrix only when fibronectin fibrils are present. Our data with collagen I are consistent with previous data showing that a polyclonal antibody to fibronectin that inhibits the establishment of a fibronectin matrix also inhibits the deposition of collagen I and III fibers (McDonald et al., 1982). Taken together, these data demonstrate that the deposition of extracellular matrix fibronectin fibrils is important in regulating the composition and organization of the extracellular matrix.
Our data also show that the maintenance of fibrillar thrombospondin-1 depends on fibronectin polymerization. Thrombospondin-1 is found in the extracellular matrix of cells in culture and in a variety of tissue extracellular matrices (O'Shea and Dixit, 1988; Bornstein and Sage, 1994). However, the mechanisms that regulate the deposition and turnover of extracellular matrix thrombospondin have not been previously reported. Our data indicate that fibronectin polymerization maintains fibrillar thrombospondin-1 by stabilizing extracellular matrix fibronectin fibrils (Figure 9). Recent evidence has shown that thrombospondin-1 is an important endogenous inhibitor of angiogenesis (Good et al., 1990; Tolsma et al., 1993). These data suggest that fibronectin polymerization may regulate thrombospondin's antiangiogenic properties by controlling the amount and localization of extracellular matrix thrombospondin. Our data also demonstrate the maintenance of collagen I fibrils in the extracellular matrix requires fibronectin polymerization. Thus, fibronectin and fibronectin polymerization regulate both the deposition and maintenance of collagen I and thrombosponin-1 matrix fibrils.
Fibronectin has long been thought to be a stable component of the extracellular matrix. In vitro studies using cultured fibroblasts indicated that there was little turnover of 125I-fibronectin from the extracellular matrix over a 28-h period (McKeown-Longo and Mosher, 1983). These studies were done in cells that produced fibronectin and in which the culture medium was supplemented with unlabeled fibronectin (McKeown-Longo and Mosher, 1983). In the present study, we used fibronectin-null cells to examine the turnover of extracellular matrix fibronectin. Fibronectin-null cells do not produce fibronectin and are maintained in serum-free medium, thus allowing us to precisely control the levels of fibronectin that are present. Our studies demonstrate that the continual presence of fibronectin in the culture medium can stabilize preexisting fibronectin fibrils and that inhibition of fibronectin polymerization can disrupt preestablished fibronectin fibrils in cells that either produce or lack fibronectin. Our data showing rapid fibronectin matrix turnover by cultured cells are consistent with a report documenting fibronectin turnover in tissues in vivo (Rebres et al., 1995). 125I-fibronectin infused intravenously into rats was incorporated into many tissues, including liver, lung, and skin (Rebres et al., 1995). As much as 30–50% of the labeled “tissue pool” of fibronectin was lost over 24 h (Rebres et al., 1995).
Fibronectin degradation by chymases has been reported in peritoneal cells, in a process that depends on the presence of sulfated heparin (Tchougounova et al., 2000). In this system, fibronectin degradation was inhibited by protamine, and by serine protease inhibitors (Tchougounova et al., 2000). It is unlikely that the loss of matrix fibronectin in our system is mediated by chymases, because chymases are produced by mast cells (Yong, 1997; Krishnaswamy et al., 2001) and because the addition of protamine and serine protease inhibitors does not prevent the loss of matrix fibronectin (our unpublished data). In fact, despite much effort, we were unable to show that extracellular proteases were a critical factor leading to loss of fibronectin matrix in fibronectin-null cells.
Others have shown that treatment of cells with a fragment from fibronectin's III-1 module, III-1C causes a loss of extracellular matrix fibronectin fibrils and also results in upregulation of the MAP kinase, p38 (Bourdoulous et al., 1998). Our data indicate that disruption of fibronectin fibrils by inhibiting fibronectin polymerization is not correlated with changes in the activity of p38, ERK, or JNK. Thus, the loss of matrix fibronectin in our system appears to be distinct from that reported for the III-1C fragment. It is also possible that the III-1C fragment has effects on cells in addition to those triggered by loss of matrix fibronectin, as III-1C has been reported to have direct effects on cell function (Tellier et al., 2000).
Loss of fibronectin matrix does not occur as a result of loss of cell-substrate adhesion, because cells remained attached and spread, with well-developed stress fibers and focal contacts after disruption of the fibronectin matrix. Although actin stress fibers are not disrupted in cells that lack an intact fibronectin matrix, changes in actin cytoskeletal dynamics are likely to be critical for turnover of the fibronectin matrix, because treatment of cells with jasplakinolide resulted in retention of fibronectin matrix fibrils in the absence of ongoing fibronectin matrix polymerization. It is well established that agents that disrupt the actin cytoskeleton disrupt fibronectin matrix organization (Barry and Mosher, 1988; Wu et al., 1995). In turn, fibronectin matrix polymerization can also regulate the actin cytoskeleton (Hocking et al., 2000). Hence, there is a dynamic, reciprocal relationship between fibronectin polymerization and actin cytoskeletal organization. One possible mechanism by which the actin cytoskeleton could regulate fibronectin matrix turnover would be by controlling fibronectin endocytosis. The actin cytoskeleton is known to be involved in endocytosis (Lamaze et al., 1997). Studies with 125I-fibronectin indicate that trichloroacetic acid–soluble counts accumulate in the culture medium of cells in which the fibronectin matrix is turned over (our unpublished data), suggesting that fibronectin is internalized and degraded by lysosomes. In support of this, recent studies indicate that fibronectin catabolism can be regulated by the endocytic receptor, low-density lipoprotein receptor-related protein (LRP; Salicioni et al., 2002).
Others have shown that actin cytoskeletal dynamics are critical for the translocation of α5β1 integrins into cell–matrix fibrillar adhesions (Pankov et al., 2000). In this study, the authors show that treatments that prevent the translocation of α5β1 integrins into cell–matrix contacts also prevent fibronectin matrix polymerization (Pankov et al., 2000). These authors propose that the movement of α5β1 integrins into cell–matrix fibrillar adhesions promotes fibronectin fibril formation (Pankov et al., 2000). Our data indicate that α5β1 integrin does not localize to fibrillar adhesions in the absence of fibronectin or fibronectin polymerization and that fibronectin polymerization is critical for maintaining the localization of α5β1 integrins in cell–matrix fibrillar adhesions. Hence, the ability of the antiintegrin antibody, mAB16 to inhibit fibronectin polymerization (Akiyama et al., 1989; Fogerty et al., 1990) could also account for its ability to inhibit the translocation of α5β1 into fibrillar adhesions (Pankov et al., 2000). Our data also demonstrate that inhibition of fibronectin polymerization results in loss of α5β1 integrins, tensin, and phosphotyrosine-containing proteins from fibrillar adhesions. Hence, fibronectin polymerization is critical for the formation and maintenance of cell–matrix fibrillar adhesions.
Taken together, these data indicate that fibronectin polymerization is a crucial switch that regulates the composition and stability of the extracellular matrix and is also an important regulator of the formation and stability of cell–matrix fibrillar adhesions. The ability of cells to up- and downregulate fibronectin polymerization (Ignotz and Massague, 1986; Allen-Hoffmann et al., 1988; Sommers and Mosher, 1993; Zhang et al., 1994, 1999; Zhong, 1998) thus provides cells with a novel mechanism for selectively altering cell–matrix adhesion structures and cell matrix signaling events.
ACKNOWLEDGMENTS
We thank Dr. Deane Mosher for providing antibodies, Ms. Michelle Arquiett, and Mr. Jonathon Nezezon for technical assistance, and Dr. Susan LaFlamme for critically reading this manuscript. This research was supported by grants HL50549 and HL03971 (to J.S.), and HL60181 and HL64074 (to D.H.) from the National Institutes of Health.
Abbreviations used:
- DOC
deoxycholate
- FAK
focal adhesion kinase
- MMP
matrix metalloproteinase
- MAPK
mitogen-activated protein kinase
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0048. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0048.
REFERENCES
- Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Hoffmann BL, Crankshaw CL, Mosher DF. Transforming growth factor beta increases cell surface binding and assembly of exogenous (plasma) fibronectin by normal human fibroblasts. Mol Cell Biol. 1988;8:4234–4242. doi: 10.1128/mcb.8.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Barry ELR, Mosher DF. Factor XIII cross-klinking of fibronectin at cellular matrix assembly sites. J Biol Chem. 1988;263:10464–10469. [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Thrombospondins. Methods Enzymol. 1994;245:62–77. doi: 10.1016/0076-6879(94)45006-4. [DOI] [PubMed] [Google Scholar]
- Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol. 1998;143:267–276. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- Chen WT, Singer SJ. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982;95:205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousov MA, Fogerty FJ, Koteliansky VE, Mosher DF. Role of the I-9 and III-1 modules of fibronectin in formation of an extracellular matrix. J Biol Chem. 1991;266:10851–10858. [PubMed] [Google Scholar]
- Choi MG, Hynes RO. Biosynthesis and processing of fibronectin in NIL.8 hamster cells. J Biol Chem. 1979;23:12050–12055. [PubMed] [Google Scholar]
- Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J Cell Sci. 1997;110:1413–1419. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- Chung CY, Zardi L, Erickson HP. Binding of tenascin-C to soluble fibronectin and matrix fibrils. J Biol Chem. 1995;270:29012–29017. doi: 10.1074/jbc.270.48.29012. [DOI] [PubMed] [Google Scholar]
- Clark RAF, McCoy GA, Folkvord JM, McPherson JM. TGF-β1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170:69–80. doi: 10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Debelle L, Tamburro AM. Elastin: molecular description and function. Int J Biochem Cell Biol. 1999;31:261–272. doi: 10.1016/s1357-2725(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Ducharme A, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (α5β1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- George EL, Georges-Labousse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–227. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995a;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyna S, Mann DM, Argraves WS. A quantitative analysis of the incorporation of fibulin-1 into extracellular matrix indicates that fibronectin assembly is required. Matrix Biol. 1995b;14:467–477. doi: 10.1016/0945-053x(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, LeBeau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependant inhibitor of angiogenesis is immunologically and fuctionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson E, Fassler R. Insights into extracellular matrix functions from mutant mouse models. Exp Cell Res. 2000;261:52–68. doi: 10.1006/excr.2000.5042. [DOI] [PubMed] [Google Scholar]
- Hausser H, Ober B, Quentin-Hoffmann E, Schmidt B, Kresse H. Endocytosis of different members of the small chondroitin/dermatan sulfate proteoglycan family. J Biol Chem. 1992;267:11559–11564. [PubMed] [Google Scholar]
- Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Langenbach KJ. Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem. 2000;275:10673–10682. doi: 10.1074/jbc.275.14.10673. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Activation of distinct α5β1-mediated signaling pathways by fibronectin's cell adhesion and matrix assembly domains. J Cell Biol. 1998;141:241–253. doi: 10.1083/jcb.141.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Izzard CS, Lochner LR. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Katz BZ, Zamir E, Bershadsky A, Kam Z, Yamada KM, Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell. 2000;11:1047–60. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Krane SM. The turnover and degradation of collagen. Ciba Found Symp. 1985;114:97–110. doi: 10.1002/9780470720950.ch7. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G, Kelley J, Johnson D, Youngberg G, Stone W, Huang SK, Bieber J, Chi DS. The human mast cell: functions in physiology and disease. Front Biosci. 2001;6:D1109–D1127. doi: 10.2741/krishnas. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchina E, Barlati S. Degradation of human plasma and extracellular matrix fibronectin by tissue type plasminogen activator and urokinase. Int J Biochem Cell Biol. 1996;28:1141–1150. doi: 10.1016/1357-2725(96)00055-6. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab′ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982;92:485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Hanning R, Mosher DF. Binding and degradation of platelet thrombospondin by cultured fibroblasts. J Cell Biol. 1984;98:22. doi: 10.1083/jcb.98.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;97:466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmo LM, McKeown-Longo P. The αvβ5 integrin functions as an endocytic receptor for vitronectin. J Cell Sci. 1998;111:425–433. doi: 10.1242/jcs.111.4.425. [DOI] [PubMed] [Google Scholar]
- Mercurius KW, Morla AO. Inhibition of vascular smooth muscle cell growth by inhibition of fibronectin matrix assembly. Circ Res. 1998;82:548–556. doi: 10.1161/01.res.82.5.548. [DOI] [PubMed] [Google Scholar]
- Miekka SI, Ingham KC, Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982;27:1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- Mosher DF, Doyle MJ, Jaffe EA. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982;93:343. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987;105:1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea KS, Dixit VM. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988;107:2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Bourdoulous S, Koivunen E, Woods VL, Ruoslahti E. A polymeric form of fibronectin has antimetastatic effects against multiple tumor types. Nat Med. 1996;2:1197–1203. doi: 10.1038/nm1196-1197. [DOI] [PubMed] [Google Scholar]
- Pereira L, et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- Pereira M, Rybarczyk BJ, Odrljin TM, Hocking DC, Sottile J, Simpson-Haidaris PJ. The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. J Cell Sci. 2002;115:609–617. doi: 10.1242/jcs.115.3.609. [DOI] [PubMed] [Google Scholar]
- Pijuan-Thompson V, Gladson CL. Ligation of integrin α5β1 is required for internalization of vitronectin by integrin αvβ3. J Biol Chem. 1997;272:2736–2743. doi: 10.1074/jbc.272.5.2736. [DOI] [PubMed] [Google Scholar]
- Quade BJ, McDonald JA. Fibronectin's amino-terminal matrix assembly site is located within the 29 kDa amino terminal domain containing five type 1 repeats. J Biol Chem. 1988;263:19602–19609. [PubMed] [Google Scholar]
- Rebres RA, McKeown-Longo PJ, Vincent PA, Cho E, Saba TM. Extracellular matrix incorporation of normal and NEM-alkylated fibronectin: liver and spleen deposition. Am J Physiol. 1995;269:G902–G912. doi: 10.1152/ajpgi.1995.269.6.G902. [DOI] [PubMed] [Google Scholar]
- Roman J, McDonald JA. Fibulin's organization into the extracellular matrix of fetal lung fibroblasts is dependent on fibronectin matrix assembly. Am J Respir Cell Mol Biol. 1993;8:538–545. doi: 10.1165/ajrcmb/8.5.538. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Mizelle KS, Loukinova E, Mikhailenko I, Strickland DK, Gonias SL. The low density lipoprotein receptor-related protein mediates fibronectin catabolism and inhibits fibronectin accumulation on cell surfaces. J Biol Chem. 2002;277:16160–16166. doi: 10.1074/jbc.M201401200. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Wiedemann H, Matzner M, Chu M-L, Timpl R. Expression of fibulin-2 by fibroblasts and deposition with fibronectin into a fibrillar matrix. JCell Sci. 1996;109:2895–2904. doi: 10.1242/jcs.109.12.2895. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- Singer II. Association of fibronectin and vinculin with focal contacts and stress fibers in stationary hamster fibroblasts. J Cell Biol. 1982;92:398–408. doi: 10.1083/jcb.92.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Scott S, Kawka DW, Kazazis DM, Gailit J, Ruoslhti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988;106:2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CE, Mosher DF. Protein kinase C modulation of fibronectin matrix assembly. J Biol Chem. 1993;268:22277–22280. [PubMed] [Google Scholar]
- Sottile J, Hocking DC, Langenbach KJ. Fibronectin polymerization stimulates cell growth by RGD-dependent and -independent mechanisms. J Cell Sci. 2000;113:4287–4299. doi: 10.1242/jcs.113.23.4287. [DOI] [PubMed] [Google Scholar]
- Sottile J, Hocking DC, Swiatek P. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J Cell Sci. 1998;111:2933–2943. doi: 10.1242/jcs.111.19.2933. [DOI] [PubMed] [Google Scholar]
- Sottile J, Mosher DF. N-terminal type I modules required for fibronectin binding to fibroblasts and to fibronectin's III1 module. Biochem J. 1997;323:61–60. doi: 10.1042/bj3230051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Wiley S. Assembly of amino-terminal fibronectin dimers into the extracellular matrix. J Biol Chem. 1994;269:17192–17198. [PubMed] [Google Scholar]
- Tchougounova E, Forsberg E, Angelborg G, Kjellen L, Pejler G. Altered processing of fibronectin in mice lacking heparin. A role for heparin-dependent mast cell chymase in fibronectin degradation. J Biol Chem. 2000;16:16. doi: 10.1074/jbc.M008434200. [DOI] [PubMed] [Google Scholar]
- Tellier MC, Greco G, Klotman M, Mosoian A, Cara A, Arap W, Ruoslahti E, Pasqualini R, Schnapp LM. Superfibronectin, a multimeric form of fibronectin, increases HIV infection of primary CD4+ T lymphocytes. J Immunol. 2000;164:3236–3245. doi: 10.4049/jimmunol.164.6.3236. [DOI] [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin HT, Staehlin T, Grodon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. Paxillin and focal adhesion signaling. Nat Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- Wu C, Keivens VM, O'Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Yuen SM, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. Fibronectin domains and receptors. In: Mosher D F, editor. Fibronectin. New York: Academic Press; 1989. pp. 47–121. [Google Scholar]
- Yanagishita M, Hascall VC. Metabolism of proteoglycans in rat ovarian glanulosa cell culture. Multiple intracelluar degradative pathways and the effect of chloroquine. J Biol Chem. 1984;259:10270. [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Yong LC. The mast cell: origin, morphology, distribution, and function. Exp Toxicol Pathol. 1997;49:409–424. doi: 10.1016/S0940-2993(97)80129-7. [DOI] [PubMed] [Google Scholar]
- Zamir E, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Peyruchaud O, French KJ, Magnusson MK, Mosher DF. Sphingosine 1-phosphate stimulates fibronectin matrix assembly through a Rho-dependent signal pathway. Blood. 1999;93:2984–2990. [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]