Abstract
EB1 is a microtubule tip–associated protein that interacts with the APC tumor suppressor protein and components of the dynein/dynactin complex. We have found that the C-terminal 50 and 84 amino acids (aa) of EB1 were sufficient to mediate the interactions with APC and dynactin, respectively. EB1 formed mutually exclusive complexes with APC and dynactin, and a direct interaction between EB1 and p150Glued was identified. EB1-GFP deletion mutants demonstrated a role for the N-terminus in mediating the EB1-microtubule interaction, whereas C-terminal regions contributed to both its microtubule tip localization and a centrosomal localization. Cells expressing the last 84 aa of EB1 fused to GFP (EB1-C84-GFP) displayed profound defects in microtubule organization and centrosomal anchoring. EB1-C84-GFP expression severely inhibited microtubule regrowth, focusing, and anchoring in transfected cells during recovery from nocodazole treatment. The recruitment of γ-tubulin and p150Glued to centrosomes was also inhibited. None of these effects were seen in cells expressing the last 50 aa of EB1 fused to GFP. Furthermore, EB1-C84-GFP expression did not induce Golgi apparatus fragmentation. We propose that a functional interaction between EB1 and p150Glued is required for microtubule minus end anchoring at centrosomes during the assembly and maintenance of a radial microtubule array.
INTRODUCTION
EB1 was originally identified as a protein interacting with the C-terminus of the adenomatous polyposis coli (APC) tumor suppressor protein (Su et al., 1995). Truncating mutations of the APC gene occur as an early event in inherited and sporadic tumors of the colon (Polakis, 1997). Truncated APC proteins are defective in downregulating β-catenin in the WNT signaling pathway (Munemitsu et al., 1995), resulting in increased cellular β-catenin and increased transcription of proliferative genes (Korinek et al., 1997). EB1 is the prototypic member of a growing family of microtubule binding proteins (MAPs; Tirnauer and Bierer, 2000) including Bim1p in budding yeast and Mal3 in fission yeast. Bim1p (Schwartz et al., 1997) localizes to the plus ends of extending microtubules and has been suggested to play a role in regulating microtubule dynamics (Tirnauer et al., 1999). More recently Bim1p been shown to directly participate in the cortical capture of cytoplasmic microtubules during spindle positioning through an interaction with Kar9p (Korinek et al., 2000; Lee et al., 2000; Miller et al., 2000). It has also been shown to participate in a mitotic checkpoint, which delays cytokinesis in the event of abnormal spindle positioning (Muhua et al., 1998; Adames and Cooper, 2000). Mal3 was originally isolated in a screen for yeast mutants defective in chromosomal segregation (Beinhauer et al., 1997), and its loss was observed to affect microtubule length, nuclear positioning, and cell shape. Taken together, these observations suggest a dual role for EB1 proteins in yeast, the regulation of microtubule dynamics, and participation in microtubule capture events at the cell cortex.
In mammalian cells, EB1 localizes to both the growing distal tip of microtubules and the centrosome (Berrueta et al., 1998; Morrison et al., 1998; Mimori-Kiyosue et al., 2000b; Bu and Su, 2001; Morrison et al., 2002). EB1 interacts with the C-terminus of APC and may participate in either the targeting of APC to microtubule tips or the APC-dependent capture of microtubule tips at the cell cortex (Askham et al., 2000; Mimori-Kiyosue et al., 2000b; Barth et al., 2002). APC itself can directly associate with microtubules in vitro and in vivo (Munemitsu et al., 1994; Smith et al., 1994; Näthke et al., 1996; Deka et al., 1998; Askham et al., 2000; Mimori-Kiyosue et al., 2000a), and it has been suggested that it may play a role in controlling microtubule dynamics and stability during epithelial cell migration (Näthke, 2000; Zumbrunn et al., 2001; Barth et al., 2002). Recently, the APC/EB1 complex has been demonstrated to positively regulate microtubule polymerization (Nakamura et al., 2001) and has been suggested to participate in kinetochore capture and the stabilization of microtubule-kinetochore interactions (Fodde et al., 2001). Furthermore, APC mutations have been linked to chromosomal instability, a common feature of many cancerous cells, possibly as a consequence of the loss of the APC-EB1 interaction (Fodde et al., 2001; Kaplan et al., 2001; see Mimori-Kiyosue and Tsukita, 2001 for a recent review).
In addition to its interactions with microtubules and APC, EB1 has been demonstrated to interact with components of the cytoplasmic dynein/dynactin microtubule motor complex (Berrueta et al., 1999). The functional significance of this interaction remains unknown, as does the identity of the specific binding partner for EB1 in this large complex. Furthermore, β-catenin has recently been demonstrated to bind directly to dynein, suggesting a role for a β-catenin-dynein complex in microtubule attachment at adherens junctions (Ligon et al., 2001). There is therefore a multitude of potential complexes involving EB1, APC, microtubules, the dynein/dynactin complex, and β-catenin.
When this work began the microtubule interaction domain in EB1 was undetermined, the regions of EB1 involved in APC and dynein/dynactin binding were poorly defined, and no clear cellular function had been identified for EB1. The aim of this study was to further characterize human EB1, producing the information and reagents required for a rational examination of EB1 function in mammalian cells.
MATERIALS AND METHODS
Cells
HCT116, SW480, and COS-7 cells were cultured as described previously (Morrison et al., 1998; Askham et al., 2000). Nocodazole treatment and washout experiments were performed as described previously (Morrison et al., 1998).
Antibodies
Monoclonal antibodies specific for EB1 and p150Glued were obtained from Transduction Laboratories (Lexington, KY). Monoclonal antibodies specific for β-catenin, acetylated tubulin, and the 58-kDa Golgi protein, along with monoclonal and polyclonal antibodies specific for γ-tubulin, were obtained from Sigma (St. Louis, MO). A rat anti–α-tubulin antibody was obtained from Serotec (Raleigh, NC). Rabbit polyclonal and mouse monoclonal anti-GFP antibodies were obtained from Clontech (Palo Alto, CA). All secondary antibodies were Alexa 488, 568, and 633 conjugates obtained from Molecular Probes (Eugene, OR).
Plasmid Construction
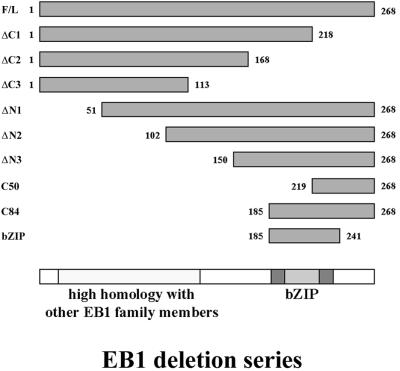
The plasmids mediating the expression of the fusion proteins GST-APC-C1 and GST-EB1 have been previously described (Askham et al., 2000). The EB1-GFP expression plasmid has also been previously described (Morrison et al., 2002). A series of GFP-tagged EB1 deletion mutants were generated by PCR amplification with Pfu DNA polymerase (Stratagene, La Jolla, CA) of sections of EB1 cDNA encoding EB1 amino acids 51–268 (EB1-ΔN1), 102–268 (EB1-ΔN2), 150–268 (EB1-ΔN3), 1–218 (EB1-ΔC1), 1–168 (EB1-ΔC2), and 1–113 (EB1-ΔC3). PCR primers incorporated restriction enzyme sites for cloning into pJMA2eGFP (Askham et al., 2000). All plasmids were sequenced to check for errors during PCR amplification. A series of GST-tagged EB1 deletion mutants were also generated by subcloning the sequenced EB1 cDNA fragments from pJMA2eGFP into pGEX-4T-A10 (Askham et al., 2000). Three further GST-tagged and GFP-tagged EB1 deletion mutants were generated by PCR amplification of sections of EB1 cDNA encoding EB1 amino acids 219–268 (EB1-C50), 185–268 (EB1-C84), and 185–241 (EB1-bZIP). PCR primers incorporated restriction enzyme sites for cloning into pGEX-4T-A10 and pJMA2eGFP. All plasmids were sequenced to check for errors during PCR amplification. A plasmid mediating the expression of a 6His-EB1 fusion protein was generated by PCR amplification with Pfu DNA polymerase of EB1 cDNA with primers incorporating BamHI and HindIII restriction sites and cloning into the same sites in pRSETA (Invitrogen, Carlsbad, CA). The resulting plasmid was sequenced to check for errors in the EB1 cDNA and was termed p6His-EB1. Plasmids mediating the expression of 6His-tagged p150Glued deletion mutants were generated by cloning rat p150Glued cDNA fragments encoding amino acids 1–811, 1–330, and 600–811 into pET21a (Novagen, Madison, WI) and fragments encoding amino acids 39–1276 and 149–811 into pET15b (Novagen) as described previously (Waterman-Storer et al., 1995).
Bacterial Protein Expression
Recombinant proteins were expressed in BL21(DE3) cells. GST fusion proteins were purified from bacterial cell extracts, using glutathione-Sepharose beads as described previously (Askham et al., 2000), and stored at −80°C until use. GST, GST-EB1, and GST-APC-C1 were recovered from glutathione-Sepharose beads by incubation in a buffer comprising 10 mM Tris-HCl, pH 8.0, 25 mM glutathione, and a cocktail of protease inhibitors (1 mM AEBSF, 100 μM leupeptin, 1 μM pepstatin) for 18 h at 4°C and stored at −80°C until use. The recovery of GST-EB1-ΔN1 and GST-EB1-ΔC3 from bacterial lysates was significantly lower than that obtained for the other fusion proteins. To avoid the introduction of artifacts associated with this discrepancy, these fusion proteins were not used in subsequent precipitation experiments. Bacteria expressing 6His-EB1 were lysed in a buffer comprising PBS, 1% Triton X-100, and protease inhibitors (PBS/TX100), and the soluble fraction was purified by nickel affinity chromatography using His-Bind cartridges (Novagen). 6His-EB1 was eluted from the cartridge in PBS/TX100, 500 mM imidazole. Imidazole was removed by dialysis, and the protein was stored at −80°C until use. For precipitation experiments, 6His-EB1 was complexed to Ni-2+Sepharose beads by incubation in PBS/TX100 for 16 h at 4°C. Bacteria expressing 6His-p150(1–330) were lysed in modified RIPA buffer (RIPA-M) comprising PBS, 1% Nonidet-P40, 1% sodium deoxycholate, 0.1% SDS, and a cocktail of protease inhibitors (1 mM AEBSF, 100 μM leupeptin, 1 μM pepstatin), and the soluble fraction was purified by nickel affinity chromatography using His-Bind cartridges (Novagen). 6His-p150(1–330) was eluted from the cartridge in RIPA-M, 500 mM imidazole.
Immunofluorescence and Confocal Microscopy
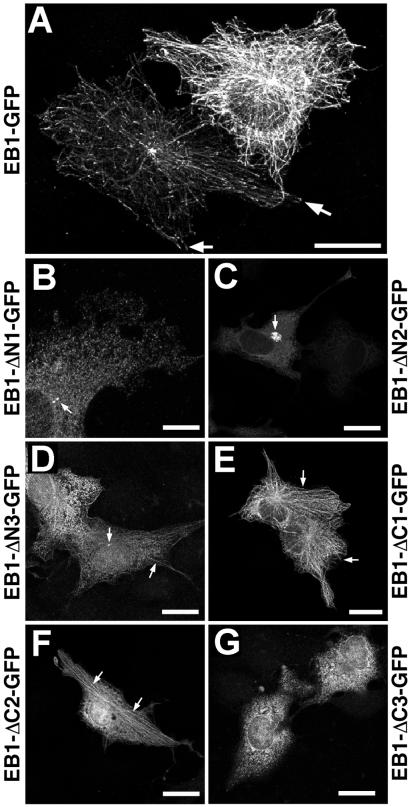
COS-7 cells were cultured on glass coverslips. Transfections were performed as described previously (Askham et al., 2000). Cell treatments with nocodazole have been previously described (Morrison et al., 1998). Eighteen hours after transfection, cultures were fixed in methanol at −20°C, processed for immunocytochemistry, and imaged using a Leica TCS-SP confocal microscope (Deerfield, IL) as described previously (Morrison et al., 1998; Askham et al., 2000). Care was taken to image cells with a relatively normal, well-spread, interphase morphology; cells exhibiting obvious signs of apoptosis or entry into mitosis (as revealed by the routine staining of chromatin using DAPI) were excluded for the purposes of this study. Comparisons of EB1-GFP fusion protein expression levels in transfected cells were performed using either the Quantitation software module of the Leica confocal microscope operating system, by exporting images to NIH Image v1.62 (obtained free of charge from the National Institutes of Health, Bethesda, MD) for further analysis or by examining confocal datasets in the Imaris suite of image analysis software (Bitplane AG, Zurich, Switzerland). The localizations identified for EB1-GFP and the deletion mutants derived from it in transfected cells (Figure 3; summarized in Table 1) are based on observations of cells expressing the lowest levels of fusion protein detectable by immunofluorescence unless otherwise stated.
Figure 3.
Distribution of EB1-GFP and deletion mutants in transfected COS-7 cells. Cells were processed for immunofluorescence with an anti-GFP antibody 18 h after transfection. (A) Distribution of EB1-GFP. The cell on the left displays microtubule tip labeling (arrows). Quantitative analysis suggested that the cell on the right was expressing about fourfold higher levels of EB1-GFP than the cell on the left, and more extensive microtubule labeling was seen. (B) Cell expressing EB1-ΔN1-GFP. No microtubule association is seen, but the centrosome is stained (arrow). (C) Cells expressing EB1-ΔN2-GFP. The image was optimized for the cell expressing higher levels of the fusion protein. At low expression levels a diffuse cytoplasmic distribution is seen. At higher levels a centrosome-associated aggregate is observed (arrow). (D) Cells expressing EB1-ΔN3-GFP. A weak microtubule association and a centrosomal localization are seen (arrows). (E) Cells expressing EB1-ΔC1-GFP. A robust microtubule associated staining pattern is seen (arrows), although it appears less polarized toward microtubule tips than that seen for full-length EB1. (F) Cell expressing EB1-ΔC2-GFP. A similar microtubule association is again apparent (arrows). (G) Cells expressing EB1-ΔC3-GFP. A diffuse cytoplasmic staining pattern is seen. Bars in all but B, 20 μm. Bar in B, 10 μm.
Table 1.
Summary of characteristics of EB1 and EB1 deletion mutant fusion proteins
| EB1 deletions | Amino acids | APC binding | Dynactin binding | EB1 binding | MT association in transfected cells | MT bundling when over-expressed | Centrosome association in transfected cells |
|---|---|---|---|---|---|---|---|
| EB1 | 1–268 | 213 | 213 | − | 213 | 213 | 213 |
| EB1-ΔN1 | 51–268 | 213 | ND | ND | − | − | 213 |
| EB1-ΔN2 | 102–268 | 213 | 213 | ND | − | − | 213 |
| EB1-ΔN3 | 150–268 | 213 | 213 | ND | 213/− | − | 213 |
| EB1-ΔC1 | 1–218 | − | − | ND | 213 | − | − |
| EB1-ΔC2 | 1–168 | − | − | ND | 213 | − | − |
| EB1-ΔC3 | 1–113 | − | ND | ND | − | − | − |
| EB1-C50 | 219–268 | 213 | − | ND | − | − | 213/− |
| EB1-C84 | 185–268 | 213 | 213 | ND | − | − | 213 |
| EB1-bZIP | 185–241 | 213 | − | ND | ND | ND | ND |
, positive association; −, negative association; +/−, slight positive association; ND, no data.
SDS-PAGE and Western Blotting
SDS-PAGE and Western blotting were performed as described previously (Morrison et al., 1998).
GST Fusion Protein Precipitations from Cell Extracts
Confluent cells in a 150-cm2 flask were scraped into PBS and pelleted by centrifugation, and the pellet was lysed in 3 ml of ice-cold PBS/TX100, 2 mM EDTA, 50 mM sodium fluoride, and 100 μM sodium orthovanadate (PBS/TX100-E). Insoluble material was pelleted by centrifugation at 12,000 ×g and discarded. Supernatants were precleared using glutathione-Sepharose beads before addition of GST fusion proteins bound to glutathione-Sepharose beads and incubation for 18 h at 4°C. Captured complexes were collected by centrifugation and washed five times for 15 min in 20 volumes of PBS/TX100-E. Finally, the complexes were resuspended in Laemmli SDS-PAGE loading buffer containing 10 mM DTT and boiled for 5 min before analysis by SDS-PAGE and Western blotting.
6His-EB1 Precipitations from Cell Extracts
Ni-2+Sepharose bound 6His-EB1 or Ni-2+Sepharose beads alone were preincubated in PBS/TX100 with GST, GST-APC-C1, or buffer alone for 1 h at 4°C with mixing. Cell extract, prepared as described above (but without EDTA), was then added, and incubation was continued for a additional 18 h. Captured complexes were processed and analyzed as described above.
In Vitro Binding Assays
One microgram each of plasmid DNA (encoding EB1-GFP and EB1-GFP deletion mutants or GFP-p150Glued, GFP alone, or 6His-p150Glued deletion mutants, as appropriate) were transcribed and translated in vitro in the presence of [35S]cysteine/methionine using the Promega TNT coupled transcription/translation system according to the manufacturers instructions (Madison, WI). The reaction mix was diluted in PBS/TX100, and a precipitation was performed using GST-APC-C1, GST-EB1, or GST alone as appropriate, essentially as described above. Precipitates were subjected to SDS-PAGE, and the gels were dried and exposed to photographic film to visualize captured fusion proteins.
Approximately 2 μg of purified 6His-p150(1–330) was incubated with ∼2 μg of purified, glutathione-Sepharose–bound GST or GST-EB1 in 1 ml RIPA-M for 18 h at 18°C. Captured proteins were recovered by centrifugation and washed four times for 15 min in 1 ml RIPA-M. Finally, the complexes were resuspended in Laemmli SDS-PAGE loading buffer containing 10 mM DTT and boiled for 5 min before analysis by SDS-PAGE and Western blotting.
Approximately 2 μg of purified 6His-p150(1–330) was incubated with ∼2 μg of purified GST or GST-EB1 in 1 ml RIPA-M (0.35% SDS) for 18 h at 18°C. Protein complexes were captured using His-bind magnetic beads (Novagen) and washed four times for 15 min in RIPA-M (0.35%) SDS. Finally, the complexes were resuspended in Laemmli SDS-PAGE loading buffer containing 10 mM DTT and boiled for 5 min before analysis by SDS-PAGE and Western blotting.
RESULTS
Identification of Functional Domains in EB1
The EB1 interactions with APC, dynein/dynactin, and microtubules have been described previously (Su et al., 1995; Berrueta et al., 1998; Morrison et al., 1998; Berrueta et al., 1999; Askham et al., 2000; Nakamura et al., 2001). To identify the functional domains in EB1 responsible for these interactions, a series of deletion mutants were created by the sequential removal of 50 aa from the N- and C-termini of the protein, creating six mutant proteins in total (see Figure 1). These deletions, along with full-length EB1, were cloned into a plasmid driving their expression as C-terminal fusions with GFP. We have previously described a GST fusion protein derived from the C-terminal 170 aa of APC, which efficiently precipitates EB1 from cell extracts (GST-APC-C1; Askham et al., 2000). We therefore used this tool to define the APC-interacting region in EB1. The EB1-GFP fusion proteins were in vitro transcribed/translated, and a precipitation reaction was performed using GST-APC-C1. As shown in Figure 2A, EB1-GFP and the EB1-ΔN1, -ΔN2 and -ΔN3-GFP fusion proteins were precipitated by GST-APC-C1, demonstrating that the C-terminal addition of GFP to EB1 did not inhibit the interaction with GST-APC-C1. None of the C-terminal deletion mutants were precipitated, suggesting that the final 50 aa in EB1 are essential for APC binding. To confirm this finding N-terminal GST fusion proteins were produced using the same deletion series used to make the GFP fusions (see Figure 1). All except GST-EB1-ΔN1 and GST-EB1-ΔC3 were efficiently purified from bacterial lysates. The remaining fusion proteins were used in precipitation experiments from lysates of HCT116 and SW480 cells.
Figure 1.
Schematic of EB1 deletion mutants used in this study. F/L, full-length. bZIP, basic leucine zipper motif.
Figure 2.
Identification of protein–protein interaction regions in EB1. (A) EB1-GFP, a series of EB1-GFP deletion mutants, and luciferase were in vitro transcribed/translated using radiolabeled amino acids. The top panel shows an autoradiograph of the products of the translation resolved by SDS-PAGE. Minor bands are likely to have arisen from translational initiation at internal methionines in the EB1 cDNA. The reaction mix was incubated with GST-APC-C1 coupled to glutathione-Sepharose beads, and washed, and interacting species were resolved by SDS-PAGE and autoradiography. EB1-GFP and the deletion mutants EB1-ΔN1, -ΔN2, and -ΔN3 were all precipitated by GST-APC-C1. The negative control luciferase and the deletion mutants EB1-ΔC1, -ΔC2, and -ΔC3-GFP did not interact with GST-APC-C1. (B) GST, GST-EB1, and a series of EB1 deletion mutants fused to GST were coupled to glutathione-Sepharose beads and used in precipitations from cell extracts. The detection of β-catenin in precipitates from HCT116 cells indicates APC binding by GST-EB1, GST-EB1-ΔN2, and GST-EB1-ΔN3. No specific precipitation of β-catenin was obtained from extracts of SW480 cells; this blot is also shown grossly overexposed to reveal nonspecific binding. GST-EB1, GST-EB1-ΔN2, and GST-EB1-ΔN3 also precipitated p150Glued from cell extracts. (C) Glutathione-Sepharose–bound GST, GST-EB1, and three additional EB1 deletion mutants were used to precipitate p150Glued and β-catenin (demonstrating an interaction with APC) from HCT116 cell extracts. The only deletion mutant to precipitate p150Glued from cell extracts was GST-EB1-C84. All three deletion mutants were capable of precipitating β-catenin.
We and others have previously shown that APC simultaneously binds EB1 and β-catenin (Morin et al., 1996; Askham et al., 2000), allowing the detection of precipitated APC-EB1 complexes by immunoblotting for β-catenin. β-Catenin was detected in GST-EB1, GST-EB1-ΔN2, and GST-EB1-ΔN3 precipitates (Figure 2B) from HCT116 cells, which contain a full-length APC capable of interacting with EB1 but not in precipitates from SW480 cells, which express a truncated APC protein that cannot bind to EB1 (Figure 2B). C-terminal GST-EB1 deletion mutants did not precipitate β-catenin. These results were entirely consistent with the in vitro interaction studies. GST-EB1 and a fragment comprised of aa 136–268 have previously been shown to interact with components of the cytoplasmic dynein/dynactin complex in cell extracts (Berrueta et al., 1999). GST-EB1 precipitates from SW480 cell extracts were therefore probed using an antibody specific for the p150Glued subunit of dynactin. The results indicated that dynactin binding also required the final 50 aa of EB1. Furthermore, the interaction was not dependent on APC (Figure 2B).
A recent study has shown that dynein can interact directly with β-catenin (Ligon et al., 2001). Because EB1 can interact with dynein/dynactin, it was possible that β-catenin present in GST-EB1 precipitates from cell extracts could result from an EB1/dynein/dynactin/β-catenin complex as well as from an EB1/APC/β-catenin complex. The observation that no β-catenin was specifically precipitated from cell extracts in the absence of an EB1/APC interaction suggests that EB1 does not interact with β-catenin via dynein/dynactin (Figure 2B). To check whether there was even a minor component of the EB1/β-catenin interaction was via dynein/dynactin, the Western blot showing β-catenin precipitations from SW480 cell extracts was overexposed (Figure 2B). A small amount of background, nonspecific binding of β-catenin was observed in all precipitations. Crucially, there was no detectable decrease in the amount of background β-catenin in GST-EB1-ΔC1, -ΔC2 precipitations when compared with GST, full-length GST-EB1 and the N-terminal deletion mutant precipitations. This result suggests that EB1 is not a significant component of any β-catenin/dynein/dynactin complex.
To test which C-terminal EB1 residues were sufficient to bind APC and dynactin, three further EB1 fusion proteins were created and used in precipitation experiments. GST-EB1-C50, GST-EB1-C84, and GST-EB1-bZIP all precipitated β-catenin from cell extracts, demonstrating an association with APC, whereas only GST-EB1-C84 precipitated p150Glued (Figure 2C). This refines the minimal dynactin interaction region in EB1 to aa 185–268. In addition, the overlapping region of these three fusion proteins suggests a minimal APC binding region between aa 219 and 241.
The EB1 C-terminus contains a sequence with limited homology to leucine zipper domains known to mediate protein–protein interactions in other proteins, and it has been suggested that this region may facilitate homodimerization of EB1 molecules (Juwana et al., 1999). We performed an in vitro transcription/translation of EB1-GFP followed by precipitation using GST or GST-EB1 coupled to glutathione-Sepharose beads to test this hypothesis. No specific interaction between GST-EB1 and EB1-GFP was detected, suggesting that EB1 does not homodimerize in this in vitro assay.
To define the region of EB1 involved in the association with microtubules, EB1-GFP and the EB1 deletion mutants were transiently transfected into COS-7 cells and the distribution of the fusion proteins examined by fluorescence microscopy. At lower expression levels, EB1-GFP localized to a subset of microtubule tips in a manner identical to endogenous EB1 as well as to the centrosome (Figure 3A, left-hand cell). At higher expression levels, more extensive microtubule labeling was observed (Figure 3A, right-hand cell). Removal of the N-terminal 50 aa of EB1 (EB1-ΔN1-GFP) abolished the microtubule association, but the centrosomal association was still observed (Figure 3B). Removal of the N-terminal 100 aa of EB1 (EB1-ΔN2-GFP) also abolished the microtubule association, but at higher levels of expression the fusion protein was seen to accumulate around the centrosome of the cell (Figure 3C). When the N-terminal 150 aa of EB1 were removed (EB1-ΔN3-GFP), weak microtubule labeling was seen in some cells, along with a centrosomal association (Figure 3D). The EB1-ΔC1-GFP fusion protein displayed a robust microtubule association in transfected cells, which was less polarized toward the microtubule plus-end than the distributions seen for endogenous EB1 or EB1-GFP (Figure 3E). Removal of the C-terminal 100 aa of EB1 (EB1-ΔC2-GFP) gave a similar result (Figure 3F). Removal of the C-terminal 150 aa of EB1 (EB1-ΔC3-GFP) completely abolished any microtubule association (Figure 3G). Thus it appears that the first 50 aa of EB1 are essential for its microtubule association, and aa 1–168 are sufficient for this association. Furthermore, none of the C-terminal EB1 deletion mutants displayed a centrosomal localization, in contrast to EB1-GFP and the N-terminal deletion mutants.
Direct Association of EB1 with p150Glued
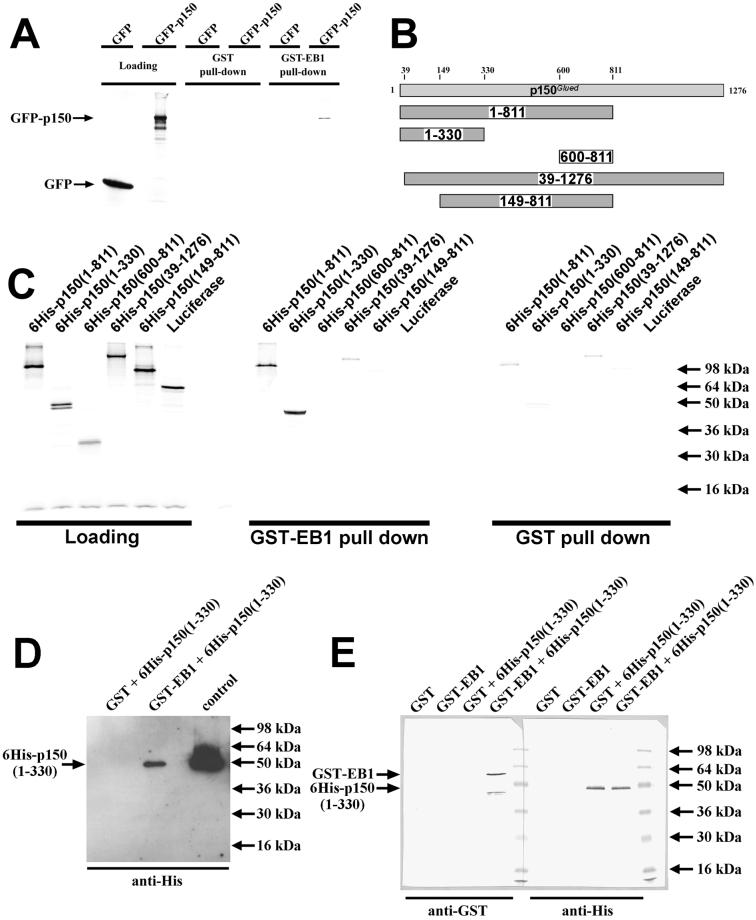
Given that EB1 could associate with complexes containing p150Glued in pull-down experiments from cell extracts (Figure 2; Berrueta et al., 1999) and that both EB1 and p150Glued localize to microtubule tips and centrosomes (Vaughan et al., 1999), we investigated whether there was a direct interaction between EB1 and p150Glued. In a simple in vitro binding assay, in vitro–translated GFP-p150Glued was precipitated by GST-EB1, but not GST alone, suggesting a direct interaction between EB1 and p150Glued (Figure 4A). To define the region of p150Glued responsible for the interaction, the binding assay was repeated using in vitro–translated p150Glued deletion mutants (Figure 4B). Significantly greater amounts of 6His-p150(1–811) and 6His-p150(1–330) were precipitated by GST-EB1 than GST alone, suggesting that the EB1 binding domain lies within the N-terminal 330 aa of p150Glued (Figure 4C). Furthermore, 6His-p150(39–1276) and 6His-p150(149–811) showed no specific binding to GST-EB1 above background, demonstrating that the N-terminal 39 aa are required for binding to EB1 (Figure 4C).
Figure 4.
(A) EB1 binds directly to p150Glued. In vitro–translated GFP-p150 or GFP alone was incubated with purified, glutathione-Sepharose–bound GST-EB1 or GST alone. Protein complexes were harvested by centrifugation, washed, and analyzed by SDS-PAGE and autoradiography. GST-EB1 precipitated GFP-p150 but not GFP alone demonstrating an EB1-p150 interaction. No proteins were precipitated with GST alone. (B) Schematic representing the in vitro–translated p150Glued deletion mutants used in subsequent experiments. (C) In vitro–translated p150 deletion mutants were incubated with purified, glutathione-Sepharose–bound GST-EB1 or GST alone. Protein complexes were harvested by centrifugation, washed extensively, and analyzed by SDS-PAGE and autoradiography. Significantly greater amounts of 6His-p150(1–811) and 6His-p150(1–330) were precipitated by GST-EB1 than GST alone, indicating that these deletion mutants specifically interacted with EB1. (D) Purified bacterially expressed 6His-p150(1–330) was incubated with purified, glutathione-Sepharose–bound GST-EB1 or GST alone. Protein complexes were harvested by centrifugation, washed, and analyzed by SDS-PAGE and Western blotting using an anti-His tag antibody. GST-EB1 precipitated 6His-p150(1–330), whereas GST did not, demonstrating a direct EB1-p150 interaction. The control protein was purified 6His-p150(1–330). (E) Purified bacterially expressed GST-EB1 or GST was incubated with purified 6His-p150(1–330). Protein complexes were harvested using Ni-2+ magnetic beads, washed, and analyzed by SDS-PAGE and Western blotting using anti-GST and anti-His tag antibodies. 6His-p150(1–330) was successfully precipitated using the magnetic beads. GST-EB1 coprecipitated with 6His-p150(1–330), whereas GST did not. The band detected with the anti-GST antibody at ∼45 kDa (immediately below the GST-EB1 band) represents a breakdown product of GST-EB1. No proteins were precipitated by the magnetic beads alone. This demonstrates a direct interaction between EB1 and p150.
To confirm a direct interaction between EB1 and p150Glued, further binding assays were performed using only purified recombinant proteins. Purified 6His-p150(1–330) was precipitated by purified GST-EB1 but not GST alone (Figure 4D). Similarly, purified GST-EB1 was precipitated with purified 6His-p150(1–330), whereas GST was not (Figure 4E). These experiments demonstrate a direct interaction between EB1 and p150Glued.
Mutually Exclusive Binding of EB1 to p150Glued and APC
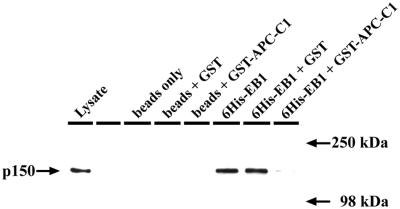
Because the interaction sites for APC and p150Glued in EB1 appeared to overlap, we next investigated whether these proteins could bind to EB1 simultaneously. We performed precipitation reactions from cell extracts using Ni-2+Sepharose–conjugated 6His-EB1, preincubated with either GST or GST-APC-C1. Preincubation with GST-APC-C1 abolished precipitation of p150Glued, showing that binding of APC to EB1 prevents the interaction with p150Glued (Figure 5). Furthermore, although EB1 is efficiently precipitated from cell extracts by GST-APC-C1 (Askham et al., 2000), no p150Glued was detected in these precipitates, again demonstrating the absence of dynactin in EB1-APC complexes.
Figure 5.
EB1 does not simultaneously interact with dynactin and APC. Ni-2+-Sepharose–bound 6His-EB1 was used to precipitate proteins from an extract of HCT116 cells. 6His-EB1 was preincubated with either GST, GST-APC-C1, or buffer alone before precipitations were performed. Precipitates were washed and probed for the presence of the dynactin subunit p150Glued. Preincubation of 6His-EB1 with GST-APC-C1 virtually abolished any interaction with p150Glued, whereas preincubation with GST had no effect.
Full-length EB1 Is Required for Microtubule Stabilization and Bundling
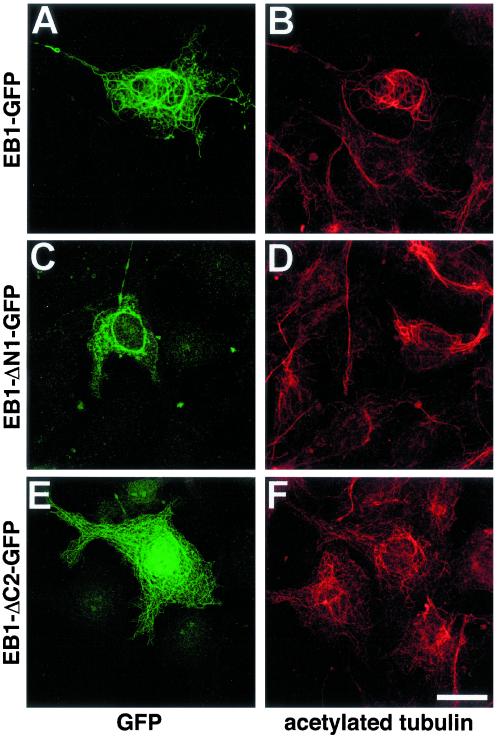
It has previously been shown that EB1 overexpression in transfected cells results in the formation of nocodazole-resistant bundles of acetylated microtubules (Bu and Su, 2001). We investigated which domains of EB1 were required for this phenomenon. At levels of expression ∼5–10-fold higher than that observed to result in microtubule tip labeling, EB1-GFP extensively labeled microtubules and induced nocodazole-resistant microtubule bundles, in agreement with the findings of Bu and Su (2001). In all cells where EB1-GFP expression induced microtubule bundling, these bundles immunostained intensely for acetylated tubulin (Figure 6, A and B). In contrast, no significant microtubule bundling was observed when any of the EB1-GFP deletion mutants were expressed in transfected cells. Furthermore, no obvious increase in acetylated tubulin immunoreactivity was seen (Figure 6, C–F; only EB1-ΔC2-GFP and EB1-ΔN1-GFP are shown for brevity), although we note that that the immunostaining for acetylated tubulin was very heterogeneous within a given population of COS-7 cells. This shows that overexpression of the whole EB1 protein is necessary to bundle and stabilize microtubules. Deletion of regions known to be essential for binding microtubules or APC and dynactin prevented this phenomenon. This finding is consistent with results from previous studies investigating the functions of these EB1 binding partners. A role for APC in stabilizing a subpopulation of cortically anchored microtubules during cell migration has been suggested (Näthke, 2000; Zumbrunn et al., 2001; Barth et al., 2002). Furthermore, a role for the APC/EB1 complex in stabilizing kinetochore microtubules has been suggested (Fodde et al., 2001), and the APC-EB1 interaction has been shown to promote microtubule polymerization (Nakamura et al., 2001). It is also well established that p150Glued overexpression induces microtubule bundling (Waterman-Storer et al., 1995; Quintyne et al., 1999).
Figure 6.
Overexpression of full-length EB1, but not EB1 deletion mutants stabilizes microtubules. Cells were transfected with EB1-GFP (A and B), EB1-ΔN1-GFP (C and D), and EB1-ΔC2-GFP (E and F), and coimmunostained with antibodies specific for GFP (A, C, and E) and acetylated tubulin (B, D, and F). EB1-GFP–induced microtubule bundles (A) showed significantly more intense staining for acetylated tubulin (B). No increase in acetylated tubulin staining was seen in cells transfected with any of the EB1-GFP deletion mutants. Bar, 20 μm.
EB1 Deletion Mutants Interfere with the Microtubule Tip Localization of the Endogenous Protein
During our characterization of the EB1-GFP deletion series, we noted that the epitope recognized by the monoclonal anti-EB1 antibody from Transduction Laboratories was destroyed by removal of the final 50 aa in EB1 (Figure 7A). Exploiting this, we investigated the effects of overexpressing the EB1-ΔC1-GFP fusion protein on the distribution of endogenous EB1 in transfected cells, because both of these proteins localized to microtubules. EB1-ΔC1-GFP displaced endogenous EB1 from microtubule tips in an expression level-dependent way (Figure 7, B–D). This suggested that this fusion protein could compete with endogenous EB1 for binding to microtubule tips. Similar results were obtained with EB1-ΔC2-GFP.
Figure 7.
EB1-ΔC1-GFP displaces endogenous EB1 from microtubules. (A) Epitope mapping of the Transduction Laboratories anti-EB1 mAb. Purified GST, GST-EB1, and all EB1-deletion mutants were Western blotted and probed with the EB1 mAb. The antibody detected full-length EB1 and EB1 proteins with N-terminal deletions. Deletion of the last 50 aa of EB1 (EB1-ΔC1) destroyed the antibody epitope, which lies within the last 84 aa of EB1 (EB1-C84). (B–D) EB1-ΔC1-GFP competes with endogenous EB1 for binding to microtubules. COS-7 cells overexpressing EB1-ΔC1-GFP (asterisk) were processed for immunofluorescence with antibodies to GFP (B) and EB1 (C). (D) The merged image. Microtubule tip labeling for endogenous EB1 was absent in cells overexpressing EB1-ΔC1-GFP. Bar, 15 μm.
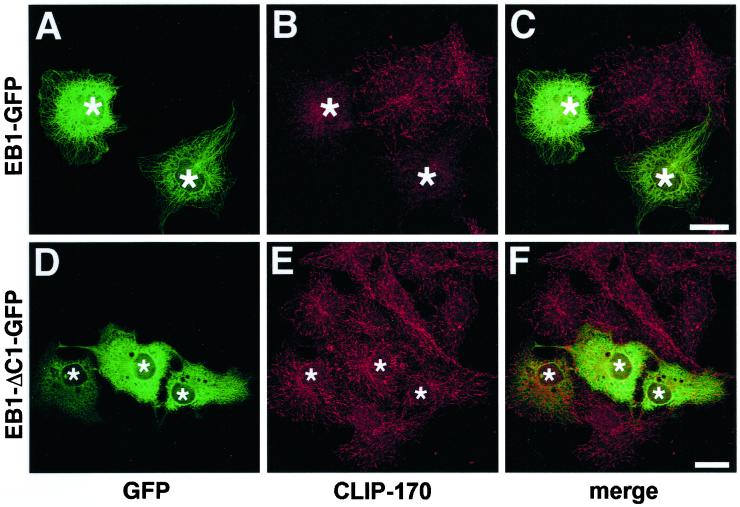
However, the observed displacement of endogenous EB1 by EB1-ΔC1-GFP could be due to either a direct competition effect or an indirect effect resulting from changes in, for example, microtubule dynamics. To resolve this question, we tested whether overexpression of these EB1 deletion mutants could displace CLIP-170, another protein that specifically localizes to growing microtubule tips (Perez et al., 1999). When overexpressed, full-length EB1-GFP (Figure 8, A–C) but not EB1-ΔC1-GFP (Figure 8, D–F) caused a loss of CLIP-170 from microtubule tips, suggesting that EB1-ΔC1-GFP can indeed displace endogenous EB1 from microtubule tips by direct competition. Similar results were obtained with EB1-ΔC2-GFP.
Figure 8.
Full-length EB1, but not EB1-ΔC1-GFP, competes CLIP-170 from microtubule tips. COS-7 cells overexpressing EB1-GFP (A–C, asterisks) and EB1-ΔC1-GFP (D–F, asterisks) were processed for immunofluorescence with antibodies to GFP (A and D) and CLIP-170 (B and E). (C and F) The merged images. Microtubule tip labeling for endogenous CLIP-170 was absent in cells overexpressing EB1-GFP but was unaffected in cells overexpressing EB1-ΔC1-GFP. Bars, 10 μm.
EB1 Is Required for Microtubule Focusing and Anchoring at Interphase Centrosomes
The results of our in vitro binding studies suggested that several EB1 deletion mutants had the potential to act as dominant-negative mutants if they were overexpressed in cells, giving us an opportunity to identify cellular functions for EB1, based on our knowledge of its interactions. In particular, the C84 fragment of EB1 would be predicted to competitively inhibit the interaction between endogenous EB1 and both APC and p150Glued, whereas the C50 fragment should only inhibit the EB1-APC interaction. These fragments were therefore overexpressed as GFP fusion proteins in COS-7 cells and the effects examined by immunofluorescence microscopy.
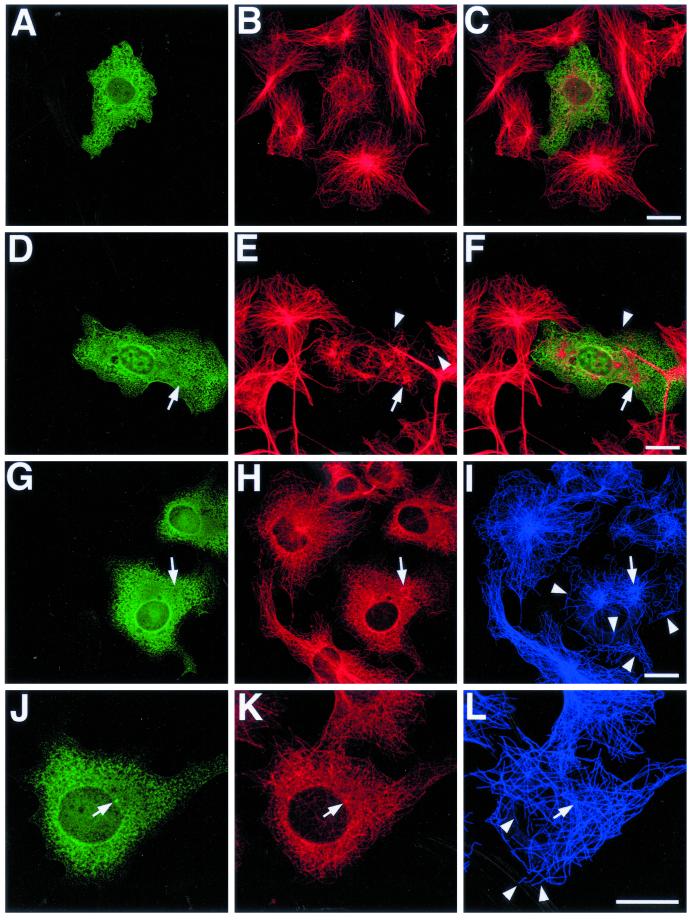
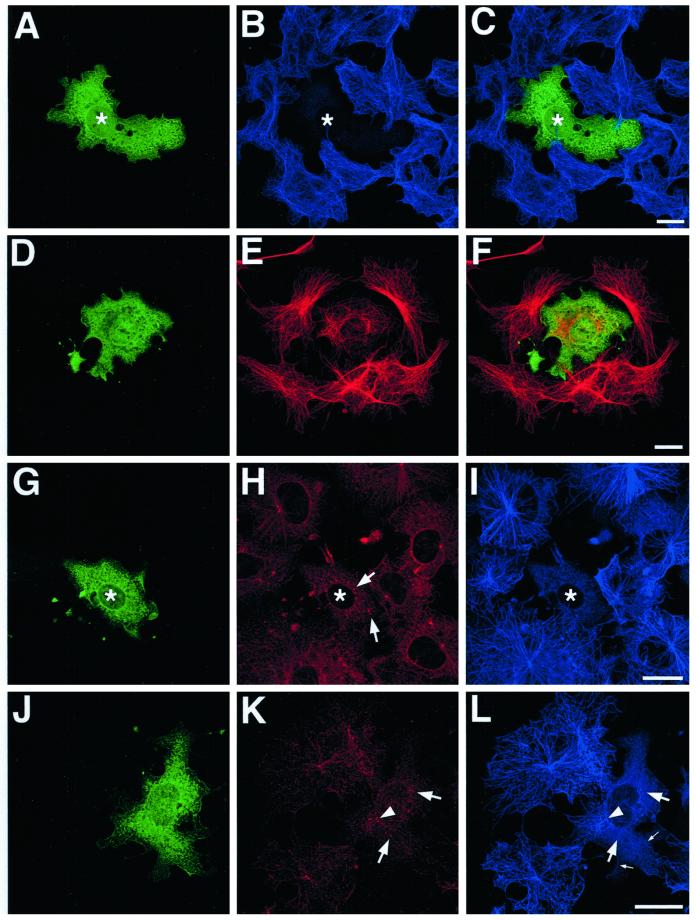
In common with the EB1 N-terminal deletions previously described in this article, EB1-C84-GFP localized to the centrosome in transfected cells (i.e., Figure 9, J–L, arrow). This association appears weaker or absent in some cells, either because background expression levels of the fusion protein are higher (Figure 9, A–C) or because centrosomal integrity appears to have been compromised (Figure 9, D–I). In cells expressing even moderate levels of EB1-C84-GFP, an inhibition of microtubule minus-end focusing at the centrosome was seen (Figure 9, A–C and J–L). In many cells a profound disruption of the normal radial microtubule array was apparent, and an increased number of free cytoplasmic microtubules was seen (Figure 9, D–L, arrowheads), implying that microtubule anchoring at the centrosome was defective. These unanchored microtubules were often short or looped. Occasionally, microtubules appeared to be loosely focused around a variable number of amorphous structures in the cytoplasm, which were also immunostained for p150Glued (Figure 9, D–I, arrows). In general, the perturbation of microtubule organization made it difficult to examine whether the microtubule tip localization of p150Glued was directly affected by EB1-C84-GFP overexpression, although our results appear to suggest that it was relatively unaffected (i.e., Figure 9, G–L). However, cells displaying a clear centrosomal EB1-C84-GFP localization appeared to have less p150Glued present at this organelle (i.e., Figure 9, J–L, arrow). Many cells expressing EB1-C84-GFP appeared to have a lower microtubule density than adjacent untransfected cells (i.e., Figure 9, A–C). This phenomenon was more marked in cells transfected for longer times. Again, this observation is consistent with a failure in microtubule anchoring at the centrosome because microtubules with free minus ends are unstable in fibroblast cells (Rodionov et al., 1999). This would lead to a progressive depletion of microtubules in cells expressing EB1-C84-GFP.
Figure 9.
EB1-C84-GFP overexpression inhibits microtubule focusing and anchoring at centrosomes. COS-7 cells overexpressing EB1-C87-GFP were processed for immunofluorescence with antibodies to GFP (A, D, G, and J), p150Glued (H and K), and/or tubulin (B, E, H, and K). Merged images are shown in C and F. EB1-C84-GFP localized to centrosomes (J, arrow), and its overexpression resulted in an unfocused microtubule array (compare transfected cell in A–C with adjacent cells). Loose cytoplasmic foci of microtubules were sometimes seen (E and I, arrows), with which p150Glued was associated (H and I, arrow). Free cytoplasmic microtubules were also seen (E, I, and L, arrowheads), suggesting a defect in centrosomal microtubule anchoring. p150Glued immunostaining at centrosomes was reduced in cells overexpressing EB1-C84-GFP, where a clear centrosomal structure was still identifiable (K, arrow). Bars, 20 μm.
Because EB1-C84-GFP overexpression appeared to disrupt normal microtubule anchoring and focusing at the centrosome, we costained EB1-C84-GFP–overexpressing cells for γ-tubulin to reveal the location of this organelle. Figure 10 shows cells in which EB1-C84-GFP overexpression again resulted in an unfocused microtubule array and the appearance of detached microtubules. In these cells, clear centrosomal γ-tubulin staining was still evident, although this staining was weaker in cells with a highly disrupted microtubule cytoskeleton. No evidence of an increase in noncentrosomal γ-tubulin foci was seen. These observations suggested that the increase in unanchored microtubules seen in cells overexpressing EB1-C84-GFP was unlikely to arise from ectopic nucleation of new microtubules at noncentrosomal sites, but instead arose from defective microtubule anchoring at centrosomes.
Figure 10.
Localization of centrosomes in cells expressing EB1-C84-GFP. COS-7 cells expressing EB1-C84-GFP were processed for immunofluorescence with antibodies to GFP (A and C, green), α-tubulin (B and D, blue), and γ-tubulin (B and D, red). Merged images of the α- and γ- tubulin staining are shown in B and D. Robust γ-tubulin immunostaining revealed a normal perinuclear localization for the centrosomes (arrowhead, A and B) in cells expressing moderate levels of EB1-C84-GFP where free cytoplasmic microtubules (arrows, B) and microtubule disorganization were clearly evident. At higher levels of EB1-C84-GFP expression, where the majority of microtubules were free in the cytoplasm and the radial microtubule array was completely destroyed, γ-tubulin immunostaining was often weaker, but the centrosomes could still be clearly identified. These were usually associated with one of the loose tangles of microtubules in these cells (arrows, C and D). Bars, 10 μm.
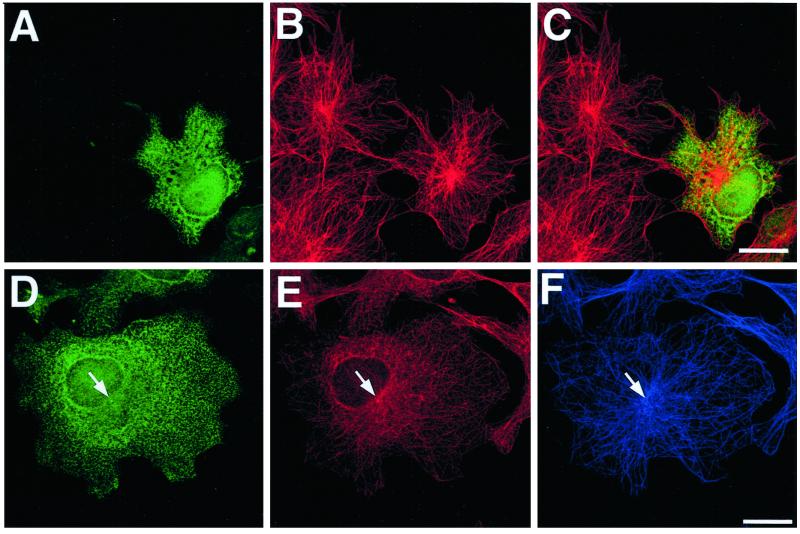
EB1-C50-GFP inconsistently displayed a weaker centrosomal localization in transfected cells (for example, this is seen in Figure 11D, arrow, but not in A). In cells overexpressing EB1-C50-GFP a relatively normal radial microtubule array was seen (Figure 11, B and F). In contrast to the effects of EB1-C84-GFP overexpression, no evidence for an increase in the proportion of free cytoplasmic microtubules was noted. Furthermore, the centrosomal localization of p150Glued was unaffected in transfected cells (Figure 11D, arrow). These findings suggested that inhibition of the EB1-p150Glued interaction and not the EB1-APC interaction underlie the defects in microtubule anchoring at the centrosome observed in cells overexpressing EB1-C84-GFP.
Figure 11.
EB1-C50-GFP overexpression does not inhibit microtubule anchoring or focusing at centrosomes. COS-7 cells overexpressing EB1-C50-GFP were processed for immunofluorescence with antibodies to GFP (A and D), p150Glued (E), and/or tubulin (B and F). A merged image is shown in C. EB1-C50-GFP localized to centrosomes in some (D, arrow) but not all cells (A). Its overexpression had little effect on microtubule focusing and anchoring at the centrosome (B and F). The localization of p150Glued to centrosomes was unaffected in transfected cells (E, arrow). Bars, 20 μm.
Because microtubule organization and centrosomal anchoring was inhibited in the presence of EB1-C84-GFP, we next investigated whether the expression of EB1 deletion mutants affected centrosomal assembly and function. Transfected cells were incubated with nocodazole to completely depolymerize the microtubule cytoskeleton then thoroughly washed to remove the drug. After increasing periods of time to allow microtubule regrowth, cells were fixed and immunostained.
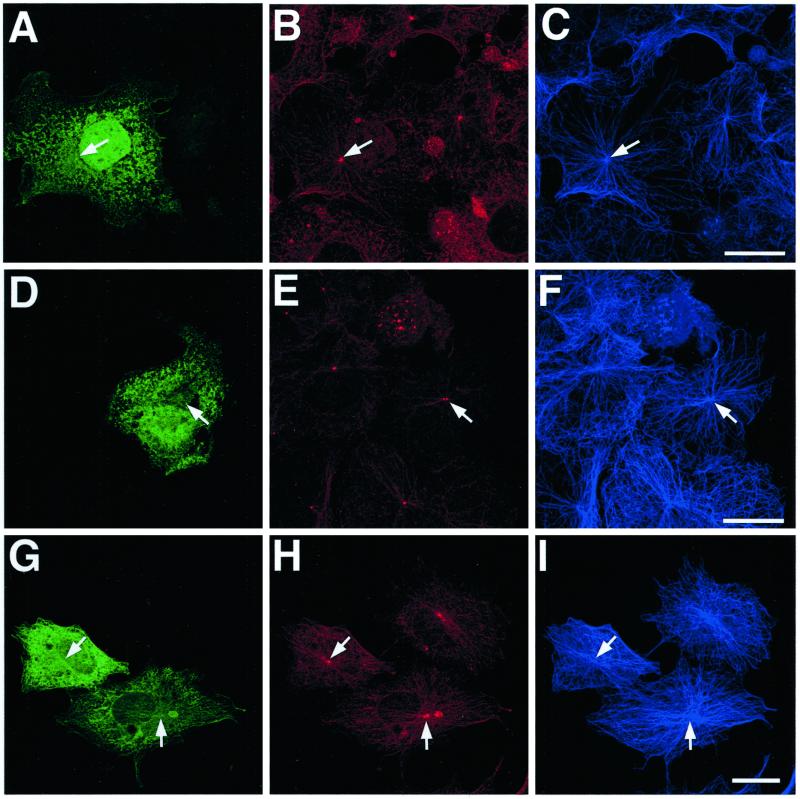
At higher expression levels, EB1-C84-GFP completely inhibited microtubule regrowth (Figure 12, A–C and G–I, asterisks). This effect was often associated with a failure to recruit p150Glued and γ-tubulin to centrosomes. Instead, both proteins were distributed in discrete foci scattered throughout the cytoplasm (Figure 12, H and K, arrows). In transfected cells where microtubule regrowth was evident (Figure 12, D–F and J–L), it often centered on a subset of cytoplasmic foci containing γ-tubulin (Figure 12, K and L, arrowheads). However, the resulting microtubule array was disorganized and unfocused (compare transfected cell in Figure 12, D–F, with surrounding cells), and evidence of defective microtubule anchoring at the centrosome was again seen (Figure 12L, small arrows).
Figure 12.
EB1-C84-GFP overexpression inhibits centrosome assembly and microtubule regrowth after nocodazole treatment. COS-7 cells transfected with EB1-C84-GFP were treated with nocodazole. Cells were fixed after increasing recovery times after nocodazole washout, and microtubule regrowth was examined by immunofluorescence microscopy. (A, D, G, and J) GFP immunostaining; (B, E, I, and L) α-tubulin; (H) p150Glued; and (K) γ-tubulin. (C) A merged image. In some cells EB1-C84-GFP expression completely abolished microtubule regrowth (A–C and G–I). This was associated with a failure to concentrate p150Glued (H, arrows) and γ-tubulin (K, arrows) at a single intracellular focus. In transfected cells where microtubule regrowth was evident, an unfocused, disorganized microtubule array was seen (D–F and J–L). Regrowth was centered around a subset of γ-tubulin foci (K and L, arrowhead), but free cytoplasmic microtubules were often seen (K and L, small arrows), implying defective microtubule anchoring at nucleation sites. Representative images from a number of different experiments and recovery times are shown. (A–C) A 45-min recovery; (D–F and J–L) a 30-min recovery; and (G–I) a 15-min recovery. All of the described effects were seen in cultures processed up to 90 min after washing. α-Tubulin was detected with an Alexa 564 secondary antibody in the experiment in D–F, and Alexa 633 elsewhere. Bars, 20 μm.
Microtubule regrowth and focusing in cells overexpressing EB1-C50-GFP was essentially indistinguishable from that in adjacent, untransfected cells (Figure 13, A–F). In addition, recruitment of p150Glued and γ-tubulin to centrosomes appeared unaffected (Figure 13, A–F, arrows). Similar results were obtained in cells overexpressing EB1-ΔC1-GFP (Figure 13, G–I), suggesting that competitive inhibition of the localization of endogenous EB1 to microtubule tips had little effect on microtubule regrowth or focusing in this system.
Figure 13.
EB1-C50-GFP and EB1-ΔC1-GFP overexpression have no effect on microtubule regrowth and focusing after nocodazole treatment. The ability of the microtubule cytoskeleton in cells transfected with EB1-C50-GFP (A–F) and EB1-ΔC1-GFP (G–I) to recover from nocodazole treatment was examined as described in Figure 12. (A, D, and G) GFP immunostaining; (B and H) p150Glued; (E) γ-tubulin; and (C, F, and I) α-tubulin. Overexpression of these deletion mutants had no effect on microtubule regrowth and centrosomal focusing or on the recruitment of either p150Glued (B and H, arrows) or γ-tubulin (E, arrow) to centrosomes. All cells shown in this figure were fixed 30 min after nocodazole washout. Bars, 20 μm.
Defects in microtubule focusing at the centrosome after inhibition of dynactin function have been previously described by other workers (Quintyne et al., 1999). We therefore wanted to assess whether the effects induced by EB1-C84-GFP overexpression were due to a similar inhibition of dynactin function. A classic hallmark of dynein/dynactin functional inhibition in interphase cells is the fragmentation and dispersal of the Golgi apparatus (Burkhardt et al., 1997). We therefore immunostained transfected cells with antibodies to a Golgi marker protein. Because Golgi dispersal can also be produced by disruption of the microtubule cytoskeleton, we coimmunostained the cells with tubulin-specific antibodies. EB1-C84-GFP expression did not induce Golgi fragmentation (Figure 14, A–F), and any observable effects on Golgi morphology and localization were entirely consistent with the severity of the defects in microtubule organization in that cell. Similar results were obtained in cells overexpressing EB1-C50-GFP (Figure 14, G–I) and EB1-ΔC1-GFP and when the Golgi apparatus in transfected cells was detected using fluorescently tagged wheat germ agglutinin.
Figure 14.
EB1-C84-GFP and EB1-C50-GFP overexpression do not induce Golgi apparatus fragmentation. COS-7 cells transfected with EB1-C84-GFP (A–F) and EB1-C50-GFP (G–I) were fixed and immunostained with antibodies to GFP (A, D, and G), the Golgi 58K protein (B, E, and H), and α-tubulin (C, F, and I). Overexpression of these fusion proteins did not result in the dispersal of the Golgi apparatus from its normal perinuclear localization (arrows), even in cells where microtubule organization was clearly impaired (D–F). Note again the presence of unanchored and looped microtubules in cells overexpressing EB1-C84-GFP (i.e., F, arrowheads). Bars, 20 μm.
DISCUSSION
Protein–Protein Interaction Domains in EB1
We initially set out to define domains in EB1 responsible for mediating the interactions with APC, the dynein/dynactin complex, and microtubules. Table 1 represents a summary of the data presented in this study.
In vitro protein–protein interaction studies localized the dynein/dynactin association region in EB1 to the C-terminal 84 aa. These data significantly refine the binding region identified in a previous study (Berrueta et al., 1999). Previously it was known that the C-terminal two thirds of EB1 was sufficient to bind APC (Su et al., 1995), and a more recent study confirmed that the binding region lay within the C-terminal half of the protein (Barth et al., 2002). Because GST-EB1-C50, -C84, and –bZIP were all able to associate with APC, our data show that the APC binding region is fully encompassed by the final 84 aa of EB1 and is likely to lie between EB1 amino acids 219 and 241. Interestingly, this is a region of EB1 highly conserved between different species and which in Bim1p overlaps with the binding region identified for Kar9p (Miller et al., 2000). However, it remains possible that EB1 has multiple APC binding regions within the last 84 amino acids.
In transfected cells the association of EB1 with microtubules required the N-terminus of the protein. At 30-kDa EB1 is much smaller than many other proteins that localize to growing microtubule tips, such as CLIP-170, CLIP-115, and p150Glued (Perez et al., 1999; Vaughan et al., 1999; Hoogenraad et al., 2000). These proteins possess a structurally related microtubule-binding region, which in the case of CLIP-170 exceeds the size of full-length EB1 (Diamantopolous et al., 1999). A requirement for the entire N-terminal half of EB1 in microtubule binding therefore appears reasonable. The weak localization of EB1-ΔN3-GFP to microtubules in transfected cells (Figure 3D) can be explained by an interaction with APC or p150Glued, because this deletion mutant can still associate with both of these proteins (Figure 2B). This raises the possibility that the EB1 microtubule association is at least partly dependent on interactions with other microtubule-binding proteins. However, given the weak nature of the EB1-ΔN3-GFP microtubule localization, we would suggest that any such contribution was minor, especially because both EB1-ΔC1-GFP and EB1-ΔC2-GFP were able to compete endogenous EB1 away from microtubule tips even though neither can interact with APC or dynein/dynactin. However, a model in which EB1 binds to microtubules directly but interactions with other MAPs contribute to a polarized localization at microtubule tips is consistent with our data.
Dissecting the Interactions between EB1, p150Glued, and APC
In vitro binding assays demonstrated a direct interaction between EB1 and p150Glued (Figure 4). The EB1 binding region in p150Glued localized within the first 330 aa of the protein. The N-terminus of p150Glued has been shown to harbor the microtubule-binding region (Waterman-Storer et al., 1995; Vaughan and Vallee, 1999), and the CDIC binding region lies between aa 150–811 (Vaughan and Vallee, 1999). Because aa 1–39 were essential for EB1 binding (Figure 4), it seems unlikely that there is much overlap between the EB1 and CDIC binding regions. This is consistent with the previously observed coimmunoprecipitation of both p150Glued and CDIC with EB1 from cell extracts (Berrueta et al., 1999).
It has recently been suggested that a dynein–β-catenin interaction plays a role in capturing and tethering microtubules at the adherens junction (Ligon et al., 2001). In this study we found no evidence for a stable EB1–β-catenin interaction mediated via dynein/dynactin (Figure 2B), although a transient interaction during microtubule capture cannot be ruled out. With this in mind we note that an EB1-p150Glued complex at the microtubule tip could contact cortically anchored, β-catenin–associated dynein, enabling the cortical capture of the microtubule via a p150Glued-dynein interaction.
In yeast a cortical microtubule capture mechanism that participates in mitotic spindle positioning has been shown to involve an interaction between microtubule-associated Bim1p and Kar9p, a protein whose cortical localization is actin dependent (see Schuyler and Pellman, 2001 for a review). Because there is some homology between the Kar9p and APC sequences in the EB1-binding region of APC, it has been suggested that Kar9p function might be provided by APC in higher eukaryotes (Bienz, 2001). This idea is supported by the observation that APC localizes to the lateral plasma membrane of epithelial cells in an actin-dependent manner (Rosin-Arbesfeld et al., 2001). A model in which EB1 at astral microtubule tips (Morrison and Askham, 2001) is involved in the capture of these microtubules at the cortex via an interaction with APC during mitosis can therefore be constructed. This model is supported by observations that implicate Drosophila APC and EB1 homologues in the orientation of cell divisions in neuroepithelial tissue (McCartney et al., 1999; Lu et al., 2001).
To complicate matters, however, the dynein/dynactin complex is already known to be required for the spindle movements arising from the cortical capture of astral microtubules in mammalian cells (Busson et al., 1998; O'Connell and Wang, 2000). A case can therefore be made for a simple model in which EB1 interacts directly with cortically anchored dynein/dynactin without any APC involvement. A direct EB1-dynein/dynactin interaction has the potential to both tether microtubules and exert force on them, an essential feature for subsequent spindle movement. Interestingly, APC is now known to interact with kinesin motor proteins (Jimbo et al., 2002). It may be possible that EB1 participates in two separate mechanisms linking cortical microtubule capture to spindle movement in animal cells, one involving an APC interaction with force generation provided by kinesins and the other an interaction with dynactin with force generation provided by dynein. This would reflect the situation in budding yeast, where dynein and the kinesin protein Kip3p define redundant mechanisms for orienting the mitotic spindle. The Bim1p-Kar9p interaction is known to act in the Kip3p-dependent pathway (Schuyler and Pellman, 2001). The potential existence and significance of these currently hypothetical interactions for EB1 at the cell cortex in mammalian cells requires careful examination.
EB1: A Microtubule Plus-end–associated Protein Required for Microtubule Anchoring at Centrosomes
Initially, the lack of microtubule focusing and retention at the centrosome in cells overexpressing EB1-C84-GFP seemed consistent with a profound inhibition of dynein/dynactin function. Previous investigators have carefully examined the function of dynactin at centrosomes using the same cell system and similar methods to those used here (Quintyne et al., 1999). Of particular interest were the effects of inhibiting p150Glued function by overexpression of the CDIC and Arp1 binding regions of the protein and the effects of a general blockade of dynein/dynactin function by p50Dynamitin overexpression. In all three cases both microtubule focusing and the p150Glued localization to centrosomes was inhibited.
However, the effects of EB1-C84-GFP expression are significantly more severe than those reported in this earlier study. For example, inhibition of dynactin function had no effect on microtubule regrowth and organization during recovery from nocodazole treatment until several hours after nocodazole washout, when a progressive defect in microtubule focusing at the centrosome became apparent. It is possible that the effects of EB1-C84-GFP overexpression arise from the combined inhibition of the EB1 interactions with APC and p150Glued. However, as any consequences of EB1-C50-GFP expression on microtubule organization were not obvious in this study, a powerful synergistic effect would be needed to produce the severe defects induced by EB1-C84-GFP. We also note that EB1-ΔC1-GFP overexpression might be expected to inhibit any EB1 interactions at microtubule plus-ends by displacing endogenous EB1 from microtubule tips, but this had no effect on microtubule regrowth and centrosomal focusing after nocodazole treatment. Taking this into account, and given the previously identified role for centrosomal p150Glued in microtubule focusing, we propose that the effects of EB1-C84-GFP overexpression arise primarily from the competitive inhibition of an EB1-p150Glued interaction at the centrosome. However, we would not rule out the possibility that the inhibition of EB1 interactions at microtubule plus-ends could also contribute to these effects.
The specific role that an EB1-p150Glued interaction might play in promoting microtubule anchoring at centrosomes remains unclear. The severity of the effects induced by EB1-C84-GFP overexpression when compared with those seen by previous workers suggests that a simple inhibition of dynein/dynactin function cannot fully account for them. Indeed, the observation that EB1-C84-GFP overexpression did not induce Golgi fragmentation demonstrates that inhibition of the EB1-p150Glued interaction does not affect all cytosolic dynein/dynactin functions (Figure 14). In the study by Quintyne et al. (1999), defects in microtubule organization were tightly correlated with the loss of p150Glued from centrosomes. The authors proposed that p150Glued was directly involved in the anchoring of microtubules at the centrosome, a function distinct from its established role as a dynein-associated dynactin subunit involved in intracellular membrane transport. We might therefore speculate that the EB1 interaction plays a fundamental role in the centrosomal recruitment and retention of p150Glued, a hypothesis consistent with our observations in this article (Figures 9 and 12). Another possibility is that EB1-C84-GFP overexpression inhibits the interaction between EB1 and an, as-yet unidentified binding partner. Although it cannot be excluded, we have tried to reduce the likelihood of this by the use of fusion proteins representing defined minimal protein–protein interaction regions within EB1 in our experiments.
Regulated microtubule release from centrosomes is a normal physiological event during the differentiation of a number of cell types, although the mechanisms underlying this phenomenon remain poorly defined (Keating and Borisy, 1999). It is tempting to speculate that modulation of the interaction between EB1 and p150Glued might play a role in this process. The interaction between EB1 and APC is known to be regulated by APC phosphorylation (Askham et al., 2000; Nakamura et al., 2001). Future studies should reveal whether the EB1-p150Glued interaction is regulated in a similar way.
ACKNOWLEDGMENTS
This work was supported by Yorkshire Cancer Research, the Medical Research Council (UK), and Cancer Research UK. E.E.M. was an MRC Fellow during the majority of this work.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0061. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0061.
REFERENCES
- Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J Cell Biol. 2000;149:863–874. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askham JM, Moncur PM, Markham AF, Morrison EE. Regulation and function of the interaction between the APC tumor suppressor protein and EB1. Oncogene. 2000;19:1950–1958. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- Barth AIM, Siemers KA, Nelson WJ. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J Cell Sci. 2002;115:1583–1590. doi: 10.1242/jcs.115.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinhauer JD, Hagan IM, Hegemann JH, Fleig U. Mal3, the fission yeast homologue of the APC-interacting protein EB1 is required for microtubule integrity and the maintenance of cell form. J Cell Biol. 1997;139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Kraeft S-K, Tirnauer JS, Schuyler SC, Chen LB, Hill DE, Pellman D, Bierer BE. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc Natl Acad Sci USA. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- Bienz M. Spindles cotton on to junctions, APC and EB1. Nat Cell Biol. 2001;3:E67–E68. doi: 10.1038/35060140. [DOI] [PubMed] [Google Scholar]
- Bu W, Su LK. Regulation of microtubule assembly by human EB1 family proteins. Oncogene. 2001;20:3185–3192. doi: 10.1038/sj.onc.1204429. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Deka J, Kuhlmann J, Muller O. A domain within the tumor suppressor protein APC shows very similar biochemical properties as the microtubule-associated protein tau. Eur J Biochem. 1998;253:591–597. doi: 10.1046/j.1432-1327.1998.2530591.x. [DOI] [PubMed] [Google Scholar]
- Diamantopolous GS, Perez F, Goodson HV, Batelier G, Melki R, Kreis TE, Rickard JE. Dynamic localization of CLIP-170 to microtubule plus ends is coupled to microtubule assembly. J Cell Biol. 1999;132:617–633. doi: 10.1083/jcb.144.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, et al. Mutations in the APC tumor suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A, Grosveld F, De Zeeuw CI, Galjart N. Functional analysis of CLIP-115 and its binding to microtubules. J Cell Sci. 2000;113:2285–2297. doi: 10.1242/jcs.113.12.2285. [DOI] [PubMed] [Google Scholar]
- Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, Akiyama T. Identification of a link between the tumor suppressor APC and the kinesin superfamily. Nat Cell Biol. 2002;4:323–327. doi: 10.1038/ncb779. [DOI] [PubMed] [Google Scholar]
- Juwana JP, et al. EB/RP gene family encodes tubulin binding proteins. Intl J Cancer. 1999;81:275–284. doi: 10.1002/(sici)1097-0215(19990412)81:2<275::aid-ijc18>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Näthke IS. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Borisy GG. Centrosomal and non-centrosomal microtubules. Biol Cell. 1999;91:321–329. [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Korinek WS, Copeland MJ, Chaudhuri A, Chant J. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science. 2000;287:2257–2259. doi: 10.1126/science.287.5461.2257. [DOI] [PubMed] [Google Scholar]
- Lee L, Tirnauer JS, Li J, Schuyler SC, Liu JY, Pellman D. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science. 2000;287:2260–2262. doi: 10.1126/science.287.5461.2260. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- Lu B, Roegiers F, Jan LY, Jan YM. Adherens junctions inhibit asymmetric division in the Drosophila neuroepithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Dierick HA, Kirkpatrick C, Moline MM, Baas A, Peifer M, Bejsovec A. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Cheng SC, Rose MD. Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of microtubules. Mol Biol Cell. 2000;11:2949–2959. doi: 10.1091/mbc.11.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. APC protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol. 2000a;148:505–517. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000b;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Tsukita S. Where is APC going? J Cell Biol. 2001;154:1105–1109. doi: 10.1083/jcb.200106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE, Wardleworth B, Askham J, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumor suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Askham JM. EB1 immunofluorescence reveals an increase in growing astral microtubule length and number during anaphase in NRK-52E cells. Eur J Cell Biol. 2001;80:749–753. doi: 10.1078/0171-9335-00221. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Moncur P, Askham JM. EB1 identifies sites of microtubule extension during neurite formation. Mol Brain Res. 2002;98:145–152. doi: 10.1016/s0169-328x(01)00290-x. [DOI] [PubMed] [Google Scholar]
- Muhua L, Adames NR, Murphy MD, Shields CR, Cooper JA. A cytokinesis checkpoint requiring the yeast homolog of an APC-binding protein. Nature. 1998;393:487–491. doi: 10.1038/31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Zhou XZ, Lu KP. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol. 2001;11:1062–1067. doi: 10.1016/s0960-9822(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Näthke IS, Adams CL, Polakis P, Sellin JH, Nelson JW. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS. The adenomatous polyposis coli protein. Mol Pathol. 2000;52:169–173. doi: 10.1136/mp.52.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell CB, Wang Y-L. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol Biol Cell. 2000;11:1765–1774. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Diamantopolous GS, Stalder R, Kreis TE. CLIP-170 highlights growing microtubule ends in vivo. Cell. 1999;96:517–527. doi: 10.1016/s0092-8674(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–F147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Nadezhdina E, Borisy G. Centrosomal control of microtubule dynamics. Proc Natl Acad Sci USA. 1999;96:115–120. doi: 10.1073/pnas.96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R, Ihrke G, Bienz M. Actin-dependent membrane association of the APC tumor suppressor in polarized mammalian epithelial cells. EMBO J. 2001;20:5929–5939. doi: 10.1093/emboj/20.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler SC, Pellman D. Search, capture and signal: games microtubules and centrosomes play. J Cell Sci. 2001;114:247–255. doi: 10.1242/jcs.114.2.247. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Richards K, Botstein D. BIM1 encodes a microtubule-binding protein in yeast. Mol Biol Cell. 1997;8:2677–2691. doi: 10.1091/mbc.8.12.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- Su L-K, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Tirnauer JS, O'Toole E, Berruetta L, Bierer B, Pellman D. Bim1p promotes the G1-specific dynamics of microtubules. J Cell Biol. 1999;145:993–1007. doi: 10.1083/jcb.145.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, Bierer B. EB1 proteins regulate microtubule dynamics, cell polarity and chromosome stability. J Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1999;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan KT, Tynan SH, Faulkner NE, Echeverri CJ, Vallee RB. Colocalisation of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends. J Cell Sci. 1999;112:1437–1447. doi: 10.1242/jcs.112.10.1437. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrunn J, Kinoshita K, Hyman AA, Näthke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3β phosphorylation. Curr Biol. 2001;11:44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]