Abstract
Geminin is an unstable inhibitor of DNA replication that gets destroyed at the metaphase/anaphase transition. The biological function of geminin has been difficult to determine because it is not homologous to a characterized protein and has pleiotropic effects when overexpressed. Geminin is thought to prevent a second round of initiation during S or G2 phase. In some assays, geminin induces uncommitted embryonic cells to differentiate as neurons. In this study, geminin was eliminated from developing Xenopus embryos by using antisense techniques. Geminin-deficient embryos show a novel and unusual phenotype: they complete the early cleavage divisions normally but arrest in G2 phase immediately after the midblastula transition. The arrest requires Chk1, the effector kinase of the DNA replication/DNA damage checkpoint pathway. The results indicate that geminin has an essential function and that loss of this function prevents entry into mitosis by a Chk1-dependent mechanism. Geminin may be required to maintain the structural integrity of the genome or it may directly down-regulate Chk1 activity. The data also show that during the embryonic cell cycles, rereplication is almost entirely prevented by geminin-independent mechanisms.
INTRODUCTION
The events of mitosis are controlled by a small group of unstable regulatory proteins that are destroyed at the metaphase/anaphase transition (King et al., 1996). The timely synthesis and destruction of these proteins ensures that mitosis proceeds in an orderly and irreversible manner. This family of proteins includes the anaphase inhibitor securin and the B-type cyclins, which activate the mitotic protein kinase Cdc2.
Geminin is a 25-kDa protein that was discovered by screening cDNA libraries for novel proteins that were destroyed during mitosis (McGarry and Kirschner, 1998). Except for B-type cyclins, geminin was the most abundant cDNA detected in the screen. Geminin's function was not immediately apparent because it is not homologous to any previously characterized protein. Overexpression experiments demonstrated that geminin strongly inhibits DNA replication by preventing formation of prereplication complex (preRC), a collection of essential replication factors that assembles on DNA at origins of replication (Stillman, 1996). At the molecular level, geminin prevents the incorporation of the mini-chromosome maintenance (MCM) complex into preRC. MCM complex is thought to be the helicase that separates the two strands of the double helix. Geminin seems to affect replication by inhibiting Cdt1, an essential replication factor that must be incorporated into preRC before the MCM complex can be added (Maiorano et al., 2000). Geminin binds tightly to Cdt1, and overexpression of Cdt1 overcomes the inhibition of replication caused by geminin (Wohlschlegel et al., 2000; Tada et al., 2001).
Based on these results, it has been proposed that the function of geminin is to inhibit a second round of DNA replication during S or G2 phase, and that geminin destruction during mitosis allows replication in the following cell cycle (McGarry and Kirschner, 1998). Although this model is appealing, it has been difficult to directly demonstrate that geminin is necessary to prevent rereplication in vertebrate cells. Immunodepletion of geminin from Xenopus egg extracts does not result in an extra round of replication. Furthermore, Xenopus egg extracts contain a pool of Cdt1 that is not bound to geminin, and this free pool seems to be sufficient to initiate replication (Tada et al., 2001).
Geminin was independently discovered as a molecule that induces uncommitted embryonic cells to differentiate as neurons (Kroll et al., 1998). It is not known how this activity is related to geminin's effect on the cell cycle. One possibility is that the two activities are linked and that geminin couples cell division and cell differentiation. For example, geminin might cause cells to accumulate in a cell cycle stage that is conducive to differentiation. Another possibility is that geminin has two separate and independent activities mediated by separate domains of the protein. In support of this hypothesis, the neuralizing activity of geminin can be reproduced by a small fragment of the protein that has no effect on DNA replication.
In this study, geminin was eliminated from developing Xenopus embryos by using antisense oligonucleotides to clarify its biological function. Geminin-depleted embryos were found to have a unique early embryonic lethal phenotype. They complete the embryonic cleavage divisions normally but suddenly arrest in G2 phase after the 13th cell division. The arrest occurred just after the midblastula transition (MBT), the point in development when the cell cycle slows and zygotic gene expression begins. There was no detectable overreplication of the genome in geminin-deficient embryos, and embryonic death occurs before the time of neural induction. The G2 arrest is enforced by the checkpoint kinase Chk1. There is increased Chk1 phosphorylation at the arrest point, and the arrest can be bypassed by overexpressing a dominant negative Chk1 mutant. Surprisingly, Chk1 is also phosphorylated around the time of the MBT in wild-type embryos, suggesting that the Chk1 pathway constitutively controls entry into mitosis. The results indicate that geminin has an essential function in allowing entry into mitosis, possibly by maintaining the integrity of the genome or by down-regulating Chk1 activity.
MATERIALS AND METHODS
Host Transfer Technique
To generate geminin-depleted embryos by the host transfer technique (Heasman et al., 1991), oocytes were injected near the vegetal pole with 5 ng of geminin antisense oligonucleotide (Bio-Synthesis, Lewisville, TX) in a total volume of 20 nl of H2O. The oligonucleotide had the sequence G*C*T*GGATTACTTTAA*G*T*G, where the asterisk indicates a phosphorothioate bond between the nucleotides. This oligonucleotide is complementary to a sequence encoding amino acids 35–41 that is completely conserved between geminin H and geminin L, the two isoforms of Xenopus geminin. It gave the most complete depletion of geminin of the three that were tested. Control embryos were injected with the sense version of the antisense oligonucleotide (C*A*C*TTAAAGTAATCC*A*G*C).
Embryo Injection Technique
To generate geminin-depleted embryos by the embryo injection technique (Heasman et al., 2000), each blastomere of a two-cell embryo was injected with 8 ng of an equal mixture of two morpholino oligonucleotides (Gene Tools, Corvallis, OR) in a volume of 10 nl. The antigeminin H oligonucleotide had the sequence ATCTCTGCTTCTTGTTGGTATTCAT and the antigeminin L oligonucleotide had the sequence TAAGCCTCTGCTCTTTTCACGCACA. After injection, embryos were cultured in 1× MMR/5% Ficoll for 2–3 h and then in 0.1× MMR. Control embryos were injected with a standard morpholino oligonucleotide that bore no sequence relationship to geminin (CCTCTTACCTCAGTTACAATTTATA).
Plasmid Construction and RNA Synthesis
Cdc2 WT and Cdc2AF genes were amplified using the primers GGGCCCGAATTCATGGACGAGTACAC and GGGCCCTCTAGATTAGTTTCTAATCTGATTG. After amplification, the fragments were digested with EcoRI and XbaI and subcloned into the vector pCS2(+), which had been digested with the same enzymes. The sequence of the Cdc2 coding region in each construct was confirmed by dideoxy sequencing. The isoform used in these experiments did not contain an internal EcoRI site.
pCS2-Cdc25 WT and pCS2-Cdc25 S287A were constructed by amplifying the Cdc25 gene from pSKCdc25-1 and pEGFP Cdc25 S287A, respectively (Kumagai and Dunphy, 1992; Kumagai and Dunphy, 1999). The primers were GGGCCCAGATCTATGGCAGAG-AGTCACATAATG and GGGCCCCTCGAGTTAAAGCTTCATTATGCGGGC. The amplified fragments were digested with BglII and XhoI and ligated to the vector pCS2(+), which had been digested with BamHI and XhoI.
pCS2-Gemininwobble was constructed by amplifying the Xenopus geminin H gene from the original cDNA clone of geminin H, p6.42.152 (McGarry and Kirschner, 1998). The two primers were GGGCCCGAATTCATGAACACAAATAAAAAACAACGC-TTGGATATGGAGAAGCC and GGGCCCCTCGAGCTAGACAGTATGTGCATC. The amplified fragment was digested with EcoRI and XhoI and inserted into the vector pCS2(+) that had been cut with the same enzymes. The sequence of the amplified geminin gene was confirmed by dideoxy sequencing.
pCS2-his6-Chk1ΔKD was constructed by digesting a pET28 clone of Chk1ΔKD (Michael et al., 2000) with NcoI, blunting the ends with the Klenow fragment, and digesting with XhoI. The fragment was ligated to the vector pCS2(+) that had been digested with BamHI, treated with Klenow fragment, and digested with XhoI.
To make mRNA, template plasmids were linearized with NotI and transcribed in vitro by using SP6 or T7 polymerase in the presence of ribonucleotide triphosphates and pGpppG cap analog (Pharmacia, Peapack, NJ). RNA was resuspended and diluted in H2O before injection. Chk1 D148A RNA was synthesized using pT7-G DA-Chk1 (Nakajo et al., 1999) as a template, and Myc RNA was synthesized using pMT-CS2.
Rescue with Cdc2, Cdc25C, and Chk1 D148A
Two-cell embryos were injected sequentially with morpholino antisense oligonucleotides and RNA. The time between the two injections was 20–30 min. It was found that the RNAs would not be expressed well when coinjected with the morpholino oligonucleotide. The embryos were scored for rescue at the late blastula stage when geminin-depleted embryos from the same clutch of eggs had arrested. Arrest was determined by judging the size and the appearance of the cells (Figure 2, B and D). In many instances one side of the embryo would be rescued and the other side would not. Rescue frequencies are calculated as the percentage of half-embryos rescued. Embryos were injected and scored blindly to avoid bias in the results. Geminin-depleted embryos injected with Cdc2 WT, Cdc2 AF, Cdc25C WT, Cdc25C S287A, or Chk1 D148A RNA did not develop past the late blastula stage, presumably because of the toxic effects of improperly regulated Cdc2 activity.
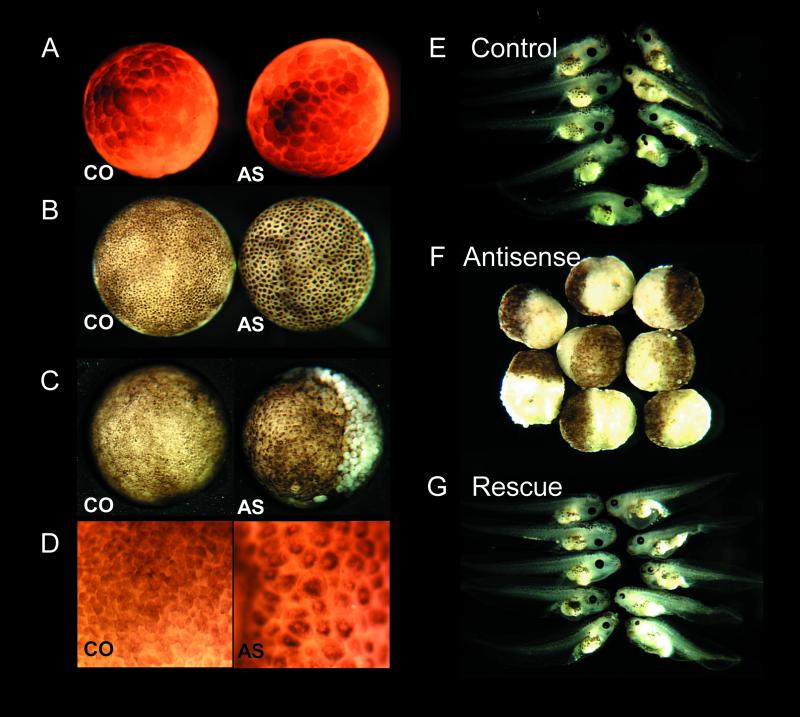
Figure 2.
Development of geminin-depleted embryos. (A–D) Geminin depletion causes a cell cycle arrest. Two-cell embryos were injected with morpholino control oligonucleotide (CO) or antisense-geminin oligonucleotide (AS). (A) Early blastula stage. (B) Shortly after the midblastula transition. (C) Gastrula stage 12, animal view. (D) Stage 12, detail of animal view. (E–G) Geminin RNA rescues geminin depletion. Stage VI oocytes were injected with control phosphorothioate oligonucleotide (control), antigeminin oligo (antisense), or antigeminin oligo followed by 240 pg of WT geminin RNA (rescue). Oocytes were fertilized by the host transfer technique and cultured in vitro. The embryos in the middle panel were fixed at stage 12, those in the top and bottom panels were fixed at stage 41–45.
Antibodies
Affinity-purified anti-geminin H antibody was described previously (McGarry and Kirschner, 1998). Anti-Cdc2 (sc-54) and His-Probe (sc-804) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphospecific anti-Cdc2 pY15 antibody was purchased from New England Biolabs (Beverly, MA). Anti-Cdc25C and anti-Cds1 antibodies were provided by Akiko Kumagai and William Dunphy (Kumagai and Dunphy, 1992; Guo and Dunphy, 2000). Anti-cyclin B1 antibody was provided by James Maller (Hartley et al., 1996). Anti-xChk1 antibody was provided by Jill Sible (Kappas et al., 2000). Monoclonal anti-actin antibody AC-40 was purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
Depletion of Geminin by Antisense Oligonucleotides
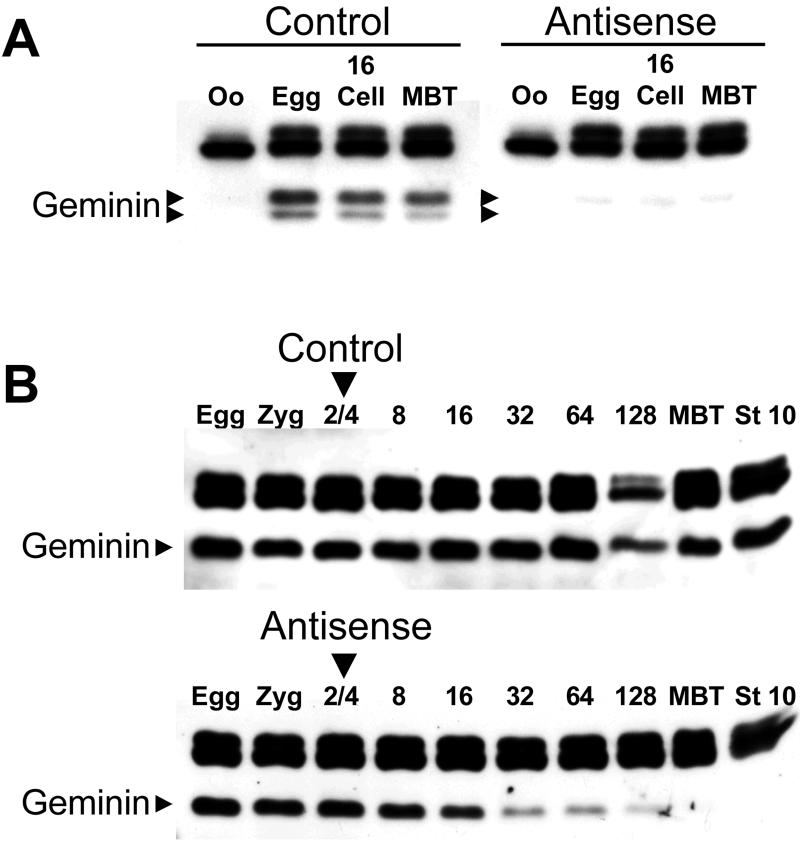
Two different techniques were used to deplete geminin from Xenopus embryos. In the host transfer technique (Heasman et al., 1991), stage VI oocytes were injected with either control or antisense-geminin phosphorothioate oligonucleotides. The injected oocytes were transplanted into the abdomen of a host female, fertilized, and cultured in vitro. In control embryos, geminin synthesis is induced during oocyte maturation and the level of the protein stays roughly constant throughout early development (Figure 1A, left). The antisense oligonucleotide eliminates 90–95% of the geminin protein induced during oocyte maturation and the geminin concentration remains low until at least the late blastula stage (Figure 1A, right). In the embryo injection technique (Heasman et al., 2000), fertilized Xenopus eggs were injected with either control or antisense-geminin morpholino oligonucleotides at the two-cell stage. The antisense oligonucleotide causes a rapid reduction in the geminin concentration and the protein is barely detectable at the 128-cell stage (Figure 1B, bottom). The concentration remains low until at least the beginning of gastrulation (stage 10; Nieuwkoop and Faber, 1967). Injection of control oligonucleotides has no effect (Figure 1B, top). The embryo injection technique was used in most of the experiments because it is much easier to perform and produces greater numbers of geminin-depleted embryos than the host transfer technique.
Figure 1.
Depletion of geminin by antisense oligonucleotides. (A) Stage VI Xenopus oocytes were injected with a phosphorothioate antigeminin or control oligonucleotide and fertilized by the host transfer technique. At various points in development, the amount of geminin was determined by immunoblotting (arrowheads). The higher molecular weight bands are unrelated proteins that cross-react with the antibody. Oo, oocyte before progesterone treatment; Egg, oocyte after progesterone treatment; 16, 16-cell embryo; MBT, mid-blastula transition. (B) Same as A, except two-cell embryos were injected with morpholino antigeminin or control oligonucleotide; 8–128, cell number at time of harvest; stage 10, onset of gastrulation.
Geminin Depletion Causes an Early Embryonic Cell Cycle Arrest
Geminin-depleted embryos generated by either technique display a characteristic early embryonic lethal phenotype. The early cell divisions seem to be normal, with normal timing and normal positioning of the cleavage furrows (Figure 2A). The first deviation from normal development becomes manifest at the late blastula stage, when the cells of geminin-depleted embryos seem to be noticeably larger than the cells of control embryos (Figure 2B). As development proceeds, the surface pigment of each cell condenses and develops a central white spot, giving the embryo a characteristic “leopard skin” appearance (Figure 2D). The larger cells of geminin-depleted embryos fail to execute normal gastrulation movements. The blastopore is misshapen and its diameter does not shrink normally. A wave of large detached white cells emerges from the region of the blastopore groove and quickly sweeps over the animal pole (Figure 2C). The underlying embryo becomes a large mass of yolky vegetal cells covered by a cap of pigmented animal cells (Figure 2F) that disintegrates over the course of a few hours. Virtually all geminin-depleted embryos displayed this phenotype (Table 1). In contrast, embryos injected with control oligonucleotides typically develop normally to at least the late tadpole stages (Table 1 and Figure 2E).
Table 1.
Phenotype of control, geminin-depleted, and rescued embryos
| G2 Arrest, % | Tadpoles, % | |
|---|---|---|
| Host transfer technique | ||
| Control oligo | 0 (n = 98) | 77 (n = 13) |
| Antisense oligo | 97 (n = 65) | 2 (n = 45) |
| AS oligo + Gem RNA | 5 (n = 21) | 79 (n = 19) |
| Embryo injection technique | ||
| Control oligo | 0.7 (n = 227) | 87 (n = 52) |
| Antisense oligo | 98 (n = 468) | 0 (n = 147) |
| AS oligo + Gem RNA | 26 (n = 135) | 28 (n = 45) |
Gem, geminin; oligo, oligonucleotide.
The observation that geminin-depleted blastulae had abnormally large cells suggested that their primary defect was a cell cycle arrest. This was confirmed by making time-lapse videorecordings of 13 geminin-depleted embryos from 13 different clutches of eggs. In all cases, the embryonic cell divisions were normal but the cells suddenly stopped dividing just after the MBT, which occurs after the 12th cell cycle. There was some variability in the exact time at which cell division stopped, both between different embryos and between different cells of the same embryo. Most often (>90% of the time), cell division stopped after the 13th or 14th division, but some cells would divide a 15th time before arresting. After the arrest, the embryos seemed healthy for about an hour and remained intact for several hours before disintegrating. To confirm that cell division had ceased, the embryos were sectioned and the mitotic index was determined by staining the nuclear DNA with 4,6-diamidino-2-phenylindole and counting the number of cells with condensed chromatin. In both late blastulae and early gastrulae (stages 9 and 11), virtually none of the cells of geminin-depleted embryos were in mitosis, whereas control embryos showed a mitotic index of 6–10% (Table 2).
Table 2.
Mitotic index and DNA content of geminin-depleted and control embryos
| Mitotic index, %
|
DNA content
|
|||
|---|---|---|---|---|
| Control | Geminin-depleted | Control | Geminin-depleted | |
| Blastula stage | 9.7 (n = 1465) | 0.5 (n = 1208) | 1.0 (n = 107) | 1.4 (n = 113) |
| Gastrula stage | 6.1 (n = 1996) | 0.0 (n = 1233) | 1.0 (n = 75) | 2.1 (n = 43) |
Injection of Geminin RNA Reverses Defects caused by Geminin Depletion
Normal development can be restored by injecting geminin-depleted embryos with geminin RNA (Figure 2G and Table 1). This control demonstrates that the phenotype is due to the loss of geminin itself and excludes the possibility that the antigeminin oligonucleotide fortuitously destroys a different protein or that the oligonucleotide preparation contains a toxic substance.
To rescue embryos generated by the host transfer method, oocytes were injected with ∼240 pg of geminin RNA 3 h after injection of the antisense oligonucleotide. This amount was sufficient to restore the geminin concentration to normal (our unpublished data). Presumably, the antisense oligonucleotide is degraded during the 3-h cultivation period so that it does not destroy the injected RNA. Virtually none the embryos that developed from these oocytes showed a large-cell phenotype characteristic of a cell cycle arrest, and most of them (15/19, ∼80%) developed to the swimming tadpole stage (Table 1 and Figure 2G). Some of the tadpoles seemed to have completely normal anatomy and others had a slightly malformed body.
To rescue embryos generated by the embryo injection technique, two-cell embryos were injected with gemininwobble RNA 20–30 min after the injection of the antigeminin oligonucleotide. To allow translation of the gemininwobble RNA, eight of the 25 bases in the region that would hybridize to the oligonucleotide were mutated in a way that preserved the amino acid sequence. Embryos injected with 100–200 pg of this RNA contain a normal amount of geminin at the late blastula stage (our unpublished data). Most of these embryos (∼75%) did not show a cell cycle arrest and many of them (∼30%) developed as far as the tadpole stage (Table 1). The extent of the rescue was not as complete as with the host transfer method, perhaps because of incomplete diffusion of the rescuing geminin RNA throughout the embryo or degradation of zygotic geminin RNA because of persistence of the morpholino oligonucleotide.
Geminin-depleted Cells Arrest in G2 Phase
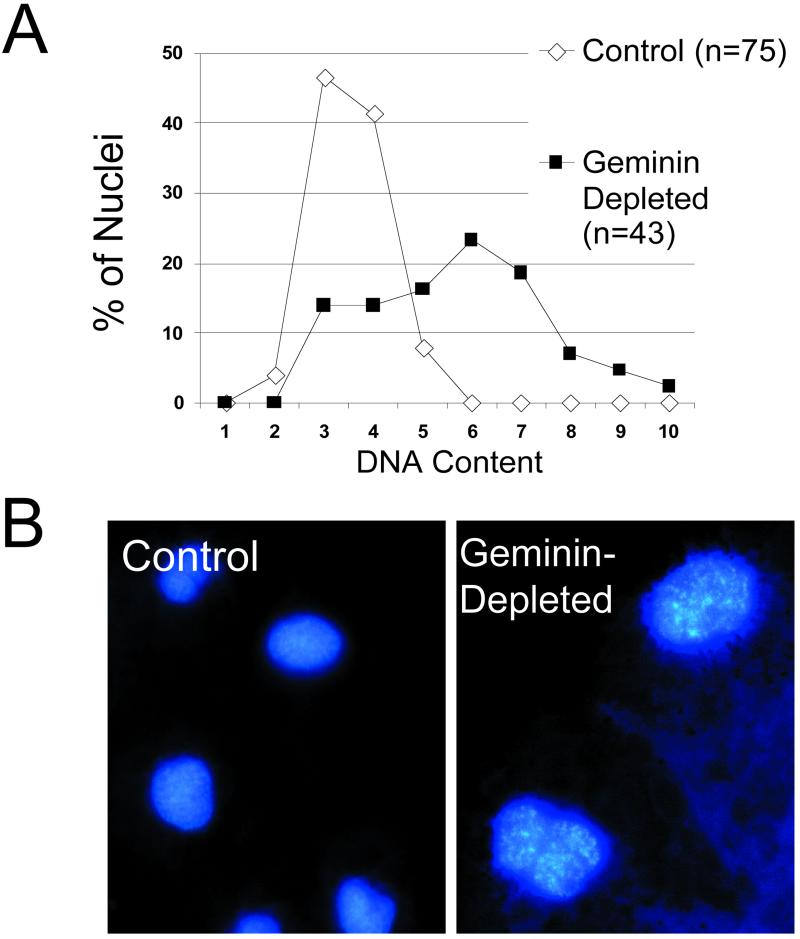
To determine the cell cycle stage of the arrest, DNA content was measured in tissue sections by quantitative fluorescence microscopy. In two separate experiments, geminin-depleted cells contained an average of 1.4 and 2.1 times as much nuclear DNA as control cells (Table 2). Figure 3A shows a histogram of DNA content for one of the experiments. The nuclei of geminin-depleted cells were noticeably larger than control nuclei and their chromatin was often partially condensed (Figure 3B). The appearance of the nuclei and the higher DNA content of the geminin-depleted cells suggested that they were arrested in G2 phase.
Figure 3.
Geminin-deficient embryos arrest in G2 phase. (A) Histogram of nuclear DNA content for control (white) and geminin-depleted cells (black). (B) Nuclei of control and geminin-depleted embryos stained with 4,6-diamidino-2-phenylindole at the gastrula stage. Both micrographs are at the same magnification.
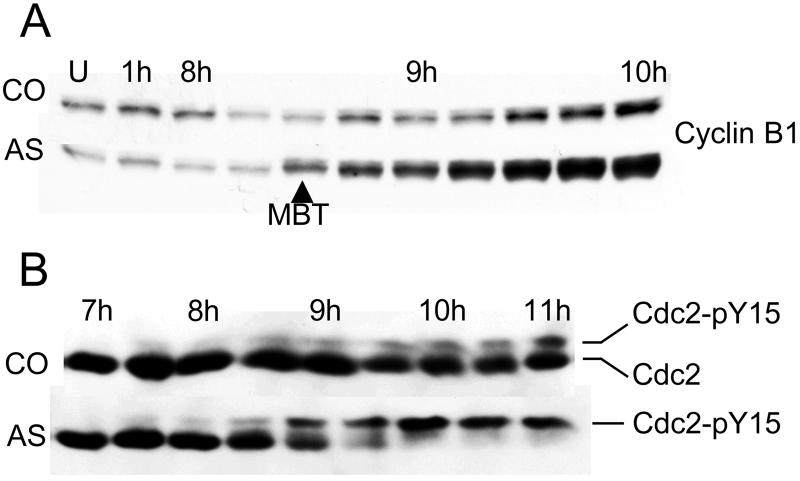
The G2 arrest was verified by demonstrating two biochemical markers that are characteristic of G2 phase cells: a high concentration of B-type cyclins and complete phosphorylation of Cdc2 on tyrosine-15 (Y15) (Draetta et al., 1988; Pines and Hunter, 1989). The B-type cyclins accumulate during S and G2 phase and bind to the mitotic protein kinase Cdc2 (King et al., 1994). Once the activating cyclin subunit is bound, Cdc2 activity is inhibited by phosphorylation of Y15 and threonine-14 (T14) by kinases in the Wee1/Myt1 family. The critical event that triggers the start of mitosis is the removal of the two inhibitory phosphates by a member of the Cdc25 family of phosphatases.
During the early embryonic cell cycles, Cdc2 is virtually unphosphorylated and S phase and mitosis directly follow each other without intervening gap phases (Graham and Morgan, 1966; Ferrell et al., 1991; Hartley et al., 1996). After the MBT, gap phases appear and cell division becomes asynchronous throughout the embryo. In normal embryos, the appearance of a population of cells in G2 phase at the MBT is reflected by the accumulation of B-type cyclins (Figure 4A, top) and the appearance of a small amount of Cdc2 that is phosphorylated on Y15 (Figure 4B, top). Both of these changes are exaggerated in geminin-depleted embryos. As the arrest point is reached, they accumulate 2–4 times as much cyclin B1 as control embryos (Figure 4A, bottom) and virtually all the Cdc2 becomes phosphorylated on Y15 (Figure 4B, bottom). These findings confirm that the arrest is in G2 phase.
Figure 4.
Cdc2 is hyperphosphorylated on tyrosine-15 in the absence of geminin. (A) Cyclin B1 accumulates in geminin-depleted embryos. Two-cell embryos were injected with antigeminin (AS) or control (CO) oligonucleotide and cultured in vitro. At various times after fertilization, the concentration of cyclin B1 was determined by immunoblotting. U, unfertilized egg. (B) Cdc2 becomes hyperphosphorylated geminin-depleted embryos. Same protocol as in A but a different experiment. The extent of Cdc2 phosphorylation was determined by immunoblotting with a mouse monoclonal Cdc2 antibody. The Y15-phosphorylated form of Cdc2 migrates through a polyacrylamide gel more slowly than the unphosphorylated form (Hartley et al., 1996).
Cdc2 AF Bypasses the Cell Cycle Arrest of Geminin-depleted Embryos
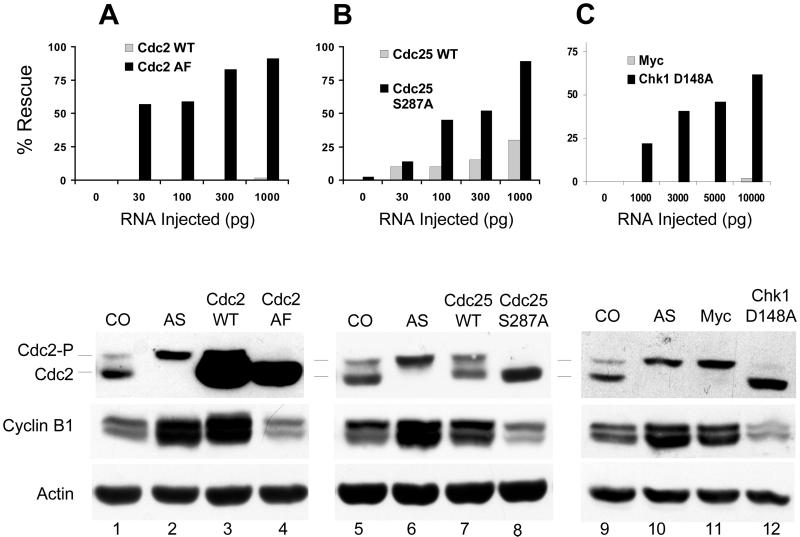
The near quantitative phosphorylation of Cdc2 on Y15 at the time of the arrest suggests that geminin-depleted embryos fail to enter mitosis because of an inability to remove the inhibitory phosphates on T14 and Y15. To test this possibility, geminin-depleted embryos were injected with RNA encoding Cdc2 AF, a mutant form of Cdc2 in which threonine-14 is replaced by alanine and tyrosine-15 is replaced by phenylalanine. Cdc2 AF cannot be phosphorylated at either of the inhibitory sites and does not require dephosphorylation for activity (Pickham et al., 1992). Injection of as little as 30 pg of Cdc2 AF RNA significantly suppressed the cell cycle arrest caused by geminin depletion, as judged by the size and appearance of the cells after the MBT (Figure 5A, top, black rectangles). The degree of suppression increased as more Cdc2 AF RNA was injected. Time-lapse videorecordings of two geminin-depleted embryos injected with 1000 pg of Cdc2 AF RNA confirmed that cell division continued throughout the embryo for several cycles after the 13th division until the cells became too small to be distinguished. In contrast, injection of as much as 1000 pg of wild-type Cdc2 RNA had no effect (Figure 5A, gray rectangles). Immunoblots showed that comparable amounts of the WT and AF proteins were expressed at each RNA concentration (our unpublished data). To confirm that the G2 arrest had been overcome, the extent of Cdc2 phosphorylation and the cyclin B1 level were measured after the MBT. Cdc2 AF expression caused the complete disappearance of tyrosine-15-phosphorylated Cdc2 and restored the cyclin B1 level to normal, indicating that the G2 arrest had been bypassed (Figure 5A, compare lanes 2 and 4). Wild-type Cdc2 had no effect (lane 3). The suppression of the arrest by Cdc2 AF indicates that geminin-depleted embryos fail to enter mitosis because of sustained inhibitory phosphorylation of Cdc2.
Figure 5.
Suppression of the Chk1 pathway rescues the cell cycle arrest caused by geminin depletion. (A) Top, Cdc2 AF rescues geminin deficiency. Two-cell embryos were sequentially injected with morpholino antigeminin oligo and RNA encoding Cdc2 WT or Cdc2 AF. The number of embryos that continued dividing past the MBT was determined by visual inspection (Figure 2, B and D). The chart shows the combined results of two independent experiments. Each data point represents 22–36 embryos. Bottom, after the MBT, the extent of Cdc2 phosphorylation and the cyclin B1 level were determined by immunoblotting. CO, uninjected embryos; AS, embryos injected with antisense oligo; AS + Cdc2 WT, embryos injected with antisense oligo and 1000 pg/side of WT Cdc2 RNA; AS + Cdc2 AF, same except Cdc2 AF RNA was injected. (B) Cdc25 S287A rescues geminin deficiency. Same as described above, except the embryos were injected with RNA encoding WT Cdc25 or Cdc25 S287A. (C) Chk1 D148A rescues geminin deficiency. Same as described above, except the embryos were injected with RNA encoding Chk1 D148A or an inert Myc tag.
Geminin Depletion Causes Increased Chk1 Phosphorylation
Checkpoint pathways prevent entry into mitosis in the presence of incompletely replicated or damaged DNA (Zhou and Elledge, 2000). Checkpoint responses are implemented by two effector protein kinases that are activated by phosphorylation, Chk1 and Cds1. Activated Chk1 and Cds1 prevent entry into mitosis in part by inhibiting Cdc25C, the phosphatase that removes the phosphates from T14 and Y15 of Cdc2 at the onset of mitosis (see below).
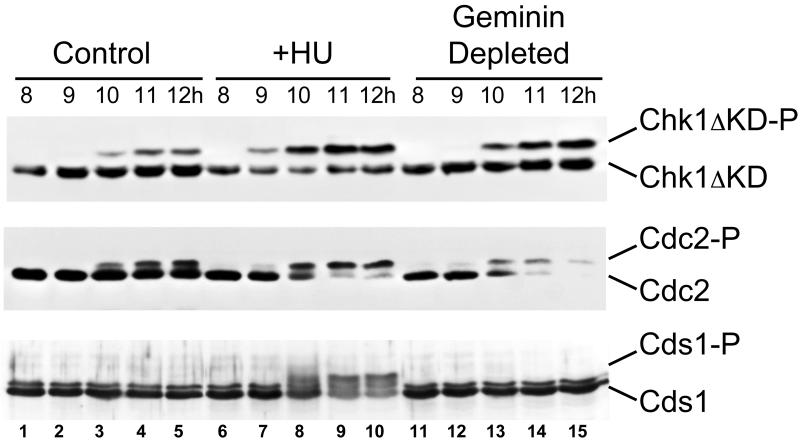
To see whether geminin depletion activates a checkpoint, the extent of Chk1 and Cds1, phosphorylation in geminin-depleted and control embryos was measured by immunoblotting (Figure 6). Phosphorylated Cds1 migrates through a polyacrylamide gel more slowly than the unphosphorylated form (bottom) (Guo and Dunphy, 2000). To monitor the phosphorylation of Chk1, embryos were injected with RNA encoding his6-tagged Chk1ΔKD, a fragment of Chk1 that undergoes an easily observable gel shift when phosphorylated. The phosphorylation of this fragment reflects endogenous Chk1 phosphorylation and is correlated with activation of the DNA replication checkpoint in Xenopus egg extracts (Michael et al., 2000). Control embryos injected with his6-Chk1ΔKD RNA develop into normal tadpoles, indicating that the protein itself is not toxic (our unpublished data). Both his6-Chk1ΔKD and Cds1 become extensively phosphorylated when the DNA replication checkpoint is induced by adding 20 mM hydroxyurea (HU) to the culture medium (Figure 6, top and bottom, lanes 6–10). Time-lapse videorecording of four HU-treated embryos showed that 80–90% of the cells stop dividing immediately after the 12th cell division, or about one division earlier than geminin-depleted embryos. The phosphorylation of his6-Chk1ΔKD and Cds1 occurs around the time of the arrest and is associated with increased phosphorylation of Cdc2 on Y15 (middle, lanes 6–10).
Figure 6.
Geminin depletion causes increased Chk1 phosphorylation. Top, Xenopus embryos were injected with RNA encoding his6-Chk1ΔKD at the two-cell stage. At various times the degree of his6-Chk1ΔKD phosphorylation was determined by immunoblotting with anti-his6-tag antibody. Injected embryos were either left untreated (control), cultured in 20 mM hydroxyurea (+HU), or injected with antigeminin oligo (geminin depleted). Times indicate hours postfertilization (p.f.). In this experiment the MBT occurred just after 9 h p.f. Middle, same experiment, but the blot was probed with anti-Cdc2 antibody. The loss of Cdc2 staining after 10 h in the geminin-depleted embryos is not reproducible (Figures 4 and 5). Bottom, same experimental protocol as described above, except the embryos were not injected with his6-Chk1ΔKD RNA and the blot was probed with anti-Cds1 antibody. In this experiment the MBT occurred around 10 h p.f.
In control embryos, Cds1 remains unphosphorylated throughout development (bottom, lanes 1–5) but his6-Chk1ΔKD becomes partially phosphorylated at the time of the midblastula transition (top, lanes 1–5). This phosphorylation is temporally correlated with the appearance of Y15-phosphorylated Cdc2 (middle, lanes 1–5), suggesting that Chk1 might constitutively control Cdc2 phosphorylation in normal post-MBT cell cycles (see DISCUSSION). His6-Chk1ΔKD becomes more extensively phosphorylated in geminin-depleted embryos than in controls (top, lanes 11–15). Quantification of the band intensities in four independent experiments showed that in control embryos Chk1 ΔKD is 22 ± 5% phosphorylated after the MBT, whereas in geminin-depleted embryos Chk1 ΔKD is 43 ± 2% phosphorylated (mean ± SE, P < 0.02). This is comparable with the amount of Chk1 DKD phosphorylated that is phosphorylated in HU-treated embryos (43 ± 5%). The increased Chk1 phosphorylation is associated with complete phosphorylation of Cdc2 on Y15 (middle, lanes 15). In contrast, Cds1 remains unphosphorylated in both control and geminin-depleted embryos throughout development. These results suggest that the Chk1 pathway is responsible for the G2 arrest that occurs when geminin is depleted.
Bypassing Chk1 Pathway Suppresses G2 Arrest Caused by Geminin Depletion
To confirm that the Chk1 pathway imposes the G2 arrest, geminin-depleted embryos were injected with RNA encoding two different proteins that bypass or inhibit the Chk1 pathway (Figure 5, B and C). Chk1 prevents Cdc2 dephosphorylation by negatively regulating Cdc25C, the phosphatase that removes the inhibitory phosphates from Cdc2. Chk1 phosphorylates Cdc25C on serine-287 (S287), generating a binding site for a member of the 14-3-3 family of proteins (Peng et al., 1997; Kumagai et al., 1998a; Kumagai and Dunphy, 1999). 14-3-3 binding masks a nuclear localization signal on Cdc25C and causes the phosphatase to be retained in the cytoplasm, where it presumably cannot dephosphorylate nuclear Cdc2.
Cdc25 S287A is a mutant in which S287 is mutated to alanine. Cdc25C S287A cannot be phosphorylated by Chk1 and induces checkpoint-arrested egg extracts to enter mitosis (Kumagai et al., 1998b). The S287A mutant efficiently suppresses the cell cycle arrest caused by geminin depletion in a dose-dependent manner, as determined by the size and appearance of the embryonic cells after the MBT (Figure 5B, top, black rectangles). Cdc25 S287A expression also causes the disappearance of Y15-phosphorylated Cdc2 and restores the cyclin level to normal (bottom, compare lanes 6 and 8), confirming that the G2 arrest has been overcome. Injection of the highest concentration of wild-type Cdc25C RNA partially suppresses the cell cycle arrest (Figure 5B, top, gray rectangles) and partially reverses the phosphorylation of Cdc2 on tyrosine-15 (bottom, lane 7). Because phosphorylation of S287 does not inhibit phosphatase activity (Kumagai et al., 1998b), wild-type Cdc25C would be able to overcome the arrest once sufficient quantities were available to saturate the amount of 14-3-3 in the cytoplasm. Immunoblots showed that comparable amounts of the Cdc25C WT and S287A proteins were expressed at each RNA concentration (our unpublished data).
Chk1 D148A is a kinase-inactive mutant that shows dominant negative effects when overexpressed with wild-type Chk1 (Nakajo et al., 1999). Injection of 1–10 ng of RNA encoding Chk1 D148A overcomes the cell cycle arrest caused by geminin depletion (Figure 5C). The D148A mutant suppresses the appearance large arrested cells after the MBT (top, black bars), eliminates the accumulation of Y15-phosphorylated Cdc2, and restores the cyclin B1 level to normal (bottom, lanes 9 and 12). Time-lapse videorecording of two embryos from two different clutches of eggs injected with 10 ng of Chk1 D148A confirmed that cell division continued past the 13th division. Injection of an inert RNA encoding a myc-epitope tag has no effect (Figure 5C, gray bars and lane 11). Immunoblots showed that the Chk1 D148A protein was vastly overexpressed relative to endogenous WT Chk1 at all RNA concentrations (our unpublished data). These experiments indicate that the G2 arrest caused by geminin depletion is Chk1 dependent.
DISCUSSION
Geminin is a recently discovered protein that inhibits DNA replication and gets destroyed during mitosis (McGarry and Kirschner, 1998). These properties strongly suggest that geminin has a key role in regulating the cell cycle, but the protein's function has been difficult to determine from overexpression experiments. It has been proposed that geminin prevents a second round of replication during S or G2 phase, yet geminin is clearly not required to prevent reinitiation in all types of cells (McGarry and Kirschner, 1998; Noton and Diffley, 2000). Furthermore, geminin seems to induce neural differentiation in some assays (Kroll et al., 1998). It is difficult to understand how a single small protein could have such diverse biological effects.
To better define the biological function of geminin, the protein was depleted from developing Xenopus embryos by using antisense oligonucleotides. Geminin-deficient embryos display a novel and unusual phenotype. The 12 early embryonic cell cycles are normal but the cells suddenly stop dividing after the 13th or 14th division, just after the midblastula transition. Several independent criteria establish that the cells are arrested in G2 phase: their mitotic index is close to zero, they have approximately twice the DNA content of normal cells, they contain a high concentration of B-type cyclins, and the mitotic kinase Cdc2 is virtually completely phosphorylated at the Y15 inhibitory site. The arrest is brought about by the Chk1-dependent checkpoint pathway, which inhibits mitosis by causing sustained inhibitory phosphorylation of Cdc2. The arrest can be overcome by expression of either a dominant negative mutant form of Chk1 or unphosphorylatable mutants of Cdc25C and Cdc2 that are not susceptible to inhibition by the Chk1 pathway.
The requirement for Chk1 in implementing the G2 arrest readily explains why geminin-depleted embryos do not stop dividing until after the midblastula transition. The MBT occurs after the 12th cell division and marks a time when the cell cycle undergoes an extensive reorganization (Newport and Kirschner, 1982a,b). The MBT is triggered when the nuclear/cytoplasmic ratio exceeds a critical threshold, but the molecular events responsible for the transition are unknown. Before the MBT, cells divide rapidly and synchronously about every 30 min. DNA synthesis and mitosis alternate in quick succession without intervening gap phases and zygotic transcription is suppressed. After the MBT, cells divide much more slowly and asynchronously. Gap phases appear and zygotic transcription begins. Most significantly, the MBT marks the time in development when the Chk1 pathway first becomes operational (Kappas et al., 2000). When the Chk1 pathway is induced by treating embryos with aphidicolin or ionizing radiation, phosphorylated Chk1 does not appear until the MBT is reached. The pathway is blocked upstream of Chk1, possibly because the concentration of DNA is too low to generate a signal sufficient to activate the kinase. Because Chk1 is required for the G2 arrest of geminin-deficient embryos, the embryos would be able to divide normally until Chk1 becomes activated at the MBT. This probably occurs at slightly different times in different cells, explaining why there is some variability in the exact number of cell cycles that can be completed in the absence of geminin.
The mechanism by which geminin deficiency leads to Chk1 activation is not clear. Studies with Xenopus egg extracts have demonstrated that the Chk1 pathway inhibits entry into mitosis in the presence of unreplicated or UV-irradiated DNA (Kumagai et al., 1998a). The simplest explanation of the phenotype is that geminin deficiency causes a subtle defect in DNA structure that activates the pathway. For example, geminin loss may cause a small amount of overreplication that cannot be detected by the techniques used in this study. This would be consistent with the hypothesis that geminin suppresses a second round of DNA synthesis during S phase.
The results confirm that during the Xenopus early embryonic cell cycles reinitiation can be almost entirely prevented by mechanisms that do not involve geminin. After 14 rounds of replication (13 cell cycles and one additional round before the arrest), geminin-deficient cells contain roughly twice as much nuclear DNA as control cells. The difference can be accounted for if the geminin-deficient cells are in G2 phase and the control cells are in G1 or S phase. Even if the difference were due to overreplication, there could be no more than (2)1/14 = 1.05 times the normal amount of replication in each cell cycle, i.e., 5% rereplication per cell cycle. In comparison, permeabilization of the nuclear envelope causes 25–50% rereplication per cell cycle (Blow and Laskey, 1988). The results reported here corroborate previous density label studies that showed a single round of replication occurs when geminin is removed from egg extracts by using specific antibodies (McGarry and Kirschner, 1998). Because of the serious consequences of overreplicating the genome, cells may use more than one mechanism to inhibit rereplication. One likely geminin-independent mechanism for preventing rereplication is the inhibition of preRC assembly by high levels of CDK activity (Kelly and Brown, 2000; Nguyen et al., 2001).
The phenotype of geminin-depleted Xenopus embryos is very different from that of Drosophila geminin mutants (Quinn et al., 2001). Geminin (−/−) Drosophila do not survive past the larval stages and the cause of the lethality has not been established. They exhibit a variety of defects, including anaphase chromosome bridges, a greater number of S-phase cells in some tissues, and a malformed peripheral nervous system. Significantly, a G2 cell cycle arrest was not apparent, neither at the MBT nor at later stages. The reasons for the difference in phenotype between Xenopus and Drosophila embryos that lack geminin are not immediately obvious. Geminin (−/−) Drosophila may survive past the MBT because of a maternal supply of geminin protein or RNA, but it is not clear why the cells do not arrest in G2 phase later in development when the maternal supply becomes exhausted. There may be differences between the two organisms in the way checkpoint controls are exercised. It has recently been reported that cultured Drosophila cells accumulate excessive amounts of DNA (up to 8n) when geminin synthesis is inhibited by interfering RNA (Mihaylov et al., 2002). This may indicate that Drosophila cells are more dependent on a geminin-requiring mechanism to inhibit rereplication than vertebrate cells. However, it has not been established that the excess DNA results from reinitiation within a single S phase.
The results do not demonstrate a primary role for geminin in inducing neural tissue. Geminin-deficient embryos die before neural induction begins during the gastrula stage (Kroll et al., 1998). Geminin might have a separate role in inducing the nervous system that is not manifest in geminin-deficient embryos because of the early mortality. Alternatively, the neural effects of geminin may be a secondary consequence of an effect on the cell cycle. For example, geminin might cause undifferentiated cells to accumulate in a cell cycle stage that is conducive to neural differentiation.
The results reported here suggest that the Chk1 pathway constitutively controls Cdc2 dephosphorylation in normal cell cycles. Phosphorylated Chk1 ΔKD appears at the late blastula stage in normal embryos that are not exposed to DNA-damaging agents or replication inhibitors. The onset of Chk1 phosphorylation coincides with the appearance of Y15-phosphorylated Cdc2 (Figure 6), and expression of dominant negative Chk1 D148A suppresses the appearance of phosphorylated Cdc2 (our unpublished data). The removal of the inhibitory phosphates from Cdc2 is the rate-limiting step for mitotic entry in most organisms (King et al., 1994). The hypothesis that Chk1 constitutively controls Cdc2 dephosphorylation is consistent with previously published reports that Chk1 is an essential protein in Drosophila and in mice (Sibon et al., 1997; Liu et al., 2000; Takai et al., 2000). The phenotype of Drosophila Chk1 (grapes) mutants suggests that the function of the protein is to prevent mitotic entry until DNA replication is complete. In maternal grapes mutants, the cells of the syncytial blastoderm continue to divide rapidly after the midblastula transition is reached, causing a catastrophic premature entry into mitosis that kills the embryo. Chk1 is also required for mouse ES cell viability, suggesting that the protein has a constitutive role in somatic cell cycles. Because Chk1 is activated in normal cell cycles, another interpretation of the data presented here is that geminin is more directly required to down-regulate Chk1. If this were the case, then one would expect active Chk1 to accumulate and cause a G2 arrest when geminin was absent. Further experiments will be required to evaluate this possibility.
ACKNOWLEDGMENTS
I especially thank Lew Cantley for outstanding support and commitment to this project. The manuscript was critically reviewed by Lew Cantley, Marc Kirschner, K. Ping Lu, Seth Field, Reuben Shaw, and Ben Turk. This work was supported by grants to T.J.M. from the Howard Hughes Medical Institute, the Warren-Whitman-Richardson Foundation, and the National Heart Lung and Blood Institute (K 08 HL03461), and by a grant to Lew Cantley from the National Institutes of Health (GM-56203).
Abbreviations used:
- MBT
midblastula transition
- S287
serine-287 of Cdc25
- T14
threonine-14 of Cdc2
- Y15
tyrosine-15 of Cdc2
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0199. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0199.
REFERENCES
- Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- Draetta G, Piwnica-Worms H, Morrison D, Druker B, Roberts T, Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988;336:738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CF, Morgan RW. Changes in the cell cycle during early amphibian development. Developmental Biology. 1966;14:439–460. [Google Scholar]
- Guo Z, Dunphy WG. Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol Biol Cell. 2000;11:1535–1546. doi: 10.1091/mbc.11.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Rempel RE, Maller JL. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Heasman J, Holwill S, Wylie CC. Fertilization of cultured Xenopus oocytes and use in studies of maternally inherited molecules. Methods Cell Biol. 1991;36:213–230. doi: 10.1016/s0091-679x(08)60279-4. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. β-Catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Kappas NC, Savage P, Chen KC, Walls AT, Sible JC. Dissection of the XChk1 signaling pathway in Xenopus laevis embryos. Mol Biol Cell. 2000;11:3101–3108. doi: 10.1091/mbc.11.9.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TJ, Brown GW. Regulation of chromosome replication. Annu Rev Biochem. 2000;69:829–880. doi: 10.1146/annurev.biochem.69.1.829. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition [see comments] Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Salic AN, Evans LM, Kirschner MW. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development. 1998;125:3247–3258. doi: 10.1242/dev.125.16.3247. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Binding of 14–3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 1999;13:1067–1072. doi: 10.1101/gad.13.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998a;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998b;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Maiorano D, Moreau J, Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Michael WM, Ott R, Fanning E, Newport J. Activation of the DNA replication checkpoint through RNA synthesis by primase. Science. 2000;289:2133–2137. doi: 10.1126/science.289.5487.2133. [DOI] [PubMed] [Google Scholar]
- Mihaylov IS, Kondo T, Jones L, Ryzhikov S, Tanaka J, Zheng J, Higa LA, Minamino N, Cooley L, Zhang H. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol Cell Biol. 2002;22:1868–1880. doi: 10.1128/MCB.22.6.1868-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Dev Biol. 1999;207:432–444. doi: 10.1006/dbio.1998.9178. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982a;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982b;30:687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nguyen VQ, Co C, Li JJ. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411:1068–1073. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- Noton E, Diffley JF. CDK inactivation is the only essential function of the APC/C and the mitotic exit network proteins for origin resetting during mitosis. Mol Cell. 2000;5:85–95. doi: 10.1016/s1097-2765(00)80405-0. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216 [see comments] Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Pickham KM, Meyer AN, Li J, Donoghue DJ. Requirement of mosXe protein kinase for meiotic maturation of Xenopus oocytes induced by a cdc2 mutant lacking regulatory phosphorylation sites. Mol Cell Biol. 1992;12:3192–3203. doi: 10.1128/mcb.12.7.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Quinn LM, Herr A, McGarry TJ, Richardson H. The Drosophila geminin homolog: roles for geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev. 2001;15:2741–2754. doi: 10.1101/gad.916201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]