Abstract
At the trans-Golgi network, clathrin coats containing AP-1 adaptor complexes are formed in an ARF1-dependent manner, generating vesicles transporting cargo proteins to endosomes. The mechanism of site-specific targeting of AP-1 and the role of cargo are poorly understood. We have developed an in vitro assay to study the recruitment of purified AP-1 adaptors to chemically defined liposomes presenting peptides corresponding to tyrosine-based sorting motifs. AP-1 recruitment was found to be dependent on myristoylated ARF1, GTP or nonhydrolyzable GTP-analogs, tyrosine signals, and small amounts of phosphoinositides, most prominently phosphatidylinositol 4,5-bisphosphate, in the absence of any additional cytosolic or membrane bound proteins. AP-1 from cytosol could be recruited to a tyrosine signal independently of the lipid composition, but the rate of recruitment was increased by phosphatidylinositol 4,5-bisphosphate. The results thus indicate that cargo proteins are involved in coat recruitment and that the local lipid composition contributes to specifying the site of vesicle formation.
INTRODUCTION
Sorting of membrane proteins is generally mediated by cytosolic coats which serve the dual role of creating a scaffold to form coated buds and vesicles and of selectively concentrating cargo proteins by interacting with cytosolic signals. The best studied systems are COPI in intra-Golgi and Golgi-to-endoplasmic reticulum (ER) transport, COPII in ER-to-Golgi transport, and clathrin with associated adaptor proteins in the formation of vesicles at the plasma membrane, the trans-Golgi network (TGN) and endosomes. There are different types of clathrin-associated adaptor proteins (APs), heterotetrameric complexes composed of two ∼100-kDa adaptins, a ∼50-kDa medium (μ), and a ∼20-kDa small (ς) chain (Robinson and Bonifacino, 2001). The adaptor complexes form the inner layer of the coat that specifies the site of coat formation and interacts with cargo molecules. AP-1 adaptors are primarily functional at the TGN generating vesicles destined for endosomes but have also been found on sorting endosomes and implicated in (basolateral) recycling to the plasma membrane (Futter et al., 1998). AP-2 adaptors are found at the plasma membrane to form coated vesicles for endocytosis. AP-3 adaptors are involved in lysosomal transport from the TGN or endosomes. The different adaptor complexes recognize similar tyrosine and dileucine signals in cargo molecules, and in many cases the same signals are recognized by several adaptor types (Bonifacino and Dell'Angelica, 1999; Heilker et al., 1999).
Recruitment of the different coats to their specific membranes appears to involve common basic mechanisms. With the exception of AP-2/clathrin coats, all the coats mentioned above require small GTPases that are activated from their soluble GDP-bound to their membrane-associated GTP-bound form by a guanine nucleotide exchange factor (GEF) at the correct membrane. For COPII coats in yeast, the GTPase Sar1p is activated by the GEF Sec12p in the ER membrane. In an assay with chemically defined liposomes containing acidic lipids like phosphatidic acid (PA), phosphatidylserine (PS), or phosphoinositides, these components were sufficient to recruit the subunits of COPII, first Sec23p/24p and then Sec13p/31p, to form coated buds and vesicles (Matsuoka et al., 1998b). In the presence of cargo membrane proteins (the v-SNAREs Sec22p or Bos1p), these were selectively incorporated (Matsuoka et al., 1998a).
For COPI coats, the GTPase ARF1 (ADP-ribosylation factor 1) is activated by a Golgi-associated GEF. On liposomes made of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) with unsaturated fatty acids or containing acidic phospholipids, ARF1·GTPγS and COPI complexes were sufficient to form coats and vesicles (Spang et al., 1998; Bremser et al., 1999). However, with saturated lipids of different compositions, COPI recruitment was only achieved in the presence of liposome-associated cargo sequences (Bremser et al., 1999).
Recruitment of the clathrin adaptors AP-1 and AP-3 also involves ARF1, together with specific GEFs (e.g., BIG2; Shinotsuka et al., 2002). ARF·GTPγS, AP-3, and clathrin were sufficient to generate coats on liposomes made from soybean lipids (containing 20% PC and various other lipids) and to bud coated vesicles (Drake et al., 2000). Based on various studies (Dittiéet al., 1996; Mallet and Brodsky, 1996; Seaman et al., 1996; Zhu et al., 1998, 1999a), the following model for AP-1/clathrin coat formation has been proposed (Zhu et al., 1998). After nucleotide exchange in ARF1 by a GEF at the site of coat initiation, ARF1·GTP will interact rapidly with putative docking protein(s) to generate high-affinity binding sites for AP-1. In turn, clathrin trimers will bind to immobilized AP-1 and laterally associate to form the characteristic lattice. Cargo molecules will associate with AP-1 despite the low affinity of interaction, because AP-1 is highly concentrated in the coat. GTP hydrolysis induced by an ARF GTPase activating protein will eventually inactivate the docking protein. As the growing coat soon interacts with multiple cargo proteins, it will stay membrane bound even as docking proteins and ARF1·GDP dissociate.
It has been proposed that the mannose-6-phosphate receptors form the major docking sites for AP-1 at the TGN (Le Borgne and Hoflack, 1997), a concept that has been challenged by studies with Golgi membranes devoid of mannose-6-phosphate receptors (Zhu et al., 1999b). In addition, the finding that AP-1 could be recruited in an ARF1-dependent manner to protein-free soybean liposomes, which can be easily pelleted, in the presence of cytosol indicated that integral membrane proteins are not necessary (Zhu et al., 1999a). Yet, the cytosol dependence of the process suggested the involvement of a soluble cytosolic factor(s) that peripherally attaches to the liposomes and functions as the AP-1 docking site. Peripheral membrane proteins have also been shown to bind to AP-1 on affinity chromatography (Mallet and Brodsky, 1996), and a Tris-strippable factor was shown to be required for AP-1 binding to immature secretory granules (Dittiéet al., 1996). AP-1 binding to liposomes was dependent on the lipid composition, which thus might play a role in the binding of a cytosolic factor to the membrane. A soybean lipid mixture containing 20% PC and acidic lipids was optimal, whereby PS, but to some extent also phosphatidylinositol (PI) or PA seemed to contribute (Zhu et al., 1999a).
In the present study, we have analyzed the minimal requirements for the recruitment of AP-1 adaptor complexes to a membrane in vitro using chemically defined liposomes in a floatation assay that does not require the liposomes to be pelletable. In particular, the contributions of cargo-sorting signals and lipids were tested. Stable AP-1 recruitment was found to require in addition to myristoylated ARF1·GTP also the presence of membrane-anchored tyrosine signals and specific phosphoinositides but no further cytosolic factors.
MATERIALS AND METHODS
Reagents
Guanylyl imidodiphosphate (GMP-PNP), guanosine 5′-O-(3-thiotriphosphate; GTPγS), and GTP were from Roche Diagnostics. Superose-6 (Prep grade) and ECL reagent were from Amersham Pharmacia Biotech (Piscataway, NJ). N-((4-maleimidylmethyl)cyclohexane1-carbonyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (MMCC-DHPE) was from Molecular Probes (Eugene, OR). Egg PC, liver PI, liver PE, and brain PS were from Avanti Polar Lipids (Alabaster, AL), phosphatidylinositol 3-phosphate (PI3P), PI5P, and PI(3,4)P2 from Echelon Research Laboratories Inc. (Salt Lake City, UT), PI(3,5)P2 from Calbiochem (La Jolla, CA), and PI(3,4,5)P3 from Matreya Inc. (Pleasant Gap, PA). mAb 100/3 (anti–α-adaptin), horseradish peroxidase–coupled anti-mouse IgG antibody, PI4P, PI(4,5)P2, soybean PC (azolectin, P-5638), mixed phosphoinositides (P-6023), GDP, and dipalmitoyl-PA were purchased from Sigma (Buchs, Switzerland). Peptides were synthesized on a Pioneer synthesizer (PerSeptive Biosystems, Framingham, MA) using Fmoc (fluorenylmethoxycarbonyl) protected amino acids with TBTU (2-(1H-benzotriazole 1-yl)-1,1,3,3 tetramethyluronium tetrafluoroborate) as coupling reagent. Cleaved and deprotected peptides were first purified via reverse phase HPLC (RP C18, Vydac, Hesperia, CA) and then verified by MALDI-TOF mass spectrometry (TOFSPEC-2E, Micromass, Manchester, UK). mAb 1D9 against ARF1 was a kind gift by Richard Kahn (Emory University, Atlanta, GA).
Purification of AP-1 and ARF1
Clathrin-coated vesicles were purified from calf brains, freshly obtained at the local slaughterhouse as described (Campbell et al., 1984). All the procedures were performed at 4°C. The coats were released by homogenizing vesicles with one volume of 1.5 M Tris-HCl (pH 7.0), 6 mM EDTA, 0.6 mM DTT, 0.5 mM phenylmethylsulphonyl fluoride (PMSF), and 10 μg/ml benzamidine and 2 μg/ml pepstatin A, leupeptin, antipain, and chymostatin. After overnight incubation at 4°C membranes were spun for 30 min at 100,000 × g, and the supernatant was loaded in 2-ml portions on a 50 × 1.6 cm Superose-6 column equilibrated with 0.5 M Tris-HCl (pH 7.0), 2 mM EDTA, 0.2 mM DTT and run at 0.5 ml/min. Mixed adaptors were collected between 55 and 64 ml of elution. To eliminate the remaining clathrin, mixed adaptors were dialyzed into 0.1 M MES, 1 mM EGTA, 0.5 mM MgCl2, 0.2 mM DTT (pH 6.6) to form clathrin cages and centrifuged for 1 h at 400,000 × g. Although clathrin was only found in the pellet with most of AP-2 and AP180, AP-1 largely stayed in solution in accordance with its lower cage-promoting activity (Keen, 1989; Lindner and Ungewickell, 1992). The supernatant was dialyzed into 20 mM ethanolamine, pH 8.9, 2 mM EDTA, 1 mM DTT (MonoQ buffer; Ahle et al., 1988) and loaded on a 2-ml CHT-II hydroxyapatite column (Bio-Rad, Cambridge, MA) that was equilibrated and washed with 0.5 M Tris-HCl, 2 mM K/PO4, pH 7.0, followed by 10 mM phosphate in the same buffer. AP-1 was eluted stepwise with 50 mM and 100 mM phosphate. Purified AP-1 was dialyzed against MonoQ buffer containing 0.5 mM PMSF and stored at 4°C with protease inhibitors. The 70-kDa protein was identified after Coomassie staining and in-gel digestion with trypsin (Perrot et al., 1999) by analysis on a Reflex III MALDI-TOF instrument (Bruker, Bremen, Germany) using α-cyano-hydroxy-cinnamic acid as matrix. Protein identification was done using the Mascot software (Matrix Science Ltd., London, UK).
Plasmids encoding bovine ARF1 with residues 3–7 from yeast Arf2p (Liang et al., 1997) and yeast N-myristoyltransferase (pBB131; Duronio et al., 1990) were generous gifts by Stuart Kornfeld and Jeffrey Gordon, respectively (both at Washington University, St. Louis, MO). After cotransformation of both plasmids into Escherichia coli BL21(DE3), myristoylated ARF1 was purified as described (Liang and Kornfeld, 1997). This ARF1 preparation bound to Golgi membranes (Martín et al., 2000), indicating its efficient myristoylation. Nonmyristoylated ARF1 was also prepared and purified and showed the expected mobility shift on SDS gel electrophoresis (Franco et al., 1995; Liang and Kornfeld, 1997). Proteins were quantified using the bicinchoninnic acid assay (BCA; Pierce, Rockford, IL) or the Bradford assay (Bio-Rad; for samples containing Tris), using bovine serum albumin as standard. Silver staining of polyacrylamide gels was performed as described (Morrissey, 1981).
Preparation of Peptidoliposomes
Five micromoles of egg PC (3.8 mg) were combined with 125 nmoles MMCC-DHPE (2.5 mol %). When indicated, other lipids were used to replace some of the PC. The organic solvent was evaporated under a stream of nitrogen. Dichloromethane was added and evaporated twice. Dried lipids were resuspended into 1 ml 10 mM HEPES (pH 6.5), 0.1 M NaCl, 0.5 mM EDTA and freeze-thawed five times in liquid nitrogen and then extruded 11 times through a 400-nm Nucleopore polycarbonate membrane (Corning, Corning, NY) using a homemade hand-driven extruder. The liposomes (0.3 ml) were immediately incubated with 120 μg of peptide (i.e., about a fourfold excess over the coupling lipid, assuming half of it is exposed) for 1 h at room temperature, and then stored at 4°C with 0.02% (wt/vol) NaN3 for up to 2 weeks. The coupling efficiency varied from ∼30 to 50% as judged by measuring the amount of peptide associated with the liposomes the bicinchoninic acid assay after extensive dialysis of the liposomes against phosphate-buffered saline. We found it unnecessary to remove free peptides from the liposomes before the AP-1 recruitment assay (negligible inhibition of adaptor binding to immobilized peptides had also been observed in surface plasmon resonance assays; Heilker et al., 1996).
Liposome Recruitment Assay
Peptidoliposomes (200 μl; 1 μmol lipid) were first incubated for 30 min at 37°C with 5 μg of ARF1 and either 0.2 mM GMP-PNP (or GTPγS), or 2 mM GTP or GDP. When GTP or GDP were used, 3 mM phosphate was also added to inhibit hydrolysis by a spurious phosphatase (Franco et al., 1995). Samples were returned to ice and 10 mM MgCl2 was added to stabilize the loaded ARF1 (Franco et al., 1995) as well as 10 μg of mixed adaptors or 0.5 μg of AP-1. After 15 min on ice, samples of 250 μl were mixed with 0.5 ml of 60% (wt/vol) sucrose in assay buffer (10 mM HEPES, pH 7.0, 150 mM NaCl, 10 mM KCl, 3 mM potassium phosphate, 2 mM MgCl2, 0.2 mM dithiothreitol; Höning et al., 1997), overlayed with 3.07 ml of 20% sucrose in assay buffer and with 0.18 ml of buffer in a 4-ml centrifuge tube, and centrifuged in a TST60 rotor (Kontron, Zurich, Switzerland) at 55,000 rpm (300,000 × gav) for 1 h at 4°C. Four 1-ml fractions were collected from the top and precipitated with 8% (wt/vol) trichloroacetic acid. Acetone-washed pellets were analyzed by 7.5–15% PAGE and immunoblotting using antibodies to γ-adaptin (100/3) or ARF1 (1D9), a peroxidase-coupled secondary antibody, and ECL reaction. Quantitation was performed using a MultiImage Light Cabinet from Alpha Innotech Corporation (San Leandro, CA).
Cytosol was obtained from calf brain or bovine adrenals (gift of Kitaru Suda, Biozentrum, Basel, Switzerland) as the high-speed supernatant after homogenization (Campbell et al., 1984), supplemented with protease inhibitors, and clarified by centrifugation before use. Peptidoliposomes (0.5 μmol lipid) were incubated for 30 min at 37°C with 0.5 mg of cytosol, 5 μg of ARF1, and 0.2 mM GMP-PNP in 200 μl of assay buffer. Samples were returned to ice and mixed with 0.4 ml of 60% (wt/vol) sucrose in assay buffer, and liposomes were floated as described above.
Nucleotide Exchange Assay
Nucleotide exchange was measured using [35S]GTPγS and the filtration assay according to Franco et al. (1995) under the experimental conditions used for the recruitment assay.
RESULTS
An Assay for AP-1 Recruitment to Model Membranes
To assess the interaction of AP-1 adaptors to sorting signals in the context of a chemically defined membrane, we coupled synthetic peptides via an N-terminal cysteine to a maleimide derivative of PE, thus creating lipid-anchored peptides. The reactive lipid was mixed with PC or various lipid mixtures at 2.5 mol %, and large unilamellar liposomes were produced by extrusion through a 400-nm pore-size filter. Peptides were then coupled via an N-terminal cysteine to the reactive lipid (Figure 1A). The peptides used (Lamp1Y and TGN38Y) corresponded to the C-terminal cytoplasmic domain of Lamp-1 (lysosome-associated membrane protein-1) and a portion of the cytoplasmic domain of TGN38 (trans-Golgi network protein of 38 kDa), two proteins with well characterized tyrosine-containing sorting signals (Figure 1B). The same peptides with the tyrosines mutated to alanine (Lamp1A and TGN38A) were used as negative controls. Lamp-1 is sorted from the TGN via endosomes to lysosomes (Hunziker and Geuze, 1996) and has been demonstrated by immunogold electron microscopy in AP-1–positive clathrin-coated buds and vesicles at the TGN (Höning et al., 1996). TGN38 cycles between the TGN and the plasma membrane. An interaction with AP-1 is less clearly established (Ohno et al., 1995; Boll et al., 1996; Stephens et al., 1997).
Figure 1.
Peptidoliposomes to assay AP-1 recruitment in vitro. The maleimide derivative of PE MMCC-DHPE was used to couple synthetic peptides via an N-terminal cysteine to a lipid (A). The peptides used correspond to the cytoplasmic domain of Lamp1 (B, Lamp1Y) or the segment of TGN38 that has previously been shown to contain the functional tyrosine motif (Boll et al., 1996). Lamp1A and TGN38A are the control peptides with the critical tyrosine mutated to alanine. After incubation of peptidoliposomes with AP-1 and with or without ARF1, they were floated from the bottom of a sucrose step gradient (C). Four fractions were collected as indicated, with fraction I containing the floated liposomes with bound proteins and fraction IV including the loading zone with unbound proteins.
Adaptor complexes were isolated from calf brain coated vesicles by releasing the coat with 1 M Tris followed by gel filtration to remove the bulk of clathrin. This mixed adaptor preparation (containing both AP-1 and AP-2) was incubated with the peptidoliposomes. The mixture, supplemented with sucrose to a concentration of 40%, was then loaded below a 20% sucrose cushion and a small amount of sucrose-free buffer (Figure 1C) and centrifuged for 1 h at 300,000 × g to separate the liposomes and bound proteins from free adaptors. The gradient was collected from the top in four fractions (I–IV), with fraction I containing the floated liposomes with recruited proteins and fraction IV containing unbound material. Aliquots of the four fractions were analyzed by SDS-gel electrophoresis and probed by immunoblot analysis.
Because in vivo recruitment of AP-1 to the TGN requires the GTPase ARF1 in its active GTP-bound form (Stamnes and Rothman, 1993; Traub et al., 1993), the potential requirement of ARF1 in our assay was tested by incubating purified ARF1 with the peptidoliposomes together with GTP or a nonhydrolyzable GTP analog (GMP-PNP or GTPγS) at 37°C for 30 min before addition of adaptors. It has previously been shown that liposomes induce guanine nucleotide exchange on ARF1 and thus activate it (Antonny et al., 1997), a function performed in vivo by specific GEFs at the TGN.
Recruitment of AP-1 Adaptors to Liposomes Requires a Tyrosine-based Signal, ARF1, and Specific Lipids
In previous in vitro assays, AP-1 was shown to bind to the cytoplasmic sequence of Lamp-1 immobilized on beads or on the sensor surface in surface plasmon resonance experiments (Höning et al., 1996). In our assay, however, no recruitment of AP-1 could be observed to Lamp1Y presented on liposomes made of PC or of a 1:1 mixture of PC and soybean lipids (azolectin; Figure 2A, lanes 1–4). γ-Adaptin, a 100-kDa subunit of AP-1 complexes, was detected exclusively in fraction IV of the step gradients, which represents the loading zone. This result is consistent with the apparent dissociation rates of adaptors from immobilized tyrosine motifs in surface plasmon resonance experiments (Heilker et al., 1996; Höning et al., 1996), which would not allow interacting adaptors to stay bound to the peptidoliposomes during a 1-h floatation.
Figure 2.
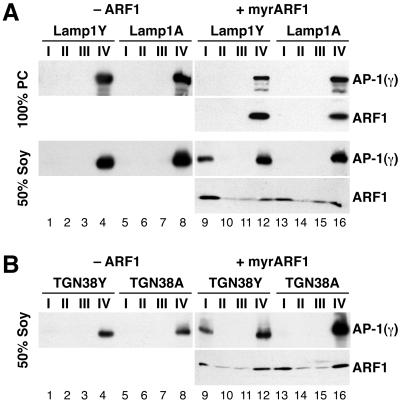
AP-1 recruitment to peptidoliposomes is signal-, ARF1- and lipid-dependent. (A) Peptidoliposomes made of 100% PC or 50% PC/50% soybean lipids and presenting Lamp1Y or Lamp1A peptides were incubated with a mixed adaptor preparation and with or without myristoylated ARF1 and GMP-PNP. After flotation on a sucrose step gradient, four fractions (I–IV, as shown in Figure 1C) were collected from the top and analyzed by immunoblotting for γ-adaptin or ARF1. (B) The same experiments were performed using peptidoliposomes made of 50% PC/50% soybean lipids and presenting TGN38Y or TGN38A peptides.
However, if purified myristoylated ARF1 with GMP-PNP was added to the Lamp1Y peptidoliposomes and incubated at 37°C before addition of adaptors, a significant fraction of AP-1 was floated to the top of the gradient (fraction I) together with liposomes containing 50% soybean lipids (Figure 2A, lanes 9–12). AP-1 was not recruited to liposomes presenting Lamp1A peptides or to liposomes composed entirely of PC (lanes 9–16) even in the presence of ARF1·GMP-PNP.
AP-1 recruitment to the membrane was rather stable, because the middle fractions II and III of the gradient were entirely devoid of γ adaptin, indicating that bound adaptors did not significantly dissociate during the floatation. This is in contrast to the interaction of the bulk of ARF1 with liposomes. On nucleotide exchange, the active ARF1 exposes its myristoyl tail, which allows it to interact with lipid bilayers (Antonny et al., 1997). The equilibrium between lipid-associated and soluble ARF1 is shifted by the addition of soy lipids in favor of the lipid-associated form: although ARF1 is not dragged out of the loading zone (fraction IV) by pure PC liposomes (in agreement with Helms et al., 1993), approximately half of ARF1 was floated to fraction I in the presence of 50% soybean lipid, with considerable trailing into fractions II and III. The residual clathrin in the adaptor preparation was not corecruited with AP-1.
Like Lamp1Y, the tyrosine motif peptide TGN38Y was similarly able to recruit AP-1 only in the presence of ARF1·GMP-PNP and with liposomes containing 50% soybean lipids (Figure 2B). Again, recruitment depended on the tyrosine signal, because TGN38A was not functional. ARF1, in contrast, was associated with liposomes irrespective of the peptides coupled to them. The results show that recruitment of AP-1 to liposomes requires activated ARF1, functional tyrosine motifs, and a particular lipid composition.
Phosphoinositides Are Required to Recruit AP-1
The soybean lipids used in Figure 2 contain 20% PC and an ill-defined mixture of other lipids. To identify which components are responsible for AP-1 recruitment, 3% of PE, PA, PS, PI, or a mixture of phosphoinositides (PIPs) were added to PC to produce peptidoliposomes presenting Lamp1Y in our assay (Figure 3A). AP-1 was not significantly recruited to the liposomes containing PE, PA, or PS and only slightly to those containing 3% PI. Most efficient recruitment was reproducibly observed to liposomes containing phosphoinositides.
Figure 3.
Lipid requirement for AP-1 recruitment to peptidoliposomes. (A) Three percent of the indicated lipid was incorporated into PC peptidoliposomes exposing Lamp1Y. After incubation with a mixed adaptor preparation and with myristoylated ARF1·GMP-PNP, fractions I and IV of a flotation gradient were analyzed by immunoblotting. PIPs indicates a commercial mixture of phosphoinositides. (B) Two percent of PI-monophosphates and 1% of PI-bis- and trisphosphates were incorporated into PC peptidoliposomes exposing Lamp1Y and analyzed as in A. (C) The recruitment of AP-1 and ARF1 to liposomes containing different phosphoinositides (2% of PI-monophosphates and 1% of PI-bis- and trisphosphates) were densitometrically quantified. The amount recovered in fraction I is expressed in percent of the total in fractions I plus IV. The average and SDs of at least three experiments, including those shown in B, are presented.
To determine which phosphoinositides are capable of stimulating AP-1 recruitment, we compared Lamp1Y/PC peptidoliposomes containing 2% of the monophosphorylated phosphoinositides PI3P, PI4P, or PI5P, or 1% of the phosphatidylinositol bisphosphates PI(3,4)P2, PI(3,5)P2, or PI(4,5)P2, or phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3). At these concentrations the phosphoinositides with one and two phosphates on the inositol ring introduce approximately the same negative charge to the membranes.
Among the monophosphorylated phosphoinositides, PI5P was the most effective in recruiting AP-1 (Figure 3, B and C), whereas PI3P and PI4P were only marginally functional. However, the most efficient AP-1 recruitment of all was obtained with PI(4,5)P2, even though it was used at only half the concentration of the monophosphorylated phosphoinositides. The other bis- or trisphosphorylated molecules were unable to sustain AP-1 recruitment. In contrast to the pronounced lipid dependence of AP-1 recruitment, the amount of ARF1 recovered in fraction I did not show significant differences for different lipids used.
AP-1 Recruitment Depends on Myristoylated ARF1 in Its Active Conformation
In the above experiments, GMP-PNP, a nonhydrolyzable analogue of GTP was used, indicating that GTP hydrolysis is not required for AP-1 recruitment to peptidoliposomes. In Figure 4, we further analyzed the nucleotide requirement using myristoylated ARF1 and liposomes with 10% mixed phosphoinositides and Lamp1Y peptides. No AP-1 recruitment and no ARF1 association with liposomes was detected when only GDP was added to the ARF1/peptidoliposome incubation (lanes 9 and 10), demonstrating that AP-1 binding required active ARF1. No significant differences in the efficiency of AP-1 recruitment were observed when GTP, GTPγS, or GMP-PNP were used as the nucleotide. In contrast, ARF1 association with liposomes reproducibly depended on the type of GTP analog used. ARF·GTPγS floated more efficiently with liposomes than ARF1·GMP-PNP, whereas ARF1·GTP did so the least (lanes 3–8). This is possibly due to slight differences in conformation and/or to some hydrolysis of GTP. Both AP-1 recruitment and ARF1 association with peptidoliposomes depended on incubation of ARF1 with liposomes at 37°C because they were almost completely abolished at 4°C (Figure 4, lanes 1–4). This reflects the fact that nucleotide exchange is temperature dependent. As expected, unmyristoylated ARF1 was not functional in the assay (lanes 11 and 12).
Figure 4.
Nucleotide dependence of AP-1 recruitment to peptidoliposomes. The indicated nucleotide was incubated with myristoylated or nonmyristoylated ARF1 and peptidoliposomes containing 3% of mixed inositides and exposing Lamp1Y. The analysis was performed as in Figure 3.
The Effect of Phosphoinositides Is Not via the Nucleotide Exchange Activity of Liposomes
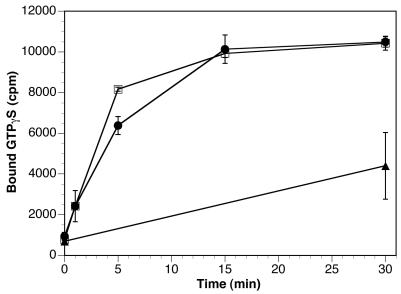
The efficiency of AP-1 binding to peptidoliposomes with different lipid compositions did not correlate with the relative or absolute amounts of ARF1 that floated with the liposomes to the top fraction of the gradient (Figure 3). It appears that all acidic lipids increased ARF1 association to the liposomes compared with pure PC, whereas AP-1 recruitment was much more specific. Nevertheless, it was conceivable that the effect of the functional phosphoinositides on AP-1 recruitment was indirect by increasing the rate or extent of nucleotide exchange in ARF1, which in our assay is performed in an unphysiological manner by the liposome surface. To test this possibility, a nucleotide exchange assay was performed using liposomes made of PC only or of PC with 10% mixed phosphoinositides. ARF1 was incubated with these liposomes and [35S]GTPγS for different times, after which the samples were filtered and the amount of radioactivity bound to ARF1 was determined. As is shown in Figure 5, the rate of nucleotide exchange in the presence of liposomes is more than 10 times higher than in the absence of membranes. Yet, there is no significant difference in the kinetics or the final extent of GTPγS loading of ARF1 in the presence or absence of phosphoinositides that could explain the dramatic difference in AP-1 recruitment observed with these lipid compositions (compare Figure 4, lanes 3 and 6, with Figure 2A, lanes 9–12, top panel). Thus, the phosphoinositides must affect other aspects of ARF1 function or must act on the AP-1 adaptors.
Figure 5.
Nucleotide exchange on ARF1. Myristoylated ARF1 was incubated at 37°C with [35S]GTPγS and either buffer only (▴), PC liposomes (●), or PC with 10% mixed phosphoinositides (□). At the indicated times, samples were quickly filtered through a nitrocellulose filter. After washing, the radioactivity on the filter, corresponding to GTPγS bound to ARF1, was counted.
A Minimal Machinery for AP-1 Recruitment
The mixed adaptor preparation used in the experiments described so far contains in addition to AP-1 also AP-2 adaptors, AP-180, and a number of unknown contaminating bands, which might directly or indirectly contribute to AP-1 recruitment. To identify the minimal set of proteins required, we purified AP-1 adaptors to near homogeneity. Figure 6A shows aliquots of the mixed adaptor preparation (lane 1) and of the purified AP-1 preparation (lane 2) containing the same amount of AP-1 (as judged by immunoblot analysis) on an SDS-gel stained with silver. All contaminating proteins except for one of ∼70 kDa were removed below detection in the purified sample. By mass spectrometry, this copurifying contaminant was identified to be hsc70, the uncoating ATPase of clathrin-coated vesicles (Schlossman et al., 1984; DeLuca-Flaherty and McKay, 1990), which is highly unlikely to contribute to coat recruitment and could not be detected in the floated fraction. Using this AP-1 preparation, again robust recruitment of AP-1 complexes was achieved to liposomes containing 1% PI(4,5)P2 presenting the Lamp1Y peptides and in the presence of myristoylated ARF1 loaded with GMP-PNP (Figure 6B, lanes 1 and 2). Using Lamp1A lacking the tyrosine, liposomes lacking the phosphoinositides, or GDP-loaded ARF, each individually abolished AP-1 association with the liposomes. This result thus defines the minimal machinery to recruit AP-1 to a membrane to consist of a peptide with a functional tyrosine motif and anchored to a lipid membrane containing a small amount of PI(4,5)P2, and myristoylated ARF1 loaded with GTP or a nonhydrolyzable GTP analog.
Figure 6.
Recruitment of pure AP-1 to peptidoliposomes. (A) Aliquots of the mixed adaptor preparation (lane 1) and of hydroxyapatite-purified AP-1 (lane 2) containing the same amount of AP-1 (as judged by immunoblot analysis) were separated by SDS-gel electrophoresis and stained with silver. AP-1 subunits β1, γ, and μ1 are indicated by filled arrowheads, whereas AP180 and AP-2 subunits αa, αc, β2, and μ2 are indicated by open arrowheads. (B) AP-1 recruitment assays were performed using liposomes made of PC with or without 1% PI(4,5)P2 and exposing Lamp1Y (LY) or Lamp1A (LA) peptides in the presence of myristoylated ARF1 loaded with GMP-PNP or GDP. The analysis was performed as in Figure 3.
Signal and Lipid Dependence of AP-1 Recruitment from Cytosol
Zhu et al. (1999a, 1999b) observed signal-independent AP-1 recruitment from cytosol to soybean liposomes in a pelleting assay. Therefore, using our floatation assay, we also investigated AP-1 recruitment from cytosol. Peptidoliposomes were mixed with cytosol supplemented with purified ARF1 and incubated for 30 min at 37°C before floatation of the liposomes as before. Consistent with the results by Zhu et al. (1999a), significant recruitment of AP-1 from brain cytosol to soybean liposomes presenting Lamp1A was observed (Figure 7A, lanes 3 and 4). This tyrosine-independent binding was even stronger using adrenal cytosol (which was used by Zhu et al. 1999a; Figure 7B, lanes 3 and 4). With both types of cytosol, however, AP-1 recruitment was clearly enhanced when functional Lamp1Y peptides were presented (Figure 7, A and B, lanes 1 and 2). If liposomes made of PC with 1% PI(4,5)P2 or of pure PC were used, recruitment to Lamp1A was detectable, but very low (lanes 7 and 8, and 11 and 12, respectively), whereas recruitment to Lamp1Y-presenting liposomes was robust with ∼40% (lanes 5 and 6, and 9 and 10).
Figure 7.
Recruitment of AP-1 from cytosol. AP-1 recruitment assays were performed using brain cytosol (A) or adrenal gland cytosol (B), and peptidoliposomes made of soybean lipids (lanes 1–4), PC with 1% PI(4,5)P2 (lanes 5–8), or pure PC (lanes 9–12), exposing Lamp1Y (LY) or Lamp1A (LA) peptides. Cytosol supplemented with purified ARF1 and GMP-PNP was incubated with the peptidoliposomes for 30 min at 37°C before separation by gradient centrifugation. (C) To determine the kinetics, AP-1 recruitment assays were performed using brain cytosol and liposomes exposing Lamp1Y peptides prepared of either PC alone (white bars) or PC containing 1% PI(4,5)P2 (dark bars) at different incubation times (average and SD of 3 determinations).
The finding that AP-1 could be recruited from cytosol to pure PC liposomes with Lamp1Y peptides (lanes 5 and 6) is in contrast to our observation with purified AP-1 derived from clathrin coats, which was not recruited to pure PC membranes (Figure 2A). However, analysis of the time-course of AP-1 recruitment from cytosol to PC liposomes with or without 1% PI(4,5)P2 revealed that the kinetics were significantly faster to peptidoliposomes containing 1% PI(4,5)P2 than to those made of PC alone (Figure 7C).
DISCUSSION
Vesicular transport requires the recruitment of coat components to the specific donor membrane in the cell and the selection and incorporation of cargo proteins as well as of proteins necessary for vesicle targeting and fusion (e.g., the appropriate v-SNAREs). Two models for how this is accomplished have been proposed for different transport steps. Coat components may be targeted to the donor compartment by binding to a specific, high-affinity docking protein. Cargo molecules will diffuse into the coated area and be trapped by specific, but rather low-affinity interactions with coat molecules. Alternatively, it is the cargo itself that induces coat formation in combination with a site-specific feature like a particular lipid composition or a GEF for an accessory GTPase.
This second concept is attractive, because cargo selection and coat recruitment are coupled. This provides a mechanism to adjust vesicle formation to the amount of cargo to be transported, as has, for example, been observed experimentally for AP-2/clathrin coats in dependence of transferrin receptor overexpression (Iacopetta et al., 1988; Miller et al., 1991). However, the two models are not mutually exclusive. A docking protein is implicated in the nucleation of AP-2/clathrin coats, and there is evidence that synaptotagmin plays this role (Zhang et al., 1994). Binding of AP-2 to synaptotagmin is stimulated by tyrosine-based endocytosis motifs, i.e., by cargo (Haucke and De Camilli, 1999). Because in addition both AP-2 and synaptotagmin bind to phosphoinositides, particularly PI(4,5)P2 (Beck and Keen, 1991; Südhof and Rizo, 1996), it was proposed that the lipid composition might be an additional level of regulating AP-2 recruitment (Takei and Haucke, 2001).
Our results using liposomes show that a docking protein is not necessary for AP-1 recruitment. The minimal machinery in our assay consists of myristoylated ARF1·GTP (or GMP-PNP or GTPγS), membrane-anchored tyrosine-containing sorting motifs of cargo proteins and a small amount of specific phosphoinositides. In the absence of any other membrane-associated proteins, ARF1 thus must interact directly with AP-1 to stimulate its recruitment. Such an interaction has recently been shown between ARF1 and the β1 and γ-adaptins of AP-1 bound to immature secretory granules by cross-linking experiments (Austin et al., 2000). Similarly, a direct interaction has been shown between ARF1 and COPI complexes (Zhao et al., 1997). ARF1·GTP may dramatically increase AP-1 affinity for tyrosine signals or alternatively induce AP-1 to oligomerize, forming a surface patch with multiple cargo interactions already before addition of clathrin. AP-1 may thus behave similarly to COPI coatomer, which is induced to polymerize by a peptide corresponding to the cytoplasmic sequence of the COPI cargo protein p23 (Reinhard et al., 1999).
The third component required for AP-1 recruitment besides ARF1 and cargo signals was a lipid composition containing phosphoinositides, particularly PI(4,5)P2 and to a lesser extent PI(5)P, at physiologically low concentrations in the range of a few mole-percent. The phosphoinositide contribution is clearly specific and does not simply correlate with charge, because different isomers showed vastly different effectiveness and other acidic phospholipids at higher concentrations were inactive.
The lipid composition also affected the equilibrium distribution of activated ARF1 between the membrane-associated and the free form, as was apparent from the amount of ARF1 that was associated with the floated liposomes. However, all acidic lipids increased membrane association of ARF1, and there was no correlation between the recruitment of AP-1 and the fraction of floated ARF1. Phosphoinositides, which stimulated AP-1 recruitment, also did not affect the rate or extent of nucleotide exchange in ARF1 (in agreement with Antonny et al., 1997). Furthermore, recruitment of AP-3 or COPI, which are also ARF1 dependent, to liposomes was largely independent of the lipid composition (Bremser et al., 1999; Drake et al., 2000). The major effect of the lipid composition on AP-1 recruitment is thus unlikely to be exerted via ARF1, but rather via AP-1.
Phosphoinositides have indeed been shown to modulate tyrosine signal recognition of both AP-1 and AP-2 using a cross-linking assay with lipid/detergent micelles in the absence of ARF1. The interactions between the TGN38 motif and AP- 2 (Rapoport et al., 1997) as well as between the Lamp-1 motif and AP-1 (Rapoport et al., 1998) were found to be enhanced by PI(3,4)P2. This phenomenon thus does not explain the lipid dependence of AP-1 recruitment in our system. However, the most efficient lipid for AP-1 recruitment, PI(4,5)P2, and the appropriate kinases for their synthesis have in fact been localized to the Golgi apparatus (Cockcroft and De Matteis, 2001). There, ARF1 was shown to regulate the synthesis of PI(4,5)P2 by recruiting, and thus activating, PI 4-kinase and PI(4)P 5-kinase from the cytosol (Godi et al., 1999; Jones et al., 2000). Activation of ARF1 at the TGN may therefore contribute to preparing the ground with respect to the optimal lipid environment for AP-1 recruitment.
When a tyrosine signal was present, recruitment of AP-1 from cytosol was found not to be absolutely dependent on the lipid composition. This either reflects a difference between cytosolic and coat-derived AP-1 adaptors or contributions by unknown cytosolic factors. Yet, even in this system, the presence of PI(4,5)P2 significantly enhanced the kinetics of the process. Generation of this phosphoinositide is thus a likely mechanism of regulating coat formation.
AP-1 recruitment in our assay is strongly dependent on tyrosine motifs presented on the membrane surface. The tyrosine motif of Lamp-1 has been shown to bind to both AP-1 and AP-2 in vitro (Höning et al., 1996; Ohno et al., 1996). The tyrosine motif of TGN38, also interacted with AP-2 adaptors in vitro (Ohno et al., 1995) but only weakly with AP-1 (Boll et al., 1996); yeast two-hybrid assays with μ1 yielded variable results (Ohno et al., 1995, 1996; Rapoport et al., 1997; Stephens et al., 1997; Stephens and Banting, 1998). There is evidence that at least some membrane proteins are transported from the TGN to the basolateral surface via endosomes rather than in a direct vesicular transport route to the plasma membrane (Futter et al., 1995; Leitinger et al., 1995; Laird and Spiess, 2000; Orzech et al., 2000). Together with the recent discovery of a μ1 isoform (μ1B) involved in basolateral sorting (Fölsch et al., 1999; Ohno et al., 1999), AP-1 adaptors are thus potentially involved in surface transport of basolateral proteins, including TGN38. AP-1 recruitment by the TGN38Y sequence in our assay might be related to this function.
In summary, our results define minimal requirements for AP-1 recruitment to a membrane and suggest the following modified model of the molecular events. Whereas in our assay ARF1 was activated by spontaneous nucleotide exchange on the lipid bilayer, ARF1 activation in the cell is a controlled and catalyzed process. Already ARF1.GDP may be concentrated at the membrane as indicated by its interaction with a putative PKA-activated receptor at the Golgi (Martín et al., 2000). It is activated to ARF1·GTP by a specific brefeldin A–sensitive GEF like BIG2 (Shinotsuka et al., 2002). The second factor specifying the site of AP-1 recruitment is likely to be the lipid composition in the TGN, i.e., the local production of PI(4,5)P2, which is further stimulated by ARF1·GTP activating appropriate lipid kinases. Productive AP-1 recruitment will only take place, when a sufficient concentration of cargo proteins with AP-1 recognition sequences is present. Interaction with ARF1, PI(4,5)P2 and tyrosine signal may induce a conformational change in AP-1 inducing AP-1 oligomerization. The resulting structures will be stably attached to the membrane by multiple low-affinity interactions with cargo molecules and lipids. In our assay, this is reflected in the fact that, unlike ARF1, AP-1 attachment to the liposomes survived a 1-h floatation through a sucrose gradient without “bleeding” into the middle fractions. Subsequent binding of clathrin will then induce coat and membrane curvature. Because ARF1 is scarce in isolated clathrin-coated vesicles (Zhu et al., 1998), it must dissociate at some point, most likely upon GTP hydrolysis. Interaction of ARF1·GTP with AP-1 might activate its GTPase activity. If AP-1 has not associated with other AP-1 complexes when GTP is hydrolyzed, it will be released from the membrane. Thus, ARF1 might function as a timer regulating coat assembly. It remains to be tested whether AP-1 acts as a GTPase-activating protein for ARF1, like the COPII components Sec23p/24p for Sar1 (Antonny et al., 2001).
Our results do not exclude that docking proteins able to recruit AP-1 exist. In fact, we have reproduced the previous finding that AP-1 can be targeted to certain lipid compositions in a signal-independent, but cytosol-dependent manner. This might provide a mechanism for generating a basal level of cargo-independent vesicle budding as might be required to guarantee transport of lipids or recycling of v-SNARES for endosome-to-Golgi transport when cargo proteins are few. Interestingly, the v-SNARE VAMP4 has been recently shown to bind AP-1 via a di-leucine motif (Peden et al., 2001). Various membrane proteins thus may be able to nucleate AP-1/clathrin coats, as has also been proposed by Springer and Schekman (1998).
ACKNOWLEDGMENTS
We thank Drs. Stuart Kornfeld, Jeffrey Gordon, Richard Kahn, and Kitaru Suda for useful reagents; Dr. Ralf Heilker for preliminary experiments; Thierry Mini for mass spectrometry analysis; and Dr. Hans-Peter Hauri for critically reading the manuscript. This work was supported by grant 31–061579.00 from the Swiss National Science Foundation (to M.S.) and by a Prof. Max Cloëtta fellowship (to J.R.).
Abbreviations used:
- AP
adaptor protein
- ARF1
ADP-ribosylation factor 1
- ER
endoplasmic reticulum
- GEF
guanine nucleotide exchange factor
- GMP-PNP
guanylyl imidodiphosphate
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- Lamp-1
lysosome-associated membrane protein-1
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PIP
phosphoinositide
- PS
phosphatidylserine
- TGN
trans-Golgi network
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0309. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0309.
REFERENCES
- Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. doi: 10.1021/bi962252b. [DOI] [PubMed] [Google Scholar]
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- Austin C, Hinners I, Tooze SA. Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J Biol Chem. 2000;275:21862–21869. doi: 10.1074/jbc.M908875199. [DOI] [PubMed] [Google Scholar]
- Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Söllner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Campbell C, Squicciarini J, Shia M, Pilch PF, Fine RE. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984;23:4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, De Matteis MA. Inositol lipids as spatial regulators of membrane traffic. J Membr Biol. 2001;180:187–194. doi: 10.1007/s002320010069. [DOI] [PubMed] [Google Scholar]
- DeLuca-Flaherty C, McKay DB. Nucleotide sequence of the cDNA of a bovine 70 kilodalton heat shock cognate protein. Nucleic Acids Res. 1990;18:5569. doi: 10.1093/nar/18.18.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittié AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Zhu Y, Kornfeld S. The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol Biol Cell. 2000;11:3723–3736. doi: 10.1091/mbc.11.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio RJ, Jackson-Machelski E, Heuckeroth RO, Olins PO, Devine CS, Yonemoto W, Slice LW, Taylor SS, Gordon JI. Protein N-myristoylation in Escherichia coli: reconstitution of a eukaryotic protein modification in bacteria. Proc Natl Acad Sci USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Franco M, Chardin P, Chabre M, Paris S. Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J Biol Chem. 1995;270:1337–1341. doi: 10.1074/jbc.270.3.1337. [DOI] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-γ-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Haucke V, De Camilli P. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science. 1999;285:1268–1271. doi: 10.1126/science.285.5431.1268. [DOI] [PubMed] [Google Scholar]
- Heilker R, Manning-Krieg U, Zuber JF, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- Heilker R, Spiess M, Crottet P. Recognition of sorting signals by clathrin adaptors. BioEssays. 1999;21:558–567. doi: 10.1002/(SICI)1521-1878(199907)21:7<558::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Helms JB, Palmer DJ, Rothman JE. Two distinct populations of ARF bound to Golgi membranes. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höning S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Höning S, Sosa M, Hille-Rehfeld A, von Figura K. The 46-kDa mannose 6-phosphate receptor contains multiple binding sites for clathrin adaptors. J Biol Chem. 1997;272:19884–19890. doi: 10.1074/jbc.272.32.19884. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. BioEssays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Iacopetta BJ, Rothenberger S, Kühn LC. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988;54:485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1, and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Keen JH. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1989;105:1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird V, Spiess M. A novel assay to demonstrate an intersection of the exocytic and endocytic pathways at early endosomes. Exp Cell Res. 2000;260:340–345. doi: 10.1006/excr.2000.5006. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JO, Kornfeld S. Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J Biol Chem. 1997;272:4141–4148. doi: 10.1074/jbc.272.7.4141. [DOI] [PubMed] [Google Scholar]
- Liang JO, Sung T-C, Morris AJ, Frohman MA, Kornfeld S. Different domains of mammalian ADP-ribosylation factor 1 mediate interaction with selected target proteins. J Biol Chem. 1997;272:33001–33008. doi: 10.1074/jbc.272.52.33001. [DOI] [PubMed] [Google Scholar]
- Lindner R, Ungewickell E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J Biol Chem. 1992;267:16567–16573. [PubMed] [Google Scholar]
- Mallet WG, Brodsky FM. A membrane-associated protein complex selective binding to the clathrin coat adaptor AP1. J Cell Sci. 1996;109:3059–3068. doi: 10.1242/jcs.109.13.3059. [DOI] [PubMed] [Google Scholar]
- Martín ME, Hidalgo J, Rosa JL, Crottet P, Velasco A. Effect of protein kinase A activity on the association of ADP-ribosylation factor 1 to Golgi membranes. J Biol Chem. 2000;275:19050–19059. doi: 10.1074/jbc.275.25.19050. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Morimitsu Y, Uchida K, Schekman R. Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol Cell. 1998a;2:703–708. doi: 10.1016/s1097-2765(00)80168-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998b;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Miller K, Shipman M, Trowbridge IS, Hopkins CR. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65:621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ohno H, et al. μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized madin-darby canine kidney cells. J Biol Chem. 2000;275:15207–15219. doi: 10.1074/jbc.275.20.15207. [DOI] [PubMed] [Google Scholar]
- Peden AA, Park GY, Scheller RH. The di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J Biol Chem. 2001;276:49183–49187. doi: 10.1074/jbc.M106646200. [DOI] [PubMed] [Google Scholar]
- Perrot M, Sagliocco F, Mini T, Monribot C, Schneider U, Shevchenko A, Mann M, Jeno P, Boucherie H. Two-dimensional gel protein database of Saccharomyces cerevisiae (update 1999) Electrophoresis. 1999;20:2280–2298. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2280::AID-ELPS2280>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the β chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Harter C, Bremser M, Brugger B, Sohn K, Helms JB, Wieland F. Receptor-induced polymerization of coatomer. Proc Natl Acad Sci USA. 1999;96:1224–1228. doi: 10.1073/pnas.96.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Schlossman DM, Schmid SL, Braell WA, Rothman JE. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ, Sowerby PJ, Robinson MS. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J Biol Chem. 1996;271:25446–25451. doi: 10.1074/jbc.271.41.25446. [DOI] [PubMed] [Google Scholar]
- Shinotsuka C, Yoshida Y, Kawamoto K, Takatsu H, Nakayama K. Overexpression of an ADP-ribosylation factor-guanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J Biol Chem. 2002;277:9468–9473. doi: 10.1074/jbc.M112427200. [DOI] [PubMed] [Google Scholar]
- Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Stephens DJ, Banting G. Specificity of interaction between adaptor-complex medium chains and the tyrosine-based sorting motifs of TGN38 and lgp120. Biochem J. 1998;335:567–572. doi: 10.1042/bj3350567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DJ, Crump CM, Clarke AR, Banting G. Serine 331 and tyrosine 333 are both involved in the interaction between the cytosolic domain of TGN38 and the μ2 subunit of the AP2 clathrin adaptor complex. J Biol Chem. 1997;272:14104–14109. doi: 10.1074/jbc.272.22.14104. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Südhof TC, Anderson RGW. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Zhao L, Helms JB, Brügger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland FT. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit β. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Drake MT, Kornfeld S. ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc Natl Acad Sci USA. 1999a;96:5013–5018. doi: 10.1073/pnas.96.9.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Traub LM, Kornfeld S. High-affinity binding of the AP-1 adaptor complex to trans-Golgi network membranes devoid of mannose 6-phosphate receptors. Mol Biol Cell. 1999b;10:537–549. doi: 10.1091/mbc.10.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]