Abstract
Phospholipase Ds (PLDs) are regulated enzymes that generate phosphatidic acid (PA), a putative second messenger implicated in the regulation of vesicular trafficking and cytoskeletal reorganization. Mast cells, when stimulated with antigen, show a dramatic alteration in their cytoskeleton and also release their secretory granules by exocytosis. Butan-1-ol, which diverts the production of PA generated by PLD to the corresponding phosphatidylalcohol, was found to inhibit membrane ruffling when added together with antigen or when added after antigen. Inhibition by butan-1-ol was completely reversible because removal of butan-1-ol restored membrane ruffling. Measurements of PLD activation by antigen indicate a requirement for continual PA production during membrane ruffling, which was maintained for at least 30 min. PLD1 and PLD2 are both expressed in mast cells and green fluorescent protein-tagged proteins were used to identify PLD2 localizing to membrane ruffles of antigen-stimulated mast cells together with endogenous ADP ribosylation factor 6 (ARF6). In contrast, green fluorescent protein-PLD1 localized to intracellular vesicles and remained in this location after stimulation with antigen. Membrane ruffling was independent of exocytosis of secretory granules because phorbol 12-myristate 13-acetate increased membrane ruffling in the absence of exocytosis. Antigen or phorbol 12-myristate 13-acetate stimulation increased both PLD1 and PLD2 activity when expressed individually in RBL-2H3 cells. Although basal activity of PLD2-overexpressing cells is very high, membrane ruffling was still dependent on antigen stimulation. In permeabilized cells, antigen-stimulated phosphatidylinositol(4,5)bisphosphate synthesis was dependent on both ARF6 and PA generated from PLD. We conclude that both activation of ARF6 by antigen and a continual PLD2 activity are essential for local phosphatidylinositol(4,5)bisphosphate generation that regulates dynamic actin cytoskeletal rearrangements.
INTRODUCTION
Phospholipase D (PLD) catalyzes the hydrolysis of phosphatidylcholine (PC), the major membrane phospholipid, to produce the putative lipid second messenger phosphatidic acid (PA) and the water-soluble choline. Signaling through PLD occurs downstream of both G protein-coupled receptors and receptor tyrosine kinases and has been implicated in multiple physiological events, including the budding of coated vesicles (Ktistakis et al., 1996; Chen et al., 1997; Kun et al., 1997; Siddhanta and Shields, 1998), regulated exocytosis (Stutchfield and Cockcroft, 1993; Fensome et al., 1996; Morgan et al., 1997; Brown et al., 1998a; Caumont et al., 1998; Way et al., 2000), endocytosis (Shen et al., 2001), superoxide generation (Palicz et al., 2000), and stress fiber formation (Cross et al., 1996; Kam and Exton, 2001).
Two isoforms of PLD have been described in mammalian cells, PLD1 and PLD2, both of which exist as two splice variants. PLD1 activity is low and can be up-regulated by multiple inputs, including ADP ribosylation factor (ARF) proteins, Rho family of GTPases, and protein kinase Cα/β with a requirement for phosphatidylinositol(4,5)bisphosphate [PI(4,5)P2] as a cofactor. In contrast to PLD1, PLD2, when assayed in vitro in the presence of PI(4,5)P2, has high basal activity that is reduced by binding to α-actinin or β-actin (Park et al., 2000; Lee et al., 2001). Moreover, PLD2 can be stimulated by two of the three regulators of PLD1, ARF proteins, and protein kinase C. PLD2 can be stimulated 30-50% in vitro with ARF proteins, and this stimulation is more pronounced when the high basal activity is reduced through removal of the N-terminal 308 amino acids (Lopez et al., 1998; Sung et al., 1999; Divecha et al., 2000). ARF is still able to activate PLD2 when bound to α-actinin or β-actin, thus the high basal activity of PLD2 seen in the presence of PI(4,5)P2 may be kept in check by cytoskeletal proteins and availability of PI(4,5)P2 within the vicinity of PLD2 (Lee et al., 2001). Phorbol 12-myristate 13-acetate (PMA) also increases PLD2 activity in cells and protein kinase Cα has been shown to directly associate with protein kinase C in vitro (Siddiqi et al., 2000; Han et al., 2002). An additional regulator of PLD2 activity is oleic acid that is not shared by PLD1 (Kim et al., 1999; Laine et al., 2000). Most cells seem to express both isoforms of PLDs with some exceptions. For example HL60 cells seem to express only PLD1, whereas L210 cells seem to express PLD2 only (Kim et al., 1999).
PI(4,5)P2 has recently emerged as a signaling molecule in addition to its well-established role as a substrate for phospholipase C and phosphoinositide 3-kinases. The intact PI(4,5)P2 molecule is a central player in actin dynamics, exocytosis, and endocytosis due to its ability to bind and regulate many proteins containing PI(4,5)P2 recognition domains such as pleckstrin homology domains, basic patches, and epsin N-terminal homology domains (Martin, 1998; Cockcroft and De Matteis, 2001). ARF proteins have been recently identified to control the levels of PI(4,5)P2 either by directly activating phosphatidylinositol 4-phosphate 5-kinase (PIP5K) or indirectly by increasing PA through PLD activation or both (Fensome et al., 1996; Honda et al., 1999; Jones et al., 2000; Way et al., 2000; Skippen et al., 2002). In vitro, PA can stimulate PIP5K activity when vesicles composed of PI(4)P are used, whereas ARF proteins can directly activate PIP5K when vesicles composed of PC:PI(4)P (9:1) are used (Honda et al., 1999; Jones et al., 2000). Additionally, PA-stimulated PIP5K activity could be further activated by ARF proteins when PI(4)P vesicles were used. Because in vitro studies do not reflect the cellular lipid environment, we have recently investigated the mechanism of PI(4,5)P2 synthesis by ARF proteins in permeabilized HL60 cells by using guanosine-5′-O-(3-thio)triphosphate (GTPγS) as a stimulus (Skippen et al., 2002). By using an ARF1 point mutant (N52R) that was impaired for stimulating PLD activity but not PIP5K activity in vitro, we concluded that direct activation of PIP5K by ARF and via PA derived from the ARF-stimulated PLD made an equal contribution to PI(4,5)P2 synthesis when GTPγS was used as a stimulus. Although the above-mentioned studies put PLD activation upstream to the activation of PIP5K, Divecha et al. (2000) suggested that PI(4,5)P2 produced by PIP5K is essential for the downstream activation of PLD2.
Engagement of the high-affinity IgE receptor with antigen in mast cells stimulates a number of lipid-signaling events, which include the activation of phospholipase Cγ, phosphoinositide 3-kinase, and PLD. These events result in granule exocytosis as well as a dramatic reorganization of the actin cytoskeleton with membrane ruffling being a very prominent feature (Barker et al., 1995). This membrane ruffling, as in many other cell types, is dependent on Rac1 GTPase (Ridley et al., 1992; Guillemot et al., 1997) and requires the presence of gelsolin, and WAVE, a Wiskott-Aldrich syndrome protein-family protein, which recruits G-actin through profilin (Miki et al., 1998; Azuma et al., 2000). We report that antigen-induced formation of lamellipodia and membrane ruffles in RBL mast cells is exquisitely dependent on continual PLD activation and, therefore, PA production. Although the activity of both PLD1 and PLD2 is regulated by antigen, only PLD2 was found with ARF6 and PI(4,5)P2 in membrane ruffles induced by antigen. PLD1 was localized to an intracellular lysosomal/endosomal compartment, and its translocation to the plasma membrane was not required for membrane ruffling. In permeabilized cells, synthesis of PI(4,5)P2 by antigen was dependent on both ARF proteins and on PLD activity. We propose that the local availability of PA and ARF6 are both required for regulating the synthesis of PI(4,5)P2 during dynamic membrane ruffling.
MATERIALS AND METHODS
Immunofluorescence Staining of Actin Cytoskeleton
RBL mast cells were grown and maintained in culture in DMEM supplemented with 10% fetal calf serum as described previously (Way et al., 2000). For the immunostaining of the cytoskeleton, the cells were plated on glass-bottomed dishes (Willco, Intracel, Royston, Herts, United Kingdom). The cells were then left to recover for approximately 6 h before the medium was replaced with fresh DMEM containing 0.5 μg/ml anti-dinitrophenol (DNP)-IgE and incubated overnight to sensitize the cells.
The cells were washed with HEPES buffer (20 mM HEPES, pH 7.2, 137 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mg/ml glucose) and stimulated with antigen (40 ng of DNP-HSA/ml) at 37°C for 10 min. For labeling of total cellular F-actin, cells were first fixed in 3% paraformaldehyde and then permeabilized with 80 μg/ml lyso-PC and incubated with 0.5 μM tetramethylrhodamine B isothiocyanate (TRITC)-phalloidin for 20 min at room temperature. The cells were then washed three times with phosphate-buffered saline and coverslips were mounted. Immunofluorescence was viewed with a confocal microscope (PerkinElmer Lifesciences, Zaventem, Belgium) by using a 100× oil immersion objective. Images were acquired using a charge-coupled device camera (PerkinElmer) cooled to −35°C. Localization of TRITC-phalloidin in single cells was captured by exciting the cells at 510 nm and taking sequential laser confocal slices (0.5 μm apart) through the whole of the cells. Random fields of control and stimulated cells were taken with a 40× oil immersion lens, and sequential confocal slices were taken from the top of the cell to the adhesion plane. The total number of cells was counted from 10 random fields and was categorized according to whether or not the cell was ruffled.
Imaging of Live Cells by Using Nomarski Optics
RBL-2H3 cells were plated on glass-bottomed dishes (39 mm in diameter; Willco) and primed overnight with anti-DNP-IgE. The cells were washed in warmed (37°C) HEPES buffer and placed on a heated stage and kept at 37°C throughout the experiment. The cells were viewed on a microscope (Olympus) by using a 100× oil immersion objective. Bright field images of the cells were acquired over a 30-min period every 10 s by using Nomarski phase contrast optics with a charge-coupled device camera (PerkinElmer) cooled to −35°C. The sequences of images were exported as audio-visual interleaves, and individual frames were selected as shown in the relevant figures.
Measurement of Phospholipase D Activity
RBL cells (500 μl, 105 cells/well) were seeded onto 24-well plates and primed overnight with anti-DNP-IgE (0.5 μg/ml) in DMEM. The following day the medium was discarded and replaced with 500 μl of DMEM containing 3 μCi/ml [3H]myristic acid for 1 h to label the cells. With this protocol the major phospholipid that is labeled is PC. The medium was discarded, and the cells were washed with HEPES buffer. Buffer (400 μl) was added to the cells, followed by butan-1-ol (0.5% final) and antigen in a final incubation volume of 500 μl. Butan-1-ol was only added when phosphatidylbutanol (PBut) was monitored as a measure of PLD activity. In experiments where PA was monitored, butan-1-ol was excluded. Antigen was present for the indicated times. At the end of the incubation, the medium was removed and replaced with 500 μl of ice-cold methanol:concentrated HCl (98:2). The cells were scraped and transferred to a clean tube. The wells were rinsed with 500 μl of methanol and combined with the cells. Chloroform (1 ml) was added followed by 1 ml of water. Unlabeled PBut and PA were added to the cells during the extraction, which allowed for subsequent detection by staining of the thin layer chromatography (TLC) plate with iodine. After phase separation, the lower chloroform phase was transferred to separate tubes, dried down, and the lipids resuspended in 15 μl of chloroform. For analysis of PA (Figures 2B and 7A), the lipid samples were analyzed by two-dimensional TLC exactly as described previously (Cockcroft, 1984). The first dimension for TLC analysis was chloroform:methanol:28% ammonia (65:35:5) and the second dimension was chloroform:methanol:acetic acid:water (75:45:3:1). The counts in PA and in PC were monitored, and the results are presented as a percentage of disintegrations per minute in PC. PBut was separated by one-dimensional TLC as described previously (Whatmore et al., 1996). The TLC plates were also imaged using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) in some experiments to confirm the location of the lipids. The silica containing PBut and PC was excised and counted in the scintillation counter, and the PBut and PA results are presented as a percentage of disintegrations per minute in PC. For the experiment shown in Figure 2D, the cells were stimulated with antigen for 10 min, or 20 min in the presence of 0.5% butan-1-ol. In the samples (denoted with a star), the cells were stimulated with antigen for 20 min, but butan-1-ol was only present for the last 10 min of the incubation.
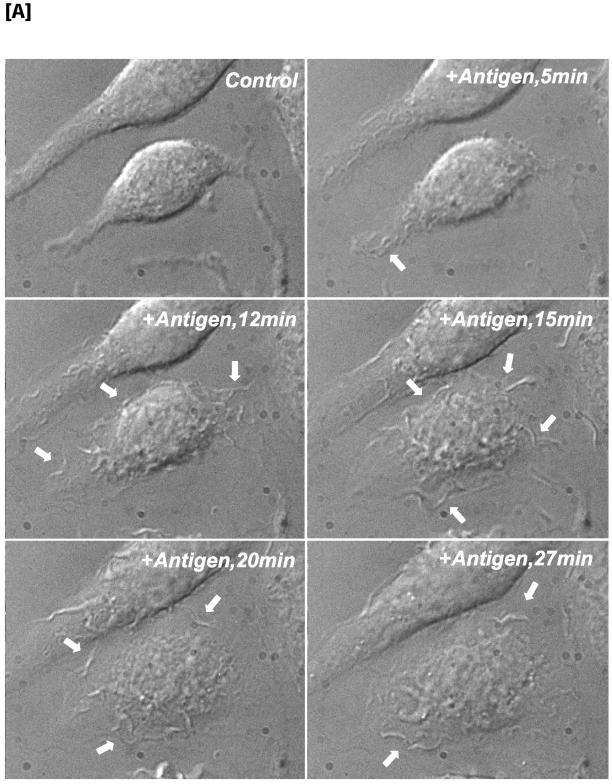
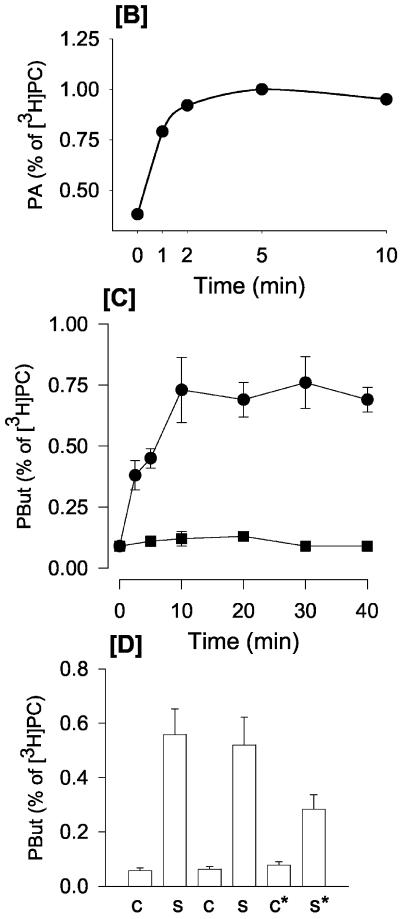
Figure 2.
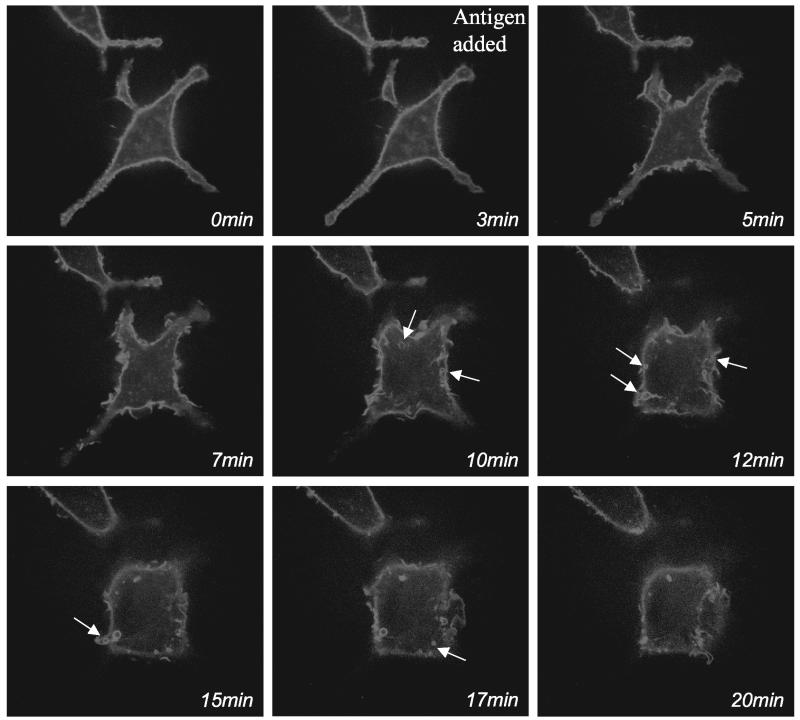
Time-dependent stimulation of membrane ruffling compared with activation of PLD activity by antigen. (A) Phase contrast, time-lapse recording of antigen-stimulated RBL mast cells, demonstrating that membrane ruffling is continuous for at least 30 min. White arrows indicate sites of ruffling. (B) Time course of PLD activation in antigen-stimulated RBL mast cells as monitored by PA formation. (C) Time course of PLD activation in antigen-stimulated RBL mast cells as monitored by PBut formation in the presence of 0.5% butan-1-ol. (D) Determination of ongoing PLD activity: RBL mast cells were incubated with butan-1-ol for 10 or 20 min with and without antigen (c, control and s, stimulated). c20* and s20*, cells incubated with buffer or antigen for 20 min with butan-1-ol present during the last 10 min.
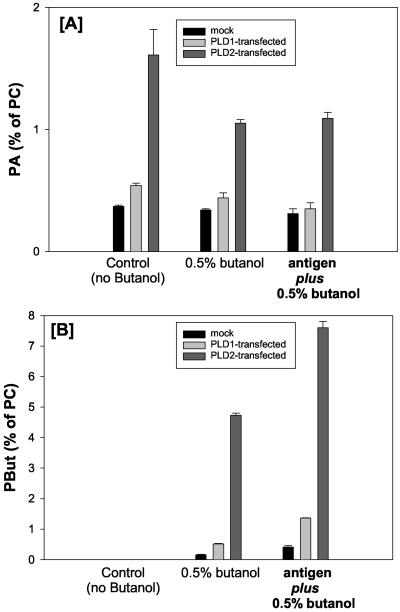
Figure 7.
Measurements of PA and PBut in PLD1- and PLD2-overexpressing RBL mast cells before and after stimulation with antigen. Cells transfected with GFP-PLD2 were stimulated with antigen for 20 min in the presence and absence of butan-1-ol. The samples were analyzed by two-dimensional TLC to separate PA (A) and PBut (B). In the absence of butan-1-ol, PA levels are enhanced fourfold in PLD2-transfected cells. Presence of butan-1-ol decreases the PA levels, and this is accompanied by a pronounced increase in PBut (note the scale for PA and PBut is different), illustrating that PLD activity is high but does not result in accumulation of PA. In the presence of antigen and butano-1-ol, levels of PA are not elevated further, although an increase in PBut is clearly observed.
Electroporation of RBL-2H3 Cells for Transient Transfection and Fluorescent Imaging of Green Fluorescent Protein (GFP)-Fusion Proteins
RBL mast cells were transiently transfected by electroporation. The cells were scraped and washed in a HEPES buffer, and the washed cells were then resuspended in 400 μl of the HEPES buffer without calcium and placed into the electroporation cuvette with 20-50 μg of plasmid DNA. After two electroporation pulses each of ∼5 ms at a voltage of 0.5 kV and a resistance of 125 Ohms, the cuvettes were placed on ice for 5 min. The cells were finally resuspended in DMEM (supplemented with 10% fetal calf serum) and plated on glass-bottomed dishes (Willco) for imaging or in six-well plates for measurements of PLD activity. The cells were then left to recover for ∼6 h before the medium was replaced with fresh DMEM containing 0.5 μg/ml anti-DNP-IgE and incubated overnight to sensitize the cells.
For imaging, the cells were washed 24 h after electroporation with warmed (37°C) HEPES buffer and placed on a heated stage. Transfected cells expressing the enhanced green fluorescent protein (EGFP)-tagged protein were identified through a 100× oil immersion objective by using an epifluorescent system that excited the cells at 490 nm. Localization of the EGFP-tagged protein in single cells was captured by taking sequential laser confocal 0.5-μm slices through the cells from the adhesion plane to the top of the cell. Stacks were taken before stimulation and subsequently at 5-min intervals after stimulation with the antigen DNP-HSA. In the case of live cell recording of a single confocal plane of an EGFP-expressing cell over time, images were recorded using LSR Ultraview temporal module software (Perkin Elmer Lifesciences), which excited the cells at 490 nm and captured confocal images every 10 s over a 30-min period.
For assay of PLD activity, transfected cells were seeded onto six-well plates and analyzed exactly as described for untransfected cells.
Measurement of β-Hexosaminidase Release
RBL cells were seeded at 2 × 105 cells/well on a 24-well plate and primed overnight with 0.5 μg/ml anti-DNP-IgE. After washing with HEPES buffer cells were preincubated for 5 min at 37°C in the presence or absence of 10 nM PMA. Cells were incubated for 25 min in the presence or absence of 40 ng/ml antigen. Reactions were quenched on ice and the cells centrifuged at 2000 × g for 5 min at 4°C. An aliquot of the supernatant was analyzed for β-hexosaminidase as described previously (Way et al., 2000).
Detection of ARF Release from Permeabilized Cells
RBL cells were washed in HEPES buffer and resuspended at 107 cells/ml. Permeabilization was initiated with the addition of 0.4 IU/ml streptolysin O. At the required time points, 107 cells were removed and centrifuged at 2000 × g for 5 min at 4°C. Proteins in the supernatant (released proteins) were trichloroacetic acid precipitated, and the pellets were treated with RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.5). Cells (105 cells/lane) were run on SDS-PAGE, blotted, and probed with peptide-specific anti-ARF1 (1:750) and anti-ARF6 (1:3000) polyclonal antibodies (Skippen et al., 2002).
Reconstitution of ARF-stimulated PIP2 Synthesis in Permeabilized RBL Mast Cells
RBL mast cells were primed with anti-DNP-IgE for 3 h. After washing in permeabilization buffer (20 mM PIPES, pH 7.2, 137 mM NaCl, 3 mM KCl, 2 mM MgCl2, 1 mg/ml glucose), the cells were permeabilized with 0.4 IU of streptolysin-O in suspension in the presence of calcium buffered at pCa 7 and MgATP (1 mM). After 10 min at 37°C, the cells were washed to remove the leaked cytosolic contents. Aliquots (50 μl) of the permeabilized cells were transferred to tubes containing antigen (40 ng/ml), ARF6 (as indicated), 1 mM MgATP, 2 μCi of γ-[32P]ATP in a final incubation volume of 100 μl. After 20 min, the samples were quenched and analyzed for their PIP2 content as described previously (Skippen et al., 2002; Way et al., 2000).
RESULTS
Butanol Inhibits Antigen-mediated Membrane Ruffling Reversibly
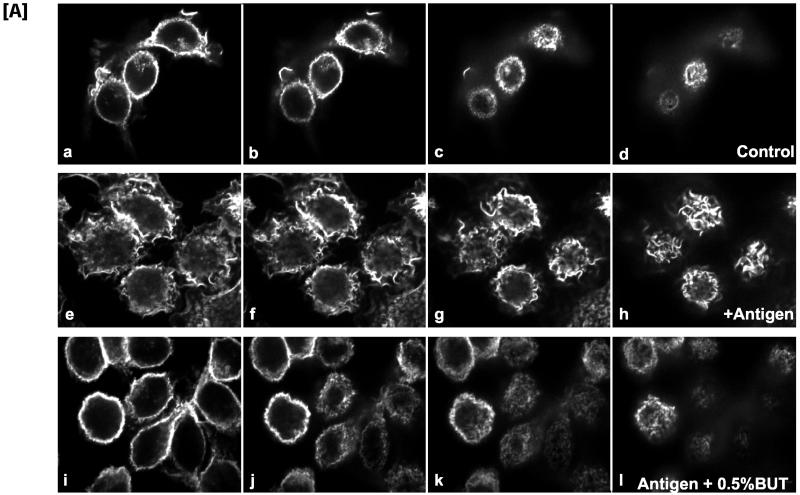
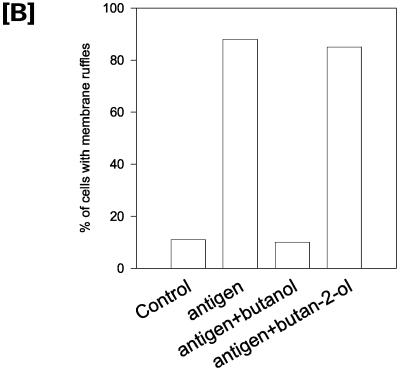
PLD hydrolyzes PC to produce PA and choline. The primary alcohol butan-1-ol can effectively replace water in this reaction to produce choline and PBut. The secondary alcohol butan-2-ol does not compete in this reaction and therefore acts as a suitable control for butan-1-ol–inhibited PA production. Using butan-1-ol, we investigated the possibility that membrane ruffling is dependent on PLD-derived PA. RBL mast cells were stimulated with antigen for 10 min in the presence and absence of 0.5% butan-1-ol (Figure 1A). In resting cells, the plasma membrane exhibits a smooth morphology with minimal membrane ruffling. F-actin, visualized by confocal microscopy after rhodamine-phalloidin staining, is localized exclusively to the cell cortex. FcεRI aggregation induces dramatic morphological changes, i.e., membrane ruffling and lamellipodial extensions that are accompanied by extensive F-actin reorganization. Confocal sectioning from the attachment plane through to the top of the cell revealed the recruitment of F-actin in membrane ruffles. This was completely inhibited when 0.5% butan-1-ol was added with antigen, whereas butan-2-ol was without effect (Figure 1B). A quantitative analysis was carried out where random fields of cells were imaged and confocal slices were obtained (Figure 1B). Under control conditions, ∼10% of the cells had a ruffled morphology. On antigen addition, >80% of the cells showed a ruffled morphology. The presence of 0.5% butan-1-ol inhibited antigen-stimulated ruffling, whereas butan-2-ol did not.
Figure 1.
Inhibition of antigen-induced membrane ruffling by butan-1-ol. (A) Confocal slices of RBL mast cells fixed and stained with TRITC-phalloidin before and after stimulation with antigen. Control cells (a–d), stimulated with antigen for 10 min (e–h), or stimulated with antigen for 10 min in the presence of 0.5% butan-1-ol (i–l). (B) Cells were treated with antigen, antigen plus 0.5% butan-1-ol, and antigen plus 0.5% butan-2-ol. For each condition, >250 cells were examined for their morphology. The results are from three separate experiments and are expressed as a percentage of total cells.
We characterized the duration of membrane ruffling and of PLD activation upon stimulation with antigen to provide an indication of the relationship between the two events. Once cells were activated, membrane ruffling was continuous for at least 30 min as observed by imaging live cells and observing their morphology by using Nomarski optics (Figure 2A). Membrane ruffling was very dynamic because new ruffles continuously formed and collapsed.
The time course of PLD activation by antigen was also examined under identical conditions by monitoring the formation of [3H]PA (Figure 2B). The RBL mast cells were labeled with [3H]myristate, which predominantly labels the PC pool (our unpublished data). PA levels increased promptly within the first minute of antigen addition and by 2 min a plateau was observed, and remained at this level for 10 min, the longest time examined. Because the plateau probably reflects increased formation coupled to increased degradation of PA, an alternative method to examine the time-dependent activation of PLD is to monitor the accumulation of the metabolically stable [3H]PBut. In the presence of butan-1-ol, [3H]PBut accumulation by antigen was linear for the first 10 min after which a plateau was observed (Figure 2C).
Because membrane ruffling was continuous over a 30-min period, whereas PLD activation reached an apparent plateau at 10 min, this would suggest that these two events were not tightly coupled. However, we were concerned that the method of monitoring PLD activation may not reflect the true events, because of the possibility that PA-stimulated PIP2 synthesis may be required to maintain continual PLD activation (see INTRODUCTION; Divecha et al., 2000). Because butan-1-ol inhibits PA formation, this would interfere with PIP2 synthesis and further PLD activation. Therefore, a different strategy was used. PLD activation was allowed to occur normally for 10 min in the absence of any butan-1-ol. Butan-1-ol was added subsequently at 10 min and the cells were incubated for an additional 10 min. If PLD was active during the 10-20-min interval, [3H]PBut would increase, and this is indeed the case (Figure 2D). Likewise, active PLD could also be monitored between the interval of 20-30 min when butan-1-ol addition was added at 20 min (our unpublished data).
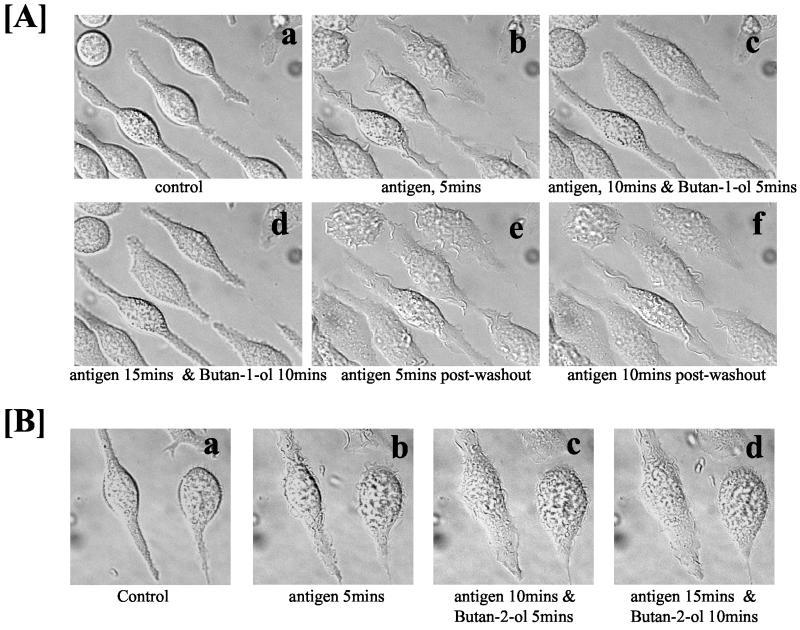
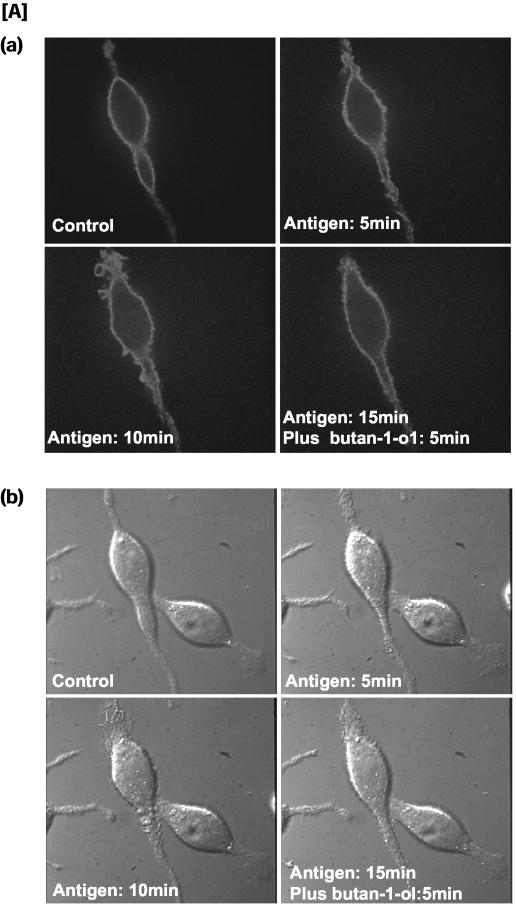
To examine that the continual production of PA during membrane ruffling was essential, butan-1-ol was added 5 min after membrane ruffling was initiated with antigen. Membrane ruffling came to a halt within 2 min and cells remained quiescent as long as the cells remained in contact with butan-1-ol (10 min in this experiment; Figure 3A, a–d). We next examined whether membrane ruffling would resume after butan-1-ol was removed. The medium containing the butan-1-ol was replaced with buffer without any butan-1-ol but with antigen, and recovery of membrane ruffling was observed (Figure 3A, e and f). It should be noted that accumulation of PBut during the initial 10-min period with antigen and butan-1-ol did not exert any inhibitory effects, confirming that it is PA that is the bioactive metabolite of PC hydrolysis, and PBut does not antagonize the effects of PA. Butan-2-ol, which is unable to participate in transphosphatidylation, was unable to inhibit membrane ruffling when added after 5 min. The cells continued to ruffle in the presence of butan-2-ol when examined 10 and 15 min later (Figure 3B, a–d).
Figure 3.
Butano-1-ol but not butan-2-ol inhibits membrane ruffling when added after membrane ruffling is initiated. Inhibition by butan-1-ol is reversible. (A) RBL mast cells were stimulated with antigen and bright field images of the cells were acquired over a 30-min period by using Nomarski phase contrast optics. Control cells (a), same cells imaged 5 min after antigen addition (b); at 5 min after antigen addition, butan-1-ol was added to halt membrane ruffling and the cells imaged at 10 min (c); the same cells imaged at 15 min to illustrate that membrane ruffling is still inhibited in the presence of butan-1-ol (d). At this point the cells were washed free of butan-1-ol and antigen added. Membrane ruffling 5 min after washout of butan-1-ol (e) and membrane ruffling still ongoing 10 min after washout of butan-1-ol (f). (B) Butan-2-ol does not inhibit membrane ruffling. Control cells (a); same field of cells after 5 min with antigen (b); 10 min with antigen and 5 min with butan-2-ol (c); 15 min with antigen and 10 min with butan-2-ol (d).
Antigen Stimulates Both PLD Isoforms but Only PLD2 Is Found in Membrane Ruffles upon Stimulation
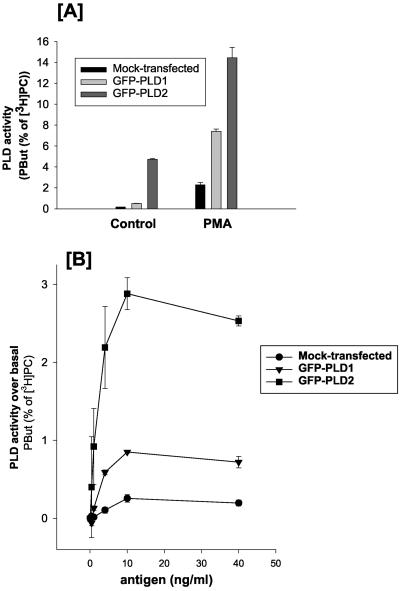
Two isoforms of PLD have been identified in mammalian cells, PLD1 and PLD2 (Liscovitch et al., 2000; Cockcroft, 2001). To examine whether both PLD1 and PLD2 are stimulated by antigen, we transfected RBL mast cells with GFP-PLD1 or GFP-PLD2 and subsequently stimulated the cells with either PMA or with antigen. Cells transfected with PLD1 or with PLD2 show an increase in basal activity, with PLD2-transfected cells showing a much greater increase compared with PLD1-transfected cells. When cells were stimulated with PMA (Figure 4A) or with antigen (Figure 4B), both PLD1- and PLD2-transfected cells show increased PLD activity compared with mock-transfected cells.
Figure 4.
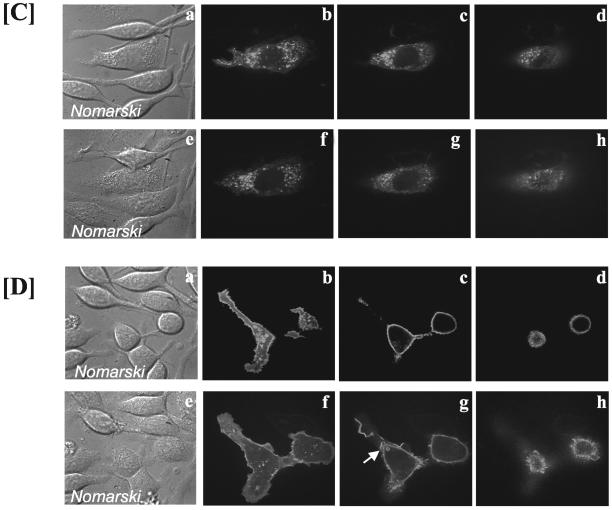
Antigen or PMA can regulate the activity of PLD1 and PLD2 in RBL mast cells. (A) Basal and PMA-stimulated PLD activity measured by formation of PBut is enhanced in GFP-PLD1– and GFP-PLD2–transfected cells. PMA, 100 nM for 20 min. (B) Antigen stimulates PLD activity as measured by formation of PBut in a concentration-dependent manner. The cells were stimulated for 20 min with antigen. (C) PLD1-transfected RBL mast cells: Top, control cells and bottom, same field of cells after stimulation with antigen for 10 min. The Nomarski image (a) of the field of cells is shown together with three confocal slices, showing the adhesion plane (b), the midplane (c), and the top of the cell (d). PLD1 localizes mainly to intracellular vesicles in unstimulated cells, and this pattern of staining is unchanged after 10 min of stimulation. No change in staining was observed at 5 min or 20 min poststimulation either. (D) PLD2-transfected RBL mast cells: Top, control cells and bottom, same field of cells after stimulation with antigen for 10 min. The Nomarski image (a) of the field of cells is shown together with three confocal slices, showing the adhesion plane (b), the midplane (c), and the top of the cell (d). PLD2 localizes to the plasma membrane and upon antigen addition, PLD2 is found in membrane ruffles and in pinocytic vesicles (indicated by arrow) that accompany membrane ruffling.
The overexpressed PLD proteins were localized by confocal microscopy in live cells, and PLD1 was found predominantly in vesicular structures in resting cells (Figure 4C, b–d), and upon stimulation with antigen, the localization of PLD1 was not altered dramatically (Figure 4C, f–h). From the Nomarski images, it is obvious that the cells were activated by antigen (Figure 4C, compare a and e). In contrast to PLD1, PLD2 was found exclusively at the plasma membrane in the resting cells (Figure 4D, b–d) and after antigen stimulation, PLD2 was found in membrane ruffles and in intracellular vesicles (Figure 4D, f–h). The PLD2-overexpressing cells were imaged live for 20 min after stimulation with antigen (Figure 5). Despite the increase in basal PLD activity (Figure 4A), the cells did not show any changes in morphology in the absence of antigen. Membrane ruffling was clearly evident within 2 min of stimulation with antigen and was maintained for at least 20 min. At 10 min postantigen, circular structures containing PLD2 could also be observed (Figure 5, white arrows). Transient transfection of PLD2 did not influence the pattern or the duration of membrane ruffling when the cells were compared with their neighboring nontransfected population (Figure 6B, c and d).
Figure 5.
Membrane ruffling in PLD2-transfected cells stimulated with antigen. GFP-PLD2–transfected RBL mast cells were imaged live at 37°C. Still images from time-lapse recording are shown. Arrows point to PLD2-containing circular structures forming over time.
Figure 6.
Membrane ruffling in PLD2-transfected cells is sensitive to inhibition by butan-1-ol but not butan-2-ol. (A, a) PLD2-transfected cell. The cells were stimulated with antigen for 10 min (images at 5 and 10 min postantigen are shown) to illustrate the pattern of membrane ruffling. Butan-1-ol (0.5%) was added at 10 min and the cells were imaged for another 5 min. Membrane ruffling was inhibited upon addition of butan-1-ol. (b) Same field of cells as observed by Normaski optics. (B, c) PLD2-transfected cell. The cells were stimulated with antigen for 10 min (images at 5 and 10 min postantigen are shown) to illustrate the pattern of membrane ruffling. Butan-2-ol (0.5%) was added at 10 min, and the cells were imaged for another 10 min. Membrane ruffling was unaffected by the presence of 0.5% butan-2-ol. (d) Same field of cells as observed by Normaski optics. Note that membrane ruffling of the transfected cells is similar to the membrane ruffling observed in the nontransfected counterparts.
Transfection with PLD2 leads to an increase in basal PLD activity but does not result in an increase in basal membrane ruffling. This is most likely due to the need to activate other signaling events, which are stimulated by antigen in a PA-independent manner. In the PLD2-transfected cells, stimulation by antigen leads to a seven to eightfold increase in PBut compared with the mock-transfected cells (Figure 4B). Because transphosphatidylation is always incomplete as there is always a large concentration of water still available for some PA to be produced (Skippen et al., 2002), we anticipated that in PLD2-transfected cells, even in the presence of butanol, sufficient PA would still be produced to make membrane ruffling resistant to inhibition by butanol. Contrary to our expectations, addition of 0.5% butan-1-ol completely inhibited ongoing formation of membrane ruffles in PLD2-overexpressing cells (Figure 6A, a and b). This was not the case when butan-2-ol was added (Figure 6B, c and d). Again, circular structures containing PLD2 are clearly visible (Figure 6B, c).
The inhibition of membrane ruffling in PLD2-transfected cells by butan-1-ol prompted us to examine the levels of PA in resting and in stimulated cells in the presence of butan-1-ol. In PLD2-transfected cells, basal levels of PA were fourfold higher compared with mock-transfected cells (Figure 7A). The absolute increase in PA in these cells is 1.2% of total PC. For comparison, the increase in PA in PLD1-transfected cells is shown. Addition of butan-1-ol for 20 min significantly decreases the PA levels in the PLD2-transfected cells and the absolute increase is now 0.7% (Figure 7A). This is now accompanied by an absolute increase in PBut levels of 4.7% (Figure 7B). Thus, the combined increased in PA plus PBut in PLD2-transfected cells is 5.4% of total PC monitored over a 20-min period. Thus, in the PLD2-transfected cells, there is a high basal activity of PLD2 but that is not reflected in the accumulation of PA. The PA levels have stabilized at 4 times the level of mock-transfected cells compared with the 30-fold increase seen if PBut is measured. Thus, although PLD2-transfected cells have a high level of PLD activity, they must compensate by increasing the rate of PA removal. In the PLD2-transfected cells, after stimulation with antigen, PLD activity is further enhanced as seen in the increase in PBut with no further accumulation in PA. Thus, PLD2-transfected cells, despite exhibiting enhanced PLD activity, remain sensitive to the presence of butan-1-ol because PA levels are still tightly regulated.
Membrane Ruffling Is Not Dependent on Exocytosis of Secretory Granules
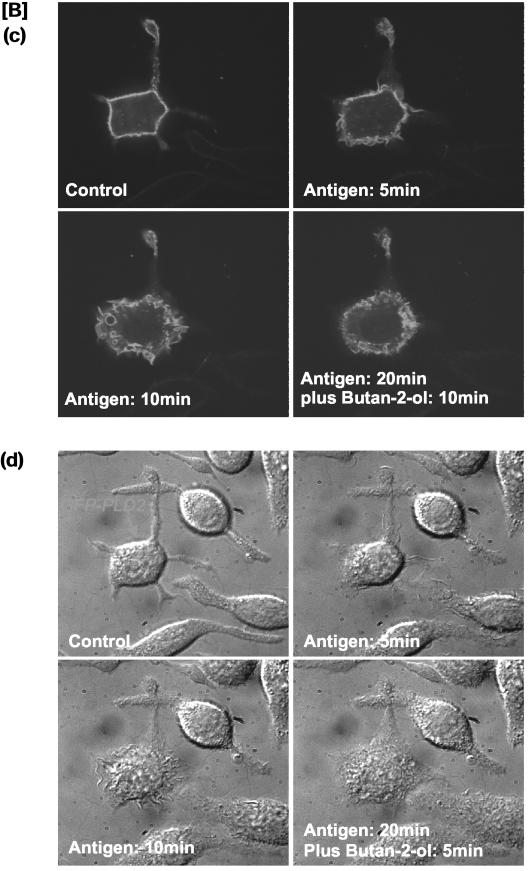
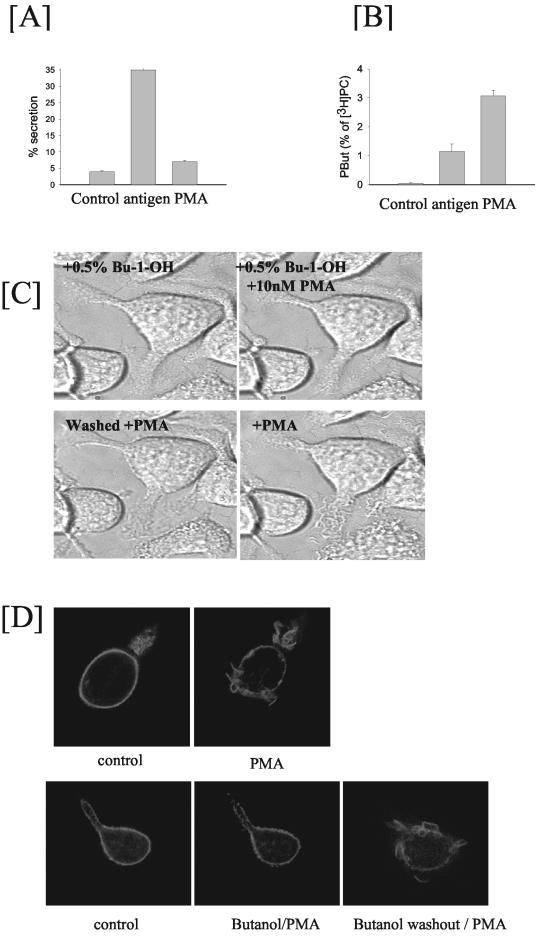
Antigen stimulation of mast cells leads to the concomitant activation of membrane ruffling and of exocytosis of secretory granules. Secretion of hexosaminidase, a marker of secretory granules, is initiated within 1 min and is completed by 10 min (our unpublished data). Typically, maximal secretion of hexosaminidase with antigen is between 25 and 35%, which is dependent on the passage number of the cells. It has been reported previously that PLD1 is localized to the secretory granules, and it completely translocates to the plasma membrane during antigen stimulation (Brown et al., 1998a). As shown in Figure 4C, PLD1 is localized to vesicular structures and upon stimulation no translocation was observed when cells were imaged live for >20 min (data for 10 min shown in Figure 4C). It is possible that some translocation did occur but the signal at the plasma membrane was too diffuse for us to monitor any change. Nonetheless, we examined whether exocytosis of secretory granules and hence translocation of PLD1 to the plasma membrane was required for membrane ruffling. We used PMA, which stimulates PLD activity but not exocytosis of secretory granules (Figure 8, A and B). PMA induces membrane ruffling in nontransfected (Figure 8C) and in PLD2-transfected cells (Figure 8D), which are sensitive to butan-1-ol (Figure 8, C and D). Like the antigen response, removal of butanol allows membrane ruffling to resume, indicating that membrane ruffling is dependent on ongoing PLD activation.
Figure 8.
PMA stimulates membrane ruffling but not exocytosis in RBL mast cells. (A) Antigen but not PMA stimulates release of hexosaminidase. (B) Antigen and PMA both stimulate PLD activity. (C) Butan-1-ol inhibits PMA-induced membrane ruffling. Cells were incubated with 0.5% butan-1-ol. PMA (10 nM) was subsequently added to the cells to stimulate membrane ruffling. No membrane ruffling was observed as long as butan-1-ol was present. After removal of butan-1-ol, membrane ruffling commenced. (D) Membrane ruffling stimulated in PLD2-transfected cells with PMA (top). Membrane ruffling is inhibited by butan-1-ol and can be restored upon washout of butan-1-ol (bottom). Butan-1-ol was washed out at 10 min after PMA addition.
Function of PA Derived from PLD Activation in Membrane Ruffling
Actin dynamics is dependent on the availability of PIP2, and previous studies have shown that antigen stimulation leads to an increase in PIP2 levels (despite the hydrolysis of PIP2 by phospholipase Cγ and conversion to PIP3 by phosphoinositide 3-kinase) (Apgar, 1995). PIP2 levels can be maintained by stimulation of the lipid kinases, in particular, the type I PIP5K. This enzyme can be stimulated by PA derived from PLD activation (indirect route), by ARF proteins (direct route), or by a combination of both routes (Honda et al., 1999; Jones et al., 2000; Skippen et al., 2002). We have recently shown that in permeabilized HL60 cells, ARF1 or ARF6 can stimulate PI(4,5)P2 synthesis, and both the direct and indirect routes are equally important when GTPγS was used as a stimulus. Because GTPγS irreversibly activates G proteins, the situation with regard to a receptor-directed stimulus may be different. This was therefore examined in antigen-stimulated, permeabilized RBL mast cells.
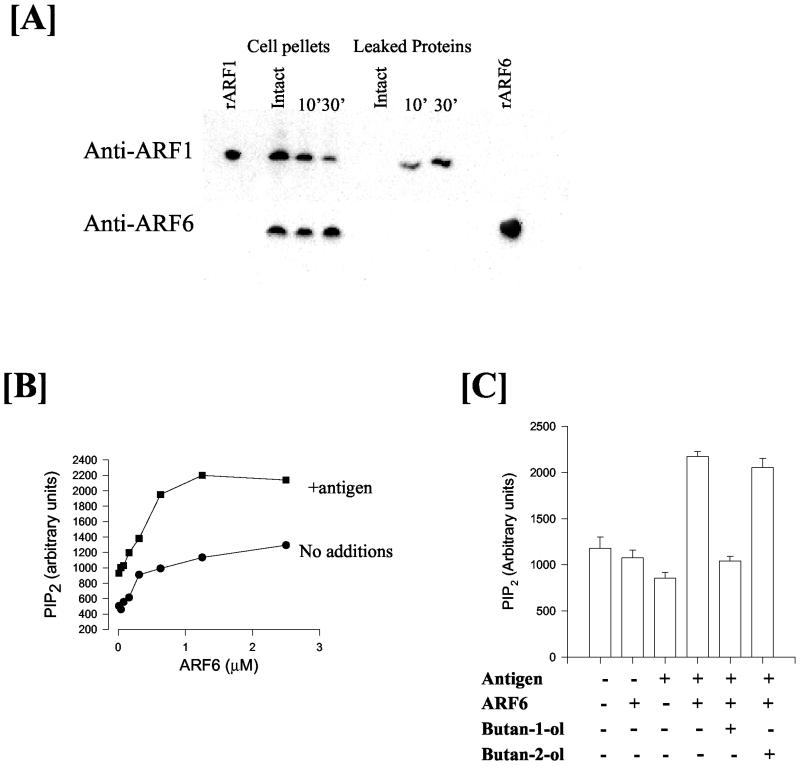
To examine the role of ARF proteins in maintaining PI(4,5)P2 levels, we used a reconstitution system with permeabilized RBL mast cells, which had lost the capacity to synthesize PI(4,5)P2 after stimulation with antigen. First, we examined whether both ARF1 and ARF6 would leak out of the cells, as shown previously for HL60 cells (Skippen et al., 2002). In contrast to HL60 cells, upon permeabilization, only ARF1 but not ARF6 was found to leak out of the cells (Figure 9A). Leakage of ARF6 presumably does not occur in RBL mast cells because of its association with intracellular structures. Despite the lack of leakage of ARF6, PI(4,5)P2 synthesis by antigen was inhibited, and this leakage could be due to the inability of ARF6-containing vesicles to fuse with the plasma membrane in the permeabilized cells. Nonetheless, antigen-stimulated PIP2 synthesis could be restored by readdition of either ARF6 (Figure 9B) or ARF1 (our unpublished data). The amount of ARF6 (or ARF1) that provided maximal synthesis of PI(4,5)P2 was 1 μM. The ARF6 used for these experiments is only partially myristoylated and thus overestimates the maximal requirement. To ensure that RBL mast cells would contain sufficient ARF6, we also estimated the relative concentration of endogenous ARF1 and ARF6 by using semiquantitative Western blotting. We estimate that ARF1 is ∼6-12 μM and ARF6 is 1-2 μM.
Figure 9.
ARF6 and PA are both required for the stimulation of PIP2 synthesis in antigen-stimulated RBL mast cells. (A) In streptolysin-O–permeabilized RBL mast cells, ARF1 but not ARF6 leaks out of cells. (B) Synthesis of PI(4,5)P2 by antigen is dependent on the addition of ARF6 proteins to permeabilized RBL mast cells. (C) ARF6 plus antigen-stimulated PIP2 synthesis requires PLD-generated PA production. Butan-1-ol but not butan-2-ol (0.5%) blocks PIP2 synthesis stimulated with antigen plus ARF6.
ARF1 was as effective in reconstituting PI(4,5)P2 synthesis as ARF6 (our unpublished data). ARF1 (and ARF6) also reconstitutes antigen-stimulated PLD activity under these conditions (Way et al., 2000). These data suggest that in the permeabilized cells, ARF specificity may be lost such that both exogenously added ARF1 or ARF6 is able to reconstitute PIP2 synthesis and PLD activation. This is similar to the in vitro situation where both ARF1 and ARF6 are able to activate PLD and PIP5K activity. To investigate whether the synthesis of PI(4,5)P2 was dependent on PLD activity or occurred independently of this pathway, we used butan-1-ol to suppress the production of PA. In Figure 9C, it is illustrated that antigen plus ARF6-stimulated PI(4,5)P2 increase is exquisitely sensitive to butan-1-ol but not butan-2-ol. From this, we conclude that ARF6 works together with PA to regulate PI(4,5)P2 levels in antigen-stimulated mast cells.
DISCUSSION
The actin cytoskeleton mediates a variety of essential processes in all eukaryotic cells, including cell motility, cell shape, phagocytosis, and cytokinesis. Three distinct kinds of actin-based structures have been identified, which are regulated by the Rho family of GTPases: Cdc42 induces filopodia, Rac regulates membranes ruffles and lamellipodia, and Rho regulates stress fiber formation (Hall, 1998). These GTPases exert their effects via specific effectors, some of which may have direct or indirect effects on lipid metabolism. In this study we provide evidence that in addition to the well-established role of Rac GTPase in membrane ruffling, there is an absolute requirement for the lipid-modifying enzyme PLD in mast cells upon stimulation with antigen. Interestingly, previous studies with endothelial cells had indicated that a PLD activity was required for Rho-dependent stress fiber formation by using lysophosphatidic acid as agonist (Cross et al., 1996). Additionally, PLD2, when overexpressed in rat embryo fibroblasts, was localized exclusively to the plasma membrane and induced irregular projections at the cellular edges and when stimulated with serum, PLD2 accumulated in restricted regions of the cell edge and redistributed to submembranous particles (Colley et al., 1997). More recently, PLD1 activity was required for actin stress fiber formation in fibroblasts (Kam and Exton, 2001). Furthermore, previous studies have suggested an interplay between Rho family and ARF family GTPases, e.g., the formation of stress fibers and focal adhesions in fibroblasts (RhoA and ARF1) (Norman et al., 1998) and the identification of an ARF6/Rac1 binding protein, POR1, involved in cytoskeletal rearrangements (D'Souza-Scharey et al., 1997).
Engagement of the high-affinity IgE receptor in mast cells elicits a rapid activation of PLD activity in addition to a number of other signaling events, including PLCγ activation and phosphoinositide 3-kinase activation. The only inhibitors of PLD-stimulated PA production that have been identified are primary alcohols, which compete with water in the transphosphatidylation reaction to make the corresponding phosphatidylalcohol. Secondary alcohols such as butan-2-ol are unable to participate in transphosphatidylation and therefore serve as a control for nonspecific effects of alcohols. A prominent feature of mast cell activation is the formation of lamellipodia and membrane ruffles, which we report to be exquisitely inhibited by butan-1-ol, but not butan-2-ol. Butan-1-ol blocks membrane ruffling at any time after stimulation, indicating that continual PLD activity is essential for the dynamics of membrane transformations. Blockade by butan-1-ol was completely reversible as demonstrated by removal of butan-1-ol. Membrane ruffling was maintained for at least 30 min, and this ruffling was accompanied by continual PLD activity. Antigen stimulation increased the activity of both PLD1 and PLD2 when overexpressed in RBL mast cells. PLD1 localized to intracellular vesicles, whereas PLD2 localized to the plasma membrane in resting cells. This pattern of PLD1 and PLD2 localization in RBL mast cells has also been described recently (Choi et al., 2002). In this study both PLD1 and PLD2 were required for exocytosis of hexosaminidase-containing secretory granules because overexpression of both PLD1 and PLD2 enhanced secretion and catalytically inactive PLD1 and PLD2 both blocked secretion stimulated by thapsigargin.
To confirm that the localization of the overexpressed PLD proteins was similar to the endogenous PLDs, we analyzed the distribution of endogenous PLDs by activity measurements and confirmed that the pattern observed for overexpressed PLD proteins was similar to the endogenous PLD proteins. PLD2 activity, monitored by stimulation with oleic acid, was localized at the plasma membrane, whereas the ARF-stimulated activity was localized intracellularly, showing partial overlap with hexosaminidase-containing secretory granules (Sarri, Pardo, Fensome, and Cockcroft; unpublished data). Overexpressed PLD2 was identified at the plasma membrane in resting cells and was found in membrane ruffles as well as the pinosomes that accompanied the membrane ruffles. In contrast, PLD1 was localized to an intracellular vesicular compartment, which did not change dramatically upon antigen addition. It has been reported previously that in stimulated mast cells, PLD1 translocates to the plasma membrane (Brown et al., 1998a; Choi et al., 2002; Powner et al., 2002). Our inability to monitor the translocation of PLD1 could be due to differences in methodology or to the degree of exocytosis triggered by antigen. In our hands, only 25-35% secretion could be triggered and mixing of the PLD1-containing granule membranes with the plasma membrane during fusion may have led to the dispersal of the GFP signal.
Membrane ruffling is accompanied by pinocytosis, and ARF6 has been implicated in coordinating the dynamics of pinosome or endocytic traffic, which accompanies membrane ruffle formation and its dissolution (Honda et al., 1999; Radhakrishna et al., 1999). Expression of a constitutively activated form of ARF6 induces actin assembly, resulting in the movement of vesicle-like particles, some of which contain markers for pinosomes (Schafer et al., 2000). The ARF6 exchange factor, EFA6, which has a pleckstrin homology domain, has also been shown to coordinate membrane recycling and the actin cytoskeleton during membrane ruffling (Franco et al., 1999). After stimulation with antigen, PLD2 was also seen in the pinosomes but PI(4,5)P2 was not. Thus, PLD2 in the pinosomes may be a means of shutting down PLD2 activity because PI(4,5)P2 was never seen in such structures.
What is the function of PLD activity during membrane ruffling? In the membrane ruffles, both PLD2 and ARF6 are found, and our data suggest that localized availability of PA via PLD2 and ARF6 coordinate the activity of PIP5K and, therefore, PIP2 production (Fensome et al., 1996; Honda et al., 1999; Divecha et al., 2000). This conclusion is supported by both in vitro studies published previously and the studies in permeabilized cells reported herein. Because antigen-stimulated PI(4,5)P2 synthesis is both ARF dependent and is inhibited by butan-1-ol, both PA and ARF proteins are required simultaneously to regulate PIP5K (Honda et al., 1999; Jones et al., 2000). During antigen stimulation, we anticipate that both ARF and PA are rate-limiting components compared with the situation when GTPγS is used as a stimulus (Skippen et al., 2002). In the case of the antigen, both pathways are required and could function as coincidence detectors. The observation that GTPγS can use both pathways for PI(4,5)P2 synthesis is most likely due to the irreversible nature of G protein activation by GTPγS compared with when antigen is used as a stimulus. Responses to GTPγS are much larger and longer sustained due to the near irreversible activation of the GTPases.
One of the most interesting facets of PLD activation that has emerged from this study is the interpretation of data when PLD activation is monitored by the formation of PBut. The majority of studies use transphosphatidylation as a means of monitoring PLD activity, and herein we demonstrate that results can be misleading. Measurements of PA as a monitor of PLD activation are also equally fraught with difficulty because PA is readily metabolized. Another difficulty in using biochemical measurements of PA is that it measures global PA rather than the PA that is topologically restricted to the site of PLD activation. We have attempted to monitor PA in living cells by using a PA-binding region of Raf-1 tagged with GFP (Rizzo et al., 2000). However, this domain localized intracellularly in a punctate staining pattern and remained so in the antigen-stimulated cells. Clearly, the availability of such a reagent would provide the ability to examine the production of PA in a topologically restricted region, and kinetics of PA production can then be directly compared with membrane ruffling.
We postulate the following sequence of events: In phase I, antigen stimulates a robust activation of PLD2 (and possibly also PLD1), generating PA. Antigen also stimulates ARF6 and together with PA stimulates the activity of PIP5K, leading to a burst of PI(4,5)P2, all within the membrane ruffle. The increase in PIP2 leads to further activation of PLD2 (phase II). Our data on PLD activity measurements provide evidence for this positive feedback model whereby ongoing local PLD activity is maintained, provided that the PA is made and can participate in a downstream event most likely stimulating the levels of PI(4,5)P2. This conclusion is deduced from the anomaly of the time course of PLD activation. An apparent plateau of PLD activity is observed after 10 min of antigen stimulation (Figure 2C), despite the demonstration that PLD is continually active over a 30-min period (Figure 2D). Butanol, by preventing phase I, would therefore prevent phase II of PLD activity. Butanol is widely used to measure PLD activity, but its effects on PA production mask the events that PA subsequently regulates, in this case the positive feedback loop. This is dependent on PA-dependent increase in PIP5K activity and thus increased PI(4,5)P2 level, leading to a further increase in PLD2 activity. A similar conclusion was suggested by recent studies overexpressing PLD2 with PIP5K (Divecha et al., 2000). Membrane ruffling and lamellipodia formation are extremely dynamic processes, and it is expected that a local ARF6 GTPase cycle operates in addition to the well-established Rac cycle. The local buildup of high levels of PA together with PI(4,5)P2 can then allow for ARF-GTPase–activating protein to deactivate ARF6, a characteristic of the ASAP and ACAP family of ARF-GTPase–activating proteins (Brown et al., 1998b; Jackson et al., 2000). In addition, PIP 5-phosphatase is also found in membrane ruffles, which suggests that turnover of PI(4,5)P2 is also taking place (Mochizuki and Takenawa, 1999).
Membrane ruffles are regions of intense actin polymerization and contain many actin-binding proteins, in particular, gelsolin and profilin, which are also PI(4,5)P2-binding proteins. Gelsolin acts to sever existing actin filaments. Profilin acts to concentrate G-actin monomer to sites of actin polymerization. PI(4,5)P2 plays an important role in regulating these two activities. We now provide evidence for a new enzymatic component, PLD2 (and possibly PLD1), whose continual activity is required for the formation and dissolution of membrane ruffles. Our results suggest that one function of PA is to modulate the activity of PIP5K together with ARF6. Local production of PI(4,5)P2 together with Rac1 regulates the activity of proteins such as gelsolin and profilin. It is therefore interesting to note that a physical interaction between PLD2 and gelsolin has been reported (Steed et al., 1996). Furthermore, it was apparent that gelsolin increased markedly the activity of PLD. Fibroblasts lacking gelsolin do not ruffle and this is assumed to be due to loss of severing activity (Azuma et al., 2000). We speculate that gelsolin-dependent membrane ruffling is due to enhanced PLD activity and subsequent increases in PI(4,5)P2 via PA-stimulated PIP5K.
Acknowledgments
We thank the Wellcome Trust and the Human Frontiers Program for support. N.O.L. was in receipt of a Wellcome Prize Studentship. We thank M. Frohman for providing the constructs of EGFP-tagged PLDs. The ARF6 antibody was a gift from J.G. Donaldson (National Institutes of Health).
Abbreviations used:
- ARF
ADP ribosylation factor
- GFP
enhanced green fluorescent protein
- PA
phosphatidic acid
- PBut
phosphatidylbutanol
- PC
phosphatidylcholine
- PI(4,5)P2
phosphatidylinositol 4,5 bisphosphate
- PLD
phospholipase D
- PIP5K
phosphatidylinositol 4-phosphate 5-kinase
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0213. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0213.
REFERENCES
- Apgar JR. Activation of protein kinase C in rat basophilic leukemia cells stimulates increased production of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: correlation with actin polymerization. Mol Biol Cell. 1995;6:97–108. doi: 10.1091/mbc.6.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J. 2000;17:1362–1370. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Hall A, Martinez AM, Pfeiffer JR, Oliver JM, Wilson BS. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by FcεR1 cross linking. Mol Biol Cell. 1995;6:1145–1158. doi: 10.1091/mbc.6.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Andrade J, Radhakrishna H, Donaldson J, Cooper JA, Randazzo P. ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998b;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Thompson N, Saqib KM, Clark JM, Powner D, Thompson NT, Solari R, Wakelam MJO. Phospholipase D1 localizes to secretory granules and lysosomes and is plasma-membrane located on cellular stimulation. Curr Biol. 1998a;8:835–838. doi: 10.1016/s0960-9822(98)70326-4. [DOI] [PubMed] [Google Scholar]
- Caumont AS, Galas MC, Vitale N, Aunis D, Bader MF. Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J Biol Chem. 1998;273:1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Kim YM, Combs C, Frohman MA, Beaven MA. Phospholipases D1, and D2 regulate different phases of exocytosis in mast cells. J Immunol. 2002;168:5682–5689. doi: 10.4049/jimmunol.168.11.5682. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMetLeuPhe or ionophore A23187. Biochim Biophys Acta. 1984;795:37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Signaling roles of mammalian phospholipase D1, and D2. Cell Mol Life Sci. 2001;58:1674–1687. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S, De Matteis MA. Inositol lipids as spatial regulators of membrane traffic. J Membr Biol. 2001;180:187–194. doi: 10.1007/s002320010069. [DOI] [PubMed] [Google Scholar]
- Colley WC, Sung T-C, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Roberts S, Ridley AJ, Hodgkin MN, Stewart A, Claesson-Welsh L, Wakelam MJO. Stimulation of actin stress fiber formation mediated by activation of phospholipase D. Curr Biol. 1996;6:588–597. doi: 10.1016/s0960-9822(02)00545-6. [DOI] [PubMed] [Google Scholar]
- D'Souza-Scharey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L. A role for POR1, a rac1 interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N, Roefs M, Halstead JR, D'Andrea S, Fernandez-Borga M, Oomen L, Saqib KM, Wakelam MJO, D'Santos C. Interaction of the Type Iα PIP kinase with phospholipase D. a role for the local generation of phosphatidylinositol 4,5-bisphosphate in the regulation of PLD2 activity. EMBO J. 2000;19:5440–5449. doi: 10.1093/emboj/19.20.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensome A, Cunningham E, Prosser S, Tan SK, Swigart P, Thomas G, Hsuan J, Cockcroft S. ARF and PITP restore GTPγS-stimulated protein secretion from cytosol-depleted HL60 cells by promoting PIP2 synthesis. Curr Biol. 1996;6:730–738. doi: 10.1016/s0960-9822(09)00454-0. [DOI] [PubMed] [Google Scholar]
- Franco M, Peters PJ, Boretto J, van Donselaar E, D'Souza-Schorey C, Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot JC, Montcourrier P, Vivier E, Davoust J, Chavrier P. Selective control of membrane ruffling and actin plaque assembly by the Rho GTPase Rac1 and CDC42 in FcεRI-activated rat basophilic leukemia (RBL-2H3) cells. J Cell Sci. 1997;110:2215–2225. doi: 10.1242/jcs.110.18.2215. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han JM, et al. Phosphorylation-dependent regulation of phospholipase D2 by protein kinase Cδ in rat pheochromocytoma PC12 cells. J Biol Chem. 2002;277:8290–8297. doi: 10.1074/jbc.M108343200. [DOI] [PubMed] [Google Scholar]
- Honda A, et al. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA. ACAPs are ARF6 GTPase-activating proteins that function in the cell periphery. J Cell Biol. 2000;151:627–638. doi: 10.1083/jcb.151.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I PIP 5-kinase directly interacts with ARF1, and is responsible for PI(4,5)P2 synthesis in the Golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Kam Y, Exton JH. Phospholipase D activity is required for actin stress fiber formation in fibroblasts. Mol Cell Biol. 2001;21:4055–4066. doi: 10.1128/MCB.21.12.4055-4066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim Y, Lee SD, Lopez I, Arnold RS, Lambeth JD, Suh PG, Ryu SH. Selective activation of phospholipase D2 by unsaturated fatty acid. FEBS Lett. 1999;454:42–46. doi: 10.1016/s0014-5793(99)00745-0. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun B, Roth MG, Ktistakis NT. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- Laine J, Bourgoin S, Bourassa J, Morisset J. Subcellular distribution, and characterization of rat pancreatic phospholipase D isoforms. Pancreas. 2000;20:323–336. doi: 10.1097/00006676-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Lee S, Park JB, Kim JH, Kim Y, Kim JH, Shin KJ, Lee JS, Ha SH, Suh PG, Ryu SH. Actin directly interacts with phospholipase D, inhibiting its activity. J Biol Chem. 2001;276:28252–28260. doi: 10.1074/jbc.M008521200. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D. molecular and cell biology of a novel gene family. Biochem J. 2000;345:401–415. [PMC free article] [PubMed] [Google Scholar]
- Lopez I, Arnold R, Lambeth JD. Cloning and initial characterization of a human phospholipase D2 (hPLD2) J Biol Chem. 1998;273:12846–12852. doi: 10.1074/jbc.273.21.12846. [DOI] [PubMed] [Google Scholar]
- Martin TFJ. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki Y, Takenawa T. Novel inositol polyphosphate 5-phosphatase localizes at membrane ruffles. J Biol Chem. 1999;274:36790–36795. doi: 10.1074/jbc.274.51.36790. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Sengelov H, Whatmore J, Borregaard N, Cockcroft S. ARF-regulated phospholipase D activity localizes to secretory vesicles and mobilizes to the plasma membrane following fMetLeuPhe stimulation of human neutrophils. Biochem J. 1997;325:581–585. doi: 10.1042/bj3250581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JC, Jones D, Holt MR, Barry ST, Cockcroft S, Critchley DR. ARF1 mediates paxillin recruitment to focal adhesions and potentiates rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palicz A, Foubert TR, Jesaitis AJ, Marodi L, McPhail LC. Phosphatidic acid, and diacylglycerol directly activate NADPH oxidase by interacting with enzyme components. J Biol Chem. 2000;276:3090–3097. doi: 10.1074/jbc.M007759200. [DOI] [PubMed] [Google Scholar]
- Park JB, Kim JH, Kim Y, Ha SH, Kim JH, Yoo JS, Du G, Frohman MA, Suh P-G, Ryu S-H. Cardiac phospholipase D2 localizes to sarcolemmal membranes, and is inhibited by α-actinin in an ADP-ribosylation factor-reversible manner. J Biol Chem. 2000;275:21295–21301. doi: 10.1074/jbc.M002463200. [DOI] [PubMed] [Google Scholar]
- Powner DJ, Hodgkin MN, Wakelam MJO. Antigen-stimulated activation of phospholipase D1b by Rac1, ARF6, and PKCα in RBL-2H3 cells. Mol Biol Cell. 2002;13:1252–1262. doi: 10.1091/mbc.01-05-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Shome K, Watkins SC, Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid, and is independent of association with Ras. J Biol Chem. 2000;275:23911–23918. doi: 10.1074/jbc.M001553200. [DOI] [PubMed] [Google Scholar]
- Schafer D, D'Souza-Schorey C, Cooper JA. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1:892–903. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu L, Foster DA. Role for phospholipase D in receptor-mediated endocytosis. Mol Cell Biol. 2001;21:595–602. doi: 10.1128/MCB.21.2.595-602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhanta A, Shields D. Secretory vesicle budding from the Trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- Siddiqi AR, Srajer GE, Leslie CC. Regulation of human PLD1, and PLD2 by calcium, and protein kinase C. Biochim Biophys Acta. 2000;1497:103–114. doi: 10.1016/s0167-4889(00)00049-5. [DOI] [PubMed] [Google Scholar]
- Skippen A, Jones DH, Morgan CP, Li M, Cockcroft S. Mechanism of ADP-ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J Biol Chem. 2002;277:5823–5831. doi: 10.1074/jbc.M110274200. [DOI] [PubMed] [Google Scholar]
- Steed PM, Nagar S, Wennogle LP. Phospholipase D regulation by a physical interaction with the actin binding protein gelsolin. Biochemistry. 1996;35:5229–5237. doi: 10.1021/bi952370j. [DOI] [PubMed] [Google Scholar]
- Stutchfield J, Cockcroft S. Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem J. 1993;293:649–655. doi: 10.1042/bj2930649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung T-C, Altshuller YM, Morris AJ, Frohman MA. Molecular analysis of mammalian phospholipase D2. J Biol Chem. 1999;274:494–502. doi: 10.1074/jbc.274.1.494. [DOI] [PubMed] [Google Scholar]
- Way G, O'Luanaigh N, Cockcroft S. Activation of exocytosis by cross-linking of the IgE receptor is dependent on ARF-regulated phospholipase D in RBL-2H3 mast cells. Evidence that the mechanism of activation is via regulation of PIP2 synthesis. Biochem J. 2000;346:63–70. [PMC free article] [PubMed] [Google Scholar]
- Whatmore J, Morgan CP, Cunningham E, Collison KS, Willison KR, Cockcroft S. ADP-ribosylation factor1-regulated phospholipase D is localized at the plasma membrane and intracellular organelles in HL60 cells. Biochem J. 1996;320:785–794. doi: 10.1042/bj3200785. [DOI] [PMC free article] [PubMed] [Google Scholar]