Abstract
Aseptic loosening of total joint arthroplastics due to periprosthetic osteolysis is a frequent cause of implant failure. The absence of clinical interventions to arrest or prevent this complication limits the use of total joint replacement especially in younger patients. Here we review recent studies implicating tumor necrosis factor (TNF)-α in periprosthetic osteolysis and the rationale for clinical studies of anti-TNF therapy and other interventions for periprosthetic loosening.
Keywords: aseptic loosening, osteolysis, pannus, prosthesis, tumor necrosis factor-α
Introduction
Joint destruction from various pathologies, most notably rheumatoid arthritis (RA) and osteoarthritis, leads many individuals to elect total joint replacement. Worldwide, more than 1.3 million total joint arthroplasties are performed each year [1]. This number can be expected to increase dramatically in the 21st century. While total joint replacement is remarkably effective in relieving pain and improving function and mobility, it it not without complications. Up to 20% of patients so treated will show evidence of osteolysis within 10 years [2,3,4]. This osteolysis usually leads to implant failure and need for revision arthroplasty, which has a poorer clinical result and a shorter duration of survival than primary total joint replacement [5,6]. Because of such failures, many younger people who would otherwise be excellent candidates for surgery are told to wait, because they might need two or three revisions in their lifetime. Therefore, a clinical intervention to prevent prosthetic implant loosening is greatly needed.
Prosthesis failure results from multiple factors, including those relating to materials, biomechanics, and host responses. The quest for more durable and wear-resistant materials, as well as for better implant designs, and the study of the forces involved in implant integration and prosthesis failure continue to be areas of active investigation. Several groups, however, have focused on the host response to debris produced by wearing of the joint, postulating that wear-debris-induced osteolysis in the main cause of failure of prosthetic implants [7]. In this model, wear debris generated from the prosthesis is phagocytosed by macrophages and initiates an inflammatory response that leads to the recruitment of activated osteoclasts and to osteolysis at the bone-implant interface. Several lines of evidence support this model. First, as many as 109 particles per gram of tissue can be recovered from the inflamed membrane attached to the failed prosthesis after revision surgery [8]. Second, ingestion of wear-debris particles induces cytokine production by mononuclear phagocytes in vitro [9]. Third, high concentrations of cytokines, including TNF-α, that are produced by macrophages are found in the fluid and tissue surrounding loose implants [10,11,12]. Fourth, conditioned medium from monocytes stimulated by wear debris can stimulate increased bone resorption in vitro [13]. Fifth, animal models of wear-debris-induced osteolysis have demonstrated the importance of cytokines in this process [14,15].
This wear-debris-induced osteolysis, which is associated with aseptic loosening, is very different from the phenomenon of stress shielding. In stress shielding, an implant takes on a portion of the mechanical load transmitted to the skeleton and shields bone from this stress [16,17,18]. Since bone metabolism is dependent upon mechanical load, bone density decreases in the affected area. Stress shielding is different in several ways from the inflammatory bone loss that occurs in response to particulate debris. First, stress shielding occurs in the absence of inflammation [18]. Second, it occurs around implants (such as rods, plates and screws) that do not release particles [19]. Third, it is not influenced by polyethylene or the bearing surface, but is reduced by using implants that have a lower modulus of elasticity so that bone takes on more of the mechanical load [16,17]. Fourth, like disuse osteopenia or osteoporosis, stress shielding increases the general porosity of bone, whereas aseptic loosening is associated with localized endosteal bone erosions [20]. Fifth, and most importantly, stress shielding has not been associated with mechanical loosening of the implant [17,18,21,22].
The first clinical manifestation of prosthesis failure is pain with associated radiographic evidence of osteolysis (Fig. 1a). If the volume of osteolysis is small (up to 2 mm in diameter), osteolysis often does not progress and the implant remains fixed. However, when the lesion is greater than 2 mm, osteolysis usually continues rapidly, leading to implant failure. In these lesions, bone is resorbed by osteoclasts and is replaced by a fibro-inflammatory membrane containing lymphocytes, macrophages, and fibroblasts (Fig. 1b) [7]. Although the histopathology and initiating mechanisms differ from those for RA, the tissue reaction in peri-implant osteolysis resembles the pannus of RA in its tendency to produce localized cytokine-mediated bone loss. Thus, a central aim in developing a therapeutic intervention for aseptic loosening is to identify a drug that will eliminate or dramatically reduce inflammation in the periprosthetic synovium-like membrane.
Figure 1.
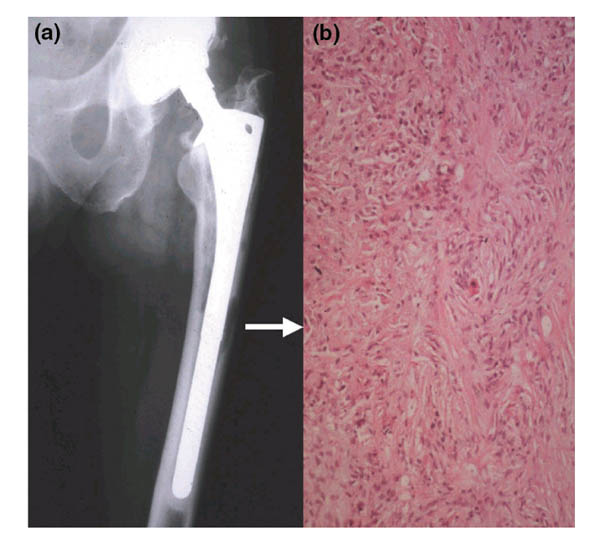
Radiographic and histologic findings in periprosthetic osteolysis and loosening of the prosthesis. (a) The radiograph demonstrates periprosthetic bone erosions along both the medial and lateral endosteal bone surfaces. The femoral head is eccentrically placed in a superior position in the acetabular cup, indicating polyethylene wear and the generation of particles. (b) The bone in the osteolytic lesions is replaced by fibro-inflammatory tissue (arrow) consisting of a background of fibroblasts with a diffuse infiltrate of inflammatory cells (lymphocytes, plasma cells, and macrophages), which is most intense in the top left-hand quadrant of this micrograph. Released particles of wear debris accumulate in this tissue, which acts as a reservoir for them and thus enhances the progression of the bone loss and further loosening. This patient underwent a revision arthroplasty.
TNF-α has been identified as a drug target in aseptic loosening for many of the same reasons it has been a focus in RA. First, since addition of anti-TNF-α antibodies inhibits the production of other pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and GM-CSF (granulocyte-macrophage colony-stimulating factor) by synovial tissue, it has been proposed that this factor is at the apex of the pro- inflammatory cytokine cascade in the synovium [23,24,25]. Another reason is that TNF-α can induce joint inflammation and proliferation of joint cells [26]. Also, it can stimulate bone resorption by inducing osteoclastogenesis and activating mature osteoclasts [27]. A fourth reason is that TNF receptor I knockout mice have virtually no osteolytic response to polymethylmethacrylate [15] or titanium [14]. And finally, in animal models, the TNF-α antagonist etanercept has been used to prevent wear-debris-induced osteolysis [28,29].
Therapies for aseptic loosening
There are currently no drugs specifically approved for the treatment of aseptic loosening of prostheses. However, the above paradigm for loosening (ie wear-debris-induced, TNF-α-mediated inflammation resulting in osteoclast activation) suggests that three categories of drugs should be tested for their ability to prevent or treat loosening of prosthetic joints. The first category is the bisphosphonates. These drugs inhibit osteoclasts, are effective, and are widely used to prevent or treat osteoporosis. A small, recent clinical study has shown that alendronate can reduce the periprosthetic bone loss that develops soon after total hip replacement [30]. However, as the authors of that study pointed out, this early bone loss is probably secondary to stress shielding rather than to wear-debris-induced inflammation. Indeed, patients who had had a total hip replacements more than 5 years previously or who were awaiting revision surgery for loosening did not have a similar increase in periprosthetic bone density when treated with alendronate. Unfortunately, periprosthetic osteolysis was not an end point in that study. The effect of bisphosphonates on inflammation-induced osteolysis has also been evaluated in patients with RA. In three studies, the effects of bisphosphonates on radiographically evaluated erosions have varied but have been predominantly negative. In one small study (a total of 27 patients randomized to either pamidronate, 1000 mg/day by mouth, or a placebo, for a year), erosions in the treated group progressed less rapidly [31]. However, no such effect was found in two larger studies (a total of 40 patients given pamidronate, 30 mg, intravenously per month or placebo by monthly infusion, and a total of 105 patients given pamidronate, 300 mg/day by mouth, or a placebo for 3 years) [32,33]. In this last study, spinal and femoral bone mineral density significantly improved in the treated group, even though the erosions progressed. Although it is possible that the doses used were inadequate to block osteolysis, these studies in humans suggest that bisphosphonates may be less effective for use against inflammation-induced osteolysis than against generalized osteoporosis. On the other hand, a report that zoledronate blocks bone resorption in a rabbit/carrageenan model of inflammatory arthritis [34] indicates that bisphosphonates may be effective in some types of inflammation-induced osteolysis.
A second category of drugs for the treatment of prosthetic loosening are those designed to inhibit TNF (etanercept and infliximab). Both of these agents are potent inhibitors of synovial inflammation [35,36] and both have been approved worldwide for the treatment of RA. Importantly, recent studies have shown that both can block erosions in this disease [37,38]. Because of their effects on erosions in RA and on wear-debris-induced asteolysis in animals [28,29], these anti-TNF agents are the most promising medications already available for the treatment of established loosening. However, they are also remarkably expensive and therefore should not be used to treat loosening until their efficacy is proven in clinical trials.
Finally, a third category of drugs to treat prosthetic loosening are the biologics being developed that interfere with RANK/RANK-ligand signaling. RANK (receptor activator of NFκB) [39] is a receptor on osteoclasts and osteoclast precursors that transmits a signal required during osteoclast and lymph node development [40]. RANK ligand (also known as OPGL, ODF, and TRANCE) is an agoinst for RANK, and is expressed on osteoblasts and activated T cells [41]; it provides the essential signal for osteoclast differentiation and survival [27]. Osteoprotegerin (OPG) is a natural decoy receptor that binds to RANK ligand and prevents its interaction with RANK [42]. The biologics being developed to inhibit osteoclasts include recombinant OPG and a soluble form of RANK. The potency of these molecules is best illustrated by the phenotype of transgenic mice that overexpress these factors and suffer from severe osteopetrosis [42,43]. Preliminary studies in an animal model indicate that a soluble chimeric RANK:Fc molecule has no effect on inflammation but completely inhibits osteoclast induction and wear-debris-induced osteolysis in vivo [29]. Thus, these new RANK-based biologics, which are even more potent inhibitors of osteoclasts than the bisphosphonates, may offer another future approach to the treatment of established loosening or to its prevention.
Based on the animal studies summarized above, a strong case can be made for the involvement of TNF in at least some models of wear-debris-induced osteolysis. We believe that clinical studies with TNF inhibitors will be a direct way of testing the validity of anti-TNF therapy in preventing prosthetic hip loosening.
Acknowledgments
Acknowledgements
We would like to thank Dr Brendan F Boyce for critical comments and assistance in preparing the manuscript. EM Schwarz is supported by a research grant from the Orthopaedic Research and Education Foundation and the National Institutes of Health (AR45971-01).
References
- Harris WH. The problem is osteolysis. Clin Orthop. 1995;311:46–53. [PubMed] [Google Scholar]
- Fender D, Harper WM, Gregg PJ. Outcome of Charnley total hip replacement across a single health region in England: the results at five years from a regional hip register. . J Bone Joint Surg Br. 1999;81:577–581. doi: 10.1302/0301-620x.81b4.9859. [DOI] [PubMed] [Google Scholar]
- Callaghan JJ, Forest EE, Olejniczak JP, et al. Charnley total hip arthroplasty in patients less than fifty years old. A twenty to twenty-five-year follow-up note. . J Bone Joint Surg Am. 1998;80:704–714. doi: 10.2106/00004623-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim JS, Cho SH. Primary total hip arthroplasty with a cementless porous-coated anatomic total hip prosthesis: 10- to 12-year results of prospective and consecutive series. . J Arthroplasty. 1999;14:538–548. doi: 10.1016/s0883-5403(99)90074-8. [DOI] [PubMed] [Google Scholar]
- Callaghan JJ, Salvati EA, Pellicci PM, et al. Results of revision for mechanical failure after cemented total hip replacement, 1979 to 1982. A two to five-year follow-up. . J Bone Joint Surg Am. 1985;67:1074–1085. [PubMed] [Google Scholar]
- Hanssen AD, Rand JA. A comparison of primary and revision total knee arthroplasty using the kinematic stabilizer prosthesis. . J Bone Joint Surg Am. 1988;70:491–499. [PubMed] [Google Scholar]
- Goldring SR, Jasty M, Roelke MS, et al. Formation of a synovial-like membrane at the bone-cement interface. Its role in bone resorption and implant loosening after total hip replacement. . Arth Rheum. 1986;29:836–842. doi: 10.1002/art.1780290704. [DOI] [PubMed] [Google Scholar]
- Margevicius KJ, Bauer TW, McMahon JT, et al. Isolation and characterization of debris in membranes around total joint prostheses. . J Bone Joint Surg Am. 1994;76:1664–75. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- Blaine TA, Rosier RN, Puzas JE, et al. Increased levels of tumor necrosis factor-alpha and interleukin-6 protein and messenger RNA in human peripheral blood monocytes due to titanium particles. . J Bone Joint Surg Am. 1996;78:1181–1192. doi: 10.2106/00004623-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Horowitz SM, Doty SB, Lane JM, Burstein AH. Studies of the mechanism by which the mechanical failure of polymethylmethacrylate leads to bone resorption. . J Bone Joint Surg Am. 1993;75:802–813. doi: 10.2106/00004623-199306000-00002. [DOI] [PubMed] [Google Scholar]
- al Saffar N, Revell PA. Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. . Br J Rheumatol. 1994;33:309–316. doi: 10.1093/rheumatology/33.4.309. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Revell PA, al-Saffar N, et al. Bone formation and bone resorption in failed total joint arthroplasties: histomorphometric analysis with histochemical and immunohistochemical technique. . J Orthop Res. 1996;14:473–482. doi: 10.1002/jor.1100140318. [DOI] [PubMed] [Google Scholar]
- Glant TT, Jacobs JJ. Response of three murine macrophage populations to particulate debris: bone resorption in organ cultures. . J Orthop Res. 1994;12:720–731. doi: 10.1002/jor.1100120515. [DOI] [PubMed] [Google Scholar]
- Schwarz EM, O'Keefe RJ. TNFα/NFκB signalling in periprosthetic osteolysis. . Arthritis Rheum. 1998;41 (suppl):S345. [Google Scholar]
- Markel KD, Erdmann JM, McHugh KP, et al. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. . Am J Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Reed KL, Robertson DD, et al. Reduced bone stress as predicted by composite beam theory correlates with cortical bone loss following cemented total hip arthroplasty. . J Orthop Res. 1999;17:525–531. doi: 10.1002/jor.1100170410. [DOI] [PubMed] [Google Scholar]
- Wan Z, Dorr LD, Woodsome T, et al. Effect of stem stiffness and bone stiffness on bone remodeling in cemented total hip replacement. . J Arthroplasty. 1999;14:149–158. doi: 10.1016/s0883-5403(99)90118-3. [DOI] [PubMed] [Google Scholar]
- Rubash HE, Sinha RK, Shanbhag AS, Kim SY. Pathogenesis of bone loss after total hip arthroplasty. . Orthop Clin North Am. 1998;29:173–186. doi: 10.1016/s0030-5898(05)70316-3. [DOI] [PubMed] [Google Scholar]
- van der Elst M, Bramer JA, Klein CP, et al. Biodegradable interlocking nails for fracture fixation. . Clin Orthop. 1998;357:192–204. doi: 10.1097/00003086-199812000-00025. [DOI] [PubMed] [Google Scholar]
- Myers MA, Casciani T, Whitbeck MG, Jr, Puzas JE. Vertebral body osteopenia associated with posterolateral spine fusion in humans. . Spine. 1996;21:2368–2371. doi: 10.1097/00007632-199610150-00012. [DOI] [PubMed] [Google Scholar]
- McAulley JP, Culpepper WJ, Engh CA. Total hip arthroplasty. Concerns with extensively porous coated femoral components. . Clin Orthop. 1998;355:182–188. [PubMed] [Google Scholar]
- McAulley JP, Moore KD, Culpepper WJ, 2nd, Engh CA. Total hip arthroplasty with porous-coated prostheses fixed without cement in patients who are sixty-five years of age or older. . J Bone Joint Surg Am. 1998;80:1648–1655. doi: 10.2106/00004623-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, Davis LS, Cush JJ, Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. . Springer Semin Immunopathol. 1989;11:123–162. doi: 10.1007/BF00197186. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Elliott M, et al. TNF alpha as a therapeutic target in rheumatoid arthritis. . Circ Shock. 1994;43:179–184. [PubMed] [Google Scholar]
- Maini RN, Brennan FM, Williams R, et al. TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. . Clin Exp Rheumatol. 1993;11 (suppl 8):S173–S175. [PubMed] [Google Scholar]
- Gitter BD, Labus JM, Lees SL, Scheetz ME. Characteristics of human synovial fibroblast activation by IL-1 beta and TNF alpha. . Immunology. 1989;66:196–200. [PMC free article] [PubMed] [Google Scholar]
- Boyce BF, Hughes DE, Wright KR, et al. Recent advances in bone biology provide insight into the pathogenesis of bone diseases. . Lab Invest. 1999;79:83–94. [PubMed] [Google Scholar]
- Clohisy JC, Teitelbaum SL, Ross FP, et al. Blockade of TNF-activation of NF-κB in osteoclast precursors prevents implant osteolysis. . J Bone Min Res. 1999;14 (suppl):S489. [Google Scholar]
- Childs LM, Goater JJ, Sanz I, et al. Efficacy of the soluble TNFa inhibitor (Enbrel) to prevent prosthetic wear debris-induced osteolysis. . Arthritis Rheum. 1999;42 (suppl):S154. [Google Scholar]
- Leung A, Scammel B, Lyons A, et al. Alendronate prevents periprosthetic bone loss - 2 year results. . Arthritis Rheum. 1999;42 (suppl):S270. [Google Scholar]
- Maccagno A, Di Giorgio E, Roldan EJ, et al. Double blind radiological assessment of continuous oral pamidronic acid in patients with rheumatoid arthritis. . Scand J Rheumatol. 1994;23:211–214. doi: 10.3109/03009749409103063. [DOI] [PubMed] [Google Scholar]
- Ralston SH, Hacking L, Willocks L, et al. Clinical, biochemical, and radiographic effects of aminohydroxypropylidene bisphosphonate treatment in rheumatoid arthritis. . Ann Rheum Dis. 1989;48:396–399. doi: 10.1136/ard.48.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggelmeijer F, Papapoulos SE, van Passen HC, et al. Increased bone mass with pamidronate treatment in rheumatoid arthritis. Results of a three-year randomized, double-blind trial. . Arthritis Rheum. 1996;39:396–402. doi: 10.1002/art.1780390307. [DOI] [PubMed] [Google Scholar]
- Pysklywec MW, Moran EL, Bogoch ER. Zoledronate (CGP 42'446), a bisphosphonate, protects against metaphyseal intracortical defects in experimental inflammatory arthritis. . J Orthop Res. 1997;15:858–861. doi: 10.1002/jor.1100150610. [DOI] [PubMed] [Google Scholar]
- Maini RN, Elliott MJ, Brennan FM, et al. Monoclonal anti-TNF alpha antibody as a probe of pathogenesis and therapy of rheumatoid disease. . Immunol Rev. 1995;144:195–223. doi: 10.1111/j.1600-065x.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. . N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Lipsky P, St. Clair W, Furst D, et al. 54-week clinical and radiographic results from the attract trial: A phase III study of infliximab in patients with active RA despite methotrexate. . Arthritis Rheum. 1999;42 (suppl):S401. [Google Scholar]
- Finck B, Martin R, fleischmann R, Moreland L, et al. A phase III trial of etanercept vs methotrexate in early rheumatoid arthritis. . Arthritis Rheum. 1999;42 (suppl):S117. [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. . Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. . Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. . Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. . Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. . Proc Natl Acad Sci USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]


