Abstract
Background
Maltose metabolism is initiated by an ATP-dependent permease system in Lactococcus lactis. The subsequent degradation of intracellular maltose is performed by the concerted action of Pi-dependent maltose phosphorylase and β-phosphoglucomutase. In some Gram-positive bacteria, maltose metabolism is regulated by a maltose operon regulator (MalR), belonging to the LacI-GalR family of transcriptional regulators. A gene presumed to encode MalR has been found directly downstream the maltose phosphorylase-encoding gene, malP in L. lactis. The purpose of this study was to investigate the physiological role of the MalR protein in maltose metabolism in L. lactis.
Results
A L. lactis ssp. lactis mutant, TMB5004, deficient in the putative MalR protein, was physiologically characterised. The mutant was not able to ferment maltose, while its capability to grow on glucose as well as trehalose was not affected. The activity of maltose phosphorylase and β-phosphoglucomutase was not affected in the mutant. However, the specific maltose uptake rate in the wild type was, at its lowest, five times higher than in the mutant. This difference in maltose uptake increased as the maltose concentration in the assay was increased.
Conclusion
According to amino acid sequence similarities, the presumed MalR is a member of the LacI-GalR family of transcriptional regulators. Due to the suggested activating effect on maltose transport and absence of effect on the activities of maltose phosphorylase and β-phosphoglucomutase, MalR of L. lactis is considered rather as an activator than a repressor.
Background
Maltose is transported by an ATP-dependent permease system in Lactococcus lactis[1]. The subsequent degradation of intracellular maltose is performed by the concerted action of Pi-dependent maltose phosphorylase (MP) and β-phosphoglucomutase (β-PGM) [2,3]. The genes encoding the MP and β-PGM of L. lactis are located in two different operons [3], (Fig. 1), and are both exposed to carbon catabolite repression [3,4]. The β-PGM encoding gene, pgmB, is located in a putative trehalose operon, including the genes likely to encode a regulatory protein and the trehalose-specific components of the phosphotransferase system as well as the gene encoding the newly characterised trehalose 6-phosphate phosphorylase [5,6]. malP, encoding the MP has been localised in an operon including the genes likely to be involved in poly-, oligo- and disaccharide degradation in L. lactis[3,5]. In addition, a gene possibly coding for a transcriptional regulator, henceforth called MalR, belonging to the LacI-GalR family has been observed downstream of malP[3,5].
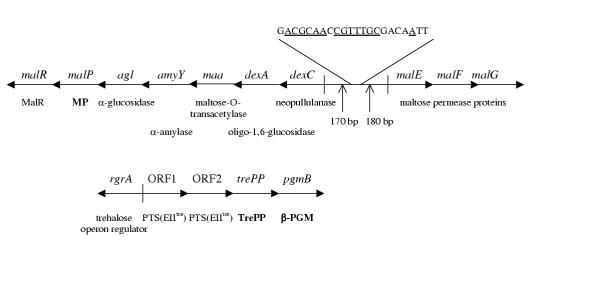
Figure 1.
The predicted maltose (above) and trehalose operons (below) of L. lactis. Genes/open reading frames are presented by arrows indicating the direction of transcription. Notations of enzymes of confirmed identity are shown in bold text. Other enzymes are from predictions based on amino acid sequence similarities. The DNA sequence shows the putative MalR binding site 170 bp and 180 bp from the translation start site of dexC and malE, respectively. The bases underlined are those that are identical to the bases found in the motifs of more than 50% of the 7 MalR binding sequences obtained from Schlösser et al. [17] and included in the current comparison. MalR, maltose operon regulator; MP, maltose phosphorylase; PTS(EIItre), trehalose-specific enzyme II component of the phosphotransferase system; TrePP, trehalose 6-phosphate phosphorylase; β-PGM, β-phosphoglucomutase. The figure is adapted from information on the genome sequence of L. lactis[5] and (GenBank accession no. Y18267) and from Nilsson and Rådström, Qian et al. and Andersson et al. [3,4,6].
In both Staphylococcus xylosus and Streptococcus pneumoniae, maltose metabolism has been shown to be dependent on a maltose operon regulator, MalR [7,8]. In S. xylosus it was shown that MalR mainly affects the maltose transport, while the corresponding regulatory protein in Strep. pneumoniae has been found to regulate two operons involved in maltose metabolism [8]. The present study highlights the physiological role of the presumed MalR in maltose metabolism in L. lactis. By investigating the activities of maltose uptake, MP and β-PGM, the influence of MalR on maltose and trehalose catabolic operons could be assessed.
Results and Discussion
MalR does not regulate the activity of MP and β-PGM in L. lactis
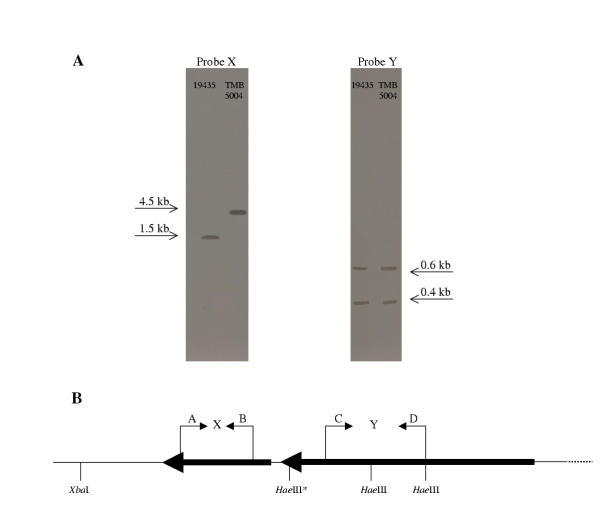
L. lactis mutants harbouring pTMB5003 were screened for impaired maltose catabolism by testing their inability to ferment maltose in liquid medium containing maltose and erythromycin. Since pTMB5003 harbours an internal fragment of malP it was supposed to integrate into the corresponding malP region of the lactococcal chromosome by a homologous recombination event and most likely negatively affect the transcription of malP (Fig. 1). Therefore, the specific activity of MP in the mutants was determined. All mutants possessed the same MP specific activity as the wild-type, 19435, and one of them, TMB5004, was chosen and further investigated for its impaired maltose catabolism. According to Southern blot analysis, the integration of pTMB5003 was localized to the left of the HaeIII* restriction enzyme recognition site in the malR-malP chromosomal region of TMB5004, since a DNA fragment of 4.5 kb was detected (Fig. 2). This DNA fragment should be expected if pTMB5003, based on the 3.2 kb integration vector pFL20 [9] was integrated. By using probe Y corresponding to an internal fragment of malP, it was confirmed that the region to the right of the HaeIII* site was unaltered when pTMB5003 integrated. We cannot further explain the recombination event in TMB5004, but this mutant was henceforth considered as a presumed MalR-deficient strain.
Figure 2.
Southern blotting of the malP-malR DNA region in L. lactis 19435 and TMB5004. A) Southern blotting results using probe X and Y (see below). B) Relevant HaeIII and XbaI restriction recognition sites in the malR-malP region of L. lactis. Probe X was constructed by using primers A and B and probe Y was constructed by using primers C and D. 10 mm corresponds to 0.2 kb.
The activities of MP and β-PGM were measured and found to be the same in the TMB5004 as in 19435 (Table 1), suggesting a negligible influence of MalR on these enzyme activities in L. lactis. In a previous study it was shown that the transcription of malP is subjected to carbon catabolite repression and that no specific sugar can be assigned for its induction [3]. β-PGM is essential for trehalose metabolism in L. lactis[9] and its encoding gene, pgmB, is located together with genes likely to encode proteins involved in the uptake and degradation of trehalose [3]. Since a gene highly likely to encode a transcriptional regulator is located in the putative trehalose operon, upstream of pgmB, it is possible that there is another system for the regulation of β-PGM activity in L. lactis[5]. In addition, the cultivation of TMB5004 on trehalose was shown not to differ from that of the wild type strain (data not shown). Thus, from the present study we may conclude that no co-regulation of the maltose and trehalose operons is exerted by the MalR, in L. lactis.
Table 1.
Specific MP and β-PGM activities in cell extracts of L. lactis 19435 and TMB5004 cultivated on different carbon sources.
| Carbon source | MP activity (U mg protein-1)a | β-PGM activity (U mg protein-1)a | ||
| 19435 | TMB5004 | 19435 | TMB5004 | |
| Maltose | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.52 ± 0.03 | 0.50 ± 0.04 |
| Glucose | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.19 ± 0.02 | 0.12 ± 0.01 |
Mean values and standard deviations are given for each cell extract analysed in triplicate in each assay. a Specific activity in cuvette. 1 U mg protein-1 corresponds to 1 μmol substrate converted per minute per milligram protein added.
MalR is crucial for active maltose translocation
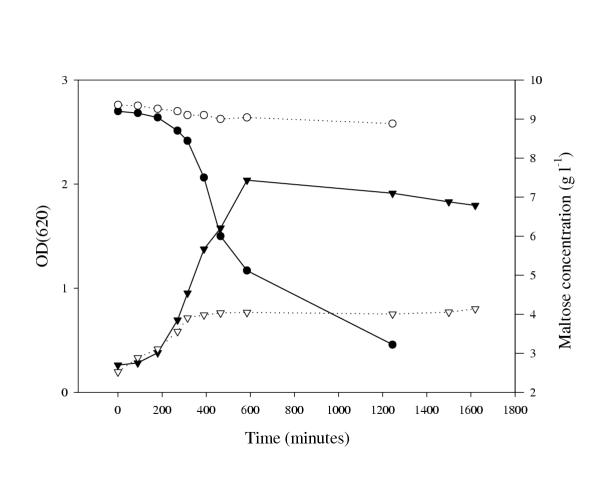
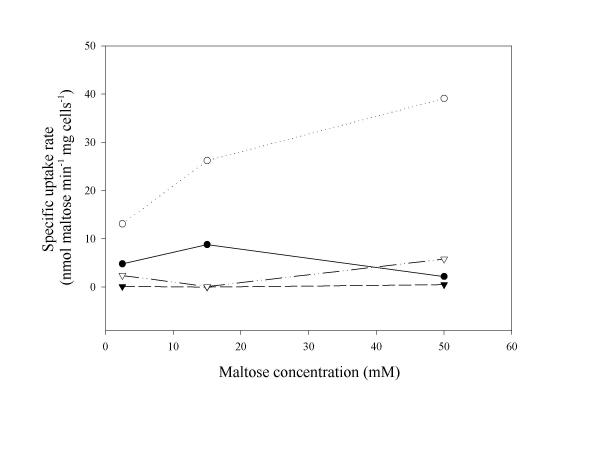
As the mutant, TMB5004, was not able to ferment maltose (Fig. 3) and since the activities of relevant maltose-degrading enzymes were unaffected (Table 1), an investigation of the maltose uptake system was performed. By monitoring the ability of the lactococcal strains to transport maltose, an explanation of the maltose-negative phenotype of TMB5004 was obtained (Fig. 4). The minimum maltose transport capacity observed for the wild type, when cultivated on maltose, was five times higher than that of the mutant. The difference in uptake rate increased when using higher maltose concentrations in the assay. Since the maltose uptake was shown to be considerably lower in glucose-cultivated lactococci, we may conclude that glucose has a repressive effect on a functional maltose permease complex, as has been reported earlier [10]. Moreover, as curves for the specific uptake rates in lactococci, cultivated in medium with no sugar added, followed those for glucose-cultivated lactococci (Fig. 4 and other data not shown), it is suggested that an active maltose translocation system requires the presence of maltose in the cultivation medium, in addition to a functional MalR protein.
Figure 3.
Cultivation of L. lactis 19435 and TMB5004 on maltose. Growth curve of L. lactis 19435 (▼); growth curve of L. lactis TMB5004 (▽); maltose concentration in cultivation medium of L. lactis 19435 (●); maltose concentration in cultivation medium of L. lactis TMB5004 (○).
Figure 4.
Specific maltose uptake rates at different maltose concentrations (2.5 mM-50 mM) used in the assay.L. lactis 19435 cultivated on glucose (●); L. lactis 19435 cultivated on maltose (○); L. lactis TMB5004 cultivated on glucose (▼); L. lactis TMB5004 cultivated on maltose (▽). The assay was run in duplicate and for every cell resuspension and results were obtained with a variation coefficient of 10%.
MalR – an exception to the LacI-GalR family of transcriptional repressors
The amino acid sequence of the MalR of L. lactis shows 30% identity to those of known MalR proteins of Strep. pneumoniae and S. xylosus (GenBank accession nos. AAK05774, Q08511, Q56201). Moreover, by studying the operator region upstream of malE, we found a putative MalR binding site (Fig. 1) sharing some identical motifs with known DNA sequences of MalR binding sites (GenBank accession no. AE006399) [11-14]. However, no MalR binding site could be found in the regions upstream of malP or pgmB. This verifies the findings of the present study, namely that the maltose transporter is the only component involved in maltose assimilation that, so far, shows any dependence of the MalR protein in L. lactis.
In contrast to the activating effect of MalR on the maltose permease complex in L. lactis, members of the LacI-GalR family have been demonstrated to be transcriptional repressors [15]. One report describes the regulation of two operons involved in maltose uptake and utilisation, by the Strep. pneumoniae MalR protein. This regulator is a repressor protein, as is the MalR of Clostridium butyricum, also a member of the LacI-GalR family [8,12]. However, another exception has been detected in S. xylosus, the MalR protein of which was shown to be required for the full activity of the maltose translocation system, as in the case of L. lactis[7]. Furthermore, the MalR-encoding gene in that strain was shown to be located directly upstream of the maltase-encoding gene, the transcription of which was not affected by the presence or absence of MalR.
Conclusions
MalR does not exert any regulation of the activity of MP and β-PGM, the key enzymes of maltose metabolism in L. lactis. MalR should be regarded as an activator, due to its suggested activating effect on the maltose transport system. This regulatory protein of L. lactis is, in addition to the MalR protein of S. xylosus, an exception to the transcriptional repressors belonging to the LacI-GalR family.
Materials and Methods
Genetic techniques
All DNA-modifying enzymes and corresponding buffers were purchased from Roche Molecular Biochemicals (Roche Diagnostics Scandinavia AB, Sweden). Polymerase chain reaction (PCR), restriction enzyme digestions, ligations, gel electrophoresis and Southern blotting were performed according to Ausubel et al and Sambrook et al [16,17]. Probe labelling was conducted using a Random Primed DNA Labelling kit (Amersham Life Science). Extraction and purification of DNA fragments or PCR products were carried out with the aid of a QIAquick kit (Qiagen). Plasmid DNA was purified from Escherichia coli using a Bio-Rad Quantum Miniprep kit (Bio-Rad Laboratories) and chromosomal DNA was extracted from L. lactis 19435 using an Easy-DNA™ kit (Invitrogen). Ultra-competent E. coli was prepared according to Inoue et al [18] and transformation was carried out using standard procedures [17]. Competent L. lactis was prepared and transformed as described earlier [19].
Origin of L. lactis TMB5004
A DNA sequence of 850 bp internally of malP (GenBank accession no. AE006398) was amplified from chromosomal DNA of L. lactis 19435 using PCR. The PCR primers used were 5'-ggcggatcctaaaggatttactgg-3' (forward), including a BamHI restriction enzyme recognition site at the 5' end, and 5'-aatcgcatgaattgaaggag-3' (reverse). The PCR product was purified and digested with the restriction enzymes BamHI and RsaI, revealing a 450 bp product, which was purified. The integration vector pFL20 [9] was digested with restriction enzyme PstI and further treated with T4 DNA polymerase, according to the instructions issued by the manufacturer, in order to generate blunt ends. Next, pFL20 was digested by BamHI and thereafter purified from an agarose gel. The construct, termed pTMB5003, resulting from ligation of the modified pFL20 and the internal DNA sequence of malP, was propagated in E. coli DH5α and finally transformed into L. lactis 19435. Selection was made for erythromycin-resistant transformants, as proof that the pTMB5003 construct had been integrated into the chromosome of L. lactis 19435 by a single crossover event. The colonies that appeared were checked for inability to grow on maltose, MP activity and for integration of pTMB5003 by PCR. All transformants showed MP activity and one of them, termed L. lactis TMB5004, was chosen for further investigations.
Southern blotting was performed to assess the location of homologous recombination in L. lactis 19435, resulting in TMB5004. The chromosomal DNA of L. lactis 19435 and TMB5004 were digested by the restriction enzymes HaeIII and XbaI. Two probes were amplified by PCR using L. lactis 19435 chromosomal DNA as template and the primers 5'-ggcggatccattggagttgtctttccc-3' (forward, A) and 5'-ggcctgcaggcacttgcccccaattgtt-3' (reverse, B) and 5'-ggcggatcctaaaggatttactgg-3' (forward, C) and 5'-aatcgcatgaattgaaggag-3' (reverse, D).
Bacterial strains, cultivation conditions and measurement of sugar consumption
Escherichia coli DH5α, (Life Technologies), used for all cloning procedures, was cultivated in Luria-Bertani liquid or agar medium at 37°C. For the selection of E. coli transformants the medium was supplied with 250 μg ml-1 erythromycin. L. lactis ssp. lactis 19435, obtained from the American Type Culture Collection, and its derivative L. lactis TMB5004 were cultivated at 30°C on M17 agar (Oxoid) or in M17 medium (Oxoid), in standing batch cultures. Erythromycin (2 μg ml-1) was added when required and carbohydrates were autoclaved and added to the culture medium separately, to a final concentration of 10 g l-1. Cell growth was monitored by measuring the optical density at 620 nm. In order to study maltose consumption, samples were taken from the cultivations, filtered through 0.2 μm filters and kept at -20°C until analysed. The maltose concentration was analysed by high performance liquid chromatography at 45°C on a cation-exchange column (Aminex HPX-87H, Bio-Rad) and quantified using a refractive index detector (Shimadzu, Japan). The mobile phase was 5 mM H2SO4 run at 0.6 ml min-1.
Cell extract preparation, determination of protein concentration and enzyme assays
Cells were collected from cultivations and harvested by centrifugation at 5 000 × g, 2°C, for 10 minutes. The cells were washed twice and resuspended in triethanolamine buffer (pH 7.2), containing 0.5 mM MgCl2 and 0.5 mM dithiotreitol. The cells were disrupted using glass beads (0.5 mm) (KEBO Lab, Sweden) and vigorous vortexing at 8°C. Cell extracts were collected after centrifugation at 19 500 × g, 2°C, for 15 minutes, while cell debris was removed. The total protein concentration in cell extracts was determined according to the method of Bradford [20], using a bovine serum albumin standard. The specific activities of MP and β-PGM were determined using coupled assays, measuring the formation of NADPH at 30°C, and 340 nm, as described earlier [2,3]. A total of 50–200 μg of protein was added to each assay mixture and all cell extracts were analysed in triplicate.
Maltose uptake measurements
The uptake of maltose by lactococcal cells was determined using a zero-trans-influx assay adapted for bacterial cells [21]. Harvested cells were washed twice in ice-cold 0.1 M potassium phosphate buffer (pH 6.5). The cells were resuspended to a dry weight of 25–30 mg ml-1 in the same buffer and kept on ice until used. Twenty microlitres each of 0.1 M potassium phosphate buffer (pH 6.5) and cell resuspension were added to a 5 ml plastic vial and incubated at 30°C, for 5 minutes in a water bath. Ten microlitres of 14C-labelled maltose (Amersham Life Science) was added to the mixture to a final specific activity of 200 counts per minute (cpm) nmol-1, and the assay was started by vortexing the vial briefly. The assay was allowed to proceed for 10 seconds, measured by the use of a metronome, and stopped by adding 3 ml of ice-cold 0.5 M maltose from a dispenser. The reaction mixture was rapidly filtered through a glass microfibre filter (GF/F, Whatman®, Merck Eurolab) and the reaction vial was washed twice with 3 ml of 0.5 M maltose. Finally, the filter equipment was washed twice with 5 ml 0.5 M maltose before the filter was removed. The filter was placed in a scintillation vial containing 5 ml scintillation solution (Ecoscint™ A, Hintze AB). Tests were run in duplicate and for every cell resuspension a background sample was prepared. The background samples were prepared and treated as the other assays, except that they were not vortexed. Instead, the reaction mixture was filtered immediately after the addition of 14C-maltose.
Authors' contributions
U. A. participated in the design of the study, carried out the laboratory work and drafted the manuscript. P. R. participated in the design of the study and its coordination. Both authors read and approved the final manuscript.
Contributor Information
Ulrika Andersson, Email: Andersson.Veb@swipnet.se.
Peter Rådström, Email: Peter.Radstrom@tmb.lth.se.
References
- Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N, Stanley GA, Hahn-Hägerdal B, Rådström P. Purification and characterization of two phosphoglucomutases from Lactococcus lactis subsp. lactis and their regulation in maltose-and glucose-utilizing cells. J Bacteriol. 1994;176:5304–5311. doi: 10.1128/jb.176.17.5304-5311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson U, Rådström P. Genetic locatisation and regulation of the maltose phosphorylase gene, malP, in Lactococcus lactis. Microbiology. 2001;147:1565–1573. doi: 10.1099/00221287-147-6-1565. [DOI] [PubMed] [Google Scholar]
- Qian N, Stanley GA, Rådström P. Product formation and phosphoglucomutase activities in Lactococcus lactis: cloning and characterization of a novel phosphoglucomutase gene. Microbiology. 1997;143:855–865. doi: 10.1099/00221287-143-3-855. [DOI] [PubMed] [Google Scholar]
- Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11:731–753. doi: 10.1101/gr.GR-1697R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Levander F, Rådström P. Trehalose 6-phosphate phosphorylase is part of a novel metabolic pathway for trehalose utilization in Lactococcus lactis. J Biol Chem. 2001;276:42707–42718. doi: 10.1074/jbc.M108279200. [DOI] [PubMed] [Google Scholar]
- Egeter O, Bruckner R. Characterization of a genetic locus essential for maltose-maltotriose utilization in Staphylococcus xylosus. J Bacteriol. 1995;177:2408–2415. doi: 10.1128/jb.177.9.2408-2415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyet A, Ibanez AM, Espinoza M. Characterization of the Streptococcus pneumoniae maltosaccharide regulator MalR, a member of the LacI-GalR family of repressors displaying distinctive genetic features. J Biol Chem. 1993;268:25402–25408. [PubMed] [Google Scholar]
- Levander F, Andersson U, Rådström P. The physiological role of β-phosphoglucomutase in Lactococcus lactis. Appl Environ Microbiol. 2001;67:4546–4553. doi: 10.1128/AEM.67.10.4546-4553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citti JE, Snadine WE, Elliker PR. Lactose and maltose uptake by Streptococcus lactis. J Dairy Sci. 1966;50:485–487. doi: 10.3168/jds.S0022-0302(67)87451-4. [DOI] [PubMed] [Google Scholar]
- Schlösser A, Weber A, Schrempf H. Synthesis of the Streptomyces lividans maltodextrin ABC transporter depends on the presence of the regulator MalR. FEMS Microbiol Lett. 2001;196:77–83. doi: 10.1016/S0378-1097(00)00566-8. [DOI] [PubMed] [Google Scholar]
- Nieto C, Espinosa M, Puyet A. The maltose/maltodextrin regulon of Streptococcus pneumoniae. J Biol Chem. 1997;272:30860–30865. doi: 10.1074/jbc.272.49.30860. [DOI] [PubMed] [Google Scholar]
- Goda SK, Eisa O, Akhter M, Minton NP. Molecular analysis of the malR gene of Clostridium butyricum NCIMB a member of the LacI-GalR family of repressor proteins. FEMS Microbiol Lett. 7423;165:193–200. doi: 10.1016/S0378-1097(98)00276-6. [DOI] [PubMed] [Google Scholar]
- Egeter O, Bruckner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- Weickert J, Adhaya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Virginia Benson Chanda. 1996.
- Sambrook J, Fitsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Press. 1989.
- Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-P. [DOI] [PubMed] [Google Scholar]
- Holo H, Nes IF. High-frequency transformation by electroporation of Lactococcus lactis ssp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitaion of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Spencer-Martins I, Van Uden N. Catabolite interconversion of glucose systems in the yeast Candida wickerhamii. Biochim Biophys Acta. 1985;812:168–172. doi: 10.1016/0005-2736(85)90535-8. [DOI] [Google Scholar]