Abstract
Background
Subtribe Artemisiinae of Tribe Anthemideae (Asteraceae) is composed of 18 largely Asian genera that include the sagebrushes and mugworts. The subtribe includes the large cosmopolitan, wind-pollinated genus Artemisia, as well as several smaller genera and Seriphidium, that altogether comprise the Artemisia-group. Circumscription and taxonomic boundaries of Artemisia and the placements of these small segregate genera is currently unresolved.
Results
We constructed a molecular phylogeny for the subtribe using the internal transcribed spacers (ITS) of nuclear ribosomal DNA analyzed with parsimony, likelihood, and Bayesian criteria. The resulting tree is comprised of three major clades that correspond to the radiate genera (e.g., Arctanthemum and Dendranthema), and two clades of Artemisia species. All three clades have allied and segregate genera embedded within each.
Conclusions
The data support a broad concept of Artemisia s.l. that includes Neopallasia, Crossostephium, Filifolium, Seriphidium, and Sphaeromeria. However, the phylogeny excludes Elachanthemum, Kaschgaria, and Stilnolepis from the Artemisia-group. Additionally, the monophyly of the four subgenera of Artemisia is also not supported, with the exception of subg. Dracunculus. Homogamous, discoid capitula appear to have arisen in parallel four to seven times, with the loss of ray florets. Thus capitular morphology is not a reliable taxonomic character, which traditionally has been one of the defining characters.
Background
Artemisia L. (Asteraceae), as broadly conceived by Linnaeus, is the largest genus in Tribe Anthemideae [1-3] and one of the largest in the family [4]. It is widespread in mid- to high-latitudes, and shrubby species dominate most cold and many warm deserts in the Northern Hemisphere [5-13]. Because of the abundance of wind-dispersed Artemisia pollen in the geological column, it is used as an indicator of steppe climates [14]. Some members are foraged by ungulates, rodents, birds, and insects [11,15-19], despite the production of sesquiterpenes that afford a bitter taste to the herbage. The woody species increase dramatically under grazing pressure, thereby excluding desirable forage [11,20,21]. Many Artemisia species are a major cause of allergies in humans [22]. All Artemisia species produce aromatic oils, and several are culinary herbs or used as flavorings, hallucinogens, vermifuges, and pharmaceuticals [23-26], and some are toxic [27]. Artemisia annua (annual wormwood) and A. mexicana produce antimalarial drugs [28-30], and artemisinin (from A. annua) appears to selectively kill human breast cancer cells [31].
Despite the well-known importance of Artemisia, there is no consensus on taxonomic relationships, which have traditionally been inferred on the basis of floral and capitular morphology. In Artemisia s.l., the typical limb of the Anthemideae-type ray florets are reduced to a membranous vestige, giving the impression that the small capitula are composed only of disk florets, referred to as disciform capitula (inflorescence) by Bremer and Humphries [2]. In other members of the genus, the ray flowers are absent, thus the capitulum is composed only of disk florets, i.e., discoid. In addition, plants with discoid capitula are considered homogamous since all florets are of one sexual form (perfect-bisexual disk florets), and plants with disciform capitula are considered heterogamous with two or more sexual forms (i.e., pistillate rays and perfect disks, or pistillate rays and staminate disks).
Taxonomic treatments for Artemisia over the past 50 years range from maintaining a single, large genus of over 500 species [32-37] to the recognition of six to eight genera from within its taxonomic boundaries [2,38,39]. Artemisia of antiquity was divided into three genera (Artemisia, Absinthium, and Abrotanum) by Tournefort [40]. However, the concept of a more inclusive genus was resurrected by Linnaeus [41], hereinafter, referred to as Artemisia s.l. Besser [42] and de Candolle [43] recognized four sections within Artemisia s.l. primarily based on the presence or absence of ray florets and the fertility/sterility of disk florets: (1) Abrotanum Besser (Artemisia of later authors) – ray florets pistillate and fertile; disk florets perfect and fertile; receptacle glabrous; (2) Absinthium (Mill.) DC – ray florets pistillate and fertile; disk florets perfect and fertile; receptacle hairy; (3) Seriphidium (Besser) Besser – ray florets absent; disk florets perfect and fertile; receptacle glabrous; and (4) Dracunculus Besser – ray florets pistillate and fertile; disk florets functionally staminate; receptacle glabrous.
The first phylogenetic treatment [44] recognized four sections within a broadly defined Artemisia s.l. with sect. Artemisia proposed as the progenitor to sect. Absinthium, Dracunculus, and Seriphidium. This phylogeny was based on two hypothesized evolutionary trends: loss of fertility in the disk florets and loss of ray florets. Sect. Artemisia and Absinthium were later united [45] and all sections were raised to the level of subgenus [46,47], i.e., subg. Artemisia, Dracunculus, and Seriphidium. In addition, a number of authors [20,21,34,35,47,48] considered the American woody sagebrushes to have an independent origin from the woody Asian species (subg. Seriphidium), and recognized sect. Tridentatae.
Poljakov [38] and others [2] segregated subg. Seriphidium as a distinct genus along with several small genera from within the boundaries of Artemisia s.l. The more recent, major classifications [2,39,49,50] have agreed with the segregation of Seriphidium on the basis of discoid-homogamous capitula and recognized the smaller segregate genera as well. Ling [39,51,52], for instance, considered Artemisia s.s. and Seriphidium to be distinct and sister to each other, and with the small segregate and allied genera in turn sister to them [53]. In their landmark monograph of Tribe Anthemideae, Bremer and Humphries [2] placed Artemisia and its allied genera in Subtribe Artemisiinae. In contrast to Ling's hypothesis [53] regarding sister group relationships between Artemisia s.s. and Seriphidium, the Bremer and Humphries [2] morphologically based cladogram placed four small genera (Neopallasia, Turaniphytum, Mausolea, Picrothamnus; with a total of seven species) as closest sisters (i.e., segregates) of Artemisia s.s. (the Artemisia-clade sensu Bremer and Humphries), whereas Seriphidium and two small genera (Kaschgaria, Crossostephium; 3 species total) were placed outside the Artemisia-clade as allies to Artemisia s.s. In addition, Sphaeromeria, Filifolium, Ajaniopsis, and Stilnolepis (13 species total) were sister to this clade of eight genera that included Artemisia s.s. and its segregates and allies. Further, Ling and Ling [54] recognized Elachanthemum as distinct from Stilnolepis, i.e., as two monotypic genera. Altogether these 12–13 genera comprise the Artemisia-group sensu Bremer and Humphries.
According to Bremer and Humphries [2], the Artemisia-group is monophyletic and defined by heterogamous-disciform capitula (disk florets usually bisexual and fertile, ray florets pistillate) or homogamous-discoid capitula (disk florets usually bisexual and fertile, ray florets absent), pollen with short or no spines (associated with anemophily), and thick-walled cypselas devoid of ribs (sometimes faint ribs are present in Sphaeromeria and Crossostephium). All of the Artemisia-group genera contain species that have been variously placed within the taxonomic boundaries of Artemisia s.l. with the exceptions of Sphaeromeria, Neopallasia, Turaniphytum, and Mausolea. Nonetheless, these four genera are always considered closely related to it [2,33,37,38,55]. Artemisia s.s. and Seriphidium are composed of approximately 380 and 140 species, respectively, and are geographically widespread. Their species occur predominantly throughout Europe and temperate Asia, as well as in western North America. Sphaeromeria and Picrothamnus are endemic to western North America, whereas all remaining Artemisiinae genera occur in eastern, southwestern, or central Asia.
Several biological factors may account for the disagreement and wide range of interpretation in relation to generic circumscription and subgeneric boundaries in Artemisia and its allies, including rapid and recent diversification, reduction in floral features (associated with anemophily) with the concomitant loss of potentially useful taxonomic characters, multiple origins of woodiness [34,35,56], and chromosomal evolution and hybridization [57-60]. In addition, most studies have focused on a limited geographic region, rather than studying the taxon worldwide.
The primary objective of this study is to construct a molecular phylogeny of Subtribe Artemisiinae, including Artemisia and its allies, employing the internal transcribed spacers (ITS) of nuclear ribosomal (nr) DNA. The goals are (1) to test the monophyly of Subtribe Artemisiinae and the Artemisia-group; (2) determine the phylogenetic placements of the segregate and allied genera in relation to Artemisia s.s.; and (3) evaluate the patterns of diversification and test biogeographic hypotheses and evolutionary trends in capitular and floral morphology in the Artemisia-group [49,50]. The ITS have been demonstrated to be appropriate for examining generic relationships in many plant families including the Asteraceae [61], for Tribe Anthemideae specifically [62,63] and within Artemisia [34,36].
Results
The PCR products of the ITS were resolved as single bands on 1.0% agarose gels. Of the 520 aligned nucleotide positions for both spacers combined (excluding gaps required for alignment), 213 were phylogenetically informative. Sequence divergence (uncorrected "p") is 4–6% among members of the Artemisia-clade, 4–10% among members of Subtribe Artemisiinae, and 7–17% between Subtribe Artemisiinae and the outgroup species from other Anthemideae subtribes.
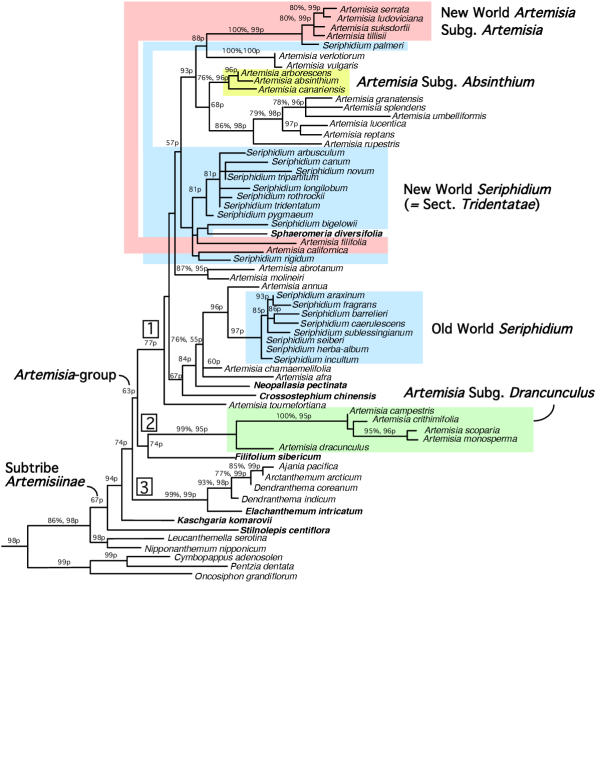
Resulting phylogenetic trees obtained by maximum parsimony (MP), maximum likelihood (ML), and Bayesian analyses were congruent in topology. All three analyses resolved three major clades for Subtribe Artemisiinae, with MP the least resolved and ML the most highly resolved. The topologies differed in the placement of Stilnolepis, which was placed outside of Subtribe Artemisiinae by MP and at the base of the subtribe by both ML and Bayes. Otherwise, the tree topologies differed only in their ability to resolve relationships within the three major clades (numbered 1, 2, 3 in Fig. 1).
Figure 1.
Maximum likelihood tree based on ITS sequence data for Subtribe Artemisiinae (Asteraceae). With the exception of Nipponanthemum, Leucanthemella, Cymbopappus, Pentzia and Oncosiphon, all outgroup genera used in analysis are not shown but are listed in Table 1 (see additional file 1). Small segregate and allied genera of Artemisia s.l. are in bold. Subgeneric assignments for Artemisia s.s. are indicated by colored boxes. All Artemisia species that are not placed in a colored box are members of subg. Artemisia and primarily occur in the Old World. Numbers followed by '%' are bootstrap values derived from 100 bootstrap replicates using parsimony criteria, and numbers followed by 'p' are posterior probabilities derived from Bayesian analysis using likelihood criteria.
Using MP, two analyses (10 replicates with TBR branch swapping on 5000 trees per replicate and 100 replicates with TBR branch swapping on 1000 trees per replicate) resulted in 30,000 equally parsimonious trees of 1056 steps (CI = 0.339, RI = 0.707, RC = 0.240). The resulting consensus trees from each MP analysis are identical in topology, with little resolution achieved (trees not shown). Three major clades of Subtribe Artemisiinae were resolved and include: 1) species of all Artemisia subgenera except subg. Drancuculus, as well as Seriphidium, Crossostephium, Neopallasia, and Sphaeromeria; 2) Filifolium and species of Artemisia subg. Dracunculus; and 3) Ajania, Arctanthemum, Dendranthema, and Elachanthemum. Kaschgaria is placed within the Subtribe Artemisiinae clade, but its position is unresolved, while Stilpnolepis is placed outside the subtribe. The closest sister genera to Subtribe Artemisiinae are Nipponanthemum and Leucanthemella (Subtribe Leucantheminae). Three genera (Oncosiphon, Eriocephalus, and Microcephala) from Subtribe Matricariinae are sister to this larger clade. Bootstrap analysis supported 17 ingroup nodes for the MP tree at values >50%, and are indicated on the ML tree (Fig. 1).
The ML analysis converged upon a single tree with a log likelihood value of -6970.3335. Likelihood parameters for the dataset were determined by Modeltest 3.06 for the TrN+G model, and included the following: base frequencies of A = 0.2482, C = 0.207, G = 0.2466, T = 0.2982; r-matrix values of A-C = 1.0000, A-G = 3.1857, A-T = 1.0000, C-G = 1.0000, C-T = 5.1655, G-T = 1.0000; and a gamma distribution of 0.5663. The ML tree resolved Subtribe Artemisiinae as monophyletic, with Leucanthemella and Nipponanthemum as its closest sisters. Within Subtribe Artemisiinae, Stilnolepis and Kaschgaria form independent clades at the base of the subtribe. Similar to the MP tree, the same three major clades are resolved (Fig. 1).
Bootstrap analysis of the data using ML criteria was not computationally feasible given the size of the data set. Therefore as an alternative, we conducted a Bayesian analysis utilizing a GTR model with 6 rate classes (A-C = 1, A-G = 3.19, A-T = 1, C-G = 1, C-T = 5.16, G-T = 1) for 1 × 106 generations sampled every 10 generations that resulted in a posterior probability distribution containing 1 × 105 samples. The analysis converged on similar log likelihood scores (mean = -2431), and reached stationarity no later than 2500 generations. The initial ('burn-in') 2500 samples from the analysis were discarded and a majority rule consensus tree of the remaining 97,500 samples was constructed to obtain posterior probabilities, and resulted in a tree with 21 ingroup nodes with a significance level >95%. Posterior probabilities are indicated on the ML tree (Fig. 1). The Bayesian tree was identical in topology to the ML tree, with the exception that the New World Seriphidium subclade was unresolved.
Discussion
Monophyly of Subtribe Artemisiinae and the Artemisia-group
The ITS data provide weak support for the monophyly of Subtribe Artemisiinae (Fig. 1), i.e., 67% posterior probability as estimated by MrBayes and unsupported as measured by parsimony bootstrap analysis (<50%). ndhF sequence data also failed to resolve or support the monophyly of Subtribe Artemisiinae [64]. In the ITS phylogeny, only one genus appears to disrupt its monophyly, Stilnolepis, which occupies the basal-most position of the ingroup. However, the next node up is supported at a 94% posterior probability, indicating that Subtribe Artemisiinae is likely monophyletic, with the possible exception of Stilnolepis.
The ITS phylogeny (Fig. 1) resolved three major clades within Subtribe Artemisiinae that correspond to the following: 1) all subgenera of Artemisia minus Dracunculus, 2) Artemisia subg. Dracunculus, and 3) the largely radiate members of the subtribe (Ajania, Arctanthemum, and Dendranthema). All three clades have segregate genera embedded within each (indicated in bold), and these clades are supported at 77, 74, and 99% posterior probabilities, respectively, with the third clade also supported by a bootstrap value of 99%. The Artemisia-group sensu Bremer and Humphries [2] is not monophyletic because three segregate genera Elachanthemum, Stilnolepis, and Kaschgaria are excluded from this clade. Additionally, Artemisia s.l. is monophyletic only with the inclusion of five segregate genera: Seriphidium, Sphaeromeria, Neopallasia, Crossostephium, and Filifolium. Additionally, this clade is only weakly supported with a 63% posterior probability and bootstrap support <50%.
Placement of segregate and allied genera
Three of the eight segregate and allied genera that we examined were placed outside of the Artemisia-group (including Stilnolepis, Elachanthemum, and Kaschgaria), whereas the remaining five were placed within it (including Seriphidium, Sphaeromeria, Neopallasia, Crossostephium, and Filifolium). However many of these placements are among the most weakly supported branches of the resulting phylogenetic trees (Fig. 1).
Stilnolepis, Elachanthemum, and Kaschgaria are excluded from the clade of the Artemisia-group, and are, in part, sister to all remaining Artemisiinae genera in the ITS phylogeny. Stilnolepis and Elachanthemum are monotypic genera that occur in Central Asia, that are often treated as a single genus, Stilnolepis [2]. However, our ITS phylogeny supports Ling's treatment of them as two distinct genera [54]. Neither genus appears to be a member of Artemisia s.l. or the Artemisia-group. Both genera are discoid with corymbose capitula, but whether either genus possesses spineless pollen (a synapomorphy for the Artemisia-group) or is wind-pollinated has not yet been determined [2,37]. Kaschgaria, a genus of two species from China and central Asia, is also placed outside of the Artemisia-group, but clearly within Subtribe Artemisiinae (94% probability) in the ITS phylogeny. Kaschgaria was removed by Poljakov from Artemisia s.l. on the basis of stellate corolla hairs, and was considered more closely related to Seriphidium than Artemisia s.s., even though it is disciform rather than discoid [2]. Kaschgaria possesses spineless pollen [2,37]. Therefore, placement of Kaschgaria and Elachanthemum outside of the Artemisia-group is intriguing because if these placements remain robust, wind-pollination may have arisen more than once in the tribe, or insect-pollination may be secondarily derived within Subtribe Artemisiinae in the radiate genera (clade 3 of Fig. 1).
Four of the small allied/segregate genera are embedded within the clade of the Artemisia-group, in addition to the large segregate genus Seriphidium (Fig. 1). Filifolium is sister to species of subg. Dracunculus, and together comprise a sister clade to all remaining Artemisia species and four segregate genera, moderately supported at 74% probability. Monotypic Filifolium occurs in China and Korea, and was previously considered a species of subg. Dracunculus on the basis of sterile disk florets. Cassini [65] segregated subg. Dracunculus as Oligosporus with the sterility of the central florets as the defining synapomorphy that unites these species (Fig. 2b). Bremer and Humphries [2] noted that Mausolea and Picrothamnus share this trait, and suggested that Oligosporus should be resurrected and defined to include these three segregate genera. Mausolea was segregated by Poljakov [38] from Artemisia s.l. by marginal flowers that lack corollas. Picrothamnus was established by Nuttall, but reduced as A. spinescens Hall and Clements, and according to Bremer and Humphries [2] is essentially Dracunculus with two autapomorphies: spiny habit and cobwebby-pilose cypselas. Thus two taxonomic treatments are viable: 1) the recognition of two subgenera within Artemisia s.l. – subg. Dracunculus and an expanded subg. Artemisia; or 2) the resurrection of Oligosporus (following Cassini) to include species of subg. Dracunculus, Filifolium, and perhaps Mausolea and Picrothamnus. Unfortunately we were unable to obtain ITS sequences for Mausolea or Picrothamnus.
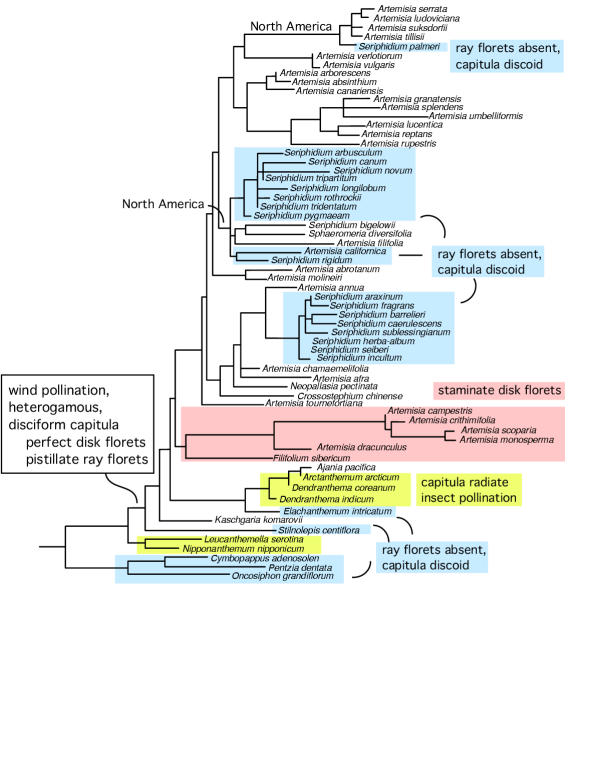
Figure 2.
Character evolution of Subtribe Artemisiinae (Asteraceae) mapped on to the Maximum Likelihood tree derived from ITS sequence data. Unless otherwise indicated by colored boxes, all Subtribe Artemisiinae species are wind-pollinated, occur primarily in Eurasia, and possess heterogamous-disciform capitula with perfect (bisexual) disk and pistillate ray florets.
Monotypic Crossostephium was treated as a segregate genus of Artemisia by Bremer and Humphries [2]. It is distinct from Artemisia by the presence of a coroniform pappus, however it was once placed near A. californica by Gray [45] and Rydberg [47] on the basis of deeply-ribbed cypselas. Crossostephium occurs in the Phillipines, Taiwan, southern Japan, and China, whereas A. californica occurs in western North America. Based on the ITS results, we conclude that Crossostephium is allied with Old World Seriphidium and several Artemisia subg. Artemisia species, and A. californica is sister to most of the New World species of Seriphidium (Fig. 1). Although placements of both are weakly supported (68 and 57% probabilities for Crossostephium and A. californica, respectively; Fig 1).
Neopallasia is a genus of three species that occur in central Asia, including southern Siberia, Mongolia, and China [2]. It was segregated from Artemisia by Poljakov [66] on the basis of several autapomorphies, including pectinate leaves, central-sterile florets at the apex of the capitulum, ovoid-lanceolate anther appendages, and a rosette-shaped arrangement of cypselas on the receptacle. Poljakov [66] and Bremer and Humphries [2] contended that Neopallasia is closely related to Artemisia subg. Drancunculus. However, the ITS data place Neopallasia sister to Asian species of Seriphidium and some members of Artemisia subg. Artemisia, close to Crossostephium. This placement is moderately supported at 87% probability.
Sphaeromeria is considered closely related to Artemisia s.l., although it was once treated as a section of Tanacetum [55]. It is a small genus of nine western North American species, and has no autapomorphies that distinguish it from Artemisia s.l. Bremer and Humphries [2] suggested that it might be more closely related to Kaschgaria, a genus composed of two species that occur in central Asia and China. They are similar in habit and have similar inflorescences that are slightly elongated. However, in the ITS phylogeny (Fig. 1), Sphaeromeria is distant to Kaschgaria. Rather its placement is unsupported within a Seriphidium subclade that contains several anomalous North American species, some of which have been placed in sect. Tridentatae. However, because of the lack of support for placement of Sphaeromeria, we are hesitant to suggest that it be submerged in Artemisia s.l. without further study. However, it does not appear closely related to Kaschgaria.
Seriphidium palmeri is an anomalous species that was once treated as segregate genus Artemisiastrum. The ITS phylogeny places it among New World subg. Artemisia species, in agreement with previous studies [34,35] and not in agreement with Bremer and Humphries [2] who place it near Seriphidium sect. Tridentatae. This is a strongly supported relationship at 100% bootstrap and 99% probability. Thus Artemisiatrum, should not be resurrected, with S. palmeri more closely related to the New World species of Artemisia subg. Artemisia rather than with the New World Seriphidium species that comprise sect. Tridentatae.
Infrageneric classification of Artemisia s.l
While this study is incomplete with regards to subgeneric boundaries because only a limited number of Artemisia species was sampled from this large genus, some preliminary conclusions can still be made on emerging patterns of the derived phylogeny. Artemisia subg. Artemisia is not supported as monophyletic, with its species placed in six different clades in the ITS tree (Fig. 1). It is apparently defined taxonomically on the basis of plesiomorphies (heterogamous, disciform capitula with perfect/fertile disk florets and pistillate ray florets), and needs to be re-circumscribed. Artemisia subg. Absinthium is monophyletic (96% probability, 76% bootstrap) in the ITS phylogeny and differs from all other subgenera by a hairy receptacle. However it is embedded within a clade of species of Old World subg. Artemisia. Thus, we agree with Gray [45] that the species of subg. Absinthium should be merged into subg. Artemisia. Similarly, Old World Seriphidium species are monophyletic (96% probability), but should also be subsumed within Artemisia subg. Artemisia.
Previously, the origin of the Tridentatae was unresolved with competing hypotheses from the New World Artemisia species vs. Old World Seriphidium species being equivocal [34,35]. The current ITS phylogeny suggests that the closest relatives of Tridentatae lie among the New World species of Artemisia, in agreement with McArthur and Plummer [20], perhaps sharing a common ancestry with A. filifolia and A. californica, in addition to Sphaeromeria, although monophyly for this subclade is unsupported by the ITS data. Seriphidium sect. Tridentatae does not share a most recent common ancestor with Old World Seriphidium species, and it should not be treated with the Old World woody artemisias, as we and others have previously suggested [34-36].
Seriphidium sect. Tridentatae is characterized by discoid-homogamous capitula, with the exception of S. bigelowii, which has occasional heterogamous-disciform capitula of one pistillate ray floret and two perfect disk florets – a character that was previously considered a plesiomorphy or secondary reversal in S. bigelowii [2]. Based on ITS and chloroplast phylogenies of sect. Tridentatae, we previously concluded [34,35] that S. bigelowii was perhaps misplaced and should be excluded from the Tridentatae. In addition, we explained [34,35] an unexpected relationship between it and A. filfifolia as possible hybridization and introgression involving bidirectional gene flow rather than recent common ancestry because heterogamous-disciform A. bigelowii differs from heterogamous-disciform A. filifolia by perfect disk florets instead of female-sterile disk florets. Sphaeromeria also has heterogamous-disciform capitula with pistillate ray and perfect disk florets, as does heterogamous-disciform S. bigelowii, suggesting that it is indeed a plesiomorphy. The occasional appearance of disciform capitula in S. bigelowii might be explained by introgression of genes or alleles from A. filfolia that control ray expression, a phenomenon observed in discoid Senecio squallidus and radiate S. vulgaris in Europe [67]. While this is speculative, it is consistent with hypotheses that hybridization and introgression are widespread in sect. Tridentatae [68], and may explain occasional heterogamous disciform capitula in S. bigelowii [34,35]. However, there may be many genetic and developmental mechanisms that affect ray formation and control floret fertility [69,70].
Artemisia subg. Drancunculus is monophyletic and is sister to all remaining Artemisia species. It, perhaps combined with Filifolium, Mausolea, and Picrothamnus, could be treated as a separate genus, Oligosporus (following Cassini), or as one of two subgenera of Artemisia. In the latter case, all remaining species of Artemisia (including subg. Absinthium and Artemisia) and Seriphidium (including sect. Tridentatae) would collectively form a much expanded subg. Artemisia. Thus, Artemisia s.l. should either be treated as two subgenera, i.e., a largely expanded subg. Artemisia (Clade 1 of Fig. 1) and Dracunculus (Clade 2 of Fig. 1), or as two distinct genera, Artemisia and Oligosporus. Both clades are moderately supported at 77 and 74% probabilities, and include several segregate genera as well. Therefore, we favor retaining the Linnean concept of the genus with subg. Dracunculus conserved, and the numerous segregate genera eventually being subsumed within Artemisia s.l.
Sister group relationships of Subtribe Artemisiinae
Sister group relationships of Subtribe Artemisiinae were unresolved on the basis of morphology [2]. However an ITS phylogeny of Francisco-Ortega et al. [62] that primarily focused on the origin of Macaronesian endemic genera of Anthemideae revealed a possible sister group relationship of Leucanthemella and Nipponanthemum (Subtribe Leucantheminae) to Subtribe Artemisiinae. Monotypic Nipponanthemum occurs in Japan and the two species of Leucanthemella occur in Eastern Europe and the Far East in Manchuria, Korea, and Japan. An ITS phylogeny of Subtribe Leucantheminae [71] also excluded these two genera from that subtribe, which is otherwise Mediterranean in distribution, congruent with a chloroplast phylogeny based on ndhF for the entire tribe [64]. The ndhF phylogeny failed to support the subtribal classification of Tribe Anthemideae sensu Bremer and Humphries, and failed to resolve the intergeneric relationships or support the monophyly of Subtribe Artemisiinae. However this study resulted in the recognition of the importance of biogeography in the evolution of the tribe as a whole, with a major divergence between relictual South African genera and Northern Hemisphere Eurasian genera where members of Subtribe Artemisiinae were placed. Our ITS phylogeny of Subtribe Artemisiinae that included 40 outgroup genera from seven Anthemideae subtribes also supports a sister group relationship of Nipponanthemum and Leucanthemella to Subtribe Artemisiinae (Fig. 1).
A close resemblance of Nipponanthemum and Leucanthemella to the Asian genus Dendranthema of Subtribe Artemisiinae was previously noted by Bremer and Humphries [2], when they provisionally placed these genera in Subtribe Leucantheminae. The ITS data clearly support a sister group relationship between these two genera and subtribe Artemisiinae and their exclusion from Subtribe Leucantheminae. Both Leucanthemella and Nipponanthemum are radiate, supporting a radiate ancestry for Subtribe Artemisiinae.
Evolution of floral traits
The genera that comprise the Artemisia-group are closely related and were considered monophyletic on the basis of ancestrally, disciform-heterogamous capitula and spineless pollen, with discoid capitula secondarily derived by the loss of ray or marginal florets (Fig. 2). Phaeostigma and Ajania, which are sister to the Artemisia-group in the Bremer and Humphries [2] cladogram, contain some species that were once classified as Artemisia s.l. since they are also disciform. However, these two genera were removed from Artemisia s.l., and on the basis of spiny pollen have been considered transitional between the Artemisia-group and four radiate genera: Tridactylina Arctanthemum, Brachanthemum, and Dendranthema. Our ITS phylogeny places disciform Ajania sister to radiate Arctanthemum derived from within the Arctanthemum-Dendranthema clade, rather than sister to the Artemisia-group. Thus, disciform Ajania does not appear to be intermediate between radiate Artemisiinae and disciform Artemisia and allies. Furthermore, the tentative placement of disciform Kaschgaria and discoid Stilnolepis sister to the entire subtribe suggests that there may have been multiple origins for disciform, as well as discoid capitula.
Biogeography
A complex scenario for the origin and diversification of Artemisia and Seriphidium, and subsequent parallel evolution of the two genera in the Old and New Worlds, was proposed by Ling [39,49]. The putative common ancestor to both "sister genera" was hypothesized to have existed in northern Asia, based on reported fossil pollen records that date the taxa to the early Oligocene or lower Eocene of the lower Tertiary, with speciation accompanied by migrations during the Tertiary and Quaternary periods – forced southward by the advancing polar ice sheets. Hypothesized migrations out of northern Asia purportedly occurred along three lines: 1) westward into Europe, with gradual migration into North America, western Asia, Asia Minor, the Mediterranean, and Africa; 2) eastward into Siberia into western North America, and into eastern Europe; and 3) further south into Asia primarily during the Quaternary. Seriphidium is clearly embedded as two distinct clades within Artemisia in the molecular phylogenies, making a sister group relationship unlikely.
According to Ling [39,49], a 'pro-Absinthium' ancestor in northern Asia gave rise to members of Absinthium, which directly gave rise to Abrotanum, which subsequently gave rise to Seriphidium. An Absinthium origin for Seriphidium has also been proposed on the basis of flavonoid data [72]. The ITS data do not support a sister group relationship of members of subg. Absinthium to the remainder of Artemisia s.l. In contrast, Absinthium is embedded within a derived clade composed of both Old and New World members of subg. Artemisia. A proposed secondary diversification occurred during the Himalayan uplift in the late Tertiary, and resulted in a center of diversity that today harbors 116 species of regional endemics of Artemisia s.s. A more detailed examination of Artemisia s.l. is necessary to more fully evaluate this hypothesis.
Hall and Clements [44] proposed a sister group relationship of subg. Artemisia to remaining subgenera. This hypothesis is also not supported because subg. Artemisia is not monophyletic. In contrast, the ITS phylogeny supports an early divergence of subg. Dracunculus from the remaining Artemisia subgenera.
It appears that the New World species of Artemisia s.l. are paraphyletic and that the North American lineages arose once with at least one back migration to the Old World by some members of subg. Artemisia and Absinthium (Fig. 2). Alternatively, the North American lineages may have arisen twice, independently. Graham [73] suggested that the most convincing earliest fossil pollen records for Artemisia are late Oligocene from Central Europe. In all likelihood, Subtribe Artemisiinae and Artemisia s. l. originated in Eurasia sharing a relatively ancient common ancestry with Asian members of Leucantheminae and South African members of Subtribe Matricariinae [64].
Conclusion
The ITS data do not support the segregation of numerous small genera or Seriphidium from within the boundaries of Artemisia. Two major clades for Artemisia s.l. are supported and include subg. Dracunculus and subg. Artemisia, with the segregate genera and subgenera of Artemisia embedded within subg. Artemisia. Additionally, three segregate genera are placed outside of the Artemisia-clade. For Subtribe Artemisiinae, the Artemisia-group of species, are sister to the radiate genera. Additional molecular markers are necessary to further resolve relationships within the Artemisiinae, before making taxonomic decisions to subsume all segregate genera within Artemisia s.l.
Methods
ITS sequences were obtained for 57 species representing 11 genera of Subtribe Artemisiinae, and 41 outgroup species from seven additional Anthemideae subtribes. Voucher specimens were deposited in herbaria (see additional file 1). Total genomic DNA was isolated from fresh or silica-gel dried leaves using the 2X CTAB procedure [74,75]. The ITS region was amplified using primers of White et al. [76], as previously described [34]. For unpublished sequences, the spacers were sequenced on an ABI PRISM 310® Genetic Analyzer using capillary sequencing and Big Dye Terminator Chemistry (Applied Biosystems, Inc., Foster City, CA). The sequence boundaries of the spacers were determined by comparison to published Artemisia sequences [34], and complete sequences were deposited in GenBank (see additional file 1). All ITS sequences were aligned using Clustal W [77], with manual gap adjustments made to improve the alignment (see additional file 2, nexus.doc).
MP analyses were performed using the software PAUP* 4.0 [78], assuming unordered character states and equal character state weighting. Gaps were treated as missing data. The HEURISTICS search option was used, ignoring invariant and uninformative characters. Random addition of sequences for 10 replicates with TREE BISECTION-RECONNECTION (TBR) branch swapping on 5000 trees per replicate, and 100 replicates with TBR swapping on 1000 trees per replicate were performed using the following settings: MULTREES, STEEPEST DESCENT, MAXTREES = 30,000, and accelerated transformation (ACCTRAN). Strict consensus trees were constructed, and bootstrap values were calculated as measures of branch support [79] using the following bootstrap parameters: 100 bootstrap replicates, 5000 MAXTREES per replicate, random addition sequence, TBR, STEEPEST DESCENT, and MULTREES. In addition, the Consistency Index (CI), Retention Index (RI), and the Rescaled Consistency Index (RC) were calculated as measures of homoplasy in the data.
An ML analysis was also conducted as a preferred alternative to MP since comparative studies have shown that in most situations MP can fail to resolve an accurate phylogeny and generally underestimates bootstrap support [80]. Model based analyses, such as ML, estimate actual site changes from observed by taking into account various aspects of molecular evolution, such as multiple hits and among site rate heterogeneity. Simulation studies have shown that ML is typically more accurate and robust than MP [81,82]. Thus an ML analysis of the data was conducted by employing an heuristic search in PAUP* using TBR branch swapping on a starting tree based on neighbor joining, ACCTRAN, and STEEPEST DESCENT under the TrN+G DNA substitution model [83]. This model was selected using a Hierarchical Likelihood Ratio Test (HLRT) in Modeltest version 3.06 [84].
Because bootstrap analysis using ML is not computationally feasible for large data sets [85], we conducted a Bayesian analysis as an alternative employing optimization parameters similar to the nucleotide substitution model that we used for ML [86,87]. The distinction between ML and Bayesian inference is that Bayesian provides probabilities for hypotheses, not probabilities of data given a hypothesis [85,87,88]. Bayesian analysis uses Markov Chain Monte Carlo (MCMC) methods to approximate posterior probability distributions that are a direct estimation of branch support because they are the true probabilities of the resulting clades under the assumed models, unlike bootstrap values [87,88]. Additionally, bootstrap values and posterior probabilities derived from Bayesian analyses for multiple data sets appear to be correlated [88]. The Bayesian analysis was conducted with the software MrBayes 2.0 [89]. A GTR substitution model with 6 rate frequencies was selected as the most similar model to the Trn+G substitution model (the latter model is not available in MrBayes). Four MCMC chains were used with initial random starting trees. The analysis was run for 1 × 106 generations with trees sampled every 10 generations resulting in 1 × 105 samples. The analysis reached stationarity at 2500 generations, and these burnin samples were excluded from the majority rule consensus tree of 97,500 samples, which was generated with PAUP* to obtain the Bayesian posterior probabilities.
Authors' contributions
LEW conceived of the project and coordinated it, as well as collected plant material, deposited new sequences in GenBank, aligned the data, analyzed the data using MP, and drafted the manuscript. PLB collected plant material, sequenced DNA samples, assisted with the alignment, and conducted the ML and Bayesian analyses. TME and MMU sequenced DNA samples; and JRE assisted with plant identifications, provided expertise on Artemisia systematics, and provided significant input on all manuscript drafts.
All authors read and approved the final manuscript.
Supplementary Material
Microsoft-Word file of list of sample species examined with source of published sequences, voucher information, and GenBank accession numbers.
Microsoft-Word file of aligned data matrix used for phylogenetic analyses in PAUP*.
Acknowledgments
Acknowledgements
The authors thank Leila Schultz, Ron Hartman, Kare Bremer, and YR. Ling for plant material; Javier Francisco-Ortega, Sara Hoot, and Amy Kornkven for sharing data and/or technical assistance; Guillermo Orti for advice on Bayesian analyses, Christopher Clark for graphics support, the National Science Foundation for financial support to LEW (DEB-9596274), and two anonymous reviewers for thoughtful and helpful comments that improved the manuscript.
Contributor Information
Linda E Watson, Email: watsonle@muohio.edu.
Paul L Bates, Email: pbates@biocomp.unl.edu.
Timothy M Evans, Email: evanst@HOPE.EDU.
Matthew M Unwin, Email: unwinmm@muohio.edu.
James R Estes, Email: jestes@unl.edu.
References
- Heywood V, Humphries C. Anthemideae-Systematic review. In: V Heywood, C Humphries, B Turner, editor. The Biology and Chemistry of the Compositae. Academic Press; 1977. [Google Scholar]
- Bremer K, Humphries C. Generic monograph of the Asteraceae-Anthemideae. London, Bull Nat Hist Museum. 1993;23:1–177. [Google Scholar]
- Oberpreiler C, Vogt R, Watson L. Tribe Anthemideae, In: J Kadereit, editor. Families and Genera of Vascular Plants. Berlin, Springer-Verlag; 2003. [Google Scholar]
- Bremer K. Asteraceae: Cladistics and Classification. Portland, OR, Timber Press. 1994.
- Shreve F. The desert vegetation of North America. Bot Rev. 1942;8:195–246. [Google Scholar]
- Petrov M. Deserts of the World. New York, Wiley. 1976.
- Ling YR. Hengduang-Himalayan Mountains (HH), A special area from the floristic point of view for Artemisia L. (Compositae). Bull Bot Res. 1990;10:73–92. [Google Scholar]
- Ling YR. The Old World Artemisia Linn. (Compositae). Bull Bot Res. 1992;12:1–108. [Google Scholar]
- Zohary M. Plant Life of Palestine. Chronica Botanica, New Series. 1962. p. 33.
- Evenari M, Shanan L, Naphtali T. The Negev. Cambridge, MA, Harvard University Press. 1971.
- Young J, Longland W, Blank R. Management of woody species of Artemisia in western North America. In: P Caligari, D Hind, editor. Compositae: Biology and Utilization. Proceedings of the International Compositae Conference 1994. Vol. 2. Royal Botanic Gardens, Kew; 1996. pp. 271–290. [Google Scholar]
- Paniza-Cabrera A. Deserts of Spain. In: MA Mares, editor. Encyclopedia of Deserts. Norman, University of Oklahoma Press; 1999. pp. 534–535. [Google Scholar]
- Benabadii N, Bouazza M. Contribution à ybe etude bioclimatique de la steppe à Artemisia herba-alba Assoc. dans l'Oranies (Algèrie occidentala). Sécheresse. 2000;11:117–123. [Google Scholar]
- Erdtman G. Handbook of palynology:morphology, taxonomy, ecology. Copenhagen, Munksgaard. 1969.
- Klebenow D. Sagegrouse versus sagebrush control in Idaho. J Range Manag. 1970;23:396–400. [Google Scholar]
- O'Gara B. Antilocapra americana. Mammalian Species. 1978;90:1–7. [Google Scholar]
- Blust M, Hopkins T. Gustatory responses of a specialist and a generalist grasshopper to terpenoids of Artemisia ludoviciana. Entom Expe Appl. 1987;45:37–46. [Google Scholar]
- Blust M, Hopkins T. Feeding patterns of a specialist and a generalist grasshopper: electronic monitoring on their host plants. Physiol Entom. 1990;15:261–267. [Google Scholar]
- MacMahon J, Mull J, Crist T. Harvester ants (Pogonomyrmex spp.). Their community and ecosystem influences. Ann Rev Ecol Syst. 2000;31:265–291. doi: 10.1146/annurev.ecolsys.31.1.265. [DOI] [Google Scholar]
- McArthur E, Plummer A. Biogeography and management of native western shrubs: a case study, section Tridentatae of Artemisia. Great Basin Naturalist Memoirs. 1978;2:229–243. [Google Scholar]
- McArthur E, Pope C, Freeman D. Chromosomal studies of subgenus Tridentatae of Artemisia: Evidence for autopolyploidy. Amer J Bot. 1981;68:589–605. [Google Scholar]
- Lewis W, Vinay D, Zenger V. Airborne and Allergenic Pollen of North America. Baltimore, MD, Johns Hopkins Press. 1983.
- Lee K, Geissman T. Sequiterpene lactones of Artemisia: Constituents of Artemisia ludoviciana ssp. mexicana. Phytochemistry. 1970;9:403–408. doi: 10.1016/S0031-9422(00)85153-5. [DOI] [Google Scholar]
- Marco J, Barbera O. Natural products from the genus Artemisia. In: X Atta-ur-Rahman, editor. Studies in Natural Products. 7A. Amsterdam, Elsevier; 1990. [Google Scholar]
- Heinrich M, Robles M, West J, Ortiz de Montellano B, Rodriguez E. Ethnopharmacology of Mexican Asteraceae (Compositae). Ann Rev Pharmacol Toxicol. 1998;38:539–565. doi: 10.1146/annurev.pharmtox.38.1.539. [DOI] [PubMed] [Google Scholar]
- Sy L, Brown G. DeoxyarteannuinB, dihydro-deoxyteannuin B and trans-5-hydroxy-2-isopropenyl-5-methylhex-3-en-l-ol from Artemisia annua. Phytochemistry. 2001;58:1159–1166. doi: 10.1016/S0031-9422(01)00358-2. [DOI] [PubMed] [Google Scholar]
- Burrows G, Tyrl R. Toxic Plants of North America. Ames, Iowa State Univ Press. 2001.
- Malagon F, Vazquez J, Delgado G, Ruiz A. Antimalarial effect of an alcoholic extract of Artemisia ludoviciana mexicana in a rodent malaria model. Parasitologia. 1997;39:3–7. [PubMed] [Google Scholar]
- Newton P, White N. Malaria: New developments in treatment and prevention. Ann Rev Medicine. 1999;50:179–192. doi: 10.1146/annurev.med.50.1.179. [DOI] [PubMed] [Google Scholar]
- Dhingra V, Rao V, Narasu L. Current state of artemisin and its derivatives as antimalarial drugs. Life Science. 2000;66:279–300. doi: 10.1016/S0024-3205(99)00356-2. [DOI] [PubMed] [Google Scholar]
- Singh N, Lai H. Selective toxicity of dihydroartemisinin and holotransferin toward human breast cancer cells. Life Sciences. 2001;70:49–56. doi: 10.1016/S0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- Cronquist A. Phylogeny and taxonomy of the Compositae. American Midland Naturalist. 1955;53:478–511. [Google Scholar]
- Cronquist A. Asterales. In: A Cronquist, A Holmgren, N Holmgren, J Reveal, P Holmgren, editor. Intermountain Flora: Vascular Plants of the Intermountain West. Vol. 5. New York, Hafner; 1988. [Google Scholar]
- Kornkven A, Watson L, Estes J. Phylogenetic analysis of Artemisia section Tridentatae (Asteraceae) based on sequences from the internal transcribed spacers (ITS) of nuclear ribosomal DNA. Amer J Bot. 1998;85:1787–1795. [PubMed] [Google Scholar]
- Kornkven A, Watson L, Estes J. A molecular phylogeny of Artemisia sect. Tridentatae (Asteraceae) based on chloroplast DNA restriction site variation. Syst Bot. 1999;24:69–84. [Google Scholar]
- Torrell M, Garcia-Jacas N, Susanna A, Vallès J. Phylogeny in Artemisia (Asteraceae, Anthemideae) inferred from nuclear DNA (ITS) sequences. Taxon. 1999;48:721–736. [Google Scholar]
- Martin J, Torrell M, Valles J. Palynological features as a systematic marker in Artemisia s.l. and related genera (Asteraceae, Anthemideae):implications for subtribe Artemisiinae delimitation. Plant Biology. 2001;4:372–378. doi: 10.1055/s-2001-16462. [DOI] [Google Scholar]
- Poljakov P. Materials and systematics, the genus Artemisia L. Trudy Inst Bot Alma-Ata. 1961;11:134–177. [Google Scholar]
- Ling YR. The genera of Artemisia L. and Seriphidium (Bess.) Poljak. in the World. Comp Newsl. 1994;25:39–45. [Google Scholar]
- Tournefort J. Institutiones rei herbariae. 1 Paris 1700.
- Linnaeus C. Species Plantarum. Stockholm 1735.
- Besser W. Synopsis Absinthiorum. Bull Soc Nat Moscou. 1829;1:219–265. [Google Scholar]
- de Candolle A. Prodromus systematis naturalis regni vegetabilis. 1837, 6 Paris.
- Hall H, Clements F. The phylogenetic method in taxonomy: the North American species of Artemisia, Chrysothamnus, and Atriplex. Wash DC, Carnegie Inst. 1923. pp. 1–156.
- Gray A. Synoptical flora of North America. New York. 1884. [DOI] [PubMed]
- Rouy G. Flore de France, 8 Paris 1903.
- Rydberg P. (Carduales), Carduaceae, Tageteae, Anthemideae. North American Flora. 1916. pp. 244–285.
- Beetle A. A study of sagebrush, the section Tridentatae of Artemisia. University of Wyoming Agricultural Experimental Station. 1960;368:1–83. [Google Scholar]
- Ling YR. The Old World Seriphidium (Bess.) Poljak. (Compositae). Bull Bot Res. 1991;11:1–40. [Google Scholar]
- Ling YR. On the floristics of Artemisia L. in the world. Bull Bot Res. 1995;15:1–37. [Google Scholar]
- Ling YR. The New World Artemisia L. In: D Hind, H Beentje, editor. Proceedings of the Kew International Compositae Conference. Vol. 1. Royal Botanic Gardens, Kew; 1994. pp. 255–281. [Google Scholar]
- Ling YR. The New World Seriphidium (Besser) Fourr. In: D Hind, H Beentje, editor. Proceedings of the Kew International Compositae Conference 1994. Vol. 1. Royal Botanic Gardens, Kew; 1996. pp. 283–291. [Google Scholar]
- Jiang L, Ling YR. Cladistic analysis of Artemisia Linn. with its allies. Bull Bot Res. 1992;12:399–406. [Google Scholar]
- Ling Y, Ling YR. Elachanthemum, genus novum familiae compositarum. Acta phytotax sin. 1978;16:61–65. [Google Scholar]
- Holmgren A, Schultz L, Lowrey T. Sphaeromeria, a genus closer to Artemisia than to Tanacetum (Asteraceae:Anthemideae). Brittonia. 1976;28:252–262. [Google Scholar]
- Carlquist S. Wood anatomy of Anthemideae, Ambrosieae, Calenduleae, and Arctotideae (Compositae). Aliso. 1966;6:1–23. [Google Scholar]
- Estes J. Evidence for autoploid evolution in the Artemisia ludoviciana complex of the Pacific Northwest. Brittonia. 1967;21:29–43. [Google Scholar]
- Ehrendorfer F. Polyploidy and distribution. In: WH Lewis, editor. Polyploidy: biological relevance. New York, Plenum Press; 1980. pp. 45–60. [Google Scholar]
- Branas M, Valles J. Karyological studies in some taxa of the genus Artemisia (Asteraceae). Can J Bot. 1993;72:1126–1135. [Google Scholar]
- Valles J, Siljak-Yakoviev S. Cytogenetic studies in the genus Artemisia L. (Asteraceae): fluorochrome-banded karyotypes of five taxa, including the Iberian endemic species Artemisia barrelieri Besser. Can J Bot. 1997;75:595–606. [Google Scholar]
- Baldwin B, Anderson M, Porter J, Wojciechowski M, Cambell C, Donoghue M. The ITS region of nuclear ribosomal DNA: a valuable source of evidence on angiosperm phylogeny. Ann Missouri Bot Gard. 1995;82:247–277. [Google Scholar]
- Francisco-Ortega J, Santos-Guerra A, Hines A, Jansen R. Molecular evidence for a Mediterranean origin of the Macaronesian endemic genus Argyranthemum (Asteraceae). Amer J Bot. 1997;84:1595–1613. [PubMed] [Google Scholar]
- Francisco-Ortega J, Barber J, Santos-Guerra A, Febles-Hernandez R, Jansen RK. Origin and evolution of the endemic genera of Gonosperminae (Asteraceae: Anthemideae) from the Canary Islands: evidence from nucleotide sequences of the internal transcribed spacers of nuclear ribosomal DNA. Amer J Bot. 2001;8:161–169. [PubMed] [Google Scholar]
- Watson L, Evans T, Boluarte T. Molecular phylogeny and biogeography of Tribe Anthemideae (Asteraceae), based on chloroplast gene ndh F. Mol Phyl Evol. 2000;15:59–69. doi: 10.1006/mpev.1999.0714. [DOI] [PubMed] [Google Scholar]
- Cassini M. Anthemidees. In: G Cuvier, editor. Dictionnaire des sciences naturelles. 2. Paris; 1816. [Google Scholar]
- Poljakov P. Duo genere novae fam. Compositae. Bot Mater Gerb Bot Inst V A Komarova. 1955;17:418–431. [Google Scholar]
- Gillies A, Cubas P, Coen E, Abbott R. Making rays in the Asteraceae: genetics and evolution of radiate versus discoid flower heads. In: Q Cronk, R Bateman, J Hawkins, editor. Developmental Genetics and Plant Evolution. London, Taylor and Francis; 2002. pp. 233–246. [Google Scholar]
- McArthur E, Sanderson S. Cytogeography and chromosome evolution of Subgenus Tridentatae of Artemisia (Asteraceae). Amer J Bot. 1999;86:1754–1775. [PubMed] [Google Scholar]
- Whitkus R, Doan H, Lowrey T. Genetics of adaptive radiation in Hawaiian species of Tetramolopium (Asteraceae). III. Evolutionary genetics of sex expression. Heredity. 2000;85:37–42. doi: 10.1046/j.1365-2540.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- Tucker S. Evolutionary lability of symmetry in early floral development. Int J Plant Sci. 1999;160:S25–S39. doi: 10.1086/314212. [DOI] [PubMed] [Google Scholar]
- Oberpreiler C, Vogt R. The position of Castrilanthemum Vogt & Oberpreiler and the phylogeny of Mediterranean Anthemideae (Compositae) as inferred from nrDNA IGS and cpDNA trnL/trnF IGS sequence variation. Plant Syst Evol. 2000;225:145–170. [Google Scholar]
- Belenovska L. Artemisia: The flavonoids and their systematic value. In: P Calagari, D Hind, editor. Compositae Systematics: Proceedings of the Kew International Compositae Conference 1994. Vol. 2. Royal Botanic Gardens, Kew; 1996. pp. 253–259. [Google Scholar]
- Graham A. A contribution to the geological history of the Compositae. In: Compositae Systematics. In: D Hind, H Beentje, editor. Proceedings of the Kew International Compositae Conference 1994. Vol. 1. Royal Botanic Gardens, Kew; 1996. pp. 123–140. [Google Scholar]
- Saghai-Maroof M, Soliman K, Jorgensen R, Allard R. Ribosomal DNA spacer length polymorphism in barley: Mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J, Doyle J. A rapid DNA isolation procedure for small amounts of leaf tissue. Phytochem Bull. 1987;19:810–815. [Google Scholar]
- White T, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal genes for phylogenies. In: M Innis, D Gelford, J Sninsky, T White, editor. PCR Protocols: A Guide to Methods and Applications. San Diego, Academic Press; 1990. pp. 315–322. [Google Scholar]
- Higgins D, Bleasby A, Fuchs R. Clustal V: improved software for multiple sequence alignment. Comp Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Swofford D. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4. Sunderland, MA, Sinauer Associates. 2002.
- Felsenstein J. Confidence limits on phylogenies: an approach to using bootstrap. Evol. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Buckley T, Cunningham C. The effects of nucleotide substitution model assumptions on estimates of nonparametric bootstrap support. Mol Biol Evol. 2002;19:394–405. doi: 10.1093/oxfordjournals.molbev.a004094. [DOI] [PubMed] [Google Scholar]
- Swofford D, Olsen G, Waddell P, Hillis D. Phylogenetic Inference. In: D Hillis, C Moritz, editor. Molecular Systematics. 2. Sunderland, MA, Sinauer Associates; 1996. pp. 407–513. [Google Scholar]
- Huelsenbeck J, Rannala B. Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science. 1997;276:227–232. doi: 10.1126/science.276.5310.227. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall K. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–817. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Lewis P. Phylogenetic systematics turns over a new leaf. TREE. 2001;16:30–37. doi: 10.1016/S0169-5347(00)02025-5. [DOI] [PubMed] [Google Scholar]
- Larget B, Simon D. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol Biol Evol. 1999;16:750–759. [Google Scholar]
- Huelsenbeck J, Ronquist F. MR-BAYES: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Leache A, Reeder T. Molecular systematics of the eastern fence lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Syst Biol. 2002;51:44–68. doi: 10.1080/106351502753475871. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J, Ronquist F. MrBayes: A program for the Bayesian inference of phylogeny, version 2.01.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microsoft-Word file of list of sample species examined with source of published sequences, voucher information, and GenBank accession numbers.
Microsoft-Word file of aligned data matrix used for phylogenetic analyses in PAUP*.