Abstract
Background
Balloon injury (BI) of the rat carotid artery (CCA) is widely used to study intimal hyperplasia (IH) and decrease in lumen diameter (LD), but CCA's small diameter impedes the evaluation of endovascular therapies. Therefore, we validated BI in the aorta (AA) and iliac artery (CIA) to compare it with CCA.
Methods
Rats underwent BI or a sham procedure (control). Light microscopic evaluation was performed either directly or at 1, 2, 3, 4 and 16 weeks follow-up. The area of IH and the change in LD (LD at 16 weeks minus LD post BI) were compared.
Results
In the BI-groups the area of IH increased to 0.14 ± 0.08 mm2 (CCA), 0.14 ± 0.03 mm2 (CIA) and 0.12 ± 0.04 mm2 (AA) at 16 weeks (NS). The LD decreased with 0.49 ± 0.07 mm (CCA), compared to 0.22 ± 0.07 mm (CIA) and 0.07 ± 0.10 mm (AA) at 16 weeks (p < 0.05). The constrictive vascular remodelling (CVR = wall circumference loss combined with a decrease in LD) was -0.17 ± 0.05 mm in CIA but absent in CCA and AA. No IH, no decrease in LD and no CVR was seen in the control groups.
Conclusions
BI resulted in: (1.) a decrease in LD in CCA due to IH, (2.) a decrease in LD in CIA due to IH and CVR, (3.) no change in LD in AA, (4.) Comparable IH development in all arteries, (5.) CCA has no vasa vasorum compared to CIA and AA, (6.) The CIA model combines good access for 2 F endovascular catheters with a decrease in LD due to IH and CVR after BI.
Keywords: balloon injury, rats, remodelling, restenosis
Background
It is acknowledged that restenosis is the 'Achilles' heel of angioplasty [1]. In vascular interventions like percutaneous transluminal (coronary) angioplasty (PT(C)A), it is well-known that balloon injury (BI) to the arterial wall induces restenosis with a typical decrease in lumen diameter (LD) as a result of dysplastic intimal hyperplasia (IH) with or without constrictive vascular remodelling (CVR), shrink or recoiling [2-7].
To improve the long-term success rate of vascular interventions, restenosis must be prevented. Many strategies have already been evaluated on their potential to prevent restenosis. Interestingly, strategies based on local endovascular techniques adjuvant to PT(C)A and stenting, like experimental brachytherapy and photodynamic therapy (PDT), showed promising results in the prevention of restenosis [8], while microwave or cryotherapy, are presently gaining interest [9].
The common animal models for the evaluation of strategies to prevent restenosis are the carotid rat artery, the dog-, the baboon-, the monkey-, the rabbit-, and the pig carotid or coronary arteries [10].
Since the early eighties, the common carotid artery (CCA) in the rat model is widely applied to study molecular mechanisms and the role of smooth muscle cells in the arterial healing after arterial injury, but it has been criticised as not representative for restenosis development [11]. This is more than likely due to abuse or misinterpretation of the rat carotid artery studies, like unjustifiably comparing various artery types. The dog model has been explored mainly because of their arterial size comparable to humans, low cost and ready availability, but they are not very reactive to diets or mechanical injury. The thrombotic activity of baboons and monkeys is more close to humans but they are expensive, difficult to handle and to maintain in the laboratory.
The larger rabbit and pig models are widely accepted models of restenosis development that mimic human disease, because they are sensitive to develop atherosclerosis after cholesterol-rich diets in large injured arteries [12,13]. However, the evaluation of endovascular strategies to prevent restenosis in the rat may be cost-effective because of a wide experience and availability of tested antibodies in contrast to other models, but data about injury in other arteries than the carotid artery are not extensive. Comparisons of the effect of balloon injury between various arteries in the rat may add to a better understanding of the arterial healing to injury.
A practical point of attention is that the relatively large diameters of the presently available local delivery devices that are used in endovascular therapies cannot be introduced in the rat CCA without causing major vascular wall injury. Until smaller and more flexible fibres and devices are produced, the present relatively large devices determine the arterial criteria of the animal model. Therefore, we developed a rat model using larger vessels with better access such as the abdominal aorta (AA) as earlier reported and common iliac artery (CIA) as never reported.
A comparison was made with the widely used CCA model to validate the model and to assess artery-type-dependent differences in the healing after injury that may be of value in closely related research topics.
Methods
The experimental protocol was approved by The Committee on Animal Research of the Erasmus University of Rotterdam, The Netherlands and complied with 'Principles of Good Laboratory Practice' and 'The guide for the Care and Use of Laboratory Animals'. Ninety-five inbred Wistar rats (Harlan CPB, Austerlitz, The Netherlands) weighing 250–300 grams (g) were used. The animals had free access to rat chow (AM II, Hope Farms, Woerden, The Netherlands) and tap water acidified to pH 4.0, and were maintained in a standard 12-hr light/dark cycle.
Study design
The rats were randomly subdivided into 4 groups. In 3 groups a balloon injury was performed of either the right CCA-group (n = 30), the AA-group (n = 30) or the right CIA-group (n = 30). A fourth group (= untreated controls; n = 5) underwent a sham procedure without BI to serve as a baseline control in the CCA, AA and CIA. The animals were sacrificed immediately after intervention or after 1, 2, 3, 4 or 16 weeks for pressure fixation of the artery. Cross-sections were used for planimetric analysis. The weight of the rat was determined pre-operatively and at the time of harvesting.
Pre-operative procedure
Ketamine (35 mg/kg), atropine (40 ug/kg) and xylazine (5 mg/kg) were given intramuscularly for general anaesthesia.
Surgical techniques
Before the study was started, extensive experiments were executed to gain skill in handling the operative procedure.
The common carotid-group
A median neck incision was performed and the wound area was kept wet with gauzes embedded in 0.9% NaCl. The skin, sternocleidomastoid and omo-/thyrohyoid muscles were retracted with 2 Luer retractors. Then, 20 mm of the right CCA was isolated from the carotid sheet. The internal, external and the proximal CCA were temporary occluded with vascular clamps (B-2 Hemoclips) to prevent retrograde blood loss. After proximal arteriotomy, the lumen was flushed with 1 ml heparin solution (50 IU/ml) and antegrade balloon inflation of a 15 mm long CCA segment performed. Xylocaine (5 mg/kg) was applied to prevent vasospasm. The arteriotomy was closed in 2 layers (interrupted 8–0 prolene sutures) and the clamps removed to restore perfusion. Finally, the neck wall was closed in 1-layer (continuous 2–0 Vicryl sutures).
The abdominal aorta-group
A medial laparotomy was performed and the intestines were wrapped in a wetted gauze (0.9% NaCl). The retroperitoneal fascia was opened medially to expose 20 mm of AA from the right renal artery to the iliac bifurcation. Then, the AA was isolated from the inferior caval vein, followed by temporary occlusion of the left and right ilio-lumbar arteries, the proximal and caudal abdominal aorta, respectively. After the arteriotomy of the AA, a 15-mm segment from the renal artery to the bifurcation was balloon injured (see balloon injury). The lumen was flushed with heparin, followed by a 1-layer closure (Prolene 8–0, interrupted) of the arteriotomy and a 2-layer closure (continuous 4–0 and 2–0 Vicryl sutures) of the abdominal wall.
The common iliac artery-group
The surgical procedure was identical to the AA-group. The CIA was prepared from the aortic bifurcation to the femoral bifurcation. Both femoral bifurcations, left lumbosacral, inferior mesenteric and iliac bifurcation were temporarily occluded. After the arteriotomy, balloon-injury of a 15-mm long CIA segment was performed [Fig. 1]. The lumen was flushed with heparin, followed by a 1-layer closure (Prolene 8–0, interrupted) of the arteriotomy and a 2-layer closure (continuous 4–0 and 2–0 Vicryl sutures) of the abdominal wall.
Figure 1.
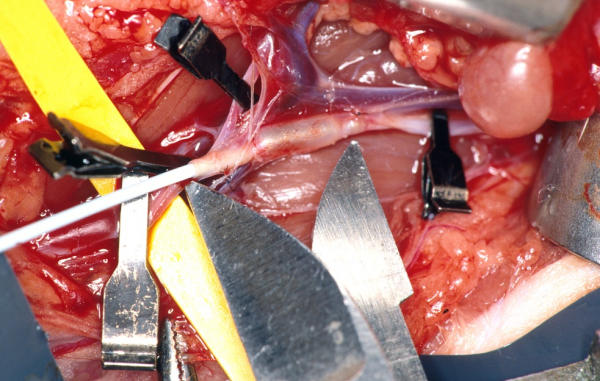
A photograph representing the balloon injury at 2 bar of the iliac artery. The metal points (*) demarcate the injured segment of 15 mm (white arrow). The black clips occlude the neighbouring arteries.
Balloon injury
A 2 F Fogarty Embolectomy catheter (Baxter Health Care Corp., Edward's Div., Irvin, Ca., USA) was inserted to denude the arterial wall at a pressure of 2 bar (manual barometer) by pulling and rotating the inflated balloon from distally to proximally over a 15-mm long segment for 3 successive times [14]. The balloon was deflated proximally and inflated distally. Then, the arteries were flushed with heparin, closed with Prolene 8–0 and marked halfway the denuded segment with a suture (Prolene 8–0).
Post-operative procedure
The rats recovered under an infrared heater to keep the body temperature at 37°C.
Specimen handling
After sacrifice, a standard perfusion-fixation procedure via the thoracic aorta was performed under ether anaesthesia at respectively 0, 1, 2, 3, 4 and 16 weeks. The lumen was flushed with a phosphate buffer solution (PBS) at pH 7.45 for 2 min and perfusion-fixed with formaldehyde 1.8% for 10 minutes via a silicon tube at 100 mm Hg for paraffin embedding light microscopy. The distally and half-way marked segment (single suture, 8–0 black nylon, B/Braun, München, Germany) of 20 mm long was harvested after length measurement and stored in fresh 1.8% formaldehyde for at least 24 hr.
Planimetric analysis of the cross sections
The entire arterial segment was embedded in paraffin wax and cut into cross-sections of 10 μm at an interval of 3 mm with a microtome. The sections were mounted from distally to proximally on a microscopic slide and stained with haematoxylin and eosin. All cross-sections were video taped with a digital camera and geometrically analysed using a digital manual video-analyser [Fig. 2] [15]. Each rat accounted for one measurement. Therefore, for calculating time-dependent effects different rats were used. Firstly, a change in LD was calculated using the maximal lumen diameter (MLD) at 0 weeks minus MLD at 16 weeks. Secondly, the absolute area of IH was determined. The IH expressed in mm2 was defined as the area between the lumen and the internal elastic lamina. Thirdly, IH-to-media-area ratio was used to compare the extent of IH contributing to the arterial wall composition between the CCA, AA and CIA, using the IH area and media area. Fourthly, the wall circumference (WALLC) was calculated in mm as an approximation of CVR (WALLC before minus WALLC after). Fifthly, the media area expressed in mm2 was defined as the area between the internal and external elastic laminae. The maximal thickness was expressed in mm. The role of animal growth during the experiment was corrected with a calculated growth factor, using the formula G = A* (x*wt)/w0, in which A has been iterated, x is the geometric value, w = weight at time 0 or at time t, [16]. Asymmetry was corrected by using the perimeter (D = circumference/π) assuming a circular configuration to calculate the MLD and MWD. The number of vasa vasorum was counted in the tunica adventitia.
Figure 2.
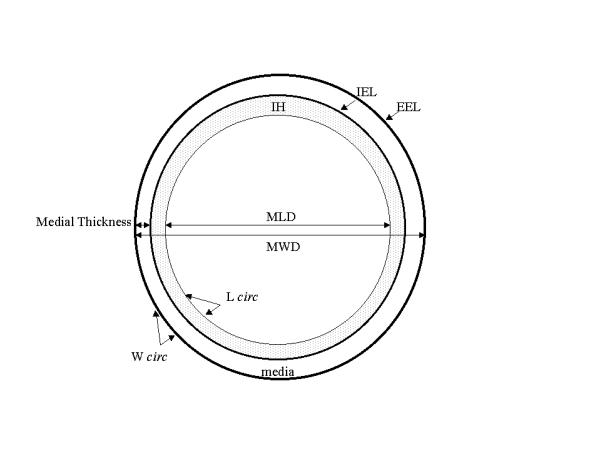
Schematic representation of an artery showing the geometric parameters used in this study. IH: Intimal Hyperplasia; media area; MLD: maximal lumen diameter; MWD: maximal wall diameter;maximal medial thickness. IEL: internal elastic lamina; EEL: external elastic lamina. The dotted line represents the lumen circumference (L circ), and the dark line the wall circumference (W circ).
Statistical analysis
Comparisons were made between different groups of rats at different time points. Each rat accounted for one measurement. Data were expressed as means ± standard error of the mean (SEM). Comparisons including the type*time relations were made using a 2-way ANOVA method with a General Linear Model regression analysis in SAS. Bivariate correlations according Pearson to describe a correlation between type*time and WALLC were used. A difference was considered to be significant at p values less than 0.05.
Results
All animals appeared healthy without significant weight loss at follow-up. No thrombosis, arterial dissection, aneurysms, paraplegia or paralysis was encountered. The wound areas appeared normal besides some mild fibrosis at the suture side. Adjacent lymph nodes frequently covered the sutured arteriotomy.
Planimetric analysis
On microscopy, the endothelial layer was absent in the arteries immediately after BI and was recovered within 2 weeks after BI. In the control arteries the endothelium layer was continuously present.
Lumen diameter
At 16 weeks after BI, LD decreased with 0.49 ± 0.11 mm (CCA), 0.22 ± 0.07 mm (CIA) and 0.07 ± 0.10 mm (AA) [Fig. 3]. The linear association of a decrease in LD in CCA and CIA was significant higher than in AA [Fig. 4] (p < 0.05). MLD was 0.86 ± 0.04 mm (CCA), 1.53 ± 0.05 mm (AA) and 0.99 ± 0.02 mm (CIA) at 0 weeks, and was 0.37 ± 0.10 mm (CCA), 1.46 ± 0.09 mm (AA) and 0.77 ± 0.7 mm (CIA) at 16 weeks [Table 1]. In time, the overall MLD differed significantly between the CCA, AA and CIA. A significantly decrease was seen at 4–16 weeks (CCA), a temporarily increase at 2 weeks (AA) and a temporarily increase at 1–3 weeks followed by a decrease at 16 weeks (CIA) [Table 1].
Figure 3.
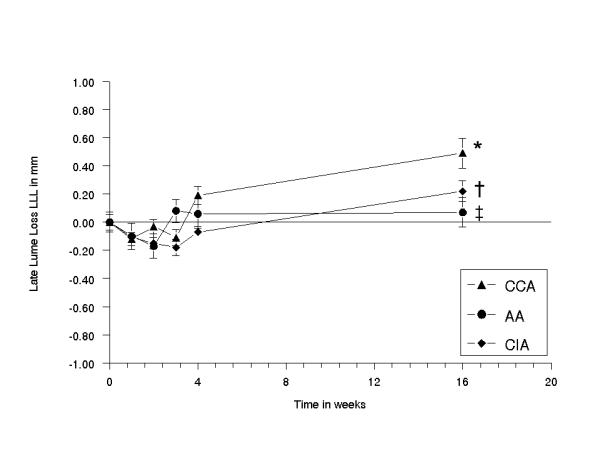
Diagram showing the mean difference in lumen diameter (MLD) as the sum of MLD at 0 weeks minus MLD at 16 weeks of the common carotid, abdominal aorta and common iliac artery, plotted against the time in weeks. The error bars are standard errors of the mean (n = 5 for each time point). At 16 weeks: (*) CCA vs AA p < 0.0002; (†) CCA vs CIA p < 0.001; AA vs CIA (‡) p < 0.027.
Figure 4.
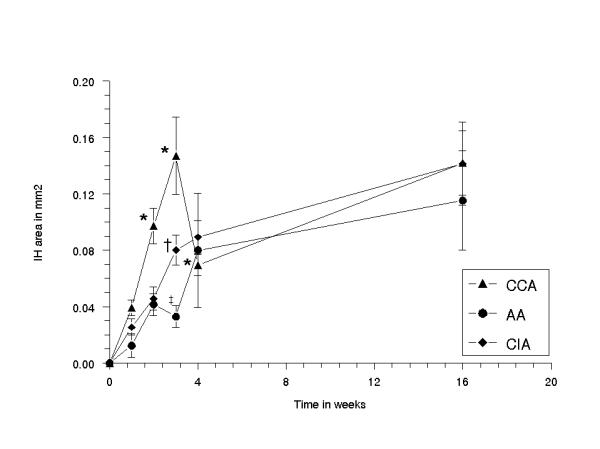
Diagram showing the mean area of intimal hyperplasia (IH) of the common carotid, abdominal aorta and common iliac artery, plotted against the time in weeks. The error bars are standard errors of the mean (n = 5 for each time point). Type*Time Difference p < 0.001: (*) CCA vs AA at 0 and 1 week NS, at 2 weeks p < 0.04, at 3 weeks p < 0.0001, at 4 weeks p < 0.0001, at 16 weeks NS; (†) CCA vs CIA at 0, 1 and 2 weeks NS, at 3 weeks p < 0.007 and at 4 and 16 weeks NS; (‡) AA vs CIA at 0, 1 and 2 weeks NS, at 3 weeks p < 0.02, at 4 weeks and at 16 weeks NS.
Table 1.
A representation of the geometric changes in time expressed in weeks
| Parameter | Time | carotid SEM | aorta SEM | iliac SEM |
| MLD in mm | 0 | 0.86 ± 0.04 | 1.53 ± 0.05 | 0.99 ± 0.02 |
| 1 | 0.98 ± 0.02 | 1.63 ± 0.08 | 1.09 ± 0.02 | |
| 2 | 0.89 ± 0.03 | 1.70 ± 0.07 | 1.14 ± 0.04 | |
| 3 | 0.97 ± 0.04 | 1.45 ± 0.07 | 1.17 ± 0.05 | |
| 4 | 0.67 ± 0.05 | 1.47 ± 0.09 | 1.06 ± 0.03 | |
| 16 | 0.37 ± 0.10 | 1.46 ± 0.09 | 0.77 ± 0.07 | |
| Media in mm2 | 0 | 0.10 ± 0.00 | 0.25 ± 0.03 | 0.13 ± 0.00 |
| 1 | 0.13 ± 0.01 | 0.32 ± 0.01 | 0.18 ± 0.01 | |
| 2 | 0.13 ± 0.01 | 0.35 ± 0.02 | 0.21 ± 0.01 | |
| 3 | 0.14 ± 0.01 | 0.40 ± 0.01 | 0.21 ± 0.01 | |
| 4 | 0.11 ± 0.00 | 0.32 ± 0.02 | 0.19 ± 0.01 | |
| 16 | 0.13 ± 0.01 | 0.28 ± 0.02 | 0.15 ± 0.01 | |
| Medial Thickness in mm | 0 | 0.06 ± 0.00 | 0.09 ± 0.01 | 0.08 ± 0.00 |
| 1 | 0.07 ± 0.00 | 0.14 ± 0.02 | 0.09 ± 0.00 | |
| 2 | 0.08 ± 0.00 | 0.12 ± 0.01 | 0.10 ± 0.01 | |
| 3 | 0.08 ± 0.01 | 0.14 ± 0.00 | 0.10 ± 0.00 | |
| 4 | 0.09 ± 0.00 | 0.13 ± 0.01 | 0.09 ± 0.01 | |
| 16 | 0.09 ± 0.00 | 0.11 ± 0.01 | 0.08 ± 0.00 |
(vertically in ascending order) after balloon injury in respectively (horizontally ordered) the right common carotid artery (CCA), abdominal aorta (AA) and right common iliac artery (CIA). The mean parameters (maximal lumen diameter (MLD), media area and maximal media thickness) are subsequently given. MLD: CCA 16 vs 0 p < 0.001; CIA 16 vs 0 p < 0.0001;CCA 1 vs 0 p < 0.0003; AA 2 vs 0 p < 0.002; CIA 3 vs 0 p < 0.004. Media area: CCA 3 vs 0 p < 0.001; AA 3 vs 0 p < 0.0001; CIA 3 vs 0 p < 0.0001. THMAX: CCA 16 vs 0 p < 0.002; AA 3 vs 0 p < 0.0001; CIA 3 vs 0 p < 0.0001
Intimal hyperplasia
In all BI groups, the absolute area of IH increased significantly in time (p < 0.017) [Fig. 5a1] [Fig. 5a2], [Fig. 5b1] and [Fig. 5b2]. A significant increase was seen at 2–16 weeks (CCA), at 3–16 weeks (AA) and at 2–16 weeks (CIA). At 16 weeks, the area of IH was 0.14 ± 0.03 mm2 (CCA), 0.14 ± 0.03 mm2 (CIA) and 0.12 ± 0.04 mm2 (AA). In all BI groups, the overall IH to media area ratio increased significantly in time (p < 0.05). A significant increase was seen at 1–16 weeks (CCA), at 4–16 weeks (AA) and at 2–16 weeks (CIA) (p < 0.05). The IH to media area ratio was higher in CCA (1.09 ± 0.15) than in AA (0.67 ± 0.23) and in CIA (0.95 ± 0.19) at 16 weeks, indicating relatively more IH development in CCA than in CIA and in AA [Fig. 6].
Figure 5.
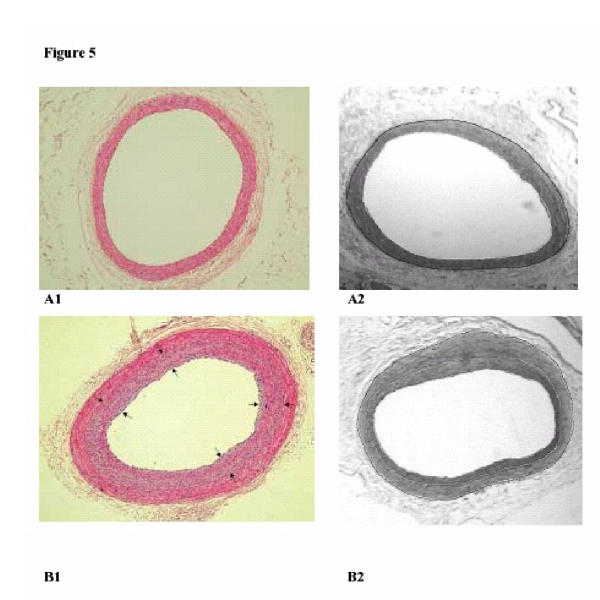
Photographs in the left column show HE-stained cross-sections of the iliac artery at 0 weeks (A1) and at 16 weeks (B1) after balloon injury (BI). The area between the black arrows indicate the area intimal hyperplasia. The digital planigraphs in the right column show the iliac artery at 0 weeks (A2) and at 16 weeks (B2) after BI. The white lines demarcate the IH area and the black line represents the external elastic lamina (border medial area = area between black and first white line).
Figure 6.
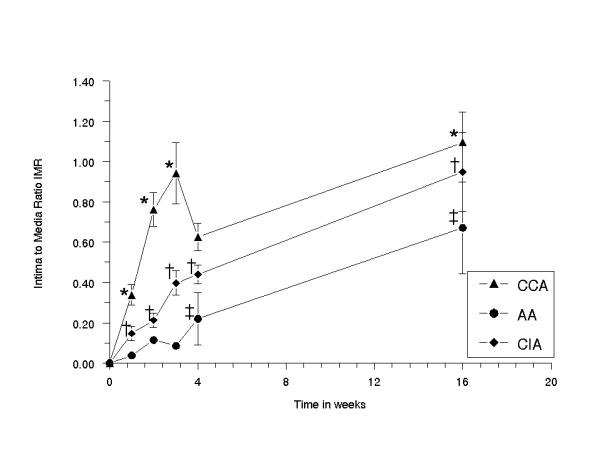
Diagram showing the mean-area-of-intimal hyperplasia-to-mean-media area ratio of the right common carotid, abdominal aorta and right common iliac artery, plotted against the time in weeks. The error bars are standard errors of the mean (n = 5 for each time point). (*) CCA vs AA at 0 and 1 week NS, at 2 weeks P < 0.0001, at 3 weeks p < 0.0001, at 4 weeks NS and at 16 weeks p < 0.009; (†) CCA vs CIA at 0 and 1 week NS, at 2 weeks p < 0.0002, at 3 weeks p < 0.0004, at 4 weeks p < 0.007 and at 16 weeks p < 0.003; (‡) AA vs CIA at 0, 1, 2 and 3 weeks NS, at 4 weeks p < 0.009 and at 16 weeks p< 0.003.
Constrictive vascular remodelling
In all BI groups, the overall wall circumferences (WALLC) varied significantly in artery type and in time [Fig. 7]. A significant increase was seen at 1–3 weeks in CCA (0.51 ± 0.13 mm), an increase at 1–16 weeks in AA (0.46 ± 0.10 mm) and an increase at 1–4 weeks (0.37 ± 0.05 mm) and a decrease at 16 weeks in CIA (-0.17 ± 0.05 mm). No significant correlation with the time was found (-0.020; p < 0.68).
Figure 7.
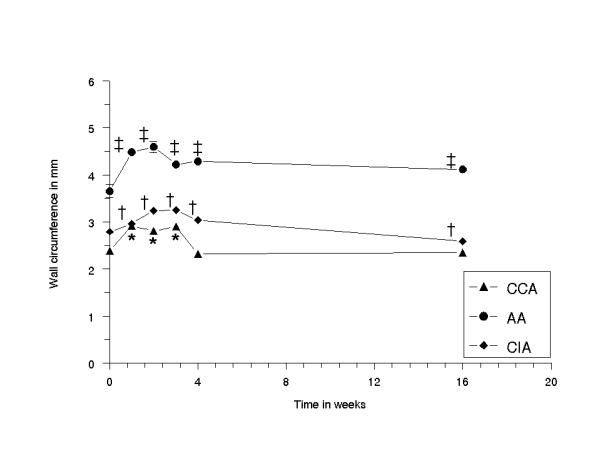
Diagram showing the wall circumference of the right common carotid, abdominal aorta and common iliac artery, plotted against the time in weeks. The error bars are standard error of the mean (n = 5 per time point). (*) CCA at 0 vs 1 week p < 0.003, 0 vs 2 weeks p < 0.004, 0 vs 3 weeks p < 0.0004, 0 vs 4 and 16 weeks NS; (‡) AA at 0 vs 1 week p < 0.004, 0 vs 2 weeks p < 0.001, 0 vs 3 p < 0.001 and 4 weeks p < 0.001, 0 vs 16 weeks p < 0.004; (†) CIA at 0 vs 1, 2, 3, 4 and 16 weeks p < 0.001.
Number of vasa vasorum in the tunica adventitia
In the CCA groups, no vasa vasorum were seen, while in the AA and CIA respectively 27 ± 2 and 13 ± 2 vasa vasorum per section at 0 weeks were seen in the tunica adventitia. A significant increase in time was assessed in the AA and CIA groups with respectively 37 ± 2 and 22 ± 2 vasa vasorum/section at 16 weeks (p < 0.05). Sporadically, some vasa vasorum in the tunica media were seen in the AA and not in the CIA and CCA groups.
Discussion
Balloon injury of the common carotid artery (CCA) in the rat is a thoroughly studied model that has logistical advantages to evaluate dysplastic intimal hyperplasia (IH) and the decrease in lumen diameter (LD) for the evaluation of strategies to prevent restenosis, like endovascular based interventions. However, CCA does not fit well to evaluate endovascular techniques due to the relatively large diameter of endovascular devices and fibre tips.
Furthermore, in contrast to human arteries, the rat carotid artery has no vasa vasorum, contains a much thinner subintimal layer and a smaller percentage of elastin in the tunica media [17].
In this study the larger – vasa vasorum containing – abdominal aorta (AA) and common iliac artery (CIA) in the rat were validated as an alternative model to be used for the evaluation of endovascular techniques.
In this study, the decrease in LD was higher in CCA and CIA compared to AA after BI. The differences in LD between CCA, AA and CIA are likely explained by an artery-type specific healing response after BI, expressed in the development of IH and constrictive vascular remodelling (CVR), due to differences in the viscoelastic composition, the vasa vasorum in the tunica adventitia and vessel diameter.
Another explanation, however, may be the lower dilatation ratio in the aorta due to its large diameter, causing less IH development and less remodelling. Indeed, we found that in CCA, AA and CIA, IH developed differently at long-term after BI. The extent of CCA IH in our model was similar to that in previous reported rat models [14,18]. Relatively more IH (expressed by the IH to media ratio) developed in CCA and CIA compared to the AA. In CCA and CIA, IH led to a higher decrease in LD.
With regard to CVR, the wall circumference temporarily increased in the CCA and CIA, and remained increased in AA. In CCA and in CIA a decrease of wall circumference was seen in contrast to the increased wall circumference of AA at 16 weeks. Apparently, AA compensated to IH by positive (= outward) remodelling underlining the blood reservoir capacity of AA, but led to shrinkage in the CCA and CIA at 16 weeks underlining the peripheral resistance capacity of CCA and CIA.
Earlier reports, using a PTA catheter or a chain-encircled balloon catheter in pig models described that IH is not correlated to CVR, suggesting that the pathogenesis is not similar [19,20]. In this study, the absence of a decrease in LD and CVR, but still the development of IH in the AA supports these findings, but are in contrast with a decrease in LD, increase in IH and an increase in CVR in the CCA and CIA.
With regard to the media area, no significant difference was found in CCA, AA and CIA in time. With regard to the maximal medial thickness, an increase was seen in CCA but not in CIA and AA after 16 weeks. Apparently the BI caused more damage in the CCA than in AA and CIA, leading to an increased proliferation in the tunica media.
A plausible explanation is likely in the viscoelastic differences between CCA, AA and CIA. The rat CCA in contrast to the human CCA is an integrating part of the circle of Willis and resembles the rat cerebral arteries characterised by a low elastin/ high collagen content and a low contraction potential. In contrast, the systemic AA and CIA have a high elastin/ moderate collagen content and a high contraction potential [21]. Then, the medial content of contractile vs synthetic SMCs varies in CCA, AA and CIA. Their role in remodelling is not fully elucidated but likely adapt the viscoelastic content in the extracellular matrix. Indeed, Goldberg et al showed in rats that an artery-type dependent variation of both synthetic and contractile SMCs determined the vascular wall response [22]. Notably, the attenuated response of the AA could be explained by the lumen diameter to BI pressure ratio as suggested earlier in a rat and rabbit model.
Furthermore, the absence of vasa vasorum in the CCA in the tunica adventitia in contrast to the AA and CIA is strikingly in this study. The increased number in the latter two may favour the arterial healing via reperfusion that may lack in the tunica adventitia of the CCA. The presence of vasa vasorum may explain the difference in arterial healing between the arteries. Indeed, Pels described that the number of vasa vasorum in the tunica adventitia changed after balloon injury in pigs, which may be a component of arterial remodelling after angioplasty [23]. Our data of the CIA, but not of the AA support this conclusion. Badimon described a discrepancy between CCA and the coronary arteries [24], while Ward described a discrepancy between the CIA and coronary artery types after standard BI [25], indicating the importance of an arterial specific healing response as emphasised in our study.
Interestingly, the arteries of rabbit, pigs and humans, are also characterised by vasa vasorum in the tunica adventitia. Thus, the CIA and AA of the rat are closer to the arteries of the larger models than the CCA of the rat.
A limitation of this study is that no vasodilator was applied. However, geometric analysis of the sections within the groups did not show any significant difference, assuming that the contribution of reversible and chronic vasodilatory spasm was negligible.
Another limitation was the difference in weight between the animals in time. The influence of increased weight on the growth of the artery is well known and was corrected in this model.
Further, the use of balloon catheters that precisely fit with the arterial lumen might be suggested for comparing BI. Indeed, the deflated catheter diameter of the 2 F balloon fitted well in CCA, CIA and moderately in AA, but the inflated balloon diameter extended for 45–60% in these arteries (NS: unpublished data). Roubin described that an increased balloon injury resulted in an increased development of restenosis needing bypass surgery in a prospectively randomised clinical trial of 336 patients [26]. In this study, the force to over-stretch the artery was standardised and comparable between the balloon injured arteries.
Besides differences in arterial morphology, the severity of injury and strain [27] – and inter-species differences [10] in the healing response and metabolism demand a careful extrapolation to the human situation.
Conclusions
In conclusion,
(1.) BI using 2 bar resulted in a decrease in LD in CCA due to IH,
(2.) In a decrease in LD in CIA due to IH and CVR,
(3.) No change in LD in AA.
(4.) Comparable IH development in all arteries,
(5.) CCA has no vasa vasorum compared to CIA and AA,
(6.) Discrepancies in changes in LD by IH development and CVR after BI depend on artery type. This should be taken into account in the interpretation of the efficacy of interventional techniques to inhibit IH and a decrease in LD.
(7.) The CIA model is suitable to evaluate fibre-based therapies, as it combines good access for 2 F endovascular catheters with the development of a decrease in LD due to IH and CVR after BI.
Abbreviations
AA, abdominal aorta; BI, balloon injury; CCA, common carotid artery; CIA, common iliac artery; CVR, constrictive vascular remodelling; EEL, external elastic lamina; IEL, internal elastic lamina; IH, intimal hyperplasia; LD, lumen diameter; MLD, maximal lumen diameter; MWD, maximal wall diameter; PT(C)A, percutenous transluminal (coronary) angioplasty; SEM, standard error of the mean; SMC, smooth muscle cell; WALLC, wall circumference.
Competing interests
None declared
Author's contributions
Author 1 E.E.E. Gabeler participated in the design, carried out the studies and drafted the manuscript. Author 2 R. van Hillegersberg participated in the design, the coordination of the study and in the surgical techniques. Author 3 R.G. Statius van Eps participated in the balloon injury technique, Author 4 W. Sluiter participated in the tissue preservation technique, Author 5. E. Gussenhoven participated in the digital analysis of the sections, Author 6 P. Mulder participated in the design and in the statistical analysis, Author 7 H. van Urk conceived of the study and participated in its design and coordination.
All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This study was supported by the Dutch Heart Foundation (project grant NHS 97.181). The authors are grateful to Jan Honkoop for expert technical assistance with the geometric analysis, Dr Marc van Sambeek for freely giving dilation catheters, Patricia Lobe and Manon Holtman for excellent staining of the cross-sections.
Contributor Information
Edward EE Gabeler, Email: EEEGabeler@hotmail.com.
Richard van Hillegersberg, Email: r.vanhillegersberg@12move.nl.
Randolph G Statius van Eps, Email: rg.vaneps@worldonline.nl.
Wim Sluiter, Email: sluiter@bc1.fgg.eur.nl.
Elma J Gussenhoven, Email: elma.gussenhoven@12move.nl.
Paul Mulder, Email: Mulder@epib.fgg.eur.nl.
Hero van Urk, Email: vanurk@hlkd.azr.nl.
References
- Bauters C, Meurice T, Hamon M, McFadden E, Lablanche JM, Bertrand ME. Mechanisms and prevention of restenosis: from experimental models to clinical practice. Cardiovasc Res. 1996;31:835–846. doi: 10.1016/0008-6363(96)00038-7. [DOI] [PubMed] [Google Scholar]
- Giraldo AA, Esposo OM, Meis JM. Intimal hyperplasia as a cause of restenosis after percutaneous transluminal coronary angioplasty. Arch Pathol Lab Med. 1985;109:173–175. [PubMed] [Google Scholar]
- Glagov S. Intimal hyperplasia, vascular modeling, and the restenosis problem. Circulation. 1994;89:2888–2891. doi: 10.1161/01.cir.89.6.2888. [DOI] [PubMed] [Google Scholar]
- Haudenschild CC. Pathogenesis of restenosis. Z Kardiol. 1989;78:28–34. [PubMed] [Google Scholar]
- Park JW, Braun P, Mertens S, Heinrich KW. Ischemia: reperfusion injury and restenosis after coronary angioplasty. Ann NY Acad Sci. 1992;669:215–236. doi: 10.1111/j.1749-6632.1992.tb17102.x. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, de Kleijn DP, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc Res. 2000;45:843–852. doi: 10.1016/S0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- Post MJ, Borst C, Pasterkamp G, Haudenschild CC. Arterial remodeling in atherosclerosis and restenosis: a vague concept of a distinct phenomenon. Atherosclerosis. 1995;118:S115–S123. doi: 10.1016/0021-9150(95)05669-6. [DOI] [PubMed] [Google Scholar]
- Teirstein PS. Prevention of vascular restenosis with radiation. Tex Heart Inst J. 1998;25:30–33. [PMC free article] [PubMed] [Google Scholar]
- Landau C, Currier JW, Haudenschild CC, Minihan AC, Heymann D, Faxon DP. Microwave balloon angioplasty effectively seals arterial dissections in an atherosclerotic rabbit model. J Am Coll Cardiol. 1994;23:1700–1707. doi: 10.1016/0735-1097(94)90678-5. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Holmes DR. Pigs, dogs, baboons and man: lessons for stenting from animal studies. J Intervent Cardiol. 1994;7:355–368. [Google Scholar]
- Carson SN, Esquivel CO, French SW. Experimental carotid stenosis due to fibrous intimal hyperplasia. Surg Gynecol Obstet. 1981;153:883–888. [PubMed] [Google Scholar]
- Muller D, Ellis S, Topol EJ. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992;19:418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- French JE, Jennings MA, Florey HW. Morphological studies on atherosclerosis in swine. Ann NY Acad Sci. 1965;127:780–794. doi: 10.1111/j.1749-6632.1965.tb49444.x. [DOI] [PubMed] [Google Scholar]
- Indolfi C, Esposito G, Di Lorenzo E, Rapacciuolo A, Feliciello A, Porcellini A, Avvedimento V, Condorelli M, Chiariello M. Smooth muscle cell proliferation is proportional to the degree of balloon injury in a rat model of angioplasty. Circulation. 1995;92:1230–1235. doi: 10.1161/01.cir.92.5.1230. [DOI] [PubMed] [Google Scholar]
- Wenguang L, Gussenhoven EJ, Bosch JG, Mastik F, Reiber JHC, Bom N. A computer-aided analysis system for the quantative assessment of intravascular ultrasound images. Proc Computers in Cardiology. 2001;1990:333–336. [Google Scholar]
- LaMuraglia GM, Klyachkin ML, Adili F, Abbott WM. Photodynamic therapy of vein grafts: suppression of intimal hyperplasia of the vein graft but not the anastomosis. J Vasc Surg. 1995;21:882–890. doi: 10.1016/s0741-5214(95)70215-6. [DOI] [PubMed] [Google Scholar]
- Sims FH. A comparison of structural features of the walls of coronary arteries from 10 different species. Pathology. 1989;21:115–124. doi: 10.3109/00313028909059547. [DOI] [PubMed] [Google Scholar]
- Schwartz RS. The vessel wall reaction in restenosis. Semin Interv Cardiol. 1997;2:83–88. [PubMed] [Google Scholar]
- Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89:2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- Holm P, Andersen HO, Nordestgaard BG, Hansen BF, Kjeldsen K, Stender S. Effect of oestrogen replacement therapy on development of experimental arteriosclerosis: a study in transplanted and balloon-injured rabbit aortas. Atherosclerosis. 1995;115:191–200. doi: 10.1016/0021-9150(94)05513-I. [DOI] [PubMed] [Google Scholar]
- Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;1995:149–173. doi: 10.1016/0163-7258(94)00071-A. [DOI] [PubMed] [Google Scholar]
- Goldberg ID, Stemerman MB, Ransil BJ, Fuhro RL. In vivo aortic muscle cell growth kinetics: Differences between thoracic and abdominal segments after Intimal Injury in the rabbit. Circ Res. 1980;47:182–189. doi: 10.1161/01.res.47.2.182. [DOI] [PubMed] [Google Scholar]
- Pels K, Labinaz M, Hoffert C, O'Brien ER. Adventitial angiogenesis early after coronary angioplasty: correlation with arterial remodeling. Arterioscler Thromb Vasc Biol. 1999;19:229–238. doi: 10.1161/01.atv.19.2.229. [DOI] [PubMed] [Google Scholar]
- Badimon JJ, Ortiz AF, Meyer B, Mailhac A, Fallon JT, Falk E, Badimon L, Chesebro JH, Fuster V. Different response to balloon angioplasty of carotid and coronary arteries: effects on acute platelet depostion and intimal thickening. Atherosclerosis. 1998;140:307–314. doi: 10.1016/S0021-9150(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Ward MR, Kanellakis P, Ramsey D, Jennings GL, Bobik A. Response to balloon injury is vascular bed specific: a consequence of de novo vessel structure?. Atherosclerosis. 2000;151:407–414. doi: 10.1016/S0021-9150(99)00407-4. [DOI] [PubMed] [Google Scholar]
- Roubin GS, Douglas JS, Jr, King SB, 3rd, Lin SF, Hutchison N, Thomas RG, Gruentzig AR. Influence of balloon size on initial success, acute complications and restenosis after percutaneous transluminal coronary angioplasty. A prospective randomized study. Circulation. 1988;78:557–565. doi: 10.1161/01.cir.78.3.557. [DOI] [PubMed] [Google Scholar]
- Assadnia S, Rapp JP, Nestor AL, Pringle T, Cerilli GJ, Gunning WT, 3rd, Webb TH, Kligman N, Allison DC. Strain differences in neointimal hyperplasia in the rat. Circ Res. 1999;84:1252–1257. doi: 10.1161/01.res.84.11.1252. [DOI] [PubMed] [Google Scholar]


