Abstract
Background
Current efforts to study the genetic underpinnings of higher brain functions have been lacking appropriate phenotypes to describe cognition. One of the problems is that many cognitive concepts for which there is a single word (e.g. attention) have been shown to be related to several anatomical networks. Recently, we have developed an Attention Network Test (ANT) that provides a separate measure for each of three anatomically defined attention networks.
Results
In this study we have measured the efficiency of neural networks related to aspects of attention using the ANT in a population of 200 adult subjects. We then examined genetic polymorphisms in four candidate genes (DRD4, DAT, COMT and MAOA) that have been shown to contribute to the risk of developing various psychiatric disorders where attention is disrupted. We find modest associations of several polymorphisms with the efficiency of executive attention but not with overall performance measures such as reaction time.
Conclusions
These results suggest that genetic variation may underlie inter-subject variation in the efficiency of executive attention. This study also shows that genetic influences on executive attention may be specific to certain anatomical networks rather than affecting performance in a global or non-specific manner. Lastly, this study further validates the ANT as an endophenotypic assay suitable for assessing how genes influence certain anatomical networks that may be disrupted in various psychiatric disorders.
Background
An inability to select and maintain mental focus is commonly observed in many heritable psychiatric disorders. For example, patients with schizophrenia exhibit difficulties in sensorimotor gating [1], smooth pursuit eye-tracking [2], set-shifting [3] and working memory tasks [4]. Children with attention-deficit/hyperactivity disorder (ADHD) exhibit abnormal performance in sustained attention tasks [5] while studies on autism reveal slowed covert orienting of visual spatial attention [6] and patients with Alzheimer's disease show covert orienting deficits [7]. Interestingly, all of these disorders show familial patterns of inheritance and increased concordance in monozygotic (MZ) vs. dizygotic (DZ) twins [8]. Recent family studies have also shown that some unaffected first degree relatives of schizophrenic patients show impaired performance in assays of auditory attention and working memory [9]. This suggests that deficits in attentional performance may contribute to the genetic susceptibility or liability [10] of complex psychiatric disorders.
Consistent with this notion, attentional performance in normal subjects appears to be influenced by genetic factors. Studies using the Continuous Performance Task (CPT) have shown that the d' signal detection component (a measure of how readily a signal can be detected above background noise) of CPT performance has a heritability among normal subjects of 0.49 [11]. The Span of Apprehension task (SPAN), a visual search task, has been shown to have an heritability among normal subjects of 0.65 [12] and the P/N ratio of the Spontaneous Selective Attention Task (SSAT) was shown to have an heritability among normal subjects of 0.41 [13]. Twin studies using normal control twins show that spatial working memory, divided attention, choice reaction time and selective attention [14] and attentional set-shifting [15] are underlain by inherited factors. Studies on infants suggest that effortful control and duration of orienting are heritable as well [16]. Lastly, molecular gene association studies on normal populations have shown that orienting of visual attention is associated with variation in the APOE gene [17] and that maternal ratings of attention in children are associated with the DRD4 gene [18]. The use of these endophenotypic measures are potentially advantageous for genetics studies since they may show increased sensitivity to specific dimensions related to complex psychiatric disorders.
Attention Network Task (ANT) as a suitable endophenotype for genetic studies
While each of the attentional measures described above is well suited for genetic studies, we have chosen an alternate assay for genetic studies of attention. Our approach is based functional neuroimaging studies which have yielded evidence on neural areas involved in aspects of attention [19,20]. Imaging data have supported the presence of three networks related to different aspect of attention. These networks carry out the functions of alerting, orienting and executive control [21,22]. Alerting is defined as achieving and maintaining a state of high sensitivity to incoming stimuli; orienting is the selection of information from sensory input; and executive control is defined as involving the mechanisms for resolving conflict among thoughts, feelings and responses. Functional imaging studies have shown that maintaining an alert state involves activation of right frontal and parietal lobes while orienting to visual stimuli activates areas of the pulvinar, superior colliculus and posterior parietal lobe. Executive function tasks activate frontal areas such as the anterior cingulate cortex and lateral prefrontal cortex, an area for which certain anatomical aspects have been shown to be highly heritable [23]. Recently, we described the Attention Network Test (ANT) that measures the efficiency of these three major neural networks [24]. The ANT is advantageous for genetic studies, insofar as it distinguishes between separate functions of attention (alerting, orienting and executive) that are correlated with the activation of these specific neuroanatomical circuits. The heritability of the ANT has been examined in a preliminary twin study using normal adult twins [25]. The efficiency of the executive network was found to be highly heritable (hF2 = 0.89) while lower heritabilities were observed for alerting and median reaction time (hF2 = 0.18 and 0.16 respectively). The heritabilities of these measures suggest that candidate gene association studies are reasonable to pursue. It remains an open question however, whether such candidate genes will relate to overall attentional performance and reaction time, or whether the candidate genes will show specific associations with specific neural networks.
As an initial assessment of this approach, we chose four candidate genes (DRD4, DAT1, COMT and MAOA) that are among the most widely studied and repeatedly associated with various psychiatric disorders where attention is found to be disrupted. The longstanding interest in these candidate genes stems mainly from pharmacological evidence implicating dopamine and norepinephrine in attentional processes as well as biochemical studies that have related alleleic variants to differences in protein activity and expression levels. Studies showing the expression of dopamine receptors in the anterior cingulate cortex [26] and that activation of mesocortical dopaminergic neurons via apomorphine enhances activity in the prefrontal cortex [27,28] suggest that dopaminergic modulation influences the efficiency of the executive attention network. Similarly, pharmacological studies with alert monkeys have related the alerting network to the brain's norepinepherine system whose cell bodies are located in the locus coeruleus. Drugs like clonidine and guanfacine act to block norepinepherine, and reduce or eliminate the normal effect of warning signals on reaction time, but have no influence on orienting to the target location [29].
Dopamine D4 receptor (DRD4)
The dopamine D4 receptor is located on chromosome 11p15 [30]. Many association studies have evaluated a 48 base-pair variable nucleotide tandem repeat (VNTR) polymorphism in exon III. The most common isoform of the DRD4 contains 4-repeats, while two less common isoforms contain 2-repeats and 7-repeats. Pharmacological studies show that the 7-repeat isoform is less responsive to dopamine stimulation [31]. Recently, a formal meta analysis [32] was conducted on the growing body of literature of this DRD4 polymorphism and its association with ADHD. Based on 7 case control studies (4 positive) and 14 family-based studies (9 positive), the meta analysis concluded that a "...statistically significant association between ADHD and the 7-repeat allele of DRD4" existed, with a relative risk of 1.9 for 7 case-control studies and 1.4 for 14 family-based studies. In addition to the exon III polymorphism, Barr and colleagues [33] examined the distributions of a 120-bp repeat 1.2 kilobases upstream to the transcription start site and a single nucleotide polymorphism (SNP) defined by a C to T substitution at position -521 in ADHD populations. The T-521 allele results in 40% less DRD4 transcription [34]. Studies looking at polymorphisms in DRD4 and infant attention [35] as well as disorders such as obsessive compulsive disorder [36], Tourette syndrome [37] and schizophrenia [38] as well as underlying dimensions of novelty seeking [39,40], attachment [41] and temperament [42] have also shown associations. In this study, we examine each of these polymorphisms.
Dopamine transporter (DAT1)
The dopamine transporter (DAT1 or SLC6A3) gene is located on chromosome 5p15.3 [43]. Methylphenidate, the primary stimulant used in pharmacological treatment of ADHD, was shown by PET imaging to block the dopamine transporter [44]. ADHD patients have also been found to show higher levels of DAT1 in the striatum [45]. The most well studied polymorphism is a VNTR in the 3' untranslated region of the DAT gene [46]. Since this VNTR is not in the coding region of the DAT gene, it does not affect the protein sequence of the dopamine transporter, but may affect the translational efficiency and thus the amount of protein expressed. Subjects homozygous for the 10-repeat allele showed significantly lower dopamine transporter binding than carriers of the 9-repeat allele [47]. Cook [48] summarized the results of an additional 11 family-based studies in a meta analysis and concluded that the association between the DAT gene and ADHD was highly significant (p < .0001). Associations with other disorders including, bipolar disorder [49], alcoholism [50] and temperamental dimensions [51] have been found.
Catecholamine-O-methyl transferase (COMT)
The catechol-O-methyltransferase (COMT) gene is located on chromosome 22q11 [52] and catalyzes the degradation of catecholamines in conjuction with monoamine oxidase. In the prefrontal cortex, the breakdown of synaptic dopamine through the COMT pathway is more critical since PFC is characterized by greater extracellular diffusion & slower clearance of dopamine than elsewhere in the brain [53]. The most widely examined polymorphisms in COMT were identified by Lachman [54] who found a G-to-A change at codons 108 and 158 of the COMT gene, resulting in Valine-to-Methionine substitutions which account for 3- to 4-fold differences in COMT activity in red blood cells and liver. A recent finding showed that COMT genotype was related to performance on the Wisconsin Card Sorting Test of executive cognition [55]. In addition, those subjects with the Methionine alleles needed less prefrontal cortical activity, as judged by fMRI, to show the same level of performance on the N-back task. The Valine allele was shown to be preferentially transmitted to ADHD probands and was associated with impulsive false alarm errors on a continuous performance task [56]. Several studies on obsessive compulsive disorder have found associations with COMT [57-60]. Associations with bipolar disorder [61] and temperamental dimensions [62] have also been reported.
Monoamine oxidase (MAOA)
The MAOA and MAOB genes are located on the X chromosome (Xp11) [63] and code for enzymes that catalyze the deamination of biogenic amines including the neurotransmitters norephinephrine, dopamine and serotonin. MAOA shows expression primarily in neurons and preferentially catalyzes the deamination of serotonin and noradrenaline. Because of its metabolic role, drugs which interfere with MAOA such as clorgyline have been used to treat ADHD [64]. The most notable of MAOA polymorphism was a nonsense mutation that led to a complete loss of MAOA function in males showing extreme forms of impulsive behavior and aggression [65]. More recently, the association with aggression was replicated in a study showing that maltreated children with certain alleles of MAOA were more likely to show aggressive behaviors [66]. MAOA male knockout mice also show high levels of aggression [67]. Less severe genetic variants are known to exist in MAOA which have been studied as candidates to explain various dimensions of psychiatric illness. The MAOA gene-linked polymorphic region (MAOA-LPR) is a 30 bp repetitive sequence that resides 1.2 kb upstream of the start codon. The 3 and 4-repeat copy alleles are the most common and the 2-, 3.5- and 5-repeat copy alleles are rare. Transfection experiments show that the 3-repeat allele results in a 5-fold lower transcriptional induction than the 4-repeat allele [68]. The second polymorphism we examined is a silent C to T change at position 1460 in exon 14. MAOA associations with ADHD [69], bipolar disorder [70-72] panic disorder [73] and alcoholism [74,75] have been reported.
Results
Population distributions of RT, alerting and executive scores
Table I summarizes attention network scores and overall reaction time (RT) for the subject population. The distributions (not shown) for each endophenotype are roughly bell shaped, symmetrical and show that approximately 70 % of the variation lies within 1 standard deviation. This suggests that standard parametric statistical tests are appropriate for subsequent analysis. An examination of pair-wise correlation coefficients for each network shows no significant correlation between alerting and orienting or executive attention and orienting, suggesting that these attention networks are largely independent. Both alerting and executive attention scores showed correlations with mean reaction time (P < 0.05 and 0.01 respectively) as well as a negative correlation with each other (r = -0.18, P < 0.01). This negative correlation appears to arise because with no warning the subjects are generally slower to respond and some conflict resolution may occur during the longer overall RT. To reduce the potential confounding effect of overall RT, raw attention network scores were divided by overall RT and resulting ratio scores were used in the genetic analysis.
Table 1.
Summary ANT values for large mixed population of normal subjects Summary means and standard deviations of attention network scores (ms) and overall reaction time.
| Alerting | Orienting | Executive | Reaction Time |
| 36 ± 22 | 55 ± 27 | 95 ± 43 | 540 ± 86 |
Since the ANT relies on RT measurements, it is important to consider how non-genetic factors such as age and intrinsic factors such as speed accuracy trade-offs might potentially contribute to overall variation before proceeding to draw genetic inferences. Previous studies have demonstrated the overall slowing of reaction times in aged subjects [76]. The mean population age here 28 ± 10 years is well below an aged range and no correlation was observed between age and reaction time (data not shown). Speed accuracy trade-offs constitute another potential confound. If a subject responds too quickly, more errors are likely to be committed. On the other hand, the same subject may intentionally slow down to reduce errors and overall reaction times will be higher. Traditionally, this phenomena is addressed by the use of d' as a dependent variable. In the study here, accuracy rates were high for all conditions (0.96–1.0) permitting the use of RT's and corresponding ratio scores. Lastly, it is also possible that gender differences exist in overall RT. The population was composed of males (40 %) and females (60%) and no significant sex differences (as judged by t-test) were seen for overall reaction time, alerting and executive attention.
DRD4
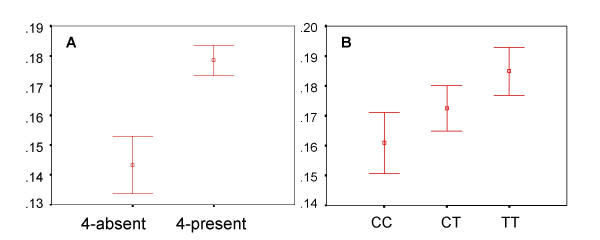
Genotypic analysis of the DRD4 gene showed that the exon III 48-bp repeat allele was present at frequencies of 0.10 (2-repeat), 0.72 (4-repeat) and 0.14 (7-repeat). Other rare variants were also present but not included in the analysis. The 120-bp repeat alleles were present at frequencies of 0.73 (long) and 0.27 (short) and the C/T SNP at -521 was present at frequencies of 0.43 and 0.57, respectively. Chi-square analyses of observed and expected frequencies of each genotypic class showed no linkage disequilibrium between the exon III 7-repeat and the -521 site or the 120 bp repeat polymorphism. The lack of disequilibrium between these sites and the 7-repeat has been observed previously [34,69]. The distribution of alerting score, executive attention score and overall reaction time as a function of each genotypic class were examined by ANOVA, linear regression and non-linear regression analysis. Each polymorphism was considered independently under a model where alleles were treated as additive (AA vs. AB vs. BB) and also as dominant (AA vs. (AB + BB)). The 120-bp repeat showed no significant association with any attention network score or overall reaction time. The C/T SNP at -521 showed an additive influence on executive attention as shown in Figure 1B where the mean executive attention scores of the C/C and T/T genotypic classes showed a nominally significant difference (P = 0.06). The exon III VNTR was also examined in this manner. In this case, each genotypic class of repeats (2/2, 2/4, 4/4, 4/7, 7/7) were treated independently. No significant associations between genotypic classes and attention network scores nor overall reaction time were observed. Each genotypic class was also grouped into various larger classes (2-present vs. 2-absent etc.). The results for one such grouping are shown in Figure 1A. A t-test for equality of means (equal variances not assumed) shows that those subjects carrying a 4-repeat show significantly (P = 0.004) higher executive attention scores than the 2,2 and 7,7 classes combined. Univariate linear regression of genotypes shows that DRD4 exon III genotypic variation contributes 3.9% (R-squared value) to the overall variation in executive attention score.
Figure 1.
DRD4 and executive attention The Y-axis shows normalized executive attention scores (mean ± SE). The X-axis shows distributions for each genotypic class. Panel A shows the distribution of executive attention score as a function of exon III VNTR genotype in the 4-repeat absent vs. 4-repeat present groups. Panel B shows distribution of executive attention score as a function of a single nucleotide genotype (CC, CT and TT) at position -521.
COMT
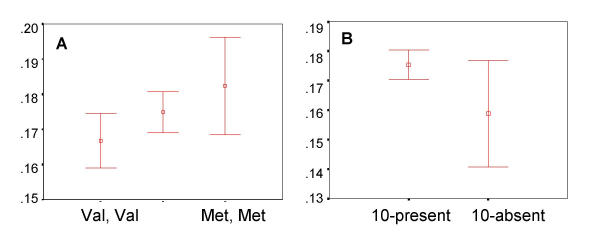
The Methionine alleles were present at a frequency of 0.39. No statistical trends or effects for overall reaction time or alerting were found. As shown in Figure 2A, a modest statistical trend toward additively higher executive attention scores was seen, and a post-hoc analysis shows that the 2 homozygous classes (Val/Val and Met/Met) show only slightly different executive attention scores (P < 0.1). Prior reports of sex differences in COMT expression in humans [77] as well as effects of gender on behavior in COMT loss-of-function mouse models [78] and reports of preferential transmission of the low activity Methionine alleles in females with OCD [79] suggested that gender could interact with ANT performance. When males and females were analyzed as independent populations, no significant associations were seen for overall reaction time or any of the network scores for either males or females. Females homozygous for the low activity Methionine allele did however, show the highest executive attention scores among all (Sex × Genotypic) classes.
Figure 2.
COMT and DAT1 and executive attention Distributions of COMT and DAT1 genotypes vs. executive attention score. The Y-axis shows normalized executive attention scores (mean + SE). The X-axis shows the distribution for each genotypic class. Panel A shows the executive attention scores for each genotypic class at the COMT Valine 108/158 Methionine polymorphism. Panel B shows the relationship between normalized executive attention scores and genotypes at the DAT1 3' UTR repeat polymorphism.
DAT1
The 10-repeat allele was present at a frequency of 0.75 and the 9-repeat allele at 0.23. As shown in Figure 2B, subjects homozygous for the rare 9-repeat allele showed modestly lower score than the pooled scores for the more common 10-repeat homozygotes and 9/10 heterozygotes. No trends were seen for accuracy, mean RT and/or the efficiency of alerting.
MAOA
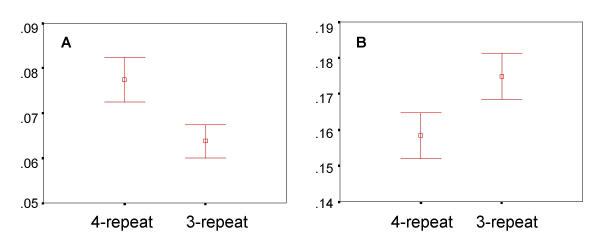
Genotypes obtained at the promoter repeat (LPR) polymorphism showed frequencies of 0.42 (3-repeat) and 0.53 (4-repeat) and genoptypes at the C1460T polymorphism showed frequencies of 0.55 (C allele) and 0.45 (T allele). Since MAOA is located on the X-chromosome, males are genetically hemizygous and females are functionally hemizygous due to random X-inactivation. For this reason, heterozygous females were excluded from the analysis. This permitted a comparison of hemizygous male and homozygous female 3-repeat vs. 4-repeat classes only. No significant main effect or trend was observed for either MAOA polymorphism and overall reaction time. As shown in Figure 3A, the MAO-LPR showed a significant influence on alerting (P < 0.01) and on executive attention (P < 0.05) as seen in Figure 3B. The C1460T polymorphism showed no significant association with alerting, but shows a modest association with executive attention (P < 0.05). When the executive attention scores for subjects carrying the C1460T (T), LPR (3-repeat) haplotype were compared with executive attention scores for subjects carrying the C1460T (C), LPR (4-repeat) haplotype, modestly significant differences (P = 0.03) were observed. This haplotype accounted for approximately 2% of the total variation.
Figure 3.
MAOA and alerting and executive attention Distributions of MAOA-LPR genotypes vs. alerting (Panel A) and executive attention (Panel B) scores. The Y-axis shows normalized alerting or executive attention scores (mean + SE). The X-axis shows the distribution for each genotypic class at the repeat polymorphism in the promoter of MAOA. Genotypic classes are a combination of males and females however only homozygous females were chosen, given the random nature of X-chromosome inactivation.
Comparison of 'high' vs. 'low' dopamine alleles
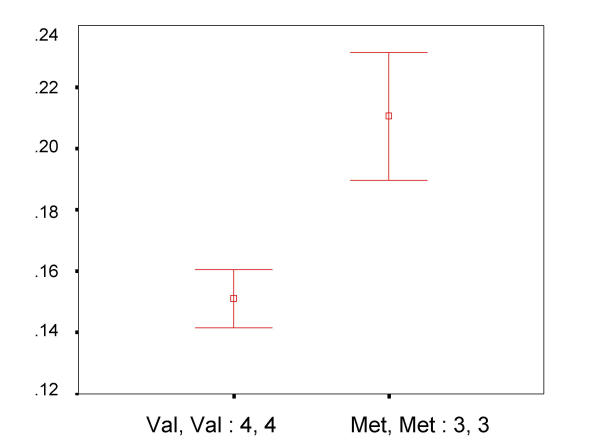
Previous biochemical studies on MAO and COMT have shown that the COMT Valine isoforms have 4-fold higher levels of enzymatic activity than the Methionine isoform. Biochemical studies on the MAO-LPR promoter repeat have shown that the 4-repeat allele has 5-fold higher levels of expression than the 3-repeat allele. This biochemical evidence permits a noninvasive inference of relative dopamine levels among subjects. In order to assess the role of dopamine on executive attention network efficiency, it would be useful to compare the distributions of executive attention scores for these 2 polymorphisms and ask whether those individuals who are expected to have higher levels of dopamine (COMT Methionine and MAOA 3-repeat) show differences in executive attention when compared to those individuals with lower levels of dopamine. Figure 4 shows the distributions for 30 subjects who carry the (Val/Val and 4/4) genotype and 20 subjects who carry the (Met/Met and 3/3) genotypes at COMT and MAOA respectively. A post-hoc analysis showed that the difference in efficiency between these subjects is highly significant (P = 0.0002) and that this particular combination of alleles contributes 3.9% to the overall variation. No significant differences were found between either the extreme 'high' or 'low' genotypic classes and each of the numerous heterozygous and compound heterozygous genotypic classes whose mean efficiency values fell within the extremes (data not shown).
Figure 4.
Effect of 'high' vs. 'low' dopamine alleles on executive attention Comparison of normalized executive attention scores in genotypic classes expected to show high and low levels of dopamine. The Y-axis shows normalized executive attention scores (mean + SE). From the entire population, 30 subjects carried the COMT (Val, Val) and MAOA-LPR (4-repeat, 4-repeat) genotypes and are expected to have relatively lower dopamine levels than 20 subjects who carried the COMT (Met, Met) and MAOA-LPR (3-repeat, 3-repeat) genotypes. These distributions are referred to as 'low' dopamine and 'high' dopamine and are shown above.
Discussion
The goal of this study was to evaluate the utility of the ANT by examining whether candidate genes frequently associated psychopathology would be related to specific attentional networks as measured by the ANT. If significant associations were found, then it would be reasonable to use the ANT for exploratory genetic studies aimed at the identification of polymorphisms that contribute to the risk of various psychiatric disorders. Genetic modelling studies suggest that when many genes underlie a complex trait or disorder, great difficulty is expected in detecting significant associations of single candidate genes [80]. In an effort to overcome this difficulty, we employed an endophenotypic measure that is (i) highly heritable (ii) highly sensitive to several core dimensions of various psychiatric disorders, and (iii) dependent on specific anatomical brain areas. As a first step in evaluating the suitability of the ANT, we chose several candidate genes that (i) have been repeatedly associated with disorders where attention is disrupted (ii) pharmacologically related to each attention network, and (iii) have been biochemically characterized so that each allelic class is associated with a difference in biochemical activity or expression level.
Variation in the DRD4 gene at the exon III VNTR and the SNP at -521 showed a modest influence on executive attentional efficiency, but no such association with reaction time or alerting. The finding that the 4-repeat absent group showed lower executive attention scores is consistent with previous findings on ADHD populations. Swanson and colleagues [81] showed that ADHD subjects with the 7-repeat allele did not show cognitive deficits on cued-detection, color-word and go-change neuropsychological tasks designed to measure various attentional functions. Although the 7-repeat allele has often been associated with ADHD, it may not contribute to a loss of attentional efficiency, but perhaps to other dimensions that underlie the development of ADHD. Moreover, recent studies on the phylogenetic history of the DRD4 exon III VNTR show that the 4-repeat is phylogenetically ancient while the 7- and 2-repeat alleles appeared recently in human populations [82] suggesting that the differences in executive attention between the 4-present vs. 4-absent groups may relate in some way to the geographic dispersal of these alleles.
Non significant trends were observed for DAT or COMT in measures of executive attention, while no trends or significant associations were observed for the other attentional network scores, mean RT and/or accuracy. The lack of any association is surprising given previous reports of associations with ADHD and other disorders. In particular, the results of Egan et al.,[55] show an influence of COMT on the efficiency of prefrontal cortical activation during an executive function task. Since functional imaging studies show that dorsolateral prefrontal cortex (DLPFC) is activated during the flanker task, and since anatomical studies [24] show that the structure of DLPFC is highly heritable, we might have expected to see a stronger association with COMT. The linear trend shown in Figure 2A does suggest that there may be some weak effect of COMT on executive attention as measured by the ANT although it is below detection in this population of normal subjects.
Polymorphisms in MAOA were found to be associated with executive attention. This was expected mainly due to the important role of MAOA in catecholamine metabolism. The additional finding of an association with alerting efficiency is consistent with the role of MAOA in the clearance of noradrenaline as well as dopamine. This role of MAOA in alerting may be related to the findings of Lim et al., [72] showing a significant association of the MAOA locus to susceptibility for bipolar disorder. Studies on depression and mood have shown deficits in simple reaction time tasks in patients that report sadness or depression [83]. These RT deficits are specific to left visual field (right hemisphere) and are consistent with the right frontal and parietal networks involved in alerting. Changes in the efficiency of the alerting network as a consequence of mood and depression are further supported by the findings of Liotti and Tucker [84] where subjects induced into sadness showed no improvement in RT when given alerting cues before target stimuli were presented.
Given that the polymorphisms examined in this study have been characterized biochemically or in other functional assays, it is worth examining in which direction (ie. more vs. less efficiency) each allele contributes. Such information might be useful for evaluating responses to treatment in disorders where medications are administered to raise or lower catecholamine levels. In the case of the DRD4 exon III polymorphism, the 4-repeat allele has been shown to have a more sensitive response to pharmacological agonists suggesting a higher response to endogenous dopamine. This allele was associated with higher (less efficient) executive attention scores. The 10-repeat allele of the DAT1 3'-VNTR polymorphism also showed higher (less efficient) executive attention scores. This is of interest since subjects with 10-repeat alleles have shown lower levels of DAT1 [47] and hence are predicted to have relatively higher levels of dopamine. While the low activity COMT Methionine allele showed no associations, the mean executive attention scores of this allele were higher (less efficient). Again, this allele should result in higher levels of endogenous dopamine. Finally, the MAOA-LPR 3-repeat allele was shown to have lower levels of transcriptional induction and thus should result in relatively higher dopamine levels. This allele showed a trend toward lower alerting scores (less efficient) and higher executive attention scores (less efficient). Interestingly, all 4 of these polymorphisms show the same directionality. That is, all of the alleles which are predicted to result in higher levels of extrasynaptic dopamine or dopamine signal transduction (DRD4-4 repeat, DAT1-10 repeat, COMT Methionine and MAOA-LPR 3 repeat) show higher (less efficient) executive attention scores. While this study demonstrates that the individual effect of each polymorphism is quite small, the combined effect of these polymorphisms could summate to exert significant behavioral effects. Since pharmacologic studies have not yet been performed on the ANT, it is not clear whether increasing relative dopamine levels results in less efficient executive attention scores. In cognitive studies of subjects and patients where dopamine levels are manipulated via medications that raise or lower dopamine levels, however, evidence of an inverted U shaped function is frequently seen [85,86].
Recommendations for molecular genetic studies on the ANT
The ANT is freely available for public download [87]. For investigators who wish to probe the genetic basis of executive attention, this study highlights many design and implementation issues. Firstly, it is evident that individual polymorphisms exert extremely weak main effects. The results of this study show that a population of even 200 subjects lacks the needed statistical power since the modest statistical associations are well below the standards set for the reporting of true association [88]. In order to adhere strictly to these guidelines, power estimates suggest that N = 600 subjects will be needed to reach these criteria for genetic studies of executive attention using the ANT. Other statistical approaches such as non-linear models or non-parametic tests may prove to be more sensitive when examining single polymorphisms or multiple polymorphic sites within a gene. Lastly, caution should be used when interpreting genetic association data on single polymorphisms since spurious associations may arise due to linkage disequilibrium at closely linked genetic loci. One encouraging aspect of this study however, is the finding that no associations or statistical trends were observed for global measures of performance such as overall reaction time. This suggests that there may be specificity in the role of genetic factors in contributing to specific neural functions.
Conclusions
Modest statistical associations of variation in executive attention were observed with genetic polymorphisms in candidate genes that affect dopaminergic signalling. These associations were not seen for global measures of performance such as reaction time, but rather for the efficiency of specific, anatomically characterised neural networks. This suggests that the ANT is a suitable endophenotypic assay for further large scale studies on the genetics of executive attention.
Methods
Subjects
Subjects were recruited in the vicinity of New York Hospital via newspaper advertisement. 25 subjects who were recruited from the vicinity of Peking University and participated in a previous heritability study were also included. Paid volunteers traveled to the Department of Psychiatry to undergo a pre-test interview. Subjects with a history of psychopathology and/or taking medication were excluded. A total of 220 adult subjects, ages 18–50 years old met inclusion criteria. All participants reported normal or corrected to normal vision. While smoking preference was recorded, only 2 subjects reported smoking regularly.
Behavioral data
The ANT was performed as previously described [1]. Briefly, participants viewed the stimuli and responses were collected via two mouse buttons. Stimuli consisted of a row of 5 visually presented horizontal black lines, with arrowheads pointing leftward or rightward, against a gray background where the target was a leftward or rightward arrowhead at the center. This target was flanked on either side by two arrows in the same direction (congruent condition), or in the opposite direction (incongruent condition), or by lines (neutral condition).
The participants' task was to identify the direction of the centrally presented arrow by pressing one button for the left direction and a second button for the right direction. Cues consisted of a 100 msec asterisk presented 400 msec before the target. There were four cue conditions: (1) no-cue, participants were shown a cross which was the same as the first fixation for 100 ms; (2) central-cue, which was at the central fixation point; (3) double-cue, in which cues were presented on the two possible target locations simultaneously (both above and below the fixation point); and (4) spatial-cue, cue was presented right on the target location (either above, below the central fixation point).
A session consisted of a 24-trial practice block and three experimental blocks of trials. Each experimental block consisted of 96 trials (12 conditions: 4 warning levels × 2 target locations × 2 target directions × 3 congruency conditions, with2 repetitions). The presentation of trials was in a random order. Participants were instructed to focus on a centrally located fixation cross throughout the task, and to response as fast, also as accurately as possible.
Calculation of attention network efficiencies
Values for attention network efficiency were calculated from the raw reaction time data as previously described. Medians were calculated for each test conditions (4 cue levels by 3 target levels, 12 conditions in total) to avoid the influence of the outliers. The alerting effect was calculated by subtracting the mean RT of the conditions with double cue from the mean RT of the conditions with no cue. Since neither of these conditions provides information on the spatial location of the target, the subtraction gives a pure measure of alerting. The orienting effect was calculated by subtracting the mean RT of the conditions with spatial (up/down) cue from the mean RT of the conditions with center cue. In both conditions the subject is alert but only the spatial cue provided spatial information on where to orient. The executive effect was calculated by subtracting the mean RT of congruent conditions from the mean RT of incongruent conditions.
Genetic sample collection and genotyping methods
Buccal swabs were obtained via buccal cell brush from consenting subjects and prepared as directed by the manufacturer. We used the MasterAMP ™ Buccal Swab DNA Extraction Kit (Epicentre Technologies, Madison, WI). Yields range from 0.5 to 3 μg of DNA from each buccal sample. Yields were determined spectrophotometrically by absorbance at 260 nm. Taq polymerase, PCR buffer and dNTPs were obtained from QIAGEN and used at recommended concentrations for a 20 ul PCR reaction. PCR reactions and restriction digests (PCR-RFLP) are optimized for each marker and performed on the PTC-100 Programmable Thermal Controller (MJ Research) outfitted with a heated lid for oil-free amplifications. For most markers, a 'touchdown' PCR cycling regimen and the addition of DMSO (10% final v:v) was used in order to automatically optimize the hybridization stringency. Gel electrophoresis in either LE or Metaphor agarose followed by staining in ethidium bromide was used to resolve and visualise DNA fragments.
For genotyping of the DRD4 exon III VNTR, primers were used at 200 uM and were designed as described in [40]. Forward: 5'-GCGACTACGTGGTCTACTCG-3'; Reverse: 5'-AGGACCCTCATGGCCTTG-3'. Many investigators have noted difficulties in obtaining reliable and specific amplification of this polymorphism when using template DNA from buccal swabs. We substituted Q-solution (QIAGEN) and an optimized 'touchdown' thermocycling regime to achieve reliable and robust amplification. For genotyping of the DRD4 120-bp tandem duplication upstream of the start-codon as described in [89], we used Forward: 5'-GTTGTCTGTCTTTTCTCATTGTTTCCATTG-3' Reverse 5'-GAAGGAGCAGGCACCGTGAGC-3' primers. For genotyping of the DRD4 C to T change at position -521 as described in [34]. Forward: 5'-CGGGGGCTGAGCACCAGAGGCTGCT-3' and Reverse 5'-GCATCGACGCCAGCGCCATCCTACC-3' were used followed by digestion with FspI. For genotyping of the DAT1 40 bp-repeat (VNTR) polymorphism in the 3 untranslated region as described in [90], Forward: 5'-TGTGGTGTAGGGAACGGCCTGAG-3' Reverse 5'-CTTCCTGGAGGTCACGGCTCAAGG-3' primers were used. For genotyping of the COMT Val to Met change at position 108 as described in [91], Forward: 5'-ACTGTGGCTACTCAGCTGTG-3' and Reverse 5'-CCTTTTTCCAGGTCTGACAA-3' primers were used followed by restriction digestion with NlaIII. For genotyping of the MAOA 30-bp repeat in promotor as described (Sabol et al, 1998), Forward: 5'-ACAGCCTGACCGTGGAGAAG-3' and Reverse 5'-GAACGGACGCTCCATTCGGA-3' primers were used. The C to T change at poition 1460 in exon 14 was genotyped according to [92]. Forward: 5'-TTAAATGGTCTCGGGAAGG-3' and Reverse 5'-GCCCAATGACACAGCCTTT-3' primers were used followed by digestion with EcoRV.
Statistical analysis of genetic vs. behavioral variation
Attention network scores were calculated using MS Excel. In order to perform genotype by network score associations, biallelic loci were assigned a '1' for A/A homozygotes, a '2' for A/B heterozygotes and a '3' for B/B heterozygotes. SPSS was used to perform one-way ANOVAs and 2-tailed t-tests where genotype was considered an independent variable. Dominant effects were assessed by grouping genotypic classes. Based on earlier analyses of the small effect size of the DRD4 exon III VNTR polymorphism, we estimated statistical power for genotypic associations for different sample sizes and the allele frequencies 4-present and 4-absent (0.7 and 0.3 respectively). A minimum significance level for type I errors (alpha) of P < 0.0001 is based on the recommendations of Lander and Kruglyak [88]. Using a computer-based power determination algorithm, a minimum sample size of N = 600 is required to achieve a power of 95%. A power of 80% was estimated for the current sample size. Linkage disequilibrium between pairs of alleles was determined using the method of Weir [93]. In this method, allele frequencies were compared to expected genotypic frequencies via chi-square analysis with a null hypothesis of no disequilibrium.
Authors' contributions
John Fossella and Tobias Sommer and Yanhong Wu were responsible for the collection of the behavioral data and genotyping. Jin Fan, James Swanson, Donald Pfaff and Michael Posner were responsible for the development and implementation of the ANT.
Acknowledgments
Acknowledgements
We wish to acknowledge the help and support of the members of the Sackler Institute of Developmental Psychobiology for helpful comments during the data collection and interpretation phases of this project.
Contributor Information
John Fossella, Email: johnfossella@hotmail.com.
Tobias Sommer, Email: tobias_sommer@t-online.de.
Jin Fan, Email: jif2004@med.cornell.edu.
Yanhong Wu, Email: wuyh@pku.edu.cn.
James M Swanson, Email: jmswanso@uci.edu.
Donald W Pfaff, Email: pfaff@mail.rockefeller.edu.
Michael I Posner, Email: mip2003@pop.med.cornell.edu.
References
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–68. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS, Lange K. The genetic transmission of schizophrenia: application of Mendelian latent structure analysis to eye tracking dysfunctions in schizophrenia and affective disorder. J Psychiatr Res. 1986;20:57–67. doi: 10.1016/0022-3956(86)90023-3. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–70. doi: 10.1016/S0920-9964(98)00156-X. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–7. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Swaab-Barneveld H, de Sonneville L, Cohen-Kettenis P, Gielen A, Buitelaar J, Van Engeland H. Visual sustained attention in a child psychiatric population. J Am Acad Child Adolesc Psychiatry. 2000;39:651–9. doi: 10.1097/00004583-200005000-00020. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci. 1999;19:5632–43. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–33. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- NIMH Genetics and Mental Disorders: Report of the NIMH's Genetics Workgroup. Book Genetics and Mental Disorders: Report of the NIMH's Genetics Workgroup (Editor ed.^eds.). City. 1999. http://www.nimh.nih.gov/publist/984268.htm
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a 4-year follow-up study. J Abnorm Psychol. 1999;108:176–81. doi: 10.1037//0021-843X.108.1.176. [DOI] [PubMed] [Google Scholar]
- Falconer Introduction to Quantitative Genetics. Harlow Essex: Addison Wesley Longman Ltd. 1996.
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–38. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Bartfai A, Pedersen NL, Asarnow RF, Schalling D. Genetic factors for the span of apprehension test: a study of normal twins. Psychiatry Res. 1991;38:115–24. doi: 10.1016/0165-1781(91)90037-P. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Coon H. Genetic and developmental factors in spontaneous selective attention: a study of normal twins. Psychiatry Res. 1997;71:163–74. doi: 10.1016/S0165-1781(97)00042-5. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–82. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo PJ, Knesevich MA, Vogler GP, Pardo JV, Towne B, Cloninger CR, Posner MI. Genetic and state variables of neurocognitive dysfunction in schizophrenia: a twin study. Schizophr Bull. 2000;26:459–77. doi: 10.1093/oxfordjournals.schbul.a033466. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS, Buss KA, Campos JJ. Genetic analyses of focal aspects of infant temperament. Dev Psychol. 1999;35:972–85. doi: 10.1037//0012-1649.35.4.972. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the varepsilon 4 allele of the apolipoprotein E gene. Proc Natl Acad Sci U S A. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH. Association of DRD4 with attention problems in normal childhood development. Psychiatr Genet. 2001;11:25–9. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–7. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–25. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Fan J. Attention as an Organ System. In: J Pomerantz, editor. To appear in Neurobiology of Perception and Communication:From Synapse to Society the IVth De Lange Conference. Cambridge UK: Cambridge University Press; [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–8. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cognitive Neurosci. 2001. [DOI] [PubMed]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neurosci. 2001;2:14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106:5–14. doi: 10.1016/S0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Savaki HE, McCulloch MC, Jehle J, Sokoloff L. The distribution of alterations in energy metabolism in the rat brain produced by apomorphine. Brain Res. 1982;243:67–80. doi: 10.1016/0006-8993(82)91121-0. [DOI] [PubMed] [Google Scholar]
- Geraud G, Arne-Bes MC, Guell A, Bes A. Reversibility of hemodynamic hypofrontality in schizophrenia. J Cereb Blood Flow Metab. 1987;7:9–12. doi: 10.1038/jcbfm.1987.2. [DOI] [PubMed] [Google Scholar]
- Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology (Berl) 1997;132:315–23. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- http://www.ncbi.nlm.nih.gov/LocusLink/LocRpt.cgi?l=1815
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–65. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1052–7. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Barr CL. Genetics of childhood disorders: XXII. ADHD, Part 6: The dopamine D4 receptor gene. J Am Acad Child Adolesc Psychiatry. 2001;40:118–21. doi: 10.1097/00004583-200101000-00025. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Ishiguro H, Toru M, Arinami T. A genetic polymorphism in the promoter region of DRD4 associated with expression and schizophrenia. Biochem Biophys Res Commun. 1999;258:292–5. doi: 10.1006/bbrc.1999.0630. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R. DRD4 related to infant attention and information processing: a developmental link to ADHD? Psychiatr Genet. 2001;11:31–5. doi: 10.1097/00041444-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Cruz C, Camarena B, King N, Paez F, Sidenberg D, de la Fuente JR, Nicolini H. Increased prevalence of the seven-repeat variant of the dopamine D4 receptor gene in patients with obsessive-compulsive disorder with tics. Neurosci Lett. 1997;231:1–4. doi: 10.1016/S0304-3940(97)00523-5. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade-Andavolu R, Gonzalez N, Wu S, Muhleman D, Blake H, Dietz G, Saucier G, MacMurray JP. Comparison of the role of dopamine, serotonin, and noradrenaline genes in ADHD, ODD and conduct disorder: multivariate regression analysis of 20 genes. Clin Genet. 2000;57:178–96. doi: 10.1034/j.1399-0004.2000.570304.x. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Konneker M, Henneken M, Dettling M, Muller-Oerlinghausen B, Roots I, Brockmoller J. Dopamine D4 receptor 48-bp repeat polymorphism: no association with response to antipsychotic treatment, but association with catatonic schizophrenia. Mol Psychiatry. 2000;5:418–24. doi: 10.1038/sj.mp.4000729. [DOI] [PubMed] [Google Scholar]
- Paterson AD, Sunohara GA, Kennedy JL. Dopamine D4 receptor gene: novelty or nonsense? Neuropsychopharmacology. 1999;21:3–16. doi: 10.1016/S0893-133X(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of Novelty Seeking. Nat Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Toth I, Ronai Z, Ney K, Sasvari-Szekely M, Gervai J. Further evidence for the role of the dopamine D4 receptor (DRD4) gene in attachment disorganization: interaction of the exon III 48-bp repeat and the -521 C/T promoter polymorphisms. Mol Psychiatry. 2002;7:27–31. doi: 10.1038/sj.mp.4001986. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of Novelty Seeking. Nat Genet. 1996;12:81–4. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- http://www.ncbi.nlm.nih.gov/LocusLink/LocRpt.cgi?l=6531
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–31. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, Hahn K, Tatsch K. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27:1518–24. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Howlett S, Earl L, White NG, McComb J, Schanfield MS, Briceno I, Papiha SS, Osipova L, Livshits G, et al. Distribution of the 3' VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol. 2000;72:295–304. [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157:1700–3. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Cook E. Molecular Genetic Studies of Attention Deficit Hyperactivity Disorder. Wenner-Gren Foundations International Symposium: Neurobiology of ADHD; Stockholm. 2000.
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, Kelsoe JR. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. Am J Med Genet. 2001;105:145–51. doi: 10.1002/1096-8628(2001)9999:9999<::AID-AJMG1161>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T, Komiyama T, Mitsushio H, Sano A, Tanabe H. Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism. Mol Psychiatry. 1999;4:552–7. doi: 10.1038/sj.mp.4000562. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Rybakowski F, Czerski P, Zakrzewska M, Stepien G, Pelka-Wysiecka J, Horodnicki J, Rybakowski JK, Hauser J. Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationship to temperamental dimensions measured by the Temperament and Character Inventory in healthy volunteers. Neuropsychobiology. 2001;43:248–53. doi: 10.1159/000054898. [DOI] [PubMed] [Google Scholar]
- http://www.ncbi.nlm.nih.gov/LocusLink/LocRpt.cgi?l=1312
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–4. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, Goldberg R, Kucherlapati R, Papolos DF. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67:468–72. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J, Mei-Tal G, Steinberg A, Tartakovsky E, Zohar A, Gritsenko I, Nemanov L, Ebstein RP. Haplotype relative risk study of catechol-O-methyltransferase (COMT) and attention deficit hyperactivity disorder (ADHD): association of the high-enzyme activity Val allele with ADHD impulsive-hyperactive phenotype. Am J Med Genet. 1999;88:497–502. doi: 10.1002/(SICI)1096-8628(19991015)88:5<497::AID-AJMG12>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Niehaus DJ, Kinnear CJ, Corfield VA, du Toit PL, van Kradenburg J, Moolman-Smook JC, Weyers JB, Potgieter A, Seedat S, Emsley RA, et al. Association between a catechol-o-methyltransferase polymorphism and obsessive-compulsive disorder in the Afrikaner population. J Affect Disord. 2001;65:61–5. doi: 10.1016/S0165-0327(00)00246-9. [DOI] [PubMed] [Google Scholar]
- Schindler KM, Richter MA, Kennedy JL, Pato MT, Pato CN. Association between homozygosity at the COMT gene locus and obsessive compulsive disorder. Am J Med Genet. 2000;96:721–4. doi: 10.1002/1096-8628(20001204)96:6<721::AID-AJMG4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, Gogos JA. Genotype determining low catechol-O-methyltransferase activity as a risk factor for obsessive-compulsive disorder. Proc Natl Acad Sci U S A. 1997;94:4572–5. doi: 10.1073/pnas.94.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear C, Niehaus DJ, Seedat S, Moolman-Smook JC, Corfield VA, Malherbe G, Potgieter A, Lombard C, Stein DJ. Obsessive-compulsive disorder and a novel polymorphism adjacent to the oestrogen response element (ERE 6) upstream from the COMT gene. Psychiatr Genet. 2001;11:85–7. doi: 10.1097/00041444-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Rotondo A, Mazzanti C, L Dell'Osso, Rucci P, Sullivan P, Bouanani S, Gonnelli C, Goldman D, Cassano GB. Catechol o-methyltransferase, serotonin transporter, and tryptophan hydroxylase gene polymorphisms in bipolar disorder patients with and without comorbid panic disorder. Am J Psychiatry. 2002;159:23–9. doi: 10.1176/appi.ajp.159.1.23. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Kotler M, Gritsenko I, Nemanov L, Belmaker RH, Ebstein RP. Association between tridimensional personality questionnaire (TPQ) traits and three functional polymorphisms: dopamine receptor D4 (DRD4), serotonin transporter promoter region (5-HTTLPR) and catechol O-methyltransferase (COMT). Mol Psychiatry. 2000;5:96–100. doi: 10.1038/sj.mp.4000640. [DOI] [PubMed] [Google Scholar]
- http://www.ncbi.nlm.nih.gov/LocusLink/LocRpt.cgi?l=4128
- Zametkin A, Rapoport JL, Murphy DL, Linnoila M, Ismond D. Treatment of hyperactive children with monoamine oxidase inhibitors. I. Clinical efficacy. Arch Gen Psychiatry. 1985;42:962–6. doi: 10.1001/archpsyc.1985.01790330042005. [DOI] [PubMed] [Google Scholar]
- Brunner NM HG, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–6. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–9. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Payton A, Holmes J, Barrett JH, Hever T, Fitzpatrick H, Trumper AL, Harrington R, McGuffin P, O'Donovan M, Owen M, et al. Examining for association between candidate gene polymorphisms in the dopamine pathway and attention-deficit hyperactivity disorder: a family-based study. Am J Med Genet. 2001;105:464–70. doi: 10.1002/ajmg.1407. [DOI] [PubMed] [Google Scholar]
- Craddock N, Roberts Q, Williams N, McGuffin P, Owen MJ. Association study of bipolar disorder using a functional polymorphism (Ser311–>Cys) in the dopamine D2 receptor gene. Psychiatr Genet. 1995;5:63–5. doi: 10.1097/00041444-199522000-00003. [DOI] [PubMed] [Google Scholar]
- Furlong RA, Ho L, Rubinsztein JS, Walsh C, Paykel ES, Rubinsztein DC. Analysis of the monoamine oxidase A (MAOA) gene in bipolar affective disorder by association studies, meta-analyses, and sequencing of the promoter. Am J Med Genet. 1999;88:398–406. doi: 10.1002/(SICI)1096-8628(19990820)88:4<398::AID-AJMG18>3.3.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Lim LC, Powell J, Sham P, Castle D, Hunt N, Murray R, Gill M. Evidence for a genetic association between alleles of monoamine oxidase A gene and bipolar affective disorder. Am J Med Genet. 1995;60:325–31. doi: 10.1002/ajmg.1320600413. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–4. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Lesch KP, Rottmann M, Smolka M, Syagailo YV, Okladnova O, Rommelspacher H, Winterer G, Schmidt LG, Sander T. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Res. 1999;86:67–72. doi: 10.1016/S0165-1781(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Yu LM, Tarter RE, Deka R. Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAOA gene with early onset alcoholism/substance abuse. Am J Med Genet. 1995;60:122–6. doi: 10.1002/ajmg.1320600207. [DOI] [PubMed] [Google Scholar]
- Melis A, Soetens E, van der Molen MW. Process-specific slowing with advancing age: evidence derived from the analysis of sequential effects. Brain Cogn. 2002;49:420–35. doi: 10.1006/brcg.2001.1508. [DOI] [PubMed] [Google Scholar]
- Ladosky W, Figueiredo BC, Schneider HT. Hypothalamic nuclei catechol-O-methyl-transferase and the process of brain sexual differentiation. Braz J Med Biol Res. 1984;17:107–17. [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–6. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsobrook JP, Zohar AH, 2nd, Leboyer M, Chabane N, Ebstein RP, Pauls DL. Association between the COMT locus and obsessive-compulsive disorder in females but not males. Am J Med Genet. 2002;114:116–20. doi: 10.1002/ajmg.10040. [DOI] [PubMed] [Google Scholar]
- Ennis S, Maniatis N, Collins A. Allelic association and disease mapping. Brief Bioinform. 2001;2:375–87. doi: 10.1093/bib/2.4.375. [DOI] [PubMed] [Google Scholar]
- Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, Wasdell M, Ding Y, Chi HC, Smith M, et al. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci U S A. 2000;97:4754–9. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Flodman P, Spence MA, Schuck S, Swanson JM, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci U S A. 2002;99:309–14. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladavas E, Nicoletti R, Umilta C, Rizzolatti G. Right hemisphere interference during negative affect: a reaction time study. Neuropsychologia. 1984;22:479–85. doi: 10.1016/0028-3932(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Liotti M, Tucker DM. Right hemisphere sensitivity to arousal and depression. Brain Cogn. 1992;18:138–51. doi: 10.1016/0278-2626(92)90075-w. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Depue RA. Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cereb Cortex. 1998;8:218–26. doi: 10.1093/cercor/8.3.218. [DOI] [PubMed] [Google Scholar]
- Luciana M, Sullivan J, Nelson CA. Associations between phenylalanine-to-tyrosine ratios and performance on tests of neuropsychological function in adolescents treated early and continuously for phenylketonuria. Child Dev. 2001;72:1637–52. doi: 10.1111/1467-8624.00370. [DOI] [PubMed] [Google Scholar]
- http://140.251.58.136/~jinfan/ant/ant.html
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–7. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Seaman MI, Fisher JB, Chang F, Kidd KK. Tandem duplication polymorphism upstream of the dopamine D4 receptor gene (DRD4). Am J Med Genet. 1999;88:705–9. doi: 10.1002/(SICI)1096-8628(19991215)88:6<705::AID-AJMG22>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4:192–6. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, Spurlock G, Riley B, Scambler P, Asherson P, et al. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry. 1996;153:268–70. doi: 10.1176/ajp.153.2.268. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Breakefield XO. Human monoamine oxidase A gene determines levels of enzyme activity. Am J Hum Genet. 1991;49:383–92. [PMC free article] [PubMed] [Google Scholar]
- Weir BS. Genetic Data Analysis II. Sinauer Associates, Inc Sunderland, MA. 1996.