Abstract
Cytokines and soluble cellular receptors are involved in inflammatory processes and probably in the pathogenesis of parasite and bacterial diseases. In a previous study, we reported increased levels of soluble receptors of interleukin-2 (sIL2-R) in children with acute Chagas' disease, one of the main parasitic infections that is endemic in Latin America. We sought to analyze the pattern of different cytokines and soluble receptors in the sera of children with chagasic infection. Children with acute and indeterminate stages of Chagas' disease, as well as nonchagasic children, were studied. Sera were assayed by enzyme-linked immunosorbent assay to measure the levels of tumor necrosis factor alpha (TNF-α), IL-6, IL-2, IL-8, IL-12, sIL-2R, and the soluble receptors of CD8 and CD4 (sCD8 and sCD4). sIL-2R and sCD8 showed the highest levels in serum in acutely infected children, decreasing after specific antiparasite therapy. Chronic children showed a pattern similar to the one of nonchagasic children. Although they were not statistically significant, TNF-α, IL-6, and sCD4 showed a tendency to reach high levels in the acutely infected group, whereas IL-2, IL-8, and IL-12 did not reveal changes with respect to the noninfected children. In summary, we report here the patterns of cytokines and soluble receptors in in the sera of children infected with Trypanosoma cruzi; we found significantly increased levels of sIL-2R and sCD8 in acute infection that decreased after therapy, and high levels of TNF-α, IL-6, and sCD4 in some of the acute patients. The measurement of sIL-2R and sCD8 may provide a useful tool in the follow-up of children with Chagas' disease.
Chagas' disease, produced by the protozoan parasite Trypanosoma cruzi, is one of the main parasitic infections that is endemic in Latin America, where near 20 million people are infected and another 60 million are at risk of infection. The evolution of the disease shows an acute period of 30 to 60 days, a long indeterminate stage and in ca. 30% of patients, a chronic disease with cardiac or digestive failure. Children below the age of 14 years have the highest incidence of infection. Hence, the diagnosis and follow-up of acute and congenital cases of disease in which the treatment of the infection is still effective is very important (13, 14). To do this, new laboratory tools are necessary to monitor therapeutic effectiveness and the outcome of the infection.
In recent years, several reports have focused their attention on molecules in serum that could serve as markers of infection. It is known that cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) are involved in inflammatory processes and possibly, in the pathogenesis of parasitic and bacterial diseases (6, 17, 23). Furthermore, Rubin et al. (19) described the shedding of the receptor of IL-2 (sIL-2R) from activated lymphocytes and its detection by immunoenzymatic methods. Measurement of the levels of cytokines and soluble receptors in plasma has been proposed as a valuable tool in the follow-up of patients with bacterial sepsis (2-4, 7). In a previous study, we reported increased levels of sIL2-R in children with acute Chagas' disease (11).
The aim of the present study was thus to analyze the patterns of cytokines and soluble receptors in the sera of children with acute Chagas' disease and to determine the possible usefulness of these cytokines and soluble receptors as immunological markers of the clinical evolution in Chagas' disease.
MATERIALS AND METHODS
Patients and controls.
Children aged between 1 to 13 were selected according the following criteria: (i) children with acute Chagas' disease (ACU), with typical clinical symptoms and parasitemia detected by the microhematocrit method (1, 24); (ii) a subset of these children, studied after 30 days of treatment with parasiticide drug benznidazol, with undetectable parasitemia (PT); (iii) children with asymptomatic, indeterminate-stage Chagas' disease (ID), as demonstrated by the presence of anti-T. cruzi antibodies and determined based on at least two serological tests (i.e., indirect hemaglutination; indirect immunofluorescence, and/or enzyme-linked immunosorbent assay [ELISA]); and (iv) nonchagasic control children (NCh), with negative serology for Chagas' disease. This last population was composed of children with respiratory diseases (n = 3), viral hepatitis A (n = 2), and diarrhea (n = 2), as well as a miscellaneous ambulatory population that came to the laboratory for routine studies. All patients were from the provinces of Santiago del Estero and Córdoba, Argentina. Informed consent was obtained from the parents of children included in the study.
Measurement of cytokines and soluble receptors.
ELISA commercial kits were used to measure the levels of TNF-α, IL-6, IL-2, IL-8, IL-12, and sIL-2R (Quantikine Immunoassay; R&D Systems, Inc., Minneapolis, Minn.) and sCD and sCD4 (Cellfree; T-cell Diagnostics, Cambridge, Mass.) in serum according to the manufacturers' instructions. Briefly, immunoplates coated with a monoclonal antibody to the studied receptors or cytokines were incubated with the samples and standards. After a washing step, a peroxidase-conjugated polyclonal antiserum was added to each cytokine or soluble receptor, and after a second step of incubation and washing, tetramethyl benzidine was added as a substrate. The reactions were stopped with 2 N H2SO4, and the optical densities at 450 nm were measured in an ELISA plate reader. Values were calculated from standard curves based on prepared dilutions of recombinant cytokines.
Statistics.
The Student t test was used to investigate the statistical significance of the differences among the different groups of patients and noninfected children. A P value of <0.05 was considered significant. A paired t test was applied between ACU and PT patients in the subpopulation that was studied before and after treatment.
RESULTS
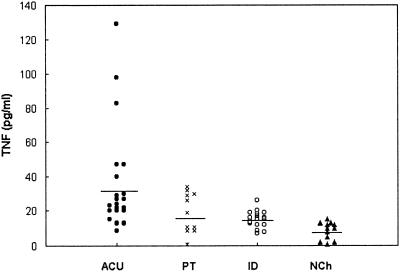
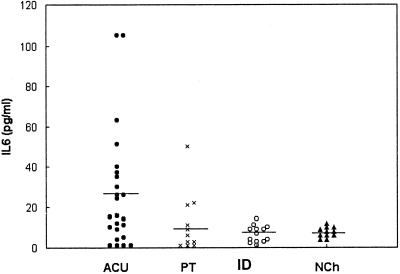
The levels of TNF-α in serum that we obtained are shown in Fig. 1. Clearly, in some of the children belonging to the acute group (6 of 21), the observed values were higher than in those from the other groups. Nevertheless, the differences of the means were not statistically significant. The mean values ± the standard deviation observed in each group were as folows: ACU (n = 21), 36 ± 30 pg/ml; PT (n = 11), 19 ± 11; ID (n = 16), 15 ± 5 pg/ml; and NCh (n = 12), 8 ± 5 pg/ml. Analysis of the IL-6 levels in serum also did not reveal any statistically significant differences among the groups (Fig. 2). Nevertheless, in the acute group, 8 of 24 of the studied children had concentrations greater than the established cutoff value. The mean values of IL-6 were as follows: ACU (n = 24), 28 ± 34 pg/ml; PT (n = 11), 11 ± 15 pg/ml; ID (n = 13), 7 ± 4 pg/ml; and NCh (n = 11), 8 ± 3 pg/ml. The paired t test did not show statistically significant differences between ACU and PT either in TNF-α or in IL-6 levels in plasma.
FIG. 1.
Levels of TNF-α in the sera of children with acute chagasic infection (ACU; n = 21), a subset of children with acute chagasic infection treated with benznidazol (PT; n = 11), children with an indeterminate stage of the infection (ID; n = 16) and nonchagasic children (NCh; n = 12). The horizontal line shows the median values of each group.
FIG. 2.
Levels of IL-6 in the sera of children with acute chagasic infection (ACU; n = 24), a subset of children with acute chagasic infection treated with benznidazol (PT; n = 11), in children with an indeterminate stage of the infection (ID; n = 13), and in nonchagasic children (NCh; n = 11). The horizontal line shows the median values of each group.
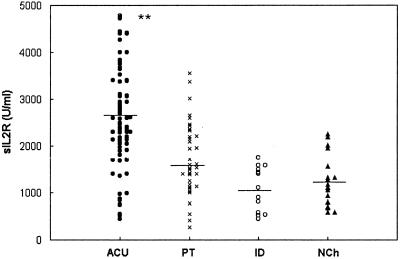
Figure 3 shows the levels of sIL-2R in the different groups of children. Compared to all of the other groups (P < 0.001), a significant increase in the levels of sIL-2R in serum in acute children was observed, which confirmed the preliminary results of a previous work (11). The median concentrations were as follows: ACU (n = 55), 2,630 ± 1,076 U/ml; PT (n = 11), 1,630 ± 666 U/ml; ID (n = 13), 1,087 ± 476 U/ml; and NCh (n = 16), 1,238 ± 536 U/ml, respectively. The study of the pairs of sera carried out before and after treatment showed, in most cases, a decrease in the levels of sIL-2R that ranged between 16 and 45% of the initial value. In children followed up after treatment (Table 1), the paired t test was used to compare values before and after treatment, and a statistically significant difference between these groups (P < 0.001) was shown.
FIG. 3.
Levels of sIL-2R in the sera of children with acute chagasic infection (ACU; n = 55), a subset of children with acute chagasic infection treated with benznidazol (PT; n = 33), children with an indeterminate stage of the infection (ID; n = 13), and nonchagasic children (NCh; n = 16). The horizontal line shows the median values of each group. ✽✽, P < 0.001.
TABLE 1.
Paired levels of IL-2R and sCD8 in the serum of children with acute chagasic infection (ACU) and after 30 days of treatment with benznidazol (PT)
| Patient | Concn (U/ml)a of:
|
|||
|---|---|---|---|---|
| IL-2 R
|
sCD8
|
|||
| ACUa | PTb | ACUc | PTd | |
| 1 | 3,667 | 1,601 | 1,214 | 552 |
| 2 | 3,704 | 2,322 | 2,490 | 791 |
| 3 | 3,281 | 1,299 | 1,511 | 868 |
| 4 | 5,108 | 1,608 | 2,600 | 1,200 |
| 5 | 2,910 | 2,432 | 1,222 | 732 |
| 6 | 3,382 | 1,247 | 2,388 | 318 |
| 7 | 1,968 | 1,712 | 1,383 | 1,064 |
| 8 | 3,077 | 1,414 | 1,946 | 335 |
| 9 | 4,410 | 998 | 2,360 | 421 |
| 10 | 2,300 | 2,149 | 2,180 | 605 |
| 11 | 2,120 | 416 | 1,600 | 588 |
| 12 | 2,987 | 2,322 | NDe | ND |
For IL-2R, the mean ± standard deviation values for the ACU and PT groups were 3,242.83 ± 607.1 (P < 0.001) and 1,626.67 ± 479.0, respectively. For sCD8, the mean ± standard deviation values for the ACU and PT group were 1,899.45 ± 282.5 (P < 0.0001) and 679.45 ± 285.6, respectively. ND, not done.
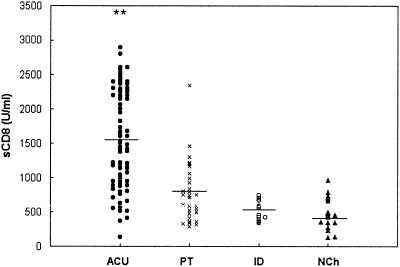
Figure 4 shows the results obtained in the levels of sCD8 in each group of patients. The pattern was similar to the one observed with sIL-2R, with significant increased levels in children with acute infection (P < 0.001), a tendency to decrease after treatment, and no differences between the indeterminate stage and the nonchagasic groups. The median levels were as follows: ACU (n = 64), 1,567 ± 682 U/ml; PT (n = 33), 742 ± 334 U/ml; ID (n = 10), 1,512 ± 139 U/ml; and NCh (n = 15), 459 ± 204 U/ml. Comparative analysis of the pair of sera obtained before and after treatment of the same children showed a decline in the levels of sCD8 by, on average, 50%. Statistical analysis by the paired t test showed significant differences between both groups (P < 0.0001; Table 1).
FIG. 4.
Levels of sCD8 in the sera of children with acute chagasic infection (ACU; n = 64), a subset of children with acute chagasic infection treated with benznidazol (PT; n = 33), children with an indeterminate stage of the infection (ID; n = 10), and nonchagasic children (NCh; n = 15). The horizontal line shows the median values of each group. ✽✽, P < 0.001
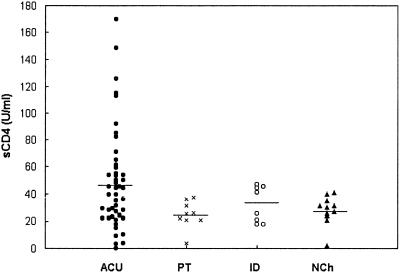
The information obtained from analysis of the concentrations of sCD4 in serum was different (Fig. 5). In fact, although significant differences among all of the groups could not be detected, the acutely infected children showed a tendency to reach high values, as observed with TNF-α and IL-6. The median levels in each group were as follows: ACU (n = 48), 48.5 ± 36.8 U/ml; PT (n = 9), 24.8 ± 10 U/ml; ID (n = 8), 32.4 ± 13 U/ml; and NCh (n = 11), 28 ± 10.6 U/ml.
FIG. 5.
Levels of sCD4 in the sera of children with acute chagasic infection (ACU; n = 48), a subset of children with acute chagasic infection treated with benznidazol (PT; n = 9), children with an indeterminate stage of the infection (ID; n = 8), and nonchagasic children (NCh; n = 11). The horizontal line shows the median values of each group.
The levels of IL-2, IL-8, and IL-12 in serum measured in the different groups of chagasic children did not differ from those of nonchagasic children (data not shown).
DISCUSSION
Cytokines have been proposed as markers in cases of neonatal sepsis (21), meningitis (16, 18), and other acute bacterial infections (5) because they correlate with the clinical outcome of the disease (10, 12, 25). In a previous study we reported elevated levels of sIL-2R in serum in children with acute infection (11). In the present study, we observed a close association between high levels of sIL-2R and sCD8 in serum with acute Chagas disease in children. The use of immunological markers could be useful in the evaluation of therapy effectiveness, as was observed in cases of bacterial infection (2, 12). In our group of children, sIL-2R and sCD8 decrease to statistically significant lower levels after treatment. Although these results do not provide unequivocal proof that the reduction in sIL-2R and sCD8 is the effect of antiparasitic treatment, at least in two cases of interruption of therapy, the levels of sIL-2R and sCD8 did not decline in the last sample (data not shown).
Taken together, the results observed in treated children and in children with indeterminate-stage infection indicate the predictive power of measuring the levels of IL-2R and sCD8 in serum in relation to the parasite load. In fact, the levels of these markers were very similar in all of the children in which the parasitemia was not detectable by microhematocrit method. TNF-α, IL-6, and sCD4 showed similar tendencies, although the differences were not statistically significant.
Noticeably, in “in vitro” experiments, Kierszenbaum et al. (8, 9) reported dramatic suppression of IL-2R on the surface of human mononuclear cells and also a clear reduction of sIL-2R in the supernatant of stimulated cells cocultivated with T. cruzi. These apparently contradictory results could be explained by the different systems studied. In fact, the conditions under which the experiments were performed are quite different from those of the human infection.
In agreement with the results of the present study, Ward et al. (22) did not found differences in the levels of IL-2, gamma interferon, and TNF in the sera of children with indeterminate and chronic forms of Chagas disease. On the other hand, Samudio et al. (20) have reported the analysis of cytokine profiles in children with Chagas' disease by studying the mRNA by reverse transcription-PCR, and these authors found that the expression of mRNA for gamma interferon, IL-2, IL-4, and IL-10 was elevated in both parasitemic and nonparasitemic children. These approaches could be useful in obtaining information about the pathogenic or resistance mechanisms of this disease. In this regard, we have seen a dramatic increase in TNF-α, IL-6, and IL-10 levels in the sera of acutely infected mice that is associated with high parasitemias and fatal outcome (L. Cervetta et al., unpublished data). In patients with acute Chagas' disease, it is possible that the levels of sIL2-R and sCD8 could increase in response to infection, such as in other infectious diseases, as result of T-cell activation and, perhaps, by increasing the shedding of soluble surface receptors. This situation has in fact been proposed as an immunosuppressive mechanism triggered by the parasite in order to facilitate its dissemination in the host (8, 9).
Finally, we did not observe increased systemic production of IL-2, IL-8, and IL-12 in none of the different populations studied here. The levels of TNF-α, IL-6, and sCD4 were increased in about one-half of the acutely infected children, suggesting that other individual factors could be involved in the development of the innate immune response in Chagas disease.
In summary, we report here that the serum pattern of cytokines and soluble receptors in children infected with T. cruzi exhibited a clear increase in the levels of sIL-2R and sCD8 in most of the acutely infected children and a decrease in these levels in the treated children. Also, several of the infected children showed high levels of TNF-α, IL-6, and sCD4 in serum. As has been proposed in the case of bacterial sepsis, the quantitative measurement of one or more of these molecules may provide an additional tool in follow-up and in the evaluation of therapeutic effectiveness in infants and children, the population in which acute Chagas' disease has been most prevalent (15).
Acknowledgments
This work was supported with funds from Servicio Nacional de Chagas, the SECYT-Universidad Nacional de Córdoba, CONICOR, and the Secretaría de Ciencia y Tecnología, San Luis.
We are grateful to Irma Castro for assistance in the selection of patients in the Servicio Nacional de Chagas and to Patricia Gil for technical assistance.
REFERENCES
- 1.Basso, B., and E. Moretti. 1984. Detección del Trypanosoma cruzi por hemocultivo en pacientes con Enfermedad de Chagas crónica. Medicina 1984:4441-4447. [PubMed] [Google Scholar]
- 2.Buck, C., J. Bundschu, H. Gallati, et al. 1996. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 93:54-58. [PubMed] [Google Scholar]
- 3.Casey, L., A. Balk, and C. Bone. 1993. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann. Intern. Med. 119:771-778. [DOI] [PubMed] [Google Scholar]
- 4.Damas, P., D. Ledoux, and M. Nys. 1992. Cytokine serum level during severe sepsis in human: IL-6 as marker of severity. Ann. Surg. 215:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fresno, M., M. Kopf, and L. Rivas. 1997. Cytokines and infectious diseases. Immunol. Today 18:56-58. [DOI] [PubMed] [Google Scholar]
- 6.Girardin, E., G. Grau, and J. Dayer. 1988. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N. Engl. J. Med. 319:397-400. [DOI] [PubMed] [Google Scholar]
- 7.Harris, M. C., A. Costarino, J. Sullivan, et al. 1994. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J. Pediatr. 124:105-111. [DOI] [PubMed] [Google Scholar]
- 8.Kierszenbaum, F., E. Moretti, and M. Sztein. 1993. Molecular basis of Trypanosoma cruzi induced immunosuppression: altered expression by activated human lymphocytes of molecules which regulate antigen recognition and progression through the cell cycle. Biol. Res. 26:197-207. [PubMed] [Google Scholar]
- 9.Kierszenbaum, F., E. Moretti, and M. Sztein. 1991. Trypanosoma cruzi induces suppression of DNA synthesis and inhibits expression of interleukin-2 receptors by stimulated human B lymphocytes. Immunology 74:317-322. [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Cortés, L., M. Cruz-Ruiz, D. Gómez-Mateos, et al. 1993. Measurement of levels of TNF-α and IL-1β in the cerebrospinal fluid of patients with meningitis of different etiologies: utility in the differential diagnosis. Clin. Infect. Dis. 16:534-539. [DOI] [PubMed] [Google Scholar]
- 11.Moretti, E., B. Basso, O. Ledesma, G. Barbieri, G. Correa, and L. Suarez. 1994. Inmunología de la enfermedad de Chagas: niveles séricos del receptor para interleukina 2 en niños con infección aguda. Rev. Asoc. Mex. Bioq. Clín. 19:152-154. [Google Scholar]
- 12.Moretti, E., S. Baigorria, L. Manzanares, P. Moya, and B. Basso. 2000. Interleuquina 6, receptor soluble de interleuquina 2 y proteina C reactiva como marcadores de sepsis neonatal. Acta Bioq. Clin. Latinoam. 34:1-14. [Google Scholar]
- 13.Moya, P., E. Moretti, R. Paolasso, B. Basso, S. Blanco, and C. Sanmartino. 1989. Enfermedad de Chagas neonatal: diagnóstico de laboratorio durante el primer año de vida. Medicina 49:595-599. [PubMed] [Google Scholar]
- 14.Moya, P., R. Paolasso, S. Blanco, M. Lapacet, C. Sanmartino, B. Basso, E. Moretti, and D. Cura. 1985. Enfermedad de Chagas: resultados terapeuticos en niños en los primeros meses de vida. Medicina 45:553-558. [PubMed] [Google Scholar]
- 15.Moya, P., and E. Moretti. 1997. Doenca de Chagas congenita. En “Clinica e terapeutica da doenca de Chagas: uma abordagem pratica para o clinico geral. Editora Fiocruz. 1997:383-410. [Google Scholar]
- 16.Offner, F., J. Philippe, D. Vogelaers, et al. 1990. Serum tumor necrosis factor levels in patients with infectious disease and septic shock. J. Lab. Clin. Med. 116:100-105. [PubMed] [Google Scholar]
- 17.Olsson, I., T. Gatanaga, U. Gullberg, et al. 1993. Tumor necrosis factor (TNF) binding proteins (soluble TNF receptor forms) with possible roles in inflammation and malignancy. Eur. Cytokine Netw. 4:169-180. [PubMed] [Google Scholar]
- 18.Roine, I., L. Foncea, J. Cofre, et al. 1992. Serum C reactive protein versus tumor necrosis factor alpha and interleukin-1β of the cerebrospinal fluid in diagnosis of bacterial meningitis with low cerebrospinal fluid cell count. Pediatr. Infect. Dis. J. 11:1057-1058. [PubMed] [Google Scholar]
- 19.Rubin, L., C. Kurman, M. Fritz, et al. 1986. Soluble IL-2 receptors are released from activated human lymphoid cells in vitro. J. Immunol. 135:3172-3177. [PubMed] [Google Scholar]
- 20.Samudio, M., S. Montenegro-James, M. Cabral, J. Martínez, A. Rojas de Arias, and M. A. James. 1998. Cytokine responses in Trypanosoma cruzi-infected children in Paraguay. Am. J. Trop. Med. Hyg. 51:119-121. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, J. S., I. Kilpatrick, A. Costarino, et al. 1992. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J. Pediatr. 120:510-515. [DOI] [PubMed] [Google Scholar]
- 22.Ward, L. S., M. E. Guariento, G. A. Fernandes, and R. M. Maciel. 1999. Serum cytokines in chronic Chagas' disease. Rev. Brasil. Med. Trop. 32:285-289. [DOI] [PubMed] [Google Scholar]
- 23.Wilson, M., R. Seymour, and B. Henderson. 1998. Bacterial perturbation of cytokine networks. Infect. Immun. 66:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo, P. T. 1969. The haematocrit centrifuge for the detection of trypanosomes in blood. Can. J. Zool. 47:921-924. [DOI] [PubMed] [Google Scholar]
- 25.Wuiteside, T. 1994. Cytokines and cytokine measurements in a clinical laboratory. Clin. Diagn. Lab. Immunol. 1:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]