Abstract
The in vitro effects of gamma interferon (IFN-γ) on the mouse CD5+ B1-cell line, TH2.52, a hybridoma between mouse B lymphoma and mouse splenic B cells that expresses a series of B1 markers, were investigated. A significant number of macrophage-like cells appeared in the cultures of TH2.52 cells exposed to IFN-γ, these adhering to plastic dishes and exhibiting phagocytic activity. Positive for esterase staining, the macrophage-like cells returned to the original TH2.52 morphology upon removal of IFN-γ. The change was prevented by treatment with SB202190, an inhibitor of p38 mitogen-activated protein (MAP) kinase and by transfection of a p38 MAP kinase dominant-negative mutant. Further, interleukin-4 (IL-4) inhibited IFN-γ-induced phosphorylation of p38 MAP kinase and the appearance of macrophage-like cells. IFN-γ and IL-4 exhibited contradictory actions on morphological change of CD5+ B1 cells into macrophage-like cells. Differential regulation of CD5+ B1 cells by IFN-γ, a Th1 cytokine, and IL-4, a Th2 cytokine, may have clear immunological significance.
The immune system features a subset of B cells that are phenotypically and functionally distinct from the conventional B-cell population (17, 18, 20, 21); these cells express a low level of CD5 and are therefore termed CD5+ B cells. The B-cell population including CD5+ B cells and CD5− B cells that are very similar is now referred to as B1 cells. Recently, there have been several reports on a close relationship between CD5+ B cells and macrophages (3-6), running counter to the current paradigm for hematopoietic lineage relationships. In the studies, CD5+ pre-B-cell lymphomas were shown to acquire macrophage characteristics, violating current textbook models of hematopoiesis in which the lymphoid and myeloid lineages diverge at the level of a very early progenitor cell. This new concept of the existence of a normal B-cell/macrophage cell (B/macrophage cell) with a CD5+ B1-cell origin has been extensively reviewed (4, 5), and it is now well established that the B/macrophage cells are morphologically distinct from classical macrophages and have unique surface characteristics in line with their B-cell origin. The TH2.52 cell line, established by fusion of a mouse B lymphoma cell with a mouse splenic B lymphocyte (14-16), is a CD5+ B1-cell line characterized by the presence of several macrophage markers (23). In the present study, the in vitro effects of gamma interferon (IFN-γ) in TH2.52 B1 cells are documented, including transformation into macrophage-like cells inhibited by interleukin-4 (IL-4).
MATERIALS AND METHODS
Reagents.
IFN-γ and IL-4 were obtained from PeproTech, London, England. SB202190 (a specific inhibitor of p38 mitogen-activated protein [MAP] kinase), its inactive analogue SB202474, AG490 (a specific inhibitor of Janus tyrosine kinase 2 [JAK2]), and PD98059 (a specific inhibitor of extracellular signal-regulated kinase 1/2 [ERK1/2]) were from Calbiochem, Darmstadt, Germany. Anti-IFN-γ antibody was purchased from Genzyme, Cambridge, Mass., and antibodies to p38, phospho-p38, the signal transducer and activator of transcription 1 (STAT1), and phospho-STAT1 were was obtained from New England Biolabs, Beverly, Mass. A pcDNA3 vector carrying the dominant-negative mutant of p38 MAP kinase (34) and the empty vector were kindly supplied by J. Han, The Scripps Research Institute (La Jolla, Calif.).
Cell culture.
The TH2.52 cell line, a hybridoma line produced by fusion between the M12.4.1 lymphoma of BALB/c mouse origin and normal splenic B lymphocytes from C57BL/6 mice (14), expresses CD5, CD11b, B220, F4/80, and CD14 (23). The cells were cultured in RPMI 1640 (Sigma Chemical, St. Louis, Mo.) supplemented with 5% heat-inactivated fetal calf serum (HyClone, Logan, Utah), 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, and antibiotics at 37°C under 5% CO2.
Identification of macrophage-like cell.
Cells were cultured at 5 × 103/100 μl in 96-well plastic plates with various stimuli for the indicated times. Macrophage-like cells were defined as spread cells having dendritic processes extending at least one cell diameter. A minimum of 200 cells were counted for the frequency of the macrophage-like morphology, and the results were expressed as the means of triplicate determinations with the standard deviation. For the detection of nonspecific esterase activity, cells were stained with an esterase staining kit (Mutou Chemical, Tokyo, Japan) according to the manufacturer's protocol. The frequency of esterase-positive cells was determined by inspecting at least 200 cells under a microscope. For the detection of phagocytic function, macrophage-like cells were incubated in culture medium containing 0.01% 6-μm latex beads (Becton Dickinson, Franklin Lakes, N.J.), washed, fixed with paraformaldehyde, and stained with Giemsa. The frequency of phagocytic cells was determined by inspecting ca. 300 cells under an inverted microscope.
Laser flow cytometric analysis of CD5 expression.
TH2.52 cells were incubated with IFN-γ (100 U/ml) for 3days and nonadherent cells were removed by washing. Macrophage-like cells were incubated in 0.05% trypsin, 0.5 mM EDTA (Gibco-BRL/Life Technologies, Carlsbad, Calif.) for 5 min and detached with a scraper. The cells were stained by a 1:200 dilution of fluorescein-conjugated anti-CD5 monoclonal antibody (PharMingen, San Diego, Calif.). The cells were washed with 0.01 M phosphate-buffered saline at pH 7.2 (PBS) and then suspended in PBS. The fluorescence intensity was analyzed with a laser flow cytometer (FACScaliber; Becton Dickinson) and expressed on a log scale.
Immunoblotting.
Immunoblotting was performed as described previously (23). Briefly, TH2.52 cells were cultured with IFN-γ (100 U/ml), washed with PBS, and lysed with a lysis buffer. The cell lysates were then diluted with an equal volume of 2× sample buffer and boiled for 5 min. Samples were separated under reducing conditions by electrophoresis with 4 to 12% polyacrylamide gels, and separated proteins were transferred to membranes by electroblotting. The membranes were blocked with 5% skim milk in PBS and, after being washed in PBS containing 0.05% Tween 20, were treated with a 1:1,000 dilution of antibodies to p38, phospho-p38, STAT1, or phospho-STAT1. The resulting immune complexes were reacted with a 1:1,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G F(ab′)2 antibody (Cappel, West Chester, Pa.). Finally, the immune complexes were detected with an enhanced chemiluminescence substrate (New England Nuclear, Boston, Mass.). A prestained low-molecular-weight standard kit from Gibco-BRL was used as a reference.
Stable transfection with the dominant-negative p38 MAP kinase mutant.
TH2.52 cells were seeded at 2 × 105/well in 24-well plates and transfected with the dominant-negative mutant of p38 MAP kinase or the empty control for 8 h with Lipofectamine 2000 reagent (Gibco-BRL) according to the manufacturer's protocol. After 40 h of incubation, fresh RPMI 1640 containing 5% fetal bovine serum and Geneticin (1 mg/ml) were added, and the cells were incubated for an additional 48 h. Stable transfectants were treated with IFN-γ.
Statistical analysis.
The statistical significance of the differences between experimental results expressed as mean values ± the standard deviation was determined by using the Student t test.
RESULTS
Appearance of macrophage-like cells in cultures of TH2.52 cells with IFN-γ.
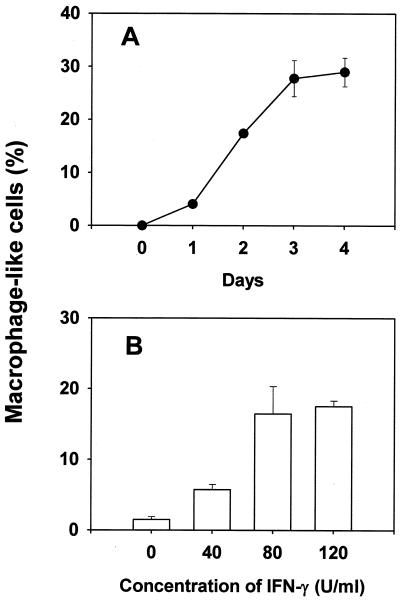
The in vitro effect of IFN-γ on TH2.52 cells was studied by incubating TH2.52 cells with IFN-γ (100 U/ml) for 3 days. A number of TH2.52 cells adhered to plastic dishes and became spindle shaped or dendritic under these conditions (Fig. 1). On the other hand, macrophage-like cells did not appear in the cultures of M12.4.1 partner cells treated with IFN-γ (100 U/ml) or in cultures of TH2.52 cells exposed to granulocyte-macrophage colony-stimulating factor (GM-CSF), M-CSF, IL-3, IL-2, tumor necrosis factor alpha, or IL-1β. Next, the time course of appearance of macrophage-like cells was monitored for 4 days. A significant number of cells was found 1 day after the addition of IFN-γ, and the number gradually increased for 3 days (Fig. 2A), with no significant increase thereafter. After 2 days, more macrophage-like cells appeared in cultures with IFN-γ at 80 U/ml than in cultures with IFN-γ at 40 U/ml. However, there was no significant increment when the concentration was 120 U/ml (Fig. 2B). Macrophage-like cells also appeared in cultures with a 500-U/ml concentration of IFN-γ for 4 days.
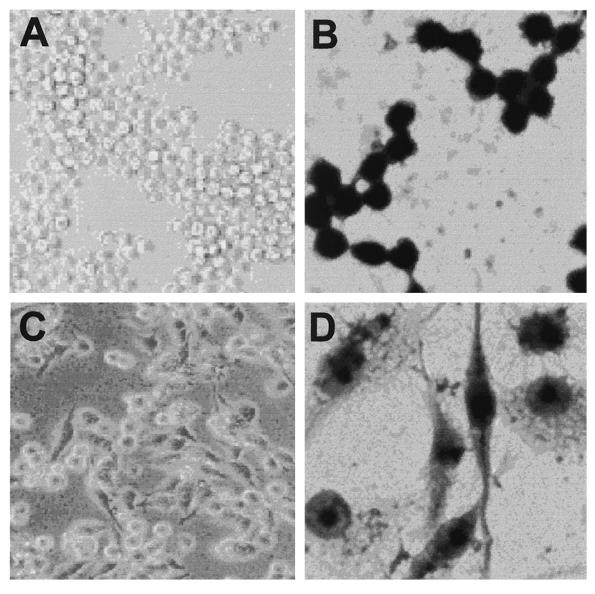
FIG. 1.
Morphology of macrophage-like cells appearing in cultures of TH2.52 cells with IFN-γ treatment. TH2.52 cells were cultured with IFN-γ (100 U/ml) for 3 days. (A and B) Untreated control cells; (C and D) IFN-γ-treated cells. Magnifications: A and C, ×100; B and D, ×400.
FIG. 2.
Appearance of macrophage-like cells in cultures of TH2.52 cells with IFN-γ treatment. TH2.52 cells were cultured with IFN-γ (100 U/ml) for various days (A) or with various concentrations of IFN-γ for 2 days (B). The frequency of macrophage-like cells was determined under a microscope.
The appearance of macrophage-like cells in cultures with IFN-γ was completely inhibited by the addition of anti-IFN-γ neutralizing antibody (1 μg/ml) but not anti-GM-CSF or IL-3 neutralizing antibody (data not shown). Supernatant from TH2.52 cells cultured with IFN-γ (100 U/ml) for 4 days did not induce the appearance of macrophage-like cells in the presence of anti-IFN-γ neutralizing antibody. Therefore, the possibility was excluded that IFN-γ might cause production of a humoral factor capable of shifting TH2.52 differentiation to a macrophage-like morphology.
Macrophage functions of macrophage-like TH2.52 cells.
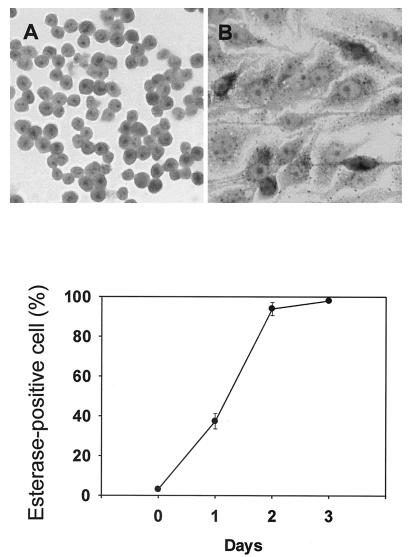
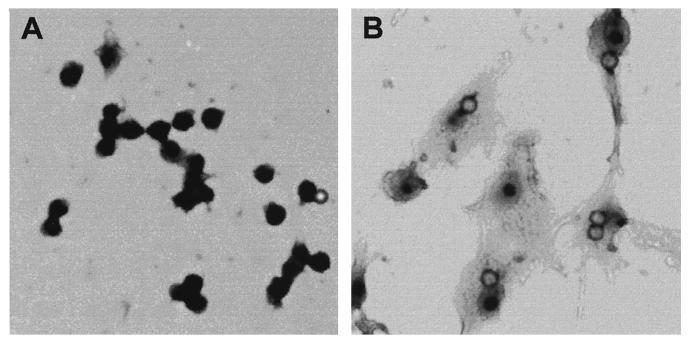
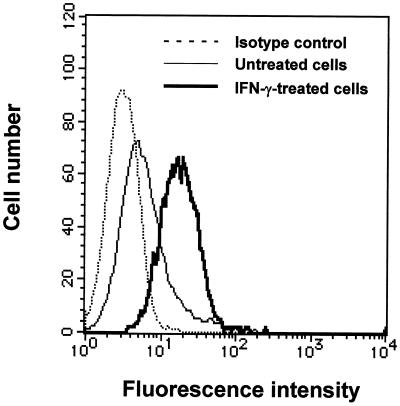
When TH2.52 cells were cultured with IFN-γ (100 U/ml) for 3 days, almost 100% were positive for nonspecific esterase staining (Fig. 3), untreated control cells being totally negative. The esterase-positive cells increased to a plateau level after 2 days (Fig. 3). Incubation with latex beads resulted in many cells engulfing one or two latex beads, but this finding was extremely rare with untreated control cells (Fig. 4). Vacuoles were also seen in the macrophage-like cells. Interestingly, IFN-γ-induced macrophage-like cells expressed a higher level of CD5 than did untreated control TH2.52 cells (Fig. 5), whereas the expression of other surface markers, including surface immunoglobulin, did not change significantly.
FIG. 3.
Nonspecific esterase staining of IFN-γ-induced macrophage-like cells. TH2.52 cells were cultured with IFN-γ (100 U/ml), and the time course in the appearance of esterase-positive cells was monitored for 4 days (top). A typical staining pattern of esterase-positive cells at day 4 is shown (bottom). (A) Untreated control cells; (B) IFN-γ-treated cells. Magnification, ×140.
FIG. 4.
Phagocytic function of IFN-γ-induced macrophage-like cells. TH2.52 cells were cultured with or without IFN-γ (100 U/ml) in culture medium containing latex beads for 4 days. (A) Untreated control cells; (B) IFN-γ-treated cells. Magnification, ×200.
FIG. 5.
Expression of CD5 on IFN-γ-induced macrophage-like cells. TH2.52 cells were incubated with IFN-γ (100 U/ml) for 3days, and nonadherent cells were removed by washing. Macrophage-like cells (bold line) and untreated control cells were stained with fluorescein isothiocyanate-conjugated anti-CD5 antibody.
Reversibility of the IFN-γ-induced change in response to a macrophage morphology.
When TH2.52 cells were cultured with IFN-γ (100 U/ml) for 3 days and macrophage-like cells were then cultured in the absence of IFN-γ for 5 days, the number of nonadherent round TH2.52 cells increased gradually, and this morphology accounted for the majority of cells by the end of the period of cultivation. There was no significant difference in the response to IFN-γ between the recovered TH2.52 cells and the original cells.
Involvement of the p38 MAP kinase pathway in IFN-γ-induced morphological change.
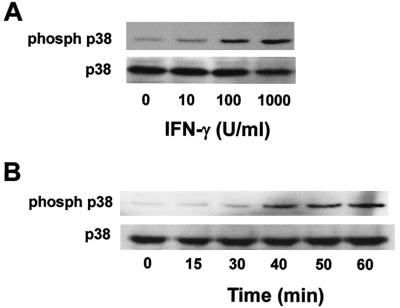
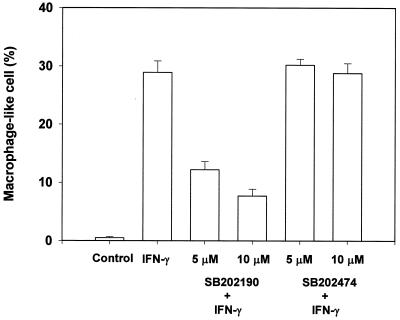
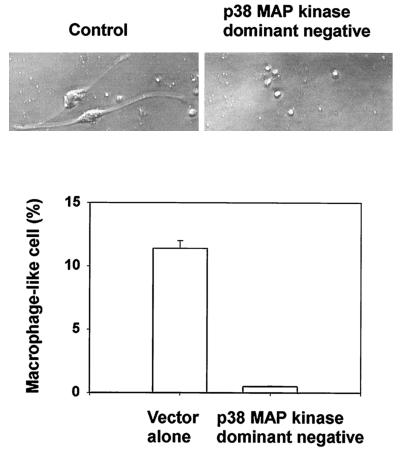
The signal pathway participating in IFN-γ-induced morphological change was characterized with a focus on p38 MAP kinase signaling, since IFN-γ is known to activate this enzyme (13, 24, 27). First, the effect of a p38 MAP kinase inhibitor (SB202190) and an inactive analogue (SB202474) on IFN-γ-induced morphological change was studied. IFN-γ-induced phosphorylation of p38 MAP kinase was confirmed by the immunoblotting with anti-phospho-p38 antibody (Fig. 6). Subsequently, pretreatment with SB202190 at 10 μM for 1 h definitely inhibited the appearance of macrophage-like cells in response to IFN-γ (Fig. 7). However, the inactive analogue, SB202474, had no effect. Moreover, transfection of a dominant-negative mutant of p38 MAP kinase into TH2.52 cells prevented the appearance of macrophage-like cells in the presence of IFN-γ (Fig. 8). IFN-γ did not induce the phosphorylation of ERK1/2 or the c-Jun-N-terminal kinase/stress-activated protein kinase (JNK/SAPK) (data not shown). Furthermore, PD98095, an ERK1/2 inhibitor, did not prevent IFN-γ-induced morphological changes.
FIG. 6.
Phosphorylation of p38 MAP kinase by IFN-γ. TH2.52 cells were cultured with various concentrations of IFN-γ for 1 h (A) or with IFN-γ at 100 U/ml for various periods (indicated in minutes) (B). Phosphorylation of p38 MAP kinase was detected by immunoblotting with an anti-phospho-p38 antibody.
FIG. 7.
Inhibition of the appearance of macrophage-like cells by the p38 MAP kinase inhibitor SB202190. TH2.52 cells were pretreated with SB202190 at 5 or 10 μM for 30 min and further cultured with IFN-γ (100 U/ml) for 4 days. A p38 MAP kinase inactive inhibitor, SB202474, was also used as a negative control.
FIG. 8.
Inhibition of the appearance of macrophage-like cells by a p38 MAP kinase dominant-negative mutant. TH2.52 cells were transfected with the p38 MAP kinase dominant-negative mutant or with the empty control vector and incubated with IFN-γ (100 U/ml) for 3 days. (Top) Morphology of TH2.52 cells transfected with the empty control vector or the p38 MAP kinase dominant-negative mutant. (Bottom) The frequency of macrophage-like cells appearing was determined under a microscope.
With regard to the JAK/STAT pathway (19), the effects of AG490, a JAK2 inhibitor (10, 19, 29, 35), on IFN-γ-induced morphological change were studied. AG490 (50 μM) blocked phosphorylation of STAT1 but not the morphological change.
Inhibition of IFN-γ-induced morphological change by IL-4.
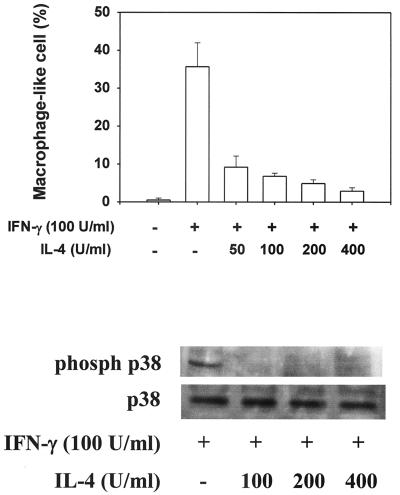
Since IFN-γ is a typical Th1 cytokine (7, 30, 31), the effects of IL-4, a representative Th2 cytokine (7, 30, 31), on IFN-γ-induced morphological change was examined (Fig. 9). When TH2.52 cells were pretreated with various concentrations of IL-4 for 30 min and cultured with IFN-γ (100 U/ml) for an additional 3 days, doses higher than 50 U/ml inhibited the appearance of macrophage-like cells. IL-4 also blocked the IFN-γ-induced phosphorylation of p38 MAP kinase.
FIG. 9.
Inhibition of the appearance of macrophage-like cells and phosphorylation of p38 MAP kinase by IL-4. TH2.52 cells were pretreated with various concentrations of IL-4 for 30 min and then further cultured with IFN-γ (100 U/ml) for 3 days. (Top) Phosphorylation of p38 MAP kinase was confirmed by the immunoblotting method. (Bottom) The frequency of macrophage-like cells was determined under a microscope.
DISCUSSION
The present study demonstrates that IFN-γ induces the morphological change of CD5+ B1 TH2.52 cells so that they become macrophage-like. Based on esterase staining and phagocytic activity, this has an apparent functional connotation. Macrophage-like cells returned to original TH2.52 B1 cells in the absence of IFN-γ, suggesting reversibility. There are several reports concerning the close relationships between malignant CD5+ B cells and macrophages (3-6). Borrello et al. (4, 5) reviewed newly identified, normal B/macrophage cells that express characteristics common to CD5+ B cells and macrophages. A minority of splenic CD5+ B cells acquire a macrophage-like phenotype in fibroblast cultures, suggesting that B/macrophage cells are derived from a subpopulation of splenic B1 cells (4, 5). This view is consistent with the fact that TH2.52 B cells are CD5+ hybridomas with splenic B cells (23) and that they are heterogeneous and individually programmed for differentiation responses (25). TH2.52 cells might thus provide a useful tool for characterizing the relation between CD5+ B1 cells and macrophages. It is of interest in this context that the morphological change of B2 cells due to lipopolysaccharide and IL-4 has been reported (8, 9).
IFN-γ and IL-4 are Th1 cytokines achieve cell-mediated immunity, whereas Th2 cytokine are responsible for developing humoral immunity (7, 30, 31). CD5+ B1 cells differentiate into macrophages in a Th1-type (IFN-γ-rich) environment and promote cell-mediated immunity as antigen-presenting cells. On the other hand, they may function as immunoglobulin-producing B cells in a Th2-type (IL-4-rich) environment and thus promote humoral immunity. Macrophage-like cells possessing antigen-specific immunoglobulin and phagocytic functions could act as antigen-specific antigen-presenting cells. B1 cells produce antibodies to multivalent antigens of bacterial cell wall components (1), such as lipopolysaccharide (33), phosphatidylcholine (28), and α1-3 dextran (11), which are also reactive with Enterobacter and Serratia spp. in the Enterobacteriaceae family (22). B1 cell-derived macrophage-like cells might become presenting cells for a restricted repertoire of antigens, such as bacterial components. Further, macrophage-like cells express high levels of CD5. Since CD5 is reported to negatively regulate B-cell receptor-mediated signaling (2, 26), this elevated expression of CD5 might attenuate the B1 character. It is of interest that IFN-γ-induced macrophage-like cells express CD5, a member of a superfamily of proteins that contains one or more extracellular domains homologous to the type I macrophage scavenger receptor cysteine-rich domain (12, 32).
The present study suggests that p38 MAP kinase might play an important role in macrophage-like morphological change in response to IFN-γ. Thus, IFN-γ and IL-4 exhibit opposing effects on p38 MAP kinase and differentially regulate the morphological change. The detailed mechanisms of how IFN-γ and IL-4 interact with the p38 MAP kinase pathway are still unclear, but there have been several reports that IFN-γ causes activation (13, 24, 27). We demonstrated its phosphorylation of p38 MAP kinase. The JAK/STAT signal pathway is unlikely to be involved in IFN-γ-induced morphological change because the inhibition of the phosphorylation of STAT1 by AG490, a JAK2 inhibitor (10, 19, 29, 35), was here found to be without influence.
It was unclear whether the phenomenon of morphological change in the CD5+ TH2.52 B1-cell line is relevant to physiological B1 cells. In a preliminary experiment, mouse splenic CD5+ B cells became Ig+ macrophage-like when isolated by a panning method and cultured in the presence of IFN-γ. This finding suggests the hypothesis that splenic B1 cells shift to a macrophage-like phenotype in response to IFN-γ and thereby play an important role in Th1- and Th2-mediated immunity through reversible transition between B1 cells and macrophages.
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture and Technology of Japan.
We are grateful to K. Takahashi and A. Morikawa for expert technical assistance.
REFERENCES
- 1.Allison, A. C., and Y. Nawata. 1992. Cytokines mediating the proliferation and differentiation of B-1 lymphocytes and their role in ontogeny and phylogeny. Ann. N. Y. Acad. Sci. 651:200-219. [DOI] [PubMed] [Google Scholar]
- 2.Bikah, G., J. Carey, J. R. Ciallella, A. Tarakhovsky, and S. Bondada. 1996. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science 274:1906-1909. [DOI] [PubMed] [Google Scholar]
- 3.Borrello, M. A., and R. P. Phipps. 1995. Fibroblasts support outgrowth of splenocytes simultaneously expressing B lymphocyte and macrophage characteristics. J. Immunol. 155:4155-4161. [PubMed] [Google Scholar]
- 4.Borrello, M. A., J. Palis, and R. P. Phipps. 2001. The relationship of CD5+ B lymphocytes to macrophages: insights from normal biphenotypic B/macrophage cells. Int. Rev. Immunol. 20:137-155. [DOI] [PubMed] [Google Scholar]
- 5.Borrello, M. A., and R. P. Phipps. 1996. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol. Today 17:471-475. [DOI] [PubMed] [Google Scholar]
- 6.Borrello, M. A., and R. P. Phipps. 1999. Fibroblast-secreted macrophage colony-stimulating factor is responsible for generation of biphenotypic B/macrophage cells from a subset of mouse B lymphocytes. J. Immunol. 163:3605-3611. [PubMed] [Google Scholar]
- 7.Clerici, M., and G. M. Shearer. 1993. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14:107-111. [DOI] [PubMed] [Google Scholar]
- 8.Davey, E. J., G. Greicius, J. Thyberg, and E. Severinson. 2000. STAT6 is required for the regulation of IL-4-induced cytoskeletal events in B cells. Int. Immunol. 12:995-1003. [DOI] [PubMed] [Google Scholar]
- 9.Davey, E. J., J. Thyberg, D. H. Conrad, and E. Severinson. 1998. Regulation of cell morphology in B lymphocytes by IL-4: evidence for induced cytoskeletal changes. J. Immunol. 160:5366-5373. [PubMed] [Google Scholar]
- 10.De Vos, J., M. Jourdan, K. Tarte, C. Jasmin, and B. Klein. 2000. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells. Br. J. Haematol. 109:823-828. [DOI] [PubMed] [Google Scholar]
- 11.Förster, I., and K. Rajewsky. 1987. Expression and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 17:521-528. [DOI] [PubMed] [Google Scholar]
- 12.Freeman, M., J. Ashkenas, D. J. Rees, D. M. Kingsley, N. G. Copeland, N. A. Jenkins, and M. Krieger. 1990. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc. Natl. Acad. Sci. USA 87:8810-8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh, K. C., S. J. Haque, and B. R. Williams. 1999. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18:5601-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamano, T., K. J. Kim, W. M. Leiserson, and R. Asofsky. 1982. Establishment of B cell hybridomas with B cell surface antigens. J. Immunol. 129:1403-1406. [PubMed] [Google Scholar]
- 15.Hamano, T., W. M. Leiserson, and R. Asofsky. 1983. Functional studies on B cell hybridomas with B cell surface antigens. II. Ia molecules on the cell membrane and the differentiative response to anti-μ antibody. J. Immunol. 131:98-102. [PubMed] [Google Scholar]
- 16.Hamano, T., W. M. Leiserson, and R. Asofsky. 1984. Functional studies on B-cell hybridomas with B-cell surface antigens. III. Differentiative response to phorbol esters. Cell. Immunol. 83:330-339. [DOI] [PubMed] [Google Scholar]
- 17.Hardy, R. R., C. E. Carmack, Y. S. Li, and K. Hayakawa. 1994. Distinctive developmental origins and specificities of murine CD5+ B cells. Immunol. Rev. 137:91-118. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa, K., and R. R. Hardy. 1988. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu. Rev. Immunol. 6:197-218. [DOI] [PubMed] [Google Scholar]
- 19.Kalina, U., D. Kauschat, N. Koyama, H. Nuernberger, K. Ballas, S. Koschmieder, G. Bug, W. K. Hofmann, D. Hoelzer, and O. G. Ottmann. 2000. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-γ production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J. Immunol. 165:1307-1313. [DOI] [PubMed] [Google Scholar]
- 20.Kantor, A. B. 1991. The development and repertoire of B-1 cells (CD5 B cells). Immunol. Today 12:389-391. [DOI] [PubMed] [Google Scholar]
- 21.Kantor, A. B., and L. A. Herzenberg. 1993. Origin of murine B cell lineages. Annu. Rev. Immunol. 11:501-538. [DOI] [PubMed] [Google Scholar]
- 22.Kearney, J. F., M. T. McCarthy, R. Stohrer, W. H. Benjamin, Jr., and D. E. Briles. 1985. Induction of germ-line anti-alpha 1-3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J. Immunol. 135:3468-3472. [PubMed] [Google Scholar]
- 23.Koide, N., T. Sugiyama, Y. Kato, D. Chakravortty, M. M. Mu, T. Yoshida, T. Hamano, and T. Yokochi. 2001. Mouse B1 cell line responds to lipopolysaccharide via membrane-bound CD14. J. Endotoxin Res. 7:39-43. [PubMed] [Google Scholar]
- 24.Kovarik, P., D. Stoiber, P. A. Eyers, R. Menghini, A. Neininger, M. Gaestel, P. Cohen, and T. Decker. 1999. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-γ uses a different signaling pathway. Proc. Natl. Acad. Sci. USA 96:13956-13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little, J., D. W. Alling, and R. Asofsky. 1993. Induction of differentiation in a B lymphoma × B lymphocyte hybrid line. II. Intraclonal heterogeneity in growth, secretion of IgM, and cytokine production in response to lipopolysaccharide. J. Immunol. 150:2129-2138. [PubMed] [Google Scholar]
- 26.Lozano, F., M. Simarro, J. Calvo, J. M. Vila, O. Padilla, M. A. Bowen, and K. S. Campbell. 2000. CD5 signal transduction: positive or negative modulation of antigen receptor signaling. Crit. Rev. Immunol. 20:347-358. [PubMed] [Google Scholar]
- 27.MacKenzie, S. J., and M. D. Houslay. 2000. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. Biochem. J. 347:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercolino, T. J., L. W. Arnold, L. A. Hawkins, and G. Haughton. 1988. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl cholin: relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J. Exp. Med. 168:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakashima, O., Y. Terada, S. Inoshita, M. Kuwahara, S. Sasaki, and F. Marumo. 1999. Inducible nitric oxide synthase can be induced in the absence of active nuclear factor-κB in rat mesangial cells: involvement of the Janus kinase 2 signaling pathway. J. Am. Soc. Nephrol. 10:721-729. [DOI] [PubMed] [Google Scholar]
- 30.Rocken, M., M. Racke, and E. M. Shevach. 1996. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol. Today 17:225-231. [DOI] [PubMed] [Google Scholar]
- 31.Seder, R. A., and W. E. Paul. 1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:635-673. [DOI] [PubMed] [Google Scholar]
- 32.Starling, G. C., M. B. Llewellyn, G. S. Whitney, and A. Aruffo. 1997. The Ly-1.1 and Ly-1.2 epitopes of murine CD5 map to the membrane distal scavenger receptor cysteine-rich domain. Tissue Antigens 49:1-6. [DOI] [PubMed] [Google Scholar]
- 33.Su, S. D., M. M. Ward, M. A. Apicella, and R. E. Ward. 1991. The primary B cell response to the O/core region of bacterial lipopolysaccharide is restricted to the Ly-1 lineage. J. Immunol. 146:327-331. [PubMed] [Google Scholar]
- 34.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934-23936. [DOI] [PubMed] [Google Scholar]
- 35.Zhu, T., and P. E. Lobie. 2000. Janus kinase 2-dependent activation of p38 mitogen-activated protein kinase by growth hormone. Resultant transcriptional activation of ATF-2 and CHOP, cytoskeletal re-organization, and mitogenesis. J. Biol. Chem. 275:2103-2114. [DOI] [PubMed] [Google Scholar]