Abstract
The immunoregulatory roles of interleukin-2 (IL-2), IL-4, IL-10, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), the soluble form of the IL-2 receptor (sIL-2R), and the soluble form of CD30 (sCD30) were evaluated in patients with hepatitis B virus (HBV) infection. Two groups of subjects were studied: 15 healthy individuals without hepatitis antecedents and 15 patients with HBV infection. Blood samples were taken during the acute and convalescent phases. The analysis of the samples was done by the enzyme-linked immunosorbent assay technique. IFN-γ and TNF-α levels decreased in the convalescent phase. IL-10, IL-2, and sIL-2R levels increased in the acute and convalescent phases, while sCD30 levels increased during the acute phase. The IL-4 concentrations decreased in both phases. During the acute phase, IFN-γ and TNF-α induced increases in IL-2, sIL-2R, IL-10, and sCD30 levels in serum, which allowed the development of immunity characterized by the nonreactivity of the HBV surface antigen, the onset of antibodies to the HBV surface antigen (anti-HBs), and normal alanine aminotransferase levels during the convalescent phase. Increased IL-2 levels during the acute phase would stimulate the activities of NK cells and CD8+ lymphocytes, which are responsible for viral clearing. The raised sIL-2R levels reveal activation of T lymphocytes and control of the IL-2-dependent immune response. The sCD30 increment during the acute phase reflects the greater activation of the Th2 cellular phenotype. Its decrease in the convalescent phase points out the decrease in the level of HBV replication. The increase in IL-10 levels could result in a decrease in IL-4 levels and modulate IFN-γ and TNF-α levels during both phases of disease, allowing the maintenance of anti-HBs concentrations.
Hepatitis B constitutes a worldwide public health problem. Approximately 350 million people are carriers. The hepatitis B virus (HBV) is directly or indirectly responsible for more than a half million deaths each year around the world (1). Venezuela has a prevalence of HBV carriers of between 2 and 7%, with three locations of high endemicity: Amerindian communities in the southern region (Amazon State) and the western region (Perijá Mountains, Zulia State) and, recently, an area in the central region of the country (Barinas State) (13, 36).
Macrophage activation represents one of the first events of innate resistance against intracellular infection. In response to pathogens, macrophages and other inflammatory cells secrete cytokines (gamma interferon [IFN-γ], interleukin-1 [IL-1], IL-6, IL-8, tumor necrosis factor alpha [TNF-α], and IFN-β). Some of these cytokines lead to activities against pathogens, activate effector cells involved in the cellular interactions that occur during inflammation, and are part of the acute and chronic stages of viral hepatitis (22, 35). The antibody response in patients with HBV infection plays a critical role in viral clearance through the formation of complexes with viral particles and their removal from the circulation (6, 8, 29).
The specific cellular immune response plays a main role in the hepatic necrosis that occurs with HBV infection and in the persistence or lack of persistence of viral infection. Certain cytokines can contribute to this process by efficiently inhibiting viral replication when the subtype Th1 cytokine secretion pattern is predominant or by facilitating the propagation of the pathogens in the patient if the subtype Th2 cytokine secretion pattern is predominant (27).
Studies carried out with cultures of peripheral blood mononuclear cells from patients with acute HBV infection showed a Th1-like cytokine pattern with increased levels of production of IFN-γ and TNF-α (2, 6). This high level of cytokine production stimulates the immune response, allowing the cure of HBV disease (21).
On the other hand, decreases in the levels of IL-2 and TNF-α synthesis and increases in the levels of IL-1 and the soluble form of the IL-2 receptor (sIL-2R) in serum have been observed in patients with chronic HBV infection (31), while high levels of IL-4 and IL-6 were found in patients with autoimmune chronic hepatitis (2).
Recent studies have demonstrated that clones of T cells can be characterized by the expression of CD30, which is a member of the TNF family (17) expressed in Th2 CD4+ T cells. Its soluble form (sCD30) is liberated by proteolysis from the outside 105-kDa portion of T cells following cellular activation (10). High serum sCD30 concentrations have been detected in patients with active illness in whom a Th2-type immune response is dominant, like patents with chronic hepatitis due to HBV (18).
The aim of this study was to analyze the immunoregulatory mechanisms participating in HBV infection through the measurement of cytokine, sIL-2R, and sCD30 levels in the sera of patients in the acute and convalescent phases of HBV infection.
MATERIALS AND METHODS
Study population.
The study population consisted of two groups. Group 1 consisted of 15 healthy individuals of both genders between 15 to 60 years of age with no clinical laboratory evidence of hepatic illness. Group 2 consisted of 15 patients (age range, 20 to 60 years; mean age ± standard deviation, 29.2 ± 8.3 years) referred by the Blood Donors Unit of the Maracaibo Blood Bank and Regional Virological Reference Laboratory, Maracaibo, Venezuela, from 1998 to 2000. Twelve of those patients showed the characteristic clinical symptoms of HBV infection: jaundice, choluria, anorexia, acholia, and serum alanine aminotransferase (ALT) concentrations above the normal value of 40 U/ml (mean ± standard deviation, 275 ± 25.2 U/ml). The remaining three patients were volunteer blood donors who had no apparent symptomatology and normal transaminase values but who had HBsAg and immunoglobulin M (IgM) antibodies against the HBV core (IgM anti-HBc).
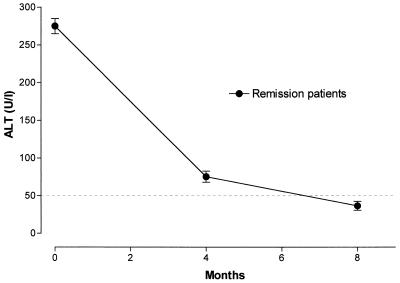
The diagnosis of acute HBV infection was based on the detection of ALT at levels 10-fold above the normal value in association with the detection of HBsAg, the HBV e antigen (HBeAg), and IgM anti-HBc antibodies (12, 25). A second sample was taken 4 months after the date of diagnosis and when clinical symptoms had disappeared. The appearance of antibodies to the HBV surface antigen (anti-HBs) and antibodies against the e antigen (anti-HBe) and the absence of HBsAg and IgM anti-HBc with normal ALT levels confirmed that the patients were in disease remission (Fig. 1).
FIG. 1.
Concentrations of ALT in the sera of our HBV-infected patients during the acute and the convalescent phases. The dashed line represents the upper limit of normal values.
Criteria for exclusion.
Pregnant women, patients with leukemia, hemophilia, or autoimmune illnesses, patients who had been vaccinated against HBV, patients with hepatitis A, C, and D virus infections, patients with human immunodeficiency virus infection, or patients under dialysis were excluded from the study.
Sample collection.
Ten milliliters of peripheral blood was withdrawn from each individual. Serum was obtained by centrifugation at 1,600 × g (Damond Mod. PR-J) for 15 min and was divided into aliquots of 500 μl each and stored at −70°C until it was analyzed.
ELISA.
The samples used for detection of HBV infection markers in the acute and convalescent phases were analyzed by the enzyme-linked immunosorbent assay (ELISA) technique with the IMX system (Abbott Laboratories Division of Diagnosis, Chicago, Ill.) (15, 16). The samples initially reactive for HBsAg and IgM anti-HBc were analyzed in duplicate to confirm the acute phase of infection.
The concentration of each cytokine (IL-2, IL-4, IL-10, IFN-γ, and TNF-α) in serum was determined by the ELISA technique (BIOTRAK cellular communication assay; Amersham, Little Chalfont, England).
Determination of sIL-2R and sCD30 levels was carried out by an ELISA (Interleukin-2-Receptor and CD30 Ki-1 Antigen ELISA kits, respectively; DAKO, Glostrup, Denmark). Both kits recognize the soluble factors in both the natural and the human recombinant forms but do not cross-react or interfere with each other or with other factors.
Statistical analysis.
The results are expressed as means ± standard errors. The data were analyzed by a one-way analysis of variance test, followed by Tukey's test to determine differences between means. The correlation coefficients among the variables were also calculated. Significance was established at a P value of <0.05.
RESULTS
The concentrations of IFN-γ and TNF-α in the sera of patients with HBV infection were significantly decreased (P < 0.001) in the convalescent phase in comparison with those in the acute phase and in comparison with those in the control group. IL-10 levels in the acute phase as well as in the convalescent phase were significantly (P < 0.001) higher than those in the control group. Significant decreases in IL-4 levels were observed (P < 0.05) in the acute phase and in the convalescent phase compared to those in the control population (Table 1).
TABLE 1.
Concentrations of cytokines, sIL-2R, and sCD30 in sera of patients with HBV infectiona
| Group | Cytokine concn (pg/ml)
|
Concn (U/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-10 | IL-4 | IL-2 | sIL-2R | sCD30 | |
| Group 1 (control) | 17.2 ± 2.0 | 9.5 ± 0.6 | 4.3 ± 0.6 | 9.3 ± 1.7 | 6.0 ± 2.1 | 443.7 ± 39.6 | 34.81 ± 6.72 |
| Group 2 | |||||||
| Acute phase | 14.9 ± 1.0 | 8.7 ± 0.6 | 10.8 ± 0.6b | 4.1 ± 0.2c | 25.7 ± 1.6b | 3478.0 ± 695.8b | 135.70 ± 36.67 |
| Convalescent phase | 2.1 ± 0.8d | 4.3 ± 0.7d | 11.9 ± 2.1b | 4.6 ± 0.8c | 22.1 ± 2.1b | 3208.0 ± 734.6b | 16.8 ± 2.47 |
The values are means ± standard errors of the means.
Significantly different from the control group (P < 0.001).
Significantly different from the control group (P < 0.05).
Significantly different from the control group and the acute phase (P < 0.001).
Significantly different from the control group and the convalescent phase (P < 0.05).
The levels of IL-2 and sIL-2R in the sera of group 2 significantly increased (P < 0.001) in the acute and convalescent phases compared to the levels in the control group (Table 1).
The results for sCD30 showed a significant increase (P < 0.05) in sCD30 concentrations during the acute phase compared to those in the convalescent phase and to those in the control group (Table 1).
DISCUSSION
HBV causes an inflammatory hepatic illness characterized by mononuclear and polymorphonuclear cellular infiltrates with evidence of hepatic macrophage activation (20). These inflammatory cells produce such cytokines as TNF-α, IFN-γ, IFN-α, IL-1α, and IL-6 (4, 20), which mediate the inflammatory process and which contribute to the successful clearance of the virus, avoiding the mechanisms of the immune response or the progression of infection and persistence of the virus (5).
In the present study, we observed normal levels of IFN-γ in the sera of patients with acute HBV infection. This could be explained by the fact the DNA viruses are poor inducers of IFN-γ (24). In contrast to our study, Penna et al. (32) and Al-Wabel et al. (2), using peripheral blood mononuclear cells, found that the Th1 cellular phenotype was prevalent; that is, they detected increases in IFN-γ levels. Similarly, Chu et al. (9) showed that serum IFN-γ levels increased significantly during the acute phase of infection. However, those studies did not find any detectable levels of IFN-γ in the healthy control population.
During the convalescent phase, the decrease in serum IFN-γ levels coincides with the increase in the levels of IL-10, which inhibits IFN-γ synthesis (33). This decrease would be related to the decrease in the levels of production of cytokines secreted by macrophages (IL-1, IL-6, IL-8, and TNF-α), which, if increased, would exacerbate the hepatic damage (35). Increased levels of these cytokines have been observed in patients with chronic hepatitis caused by HBV whose condition later evolved to a cirrhotic state (28).
In the present study, we observed that serum INF-γ and TNF-α levels had similar temporal patterns during the evolution of HBV infection. Both TNF-α and IFN-γ can be inhibited by IL-10 (33), whose levels remained high during the acute and convalescent phases. Besides the regulation possibly carried out by the inhibitory cytokine, an increase in the number of TNF-α soluble receptors (sRTNF-α) could be responsible for the maintenance of the control levels of TNF-α and for the decrease in the levels of TNF-α during the convalescent phase. According to Tilg et al. (34), increased concentrations of sRTNF-α in serum, which seem to modulate the endogenous effects of TNF-α, have been detected in patients with chronic HBV infection.
IL-10 is one of the key cytokines in the Th2 response. It is a pleiotropic cytokine able to inhibit the synthesis of other cytokines secreted by the Th1 subpopulation and the functions of the cell antigen bearers. IL-10 increases the levels of sRTNF-α released, inducing B-lymphocyte differentiation into plasmocytes and immunoglobulin synthesis (33). Therefore, IL-10 has as an important role, acting like a general suppressor of the cell-mediated response and increasing the level of humoral immunity.
The increment of IL-10 in the two phases might modulate the levels of IFN-γ and TNF-α since an exaggerated immune response by these cytokines to a viral antigen load would be responsible for the death of large numbers of hepatocytes, producing a lethal hepatitis. The persistence of high IL-10 levels in the convalescent phase is important in the secretion of surface antibodies against HBV and the development of immunity. Anti-HBs block the adherence of viral particles to noninfected cells and remove from the circulation the free antigenic particles, protecting the individual against reinfection (29).
In this study, it was determined that the serum IL-4 concentrations are decreased during the acute and convalescent phases. This finding is in agreement with those of Mansour et al. (30), who reported very low or normal levels of IL-4 in the sera of patients with HBV infection during the acute phase. Increased levels of this cytokine have been reported in patients with several parasitic and autoimmune diseases (22, 26, 30).
The decrease in IL-4 levels in patients with HBV infection could be due to the fact that this cytokine is preferentially stimulated by parasitic antigens but not by viral antigens, which mainly induce IL-10. The type of stimulus could determine the production profile for each cytokine. Also, the decrease in IL-4 levels would be a consequence of the autoregulatory mechanisms of IL-10 (7).
The increased levels of IL-2 and sIL-2R during the acute phase and until the total resolution of the HBV infection could allow for higher levels of T-lymphocyte activation during this period. IL-2 could be a stimulus for the activation of NK cells and CD8+ lymphocytes participating in the development of immunity. Investigations carried out with IL-2 report decreases in the levels of its production in patients with chronic HBV infections (3).
The increase in IL-2 levels in the acute phase of HBV infection is necessary to stimulate the activities of the NK cells and the CD8+ lymphocytes and to achieve remission. Echevarría et al. (14) demonstrated that a positive correlation between natural cytotoxicity and IL-2 levels is needed to control HBV infection before the specific cytotoxic mechanisms settle down totally. The sustained increases in IL-2 levels during the convalescent phase suggest that, despite the resolution of the infection indicated by normal ALT values and the presence of anti-HBs, an increase in hepatic damage would not be determined by the high concentrations of this cytokine. In addition, anti-HBs mask tissue surface antigens, forming immune complexes that induce a transmembrane signal able to suppress the synthesis of intracellular viral antigens (19). This would then preclude the cytotoxic actions of the CD8+ lymphocytes.
Given the necessity to use a suitable marker to determine the evolution of HBV infection, Izzo et al. (23) measured the concentrations of sIL-2R every 3 months in the sera of patients at risk for active chronic hepatitis and cirrhosis; however, they emphasized that high levels of sIL-2R are not always present in patients with severe hepatic lesions. The maintenance of high levels of sIL-2R in the convalescent phase would not be indicative of hepatic lesions, but the increased levels of detachment in the alpha chain of the IL-2 receptor in activated cells stop the immune response induced by the cytokine (11).
The present study reveals increases in sCD30 levels in the acute phase along with the presence of HBsAg and increased concentrations of IL-10, HBeAg, IgM anti-HBc, and ALT in serum, reflecting greater activation of the cellular phenotype (which promotes humoral immunity against HBV antigens) during this phase. This shows that the activity of the Th2 phenotype in the acute phase is decisive for the production of neutralizing antiviral antibodies and the resolution of infection.
The significant decrease in sCD30 levels in the convalescent phase coincides with the nonreactivity of HBsAg and the appearance of anti-HBs and normal ALT levels. The data coincide with those of Fattovich et al. (18), for whom a high sCD30 level is a sign of HBV replication and biochemical activity, and the data also explain why sCD30 levels are in the normal range in individuals without these signs. This fact could also explain the behavior of sCD30 during the convalescent phase.
The normal levels of IFN-γ and TNF-α and the increases in IL-2, sIL-2R, IL-10, and sCD30 levels during the acute phase allow the resolution of infection and the development of immunity. In the convalescent phase the decrease in IFN-γ and TNF-α levels and the increase in sIL-2R levels impede the persistence of hepatocellular damage. The increase in IL-10 levels in the convalescent phase allows the maintenance of high concentrations of anti-HBs. The decrease in sCD30 levels to control values is indicative of the decrease in the level of HBV replication and coincides with the cessation of clinical signs and the reinstitution of hepatic activity to normal.
Acknowledgments
This work was supported by grant S1-97000624 from FONACIT and Regional Laboratories of Reference, Maracaibo, Venezuela.
We are grateful to Marina Garcia for technical assistance and for collecting the specimens.
REFERENCES
- 1.Alberti, N., and F. Colina. 1995. Histología de la hepatitis B: avance en la interpretación diagnóstica y patogénica. Hepat. Clin. 2:73-82. [Google Scholar]
- 2.Al-Wabel, A., M. Al-Janadi, and S. Raziuddin. 1993. Cytokine profile of viral and auto immune chronic active hepatitis. J. Allergy Clin. Immunol. 92:902-908. [DOI] [PubMed] [Google Scholar]
- 3.Anastassakos, A., A. Wolstencroft, P. Portman, E. De Dumon, and W. Roge. 1998. Interleukin-1 and interleukin-2 activity in chronic hepatitis B virus infection. Gastroenterology 94:999-1005. [DOI] [PubMed] [Google Scholar]
- 4.Andus, T., J. Bauer, and W. Gerok. 1991. Effects of cytokines on the liver. Hepatology 13:364-375. [PubMed] [Google Scholar]
- 5.Biron, C. A. 1994. Cytokines in the generation of immune response to, and resolution of virus infection. Curr. Opin. Immunol. 6:530-538. [DOI] [PubMed] [Google Scholar]
- 6.Bocher, W. O., E. Galun, H. Marcus, N. Daudi, D. Terkieltaub, D. Shouval, H. F. Lhor, and Y. Reisner. 2000. Reduced hepatitis B virus surface antigen-specific Th1 helper cell frequency of chronic HBV carriers is associated with a failure to produce antigen-specific antibodies in the trinera mouse. Hepatology 31:480-487. [DOI] [PubMed] [Google Scholar]
- 7.Borish, L. 1998. Updates on cell and cytokine. J. Allergy Clin. Immunol. 101:293-297. [DOI] [PubMed] [Google Scholar]
- 8.Chisari, F. V. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 9.Chu, C. M., I. S. Sheen, C. Y. Yeh, S. Y. Hsieh, S. L. Tsai, and Y. F. Liaw. 1995. Serum levels of interferon-alpha and gamma in acute and chronic hepatitis B virus infection. Dig. Dis. Sci. 40:2107-2112. [DOI] [PubMed] [Google Scholar]
- 10.Del Prete, G., M. Carli, F. Almerigogna, C. K. Daniel, M. D'ekuis, G. Zancuoghi, F. Vinante, G. Pizzolo, and S. Romagnani. 1995. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 9:81-86. [PubMed] [Google Scholar]
- 11.Diamantstein, T., H. Osawa, A. Mouzaki, and A. O. Josimovic. 1986. Regulation of interleukin-2 receptor expression and receptor release. Mol. Immunol. 23:1165-1172. [DOI] [PubMed] [Google Scholar]
- 12.Echevarría, J. M., and P. Leon. 1995. Hepatitis B virus: biology, natural history, and diagnosis of the infection. Enferm. Infecc. Microbiol. Clin. 13:22-30. [PubMed] [Google Scholar]
- 13.Echevarría, J. M., L. D. Blitz, and F. Pujol. 1996. La infección por los virus causantes de hepatitis en poblaciones indígenas de suramérica: una revisión del problema. Investig. Clin. 37:191-200. [PubMed] [Google Scholar]
- 14.Echevarría, S., F. Casafont, J. L. Miera, F. De La Cruz, G. San Miguel, and F. P. Romero. 1991. Interleukin-2 and natural killer activity in acute type B hepatitis. Hepatogastroenterology 4:307-310. [PubMed] [Google Scholar]
- 15.Engvall, E., and P. Perlmann. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871-874. [DOI] [PubMed] [Google Scholar]
- 16.Engvall, E., and P. Perlmann. 1971. Enzyme-linked immunosorbent assay (ELISA). Protides of the biological fluids, p. 553-556. In H. Peeters (ed.), Proceedings of the Nineteenth Colloquium. Pergamon Press, Oxford, United Kingdom.
- 17.Falini, B., S. Pileri, G. Pizzolo, H. Durkip, L. Flenghi, F. Stirpe, F. M. Martelli, and H. Stein. 1995. CD30 (ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1:1-14. [PubMed] [Google Scholar]
- 18.Fattovich, G., F. Vinante, G. Giuestina, L. Morosato, A. Alberti, and G. Pizzolo. 1996. Serum levels of soluble CD30 in chronic hepatitis B virus infection. Clin. Exp. Immunol. 103:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujinami, R. S., and M. A. Oldstone. 1979. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature 279:529-530. [DOI] [PubMed] [Google Scholar]
- 20.Gilles, P., G. Fey, and F. V. Chisari. 1992. Tumor necrosis factor alpha negatively regulates hepatitis b virus gene expression in transgenic mice. J. Virol. 66:3955-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1:25-36. [DOI] [PubMed] [Google Scholar]
- 22.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expression of distinct helper T cell subset. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzo, F., S. Curley, P. Maio, E. Leonardi, L. Imparato, S. Giglio, F. Cremona, and G. Castello. 1996. Correlation of soluble interleukin-2 receptor levels with severity of chronic hepatitis C virus liver injury and development of hepatocellular cancer. Surgery 1:100-105. [DOI] [PubMed] [Google Scholar]
- 24.Joklik, W. K. 1991. Interferons, p. 343-361. In B. N. Fields, D. M. Knipe, et al. (ed.), Fundamental virology, 2nd ed. Raven Press, New York, N.Y.
- 25.Juszczyk, J. 2000. Clinical course and consequences of hepatitis B infection. Vaccine 2000 1:S23-S25. [DOI] [PubMed] [Google Scholar]
- 26.Kelso, A. 1995. Th1 and Th2 subsets: paradigms lost? Immunol. Today 8:374-379. [DOI] [PubMed] [Google Scholar]
- 27.Lee, M., M. Lee, S. K. Lee, M. Son, S. W. Sho, S. Park, and H. I. Kim. 1999. Expression of Th1 and Th2 type cytokines responding to HbsAg and HbxAg in chronic hepatitis B patients. J. Korean Med. Sci. 2:175-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohr, H. F., J. F. Schlaak, G. Gerken, B. Fleischer, H. P. Dienes, and Z. B. Meyer. 1994. Phenotypical analysis and cytokine release of liver infiltrating and peripheral blood T lymphocytes from patients with chronic hepatitis of different etiology. Liver 3:161-166. [DOI] [PubMed] [Google Scholar]
- 29.Machado, B. I., L. Deibis, and F. Toro. 1997. Respuesta inmunológica en hepatitis viral. Gen. 2:85-93. [Google Scholar]
- 30.Mansour, A. J., A. W. Abdulhamid, and R. Syed. 1994. Soluble CD23 and interleukin-4 levels in autoimmune chronic active hepatitis and systemic lupus erythematosus. Clin. Immunol. Immunopathol. 1:33-37. [DOI] [PubMed] [Google Scholar]
- 31.Missale, G., C. Ferrari, and F. Fiaccadori. 1995. Cytokines mediators in acute inflammation and chronic course of viral hepatitis. Ann. Ital. Med. Int. 1:14-18. [PubMed] [Google Scholar]
- 32.Penna, A., G. Del Prete, A. Cavalli, A. Bertoleti, M. D'elios, and R. Sarmiento. 1997. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cell in acute self-limited hepatitis B. Hepatitis 4:1022-1027. [DOI] [PubMed] [Google Scholar]
- 33.Raynor, B. D. 1996. Cytokines. Adv. Obstet. Gynecol. 3:27-46. [Google Scholar]
- 34.Tilg, H., A. Wilmer, W. Vogel, M. Herold, B. Nolchen, G. Julmaier, and C. Huber. 1992. Serum levels of cytokines in chronic liver disease. Gastroenterology 103:264-274. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri, G. 1997. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFNg). Curr. Opin. Immunol. 9:17-23. [DOI] [PubMed] [Google Scholar]
- 36.Vetencourt, R., and M. Vetencour. 1997. Epidemiología de las hepatitis virales en Venezuela. Gastroenterol. Endocrinol. Nutr. 2:135-140. [Google Scholar]