Abstract
Warfarin, a widely prescribed drug for preventing thrombosis, is thought to act solely through inhibition of vitamin K-dependent coagulation factors. Low concentrations of warfarin inhibit interleukin-6 production and phosphorylation of I-κB but not activation of p38 mitogen-activated protein kinase. Thus, warfarin inhibits inflammatory signal transduction, and this may contribute to clinical effects of warfarin.
Coumadin derivatives, like warfarin, are widely used for treating thrombotic complications. These effects are attributed to the effects of these compounds on vitamin K metabolism. Oral anticoagulants produce their anticoagulant effect by interfering with the cyclic interconversion of vitamin K and its 2,3 epoxide (vitamin K epoxide) (4). These compounds have now been used with great clinical success for over half a century, and annually millions of patients are treated with this class of compounds worldwide (5). Apart from their influence on vitamin K metabolism, coumadin derivatives may employ alternative molecular mechanisms. Already in 1979 Eichbaum and colleagues demonstrated that warfarin exerts an anti-inflammatory action in experimental rodents and that this effect was not related to the anticoagulant properties of this compound (3). More recently, Tummino et al. showed that human immunodeficiency virus replication was impaired by warfarin (6), whereas other studies showed that coumarin-like substances may inhibit NF-κB and stress-activated protein kinase/c-Jun NH2-terminal kinases (1, 2). Hence, we were interested to see whether the clinically most widely used coumadin derivative, warfarin, might exert action in cellular physiology independent from its action on vitamin K metabolism.
Warfarin inhibits tumor necrosis factor (TNF)-induced I-κB phosphorylation.
We studied the effect of warfarin on the TNF-induced activation of I-κBα (I-κB) in murine clone 4/4 macrophages. As expected, a 10-min administration of 50 ng of TNF/ml strongly stimulated phosphorylation of I-κB in murine macrophages as assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting and probing with rabbit polyclonal antibodies against phosphorylated I-κB (1:500; New England Biolabs). In the presence of increasing concentrations of warfarin, however, TNF-induced phosphorylation of I-κB was decreased, a maximum being reached at 0.125 mM warfarin (Fig. 1). At higher concentrations of warfarin, this coumarin derivative was less effective. The latter effect is probably the result of an effect of warfarin on I-κB phosphorylation per se, as at these concentrations warfarin stimulated I-κB phosphorylation without the presence of TNF or in the presence of TNF and etanercept, a TNF-neutralizing drug. The relevance of this effect is doubtful, as, at the highest concentration of warfarin, I-κB breakdown products appeared; thus, this warfarin effect is likelier to be induced by cell toxicity than represent a bona fide effect on cell signal transduction. Reprobing blots with anti-β-actin antibodies confirmed that the effects observed were not due to unequal protein loading. We concluded that at physiological concentrations warfarin inhibits inflammatory signal transduction.
FIG. 1.
Effects of warfarin on TNF-induced I-κB phosphorylation. Murine clone 4/4 macrophages were stimulated with TNF and increasing concentrations of warfarin for 10 min. Subsequently cell extracts were prepared, and the effects on I-κB phosphorylation were assessed by using a phospho-specific antibody.
Warfarin effects on I-κB phosphorylation are relevant for cytokine production.
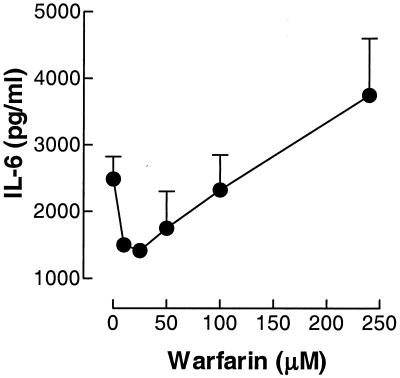
The inhibition of I-κB phosphorylation at lower concentrations of warfarin and its stimulation at higher concentrations of warfarin suggest that warfarin could directly influence inflammatory gene transcription in 4/4 macrophages. Hence, we investigated how various concentrations of warfarin would influence production of interleukin-6 (IL-6) by these cells. Figure 2 shows that warfarin influences IL-6 production in 4/4 macrophages grown in 24-well plates and stimulated for 16 h in the presence of various warfarin concentrations, as assayed by enzyme-linked immunosorbent assay (CLB, Amsterdam, The Netherlands). A dichotomal effect of warfarin was observed: high concentrations (>200 mM) of warfarin stimulated IL-6 release in murine macrophages, whereas lower warfarin concentrations (20 to 200 mM) potently inhibited TNF-induced IL-6 release. Thus, the effects seen on I-κB phosphorylation are apparently reflected in altered cytokine production. Warfarin is orally administered at a dose of 2 to 4 mg daily, resulting in physiological concentrations much lower than those necessary for the proinflammatory actions of warfarin observed in this study. Also, the induction of I-κB breakdown products at high warfarin concentrations indicates toxic effects of warfarin at this concentration and does not argue in favor of physiologically relevant effects at these concentrations. We propose that, of the effects described in the present study, only the anti-inflammatory actions may have in vivo relevance, but obviously further work is essential to establish whether such an action in vivo actually exists.
FIG. 2.
Effects of warfarin on IL-6 release. Murine clone 4/4 macrophages were stimulated with lipopolysaccharide (50 ng/ml) and increasing concentrations of warfarin for 16 h. Subsequently cell supernatants were collected, and IL-6 production was measured by using the enzyme-linked immunosorbent assay.
Warfarin acts downstream of the TNF receptor.
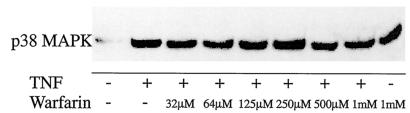
The effects seen with warfarin on TNF-induced I-κB phosphorylation may represent a specific effect of warfarin on the NF-κB signal transduction. Alternatively, warfarin may act as an inhibitor of transduction in general. To distinguish between these possibilities, we studied the effect of coumadin derivatives on TNF-induced phosphorylation of p38 mitogen-activated protein (MAP) kinase. As evident from Fig. 3, TNF strongly induced this phosphorylation. Importantly, this stimulation of p38 MAP kinase by TNF was not affected by warfarin; thus, warfarin does not interfere with TNF receptor phosphorylation per se but acts in the downstream signal transduction. Furthermore, they would seem to exclude the belief that the effects of warfarin at low concentrations are related to a general effect of warfarin on cell physiology at these concentrations. It is interesting that inhibitors of redox signaling often inhibit NF-κB-dependent signal transduction, whereas coumarins (which include warfarin) have been described as inhibitors of quinone reductases (2), quinone reductase inhibitors inhibiting both NF-κB and SAPK/JNK. In this study, however, no inhibitory effect was observed on NF-κB (2), but the concentration of warfarin used was 300 μM, which in our study stimulated, rather than inhibited, inflammatory signaling. It is tempting to suggest, therefore, that warfarin in lower concentrations, acting by inhibiting quinone reductases, interferes with the activation of NF-κB, although proving this hypothesis would have to involve the demonstration that warfarin is an in vivo inhibitor of quinone reductases at the concentrations employed in this study.
FIG. 3.
Effects of warfarin on TNF-induced p38 MAP kinase (MAPK) phosphorylation. Murine clone 4/4 macrophages were stimulated with TNF and increasing concentrations of warfarin for 10 min. Subsequently cell extracts were prepared, and the effects on p38 MAP kinase phosphorylation were assessed by using a phospho-specific antibody.
Disregarding the actual mechanism of action, the present study has shown that, in addition to its effects on the vitamin K-dependent coagulation, warfarin may also directly influence inflammatory signal transduction, in lower concentrations diminishing such signaling but in higher concentrations acting proinflammatory. At present, it is not yet clear whether these effects contribute to the clinical properties of warfarin. In view, however, of the emerging insight that vascular diseases involve an important inflammatory component, we feel that these effects warrant further study.
REFERENCES
- 1.Chen, C. C., C. L. Rosenbloom, D. C. Anderson, and A. M. Manning. 1995. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of IκB-α phosphorylation. J. Immunol. 155:3538-3545. [PubMed] [Google Scholar]
- 2.Cross, J. V., J. C. Deak, E. A. Rich, et al. 1999. Quinone reductase inhibitors block SAPK/JNK and NFkappaB pathways and potentiate apoptosis. J. Biol. Chem. 274:31150-31154. [DOI] [PubMed] [Google Scholar]
- 3.Eichbaum, F. W., O. Slemer, and S. B. Zyngier. 1979. Anti-inflammatory effect of warfarin and vitamin K1. Naunyn-Schmiedeberg's Arch. Pharmacol. 18:185-190. [DOI] [PubMed] [Google Scholar]
- 4.Hirsh, J., J. E. Dalen, D. R. Anderson, L. Poller, H. Bussey, J. Ansell, D. Deykin, and J. T. Brandt. 1998. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 114:445S-469S. [DOI] [PubMed] [Google Scholar]
- 5.Keller, C., A. C. Matzdorff, and B. Kemkes-Matthes. 1999. Pharmacology of warfarin and clinical implications. Semin. Thromb. Hemost. 5:13-16. [DOI] [PubMed] [Google Scholar]
- 6.Tummino, P. J., D. Ferguson, and D. Hupe. 1994. Competitive inhibition of HIV-1 protease by warfarin derivatives. Biochem. Biophys. Res. Commun. 30:290-294. [DOI] [PubMed] [Google Scholar]