Abstract
Human coronavirus (HCoV) strain 229E infection, but not HCoV strain OC43 infection, of monocytes/macrophages from healthy donors and patients with multiple sclerosis in remission resulted in increased apoptosis, as measured by DNA changes and annexin V staining. Apoptosis correlated with the differential release of infectious virus. HCoV strain 229E titers were 103.5 to 106 50% tissue culture-infective doses (TCID50)/ml, and HCoV strain OC43 titers were only 101.2 to 102.7 TCID50/ml.
Human coronaviruses (HCoV) that cause upper respiratory illness fall into two serogroups, 229E (group 1) and OC43 (group 2). The importance of either serogroup as a cause of disease outside of the respiratory tract has not been determined. Coronavirus-like particles were observed by electron microscopy in a perivascular lesion in the brain of a patient with multiple sclerosis (MS) (2). Sequences from the nucleocapsid genes of both serogroups are found in the brain (7). Macrophage infection contributes to the pathogenesis of other members of the order Nidovirales, such as porcine reproductive and respiratory syndrome virus, turkey hemorrhagic enteritis virus, and murine hepatitis virus (MHV), encephalomyelitis with virus-induced demyelination, a disease showing similarities to MS (8, 13, 17). Following infection with MHV strain A59, apoptosis was detected in macrophages in brain parenchyma demyelinating lesions (14). In the MHV JHM model, macrophages infiltrating the central nervous system become activated and express chemokine receptor CCR5, inducible nitric oxide synthase, and gamma interferon-inducing factor (5, 8, 18). Macrophage activation induces resistance to apoptosis, suggesting that survival is essential for the release of inflammatory mediators and reactive free radical species (4, 12). Given that both serogroups of HCoV infect macrophages, with HCoV strain 229E causing cell rounding, detachment, and occasional syncytia, we sought to determine whether apoptosis was induced (3, 10). These studies were approved by the Institutional Review Board of the State University of New York at Buffalo.
Peripheral blood monocytes/macrophages were obtained from age-matched healthy adult donors and from patients diagnosed with MS who were in remission and not receiving medication. After 48 h in culture, the adherent cells were infected with HCoV strains 229E and OC43 at a multiplicity of 1 (3, 10). Both infectivity assays were done on the same day with pairs of normal and MS monocytes/macrophages. The HCoV strains used were obtained from the American Type Culture Collection (Manassas, Va.), plaque purified twice, and grown on L-132 (229E) or HRT-18 (OC43) cells. Infectious virus titers of samples were quantitated by the immunoperoxidase assay with antiviral monoclonal antibodies (MAb) to S (spike antigen), 1.10C.3 for HCoV strain OC43 and 5.11H.6 for HCoV strain 229E (15).
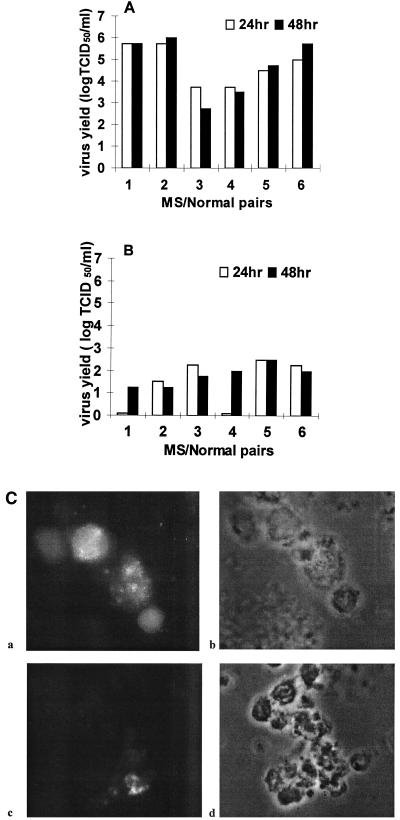
Cultures were harvested on days 1 and 2 postinfection (p.i.) and evaluated. HCoV strain 229E-infected blood monocytes/macrophages produced viral titers of 103.5 to 106 50% tissue culture-infective doses (TCID50)/ml, and HCoV strain OC43-infected cells only produced viral titers of 101.2 to 102.5 TCID50/ml (Fig. 1A and B). After HCoV strain 229E infection, the cells appeared pyknotic and granular, with swelling of the plasma membrane and a few syncytia at 16 to 18 h p.i. The number of syncytia did not increase with time. The titer and cytopathic effect were similar to those previously reported (10). HCoV strain OC43 infection proceeded without a cytopathic effect and at a low titer, comparable the amount of virus detected after 2 h of incubation at 4°C and two washes to remove the inoculum. In cultures kept for 6 days, the titer increased slightly to 102.7 TCID50/ml. Viability was comparable to that of uninfected controls. Previously, HCoV strain OC43 titers of 105.3 to 107.3 PFU/ml were obtained from umbilical cord blood monocytes/macrophages (3). For confirmation that the viral titers measured were indeed associated with infection of monocytes or macrophages, immunofluorescence microscopy was performed with MAb to S viral antigen. After blocking of Fc receptors with normal goat serum (DAKO, Carpinteria, Calif.), cells where stained for the viral S antigen expressed on the surface of paraformaldehyde (4%)-fixed cells. In cultures infected with HCoV strain 229E, the majority of cells (>55%) expressed viral antigen 2 days p.i., similar to earlier work (10). Following HCoV strain OC43 infection, very few cells (<5%) expressed viral antigen, compared to 15% of cord blood-derived monocytes/macrophages positive for viral nucleocapsid antigen (3) (Fig. 1C). This suggests that replication depends on the level of differentiation of these cells (6). Preferential viral replication in less differentiated tissues was observed with primary neurons derived from dorsal root ganglia and astrocyte cultures (1, 11). Healthy donors and MS patients gave similar responses within the parameters of our experiments.
FIG. 1.
Yield of infectious virus from HCoV-infected monocytes/macrophages derived from MS patients (lanes 1, 3, and 5) and healthy control subjects (lanes 2, 4, and 6). Panels A and B represent the same patients. Open bars: virus yields at 24 h p.i. Closed bars: virus yields at 48 h p.i. The virus titers after 2 h of incubation at 4°C and removal of the inoculum by two cell washings were 75 to 125 TCID50/ml. (A) HCoV strain 229E infections. (B) HCoV strain OC43 infections. The limit of detection was 25 TCID50/ml. (C) Detection of HCoV antigens by immunocytochemistry analysis of monocytes/macrophages infected for 48 h with an S antigen-specific MAb (5.11H.6 for 229E and 1.10C.3 for OC43). a and c are immunofluorescence images, b and d are phase-contrast images of the same field. a and b are 229E-infected cells; c and d are OC43-infected cells. Original magnification, ×600.
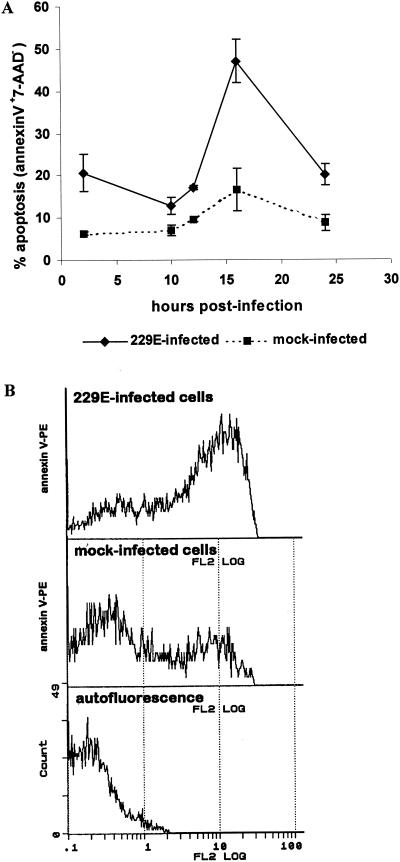
Next, apoptosis was quantified after terminal deoxynucleotidyltransferase (TdT) labeling (DeadEnd Colorimetric Apoptosis Detection System; Promega) in accordance with the manufacturer's instructions. TdT assays were done with preparations of monocytes/macrophages different from those used in infectivity assays, and the assay results are not directly comparable. The response to HCoV strain 229E inoculation was 16 to 35% of monocyte/macrophage cells positive for TdT staining (Table 1). HCoV strain OC43 infection proceeded with no significant difference in apoptosis compared to mock-infected monocytes/macrophages. Healthy subjects and MS patients gave similar responses. To verify that HCoV strain 229E infection induces apoptotic changes in monocytes/macrophages, a flow cytometric analysis for cell membrane expression of externalized phosphatidylserine was performed by double labeling of HCoV strain 229E-infected monocytes/macrophages from healthy donors with annexin V and 7-amino-actinomycin (7-AAD; a vital dye). Monocyte/macrophage cells in culture for 48 h were infected with HCoV strain 229E at a multiplicity of 1 or mock infected, incubated at 33°C for 2 h, and washed twice to remove the inoculum. After 2 h of infection and at various intervals thereafter, 0.5 × 106 to 1 × 106 cells, in duplicate, were washed in phosphate-buffered saline (PBS) and incubated with 20 μl of 7-AAD (Pharmacia). After washing in binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2), the cells were incubated with 10 μl of human annexin V-phycoerythrin (PE) (R&D Systems Inc.). A 400-μl volume of binding buffer was added. The cells were kept on ice and analyzed with a Coulter EPICS XL-MCL flow cytometer within 1 h after staining. Data from 10,000 cells was collected. Six donors were examined, and the standard deviation was calculated. Membrane externalization of phosphatidylserine, as indicated by annexin V-PE+ 7-AAD− cells, increased significantly at 16 h p.i. (Fig. 2 A). Forty-six percent of infected cells gave a 2-log displacement in annexin V-PE signal above the cutoff value for unstained cells, compared to 12% of mock-infected cells (Fig. 2B). The difference in apoptosis (46% versus 16 to 35%) between annexin V data and TdT data may be due to time-dependent factors. Plasma membrane annexin V is exposed earlier in apoptosis, before fragmentation (16). Also, in infected cultures, the number of attached cells is reduced by 50% after 24 h of infection, compared to a reduction of 6% in uninfected cultures (10). Presumably, cells become infected and detached throughout infection.
TABLE 1.
Susceptibility of monocytes/macrophages to apoptosis caused by HCoV
| Virus | % of monocyte/macrophage samples from donors positive by TdT assay ± SDa
|
|||||
|---|---|---|---|---|---|---|
| Normal | Normal | Normal | MS | MS | MS | |
| 229E | 23 ± 2.2 | 25 ± 3.4 | 34 ± 1.8 | 19 ± 2.8 | 35 ± 8 | 16 ± 1 |
| OC43 | 13 ± 3.8 | 2.7 ± 0.6 | 4.2 ± 1 | 11 ± 3.5 | NDb | 11 ± 1.5 |
| None (mock infected) | 4 ± 1.5 | 12 ± 1.5 | 4.7 ± 1.4 | 8 ± 2 | 12 ± 3.5 | 12 ± 3 |
Quantitation of TdT labeling-positive cells at 48 h p.i. Two to 400 cells per slide, in duplicate, were scored.
ND, not done.
FIG. 2.
(A) Percentage of monocytes/macrophages showing apoptosis at the times indicated after infection with HCoV strain 229E. Apoptosis was measured by dual-parameter flow cytometry for annexin V-PE staining and DNA fluorescence with 7-AAD. Data are from six healthy donors; each case is a comparison of infected cells with mock-infected control cells. (B) Flow cytometry profiles of annexin V-PE+ 7-AAD− stained cells at 16 h p.i. Top, HCoV strain 229E-infected cells; middle, mock-infected cells; bottom, cellular autofluorescence.
To further examine HCoV strain 229E-infected cells for viral spike antigen expression, monocytes/macrophages from healthy donors, in culture for 48 h, were infected with HCoV strain 229E at a multiplicity of 1, incubated for 2 h at 33°C, washed twice in PBS, and further incubated at 33°C in RPMI 1640 medium with 1% autologous serum. After 2 h and at various intervals thereafter, 0.5 × 106 to 1 × 106 cells, in duplicate, were preincubated with 10 μl of undiluted normal goat serum (DAKO) for 15 min. After one washing, 20 μl of HCoV strain 229E-specific MAb 5.11H.3 or an isotypic control antibody (1/2,000 dilution) was added. After two washings, 10 μl of fluorescein-conjugated goat anti-mouse F(ab′)2 (1/200 dilution; DAKO) was added. The cells were kept on ice in 400 μl of PBS and analyzed with a Coulter EPICS XL-MCL flow cytometer within 1 h after staining. Results of duplicate cultures were averaged.
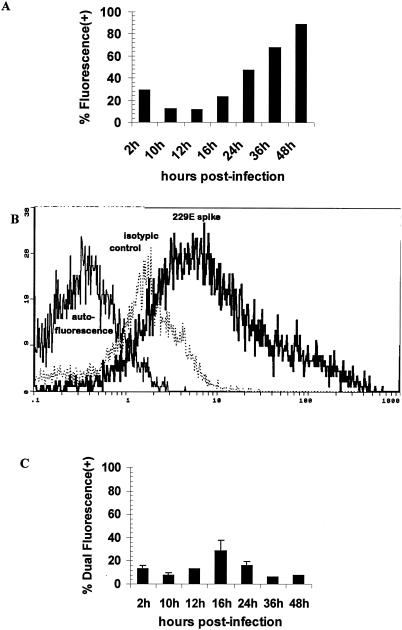
A time-dependent expression of cell membrane HCoV strain 229E S antigen on monocytes/macrophages was observed (Fig. 3A). At 24 h p.i., 47% of the infected cells showed immunofluorescence. This compares well with a previous report that about half of the attached infected cells fluoresced (10). At 48 h p.i., a >0.5-log displacement increase in signal intensity for virus-specific MAb, compared to the isotype control MAb, was seen (Fig. 3B). The percentages of viable cells, as measured by trypan blue dye exclusion, were 75, 86, 52, and 34, respectively, at 1, 2, 3, and 4 days p.i., while the uninfected cells remained 82 to 90% viable throughout. Previously, 70% viability in the infected attached cells after 24 h was reported (10).
FIG. 3.
Flow cytometric analysis of cell surface expression of viral antigen. Monocyte/macrophage cultures were inoculated with HCoV strain 229E and labeled at various times with virus-specific MAb 5.11H.6 or an isotypic control antibody. (A) Percentage of cells having a fluorescence signal displacement above the cutoff established for unstained cells. (B) Flow cytometry profile of fluorescein isothiocyanate-labeled cells at 48 h p.i. Left profile, cellular autofluorescence; middle (dotted) profile, isotypic control; right profile, virus-specific MAb. (C) Percentage of viable cells having dual fluorescence for virus-specific MAb 5.11H.6 (fluorescein isothiocyanate positive) and annexin V (PE+). Error bars represent standard deviations of data from three donors.
In order to demonstrate that the apoptotic cells were indeed the cells infected by the virus, double staining with virus-specific MAb (5.11H.6) to S antigen and annexin V-PE was performed on monocytes/macrophages from three donors and the standard deviation was calculated for the 2- to 24-h intervals. Cytofluorometric analysis showed the presence of both markers on viable cells (exclusion of 7-AAD+ cells), with 29% showing dual fluorescence at 16 h p.i. (Fig. 3C).
Overall, the increase in apoptotic cells observed in HCoV strain 229E-infected cultures at 16 h p.i. was associated with few necrotic cells and in normal cultures, there were only 4 to 12% apoptotic cells. In the mock-infected cultures, some cells likely enter the apoptotic pathway of cell death. Monocytes normally circulate in the blood for only a few days, during which time they either migrate to tissues and differentiate to macrophages or die through apoptosis (12). Apoptosis caused by HCoV in adherent monocytes/macrophages in culture might then be due to commitment to proapoptotic signals generated by the virus. Possibly, production of cytokine mediators such as tumor necrosis factor alpha was also a factor since a difference was observed in the percent apoptosis at 16 h p.i. (47% versus 29%) between cells stained only with annexin V and cells both immunostained and annexin V stained (Fig. 2A and 3C). Studies of cytokine expression in HCoV strain 229E-infected monocytes/macrophages are necessary to clarify this point. Following infection of monocytes/macrophages by HCoV strain OC43, viability remained high over 6 days and no apoptosis was observed. The low viral titers were confirmed by demonstration of viral spike antigen on the surface of cells in infected cultures by immunofluorescence microscopy. Lack of apoptosis is likely due to the very poor ability of this virus to infect the cells. Further studies of HCoV-induced apoptosis in monocytes freshly isolated from persons with clinically active MS would be appropriate because monocyte/macrophage activation was observed (acute exacerbation and progressive MS) (9, 19).
Acknowledgments
This work was supported by SUNY at Buffalo/UUP grants 068303-01 and 068303-11 and grant MT-9203 from the Medical Research Council of Canada.
I thank Marcel Desrosiers (Human Health Research Centre, INRS-Institut Armand-Frappier) for invaluable help with flow cytometry experiments, Pierre Duquette (MS Clinic, Hôpital Notre-Dame, Montréal, Québec, Canada) and Peter Kinkel (Neurology Department, State University of New York at Buffalo) for help with selecting MS patients, and Pierre Talbot (Laboratory of Neuroimmunovirology, Human Health Research Centre, INRS-Institut Armand-Frappier) for helpful discussions and the generous gift of MAb for immunoassays.
REFERENCES
- 1.Bonavia, A., N. Arbour, V. W. Yong, and P. J. Talbot. 1997. Infection of primary cultures of human neural cells by human coronaviruses 229E and OC43. J. Virol. 71:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burks. J. S., B. V. DeVald, L. D. Jankovsky, and J. C. Gerdes. 1980. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science 209:933-934. [DOI] [PubMed] [Google Scholar]
- 3.Collins, A. R. 1998. Human macrophages are susceptible to coronavirus OC43. Adv. Exp. Biol. Med. 440:635-639. [DOI] [PubMed] [Google Scholar]
- 4.Edwards, J. A., F. Denis, and P. J. Talbot. 2000. Activation of glial cells by human coronavirus OC43 infection. J. Neuroimmunol. 108:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass, W. G., M. T. Liu, W. A. Kuziel, and T. E. Lane. 2001. Reduced macrophage infiltration and demyelination of mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology 288:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho., W.-Z., J. Lioy, L. Song, J. R. Cutilli, R. A. Polin, and S. D. Douglas. 1992. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J. Virol. 66:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes, K. V. 2001. Coronaviruses, p. 1187-1203. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 8.Liu, M. T., H. S. Keirstead, and T. E. Lane. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 167:4091-4097. [DOI] [PubMed] [Google Scholar]
- 9.Oleszak, E. L., E. Zaczynska, M. Bhattacharjee, C. Butunoi, A. Legido, and C. D. Katsetos. 1998. Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin. Diagn. Lab. Immunol. 5:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson, S., and M. R. Macnaughton. 1982. Replication of human respiratory coronavirus strain 229E in human macrophages. J. Gen. Virol. 60:307-314. [DOI] [PubMed] [Google Scholar]
- 11.Pearson, J., and C. A. Mims. 1985. Differential susceptibility of cultured neural cells to the human coronavirus OC43. J. Virol. 53:1016-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perera, L. P., and T. A. Waldmann. 1998. Activation of human monocytes induces differential resistance to apoptosis with rapid down regulation of caspase-8/FLICE. Proc. Natl. Acad. Sci. USA 95:14308-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rautenschlein, S., and J. M. Sharma. 2000. Immunopathogenesis of haemorrhagic enteritis virus (HEV) in turkeys. Dev. Comp. Immunol. 24:237-246. [DOI] [PubMed] [Google Scholar]
- 14.Schwarts, T., L. Fu, and E. Lavi. 2001. Programmed cell death in MHV-induced demyelination. Adv. Exp. Med. Biol. 494:163-167. [DOI] [PubMed] [Google Scholar]
- 15.Sizun, J., N. Arbour, and P. J. Talbot. 1998. Comparison of immunofluorescence with monoclonal antibodies and RT-PCR for the detection of human coronaviruses 229E and OC43 in cell culture. J. Virol. Methods 72:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth, P. G., S. A. Berman, and S. Burstajn. 2002. Markers of apoptosis: methods for elucidating the mechanism of apoptotic cell death from the nervous system. BioTechniques 32:648-665. [DOI] [PubMed] [Google Scholar]
- 17.Thanawongnuwech, R., P. G. Halbur, and E. L. Thacher. 2000. The role of pulmonary intravascular macrophages in porcine reproductive and respiratory syndrome virus infection. Anim. Health Res. Rev. 1:95-102. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, X., D. R. Hinton, D. J. Cua, S. A. Stohlman, and M. M. Lai. 1997. Expression of interferon-gamma by a coronavirus defective interfering RNA vector and its effect on viral replication, spread and pathogenicity. Virology 233:327-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziaber, J., Z. Baj, J. Pasnik, H. Chmielewski, and H. Tchorzewski. 2000. Increased expression of neutral endopeptidase (NEP) and aminopeptidase N (APN) on peripheral blood mononuclear cells in patients with multiple sclerosis. Immunol. Lett. 71:127-129. [DOI] [PubMed] [Google Scholar]