Abstract
The participation of NK cells in the activation of splenic macrophages or in resistance to systemic candidiasis is still a matter of debate. We had previously reported that there is a correlation between natural killer cell activation and resistance to systemic candidiasis. In those experiments we had used tilorone to boost NK cell activity in mice. Here we show a mechanism elicited by tilorone in splenic macrophages which could explain their effect on mouse survival during acute disseminated Candida albicans infection. The results demonstrate that tilorone treatment elicits, by a direct effect, the production of proinflammatory cytokines (interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], and IL-12) by splenic macrophages. In addition, it increases the capacity of splenic macrophages to phagocytize C. albicans through activation of NK cells. We also demonstrate that the presence of NK cells is essential for maintaining a basal level of phagocytic activity, which characterizes splenic macrophages of naïve control mice. The results demonstrate that it is possible to identify two phenotypically and functionally peculiar cell populations among splenic macrophages: (i) cells of the “stimulator/secretor phenotype,” which show high levels of major histocompatibility complex (MHC) class II surface expression, are poorly phagocytic, and synthesize the proinflammatory cytokines IL-6, TNF-α, and IL-12, and (ii) cells of the “phagocytic phenotype,” which express low levels of MHC class II molecules, are highly phagocytic, and do not secrete proinflammatory cytokines.
The rise in the number of immunocompromised patients has dramatically increased the incidence of human systemic fungal infections in recent years. Accumulating evidence points to the pivotal role of splenic macrophages in primary resistance to systemic and disseminated candidiasis. Han et al. (14) have shown that macrophages from the marginal zone of the spleen trap Candida albicans yeast cells injected into mice. Moreover, selective elimination of mouse splenic macrophages with dichloromethylene diphosphonate correlates with increased susceptibility to experimental disseminated candidiasis (24). Furthermore, the resistance of mice to systemic infection with C. albicans is associated with activated splenic macrophages that show increased candidacidal activity in vitro (11). Thus, splenic macrophages are clearly involved in protective mechanisms during systemic infections caused by C. albicans (reviewed in reference 37), but the precise role of these macrophages has not been clearly defined.
Macrophage heterogeneity is a well-documented phenomenon (13). It has also long been recognized that macrophages isolated from different anatomical sites display a diversity of phenotypes and capabilities. The presence of functionally distinct macrophage populations gives flexibility to respond to different stimuli. Depending on the stimulus, the nature of a specific immune response is dictated in large part by the functional phenotypes of the macrophages present within the tissue. Macrophages can both regulate the immune response to C. albicans (by antigen presentation and T-helper type 1 [Th1] cell stimulation) and act as effector cells to phagocytize and kill the fungus. Macrophages have been described as producing proinflammatory cytokines (such as tumor necrosis factor alpha [TNF-α], interleukin-6 [IL-6], and IL-12) that induce the development of a Th1 cell response. It is generally accepted that a proper interaction between the innate and the adaptive immune system is required for efficient control of C. albicans infections. Furthermore, several studies provide evidence that resistance to C. albicans infection is determined by phagocytic mechanisms, the activity of which is augmented or reinforced by Th1 cytokines (26).
Immunotherapy strategies using biologic response modifiers (by themselves or in conjunction with antimycotic drugs) could be useful in improving the treatment and prognosis of yeast infections. Since it is obvious that splenic macrophages play a pivotal role in host responses to systemic candidiasis, in vivo activation of splenic macrophages by immunomodulators could be a good strategy for improving the treatment of systemic candidiasis. Therefore, the in vivo biologic response of splenic macrophages to C. albicans challenge must be extensively studied before immunomodulators or selected cytokines are used in therapeutic regimens for candidiasis. Unfortunately, at the moment, much of our knowledge about the biologic response of macrophages to C. albicans comes from in vitro studies. In vivo results have also been obtained, but in most experiments serum cytokine levels were quantitated by means of cytokine-specific enzyme-linked immunosorbent assays, so the pattern of cytokine production by the macrophage population of choice was not determined. Another important question that needs to be answered is the interaction between splenic macrophages and other immune cells (i.e., NK cells), which still remains unclear. Recently, we described a correlation between NK cell activation (induced by tilorone treatment) and resistance to experimental systemic candidiasis in mice (22). Mice that were injected intravenously (i.v.) with a lethal suspension of C. albicans and treated with tilorone remained without evidence of disease, while untreated control mice died on the third day. Tilorone is an orally active compound previously described as a potent gamma interferon (IFN-γ) inducer which is widely used to boost NK cell activity in mice.
The aim of the present study was to establish the mechanisms of protection of mice against acute disseminated C. albicans infection that are elicited by tilorone treatments. The importance of splenic macrophages for induction of a protective immune response to acute systemic infection by C. albicans is well documented; thus, we have selected this population as the best cellular choice for the study of this disease. We focus our interest on the role played by splenic macrophages of mice infected i.v. with C. albicans. The parameters that we have assessed are both phagocytic activity and production of the proinflammatory cytokines (TNF-α, IL-6, and IL-12) by splenic macrophages in response to both acute systemic C. albicans infection and tilorone treatment. We have also studied the interaction between T cells or NK cells and splenic macrophages regarding the role played in the protection of mice after lethal i.v. doses of C. albicans. The experiments were performed with BALB/c mice and athymic nude mice, and some experiments were performed with hosts previously depleted of NK cells by treatment with anti-asialo-GM1.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old male BALB/c mice were obtained from the breeding colony of the University of Jaén, and male athymic nu/nu mice were purchased from IFFA-Credo (Barcelona, Spain). The average weight of the mice was 20 g, and they were maintained under pathogen-free conditions, with free access to food and water. The experiments described in this article were performed in accordance with national guidelines on the use of experimental animals.
In vivo treatment with tilorone and anti-asialo-GM1.
The tilorone analogue R10,874DA {3,6-bis-[2-(dimethylamino)-ethoxy]-9H-xanthen-9-one dihydrochloride} (Sigma Chemical Co., St. Louis, Mo.) was used to increase in vivo NK activity. This compound is referred to below as tilorone. Each mouse receiving tilorone was treated orally via cannula with 2 mg of the compound dissolved in 200 μl of water on day −1 or −2. In some experiments, in order to abolish NK activity, mice were treated intraperitoneally on days −6 and −3 with 200 μl of a 1:50 dilution of anti-asialo-GM1 (Wako, Osaka, Japan).
Yeast culture and experimental infection with C. albicans.
C. albicans (ATCC 2091) was grown in Mueller-Hinton broth (Scharlau, Barcelona, Spain) at 37°C for 24 h, washed twice at 400 × g for 10 min, and finally adjusted to 2.5 × 106 CFU/ml in phosphate-buffered saline (PBS; Sigma). Mice were injected i.v. (through the tail vein) with 106 CFU of C. albicans.
Preparation of splenic macrophages.
At the end of the experiments, mice were anesthetized with ether and killed by cervical dislocation. Spleens were extracted under aseptic conditions, and cells were gently dispersed by using 90-mesh stainless steel screens into cold complete RPMI 1640 medium (Sigma) containing 1% penicillin G-streptomycin solution (Sigma), 1% l-glutamine (Sigma), 1% sodium pyruvate (Sigma), 1% HEPES (Flow Laboratories, Irvine, United Kingdom) and 5% fetal calf serum (FCS; Flow Laboratories). Cell suspensions were centrifuged at 200 × g and 4°C for 10 min, and erythrocytes were lysed with Red Blood Cell Lysing Buffer (Sigma) for 5 min. Remaining cells were washed twice with RPMI medium. Cell suspensions were allowed to adhere to a plastic culture flask (Nalgene Nunc International, Copenhagen, Denmark) in the absence of serum for 1 h at 37°C in 5% CO2-. Nonadherent cells were removed. Adherent cells were detached with a solution of 0.02% EDTA in PBS and finally were resuspended in RPMI medium with 10% FCS to a final concentration of 106 cells/ml. Under these conditions, adherent cells contained more than 95% macrophages, as assessed by cell staining (with Wright-Giemsa stain) and morphological analysis. The viability of cells, assessed by trypan blue exclusion, was ≥95%.
Intracellular cytokine detection.
Flow cytometric determination of intracellular cytokine production at the single-cell level was performed. In all experiments, in order to enhance the sensitivity of cytokine detection, 106 adherent spleen cells were incubated for 6 h at 37°C with brefeldin A (1 μg/ml) (GolgiPlug; BD PharMingen, San Diego, Calif.), a protein transport inhibitor that allows intracellular cytokine accumulation. Intracytoplasmic cytokine staining was performed by using the Cytofix/Cytoperm Plus kit and the manufacturer's protocol (BD PharMingen). Briefly, cells were washed in Staining Buffer, and in order to block Fc receptors, they were incubated with 1 μg of Fc Block (clone 2.4G2; antibody specific for FcγII and FcγIII receptors)/106 cells in 100 μl of Staining Buffer for 15 min at 4°C. The cells were then washed twice with Staining Buffer and resuspended in 250 μl of Cytofix/Cytoperm solution for 20 min at 4°C. Cells were subsequently washed twice with 1× Perm/Wash solution and incubated for 30 min at 4°C in the dark in 50 μl of this solution containing a predetermined optimal concentration of a fluorochrome-conjugated anticytokine antibody. After two washes, cells were resuspended in Staining Buffer prior to flow cytometric analysis on an EPICS Elite ESP flow cytometer (Coulter, Hialeah, Fla.). The fluorochrome-conjugated anticytokine antibodies (BD PharMingen) used were as follows: a fluorescein isothiocyanate-conjugated rat anti-mouse TNF-α monoclonal antibody (MAb) (clone MP6-XT22), a phycoerythrin-conjugated rat anti-mouse IL-6 MAb (clone MP5-20F3), and a phycoerythrin-conjugated rat anti-mouse IL-12 (p40/p70) MAb (clone C15.6).
In order to obtain a positive control, adherent splenic macrophages (5 × 106 viable cells/ml) obtained as described above were cocultured with lipopolysaccharide (LPS) from Escherichia coli serotype O26:B6 (Sigma) at 10 μg/ml for 24 h. Percentages reflect cytokine-positive cells.
Phagocytosis assay and flow cytometric analysis.
C. albicans phagocytosis by macrophages was assessed as previously described (21). Briefly, the yeast cells were inactivated by heating at 100°C for 1 h. In order to obtain opsonized C. albicans, 100% of non-heat-inactivated FCS was used as opsonin. The mixture was incubated with continuous shaking at 37°C for 30 min and finally washed at 400 × g and resuspended in 1 ml of PBS. In order to stain C. albicans, 25 μl of 7-amino-actinomycin D solution was added to 107 CFU of heat-inactivated C. albicans in 500 μl of PBS, and the mixture was homogenized by vortexing. The yeast cells were incubated protected from light for 20 min at 4°C and finally were pelleted by centrifugation (at 400 × g for 10 min). Adherent splenic macrophages were incubated in complete RPMI 1640 medium (supplemented with 10% heat-inactivated FCS) with heat-inactivated 7AAD-stained C. albicans (ratio, 10:1) at 37°C in a 5% CO2 incubator for 1 h with continuous shaking. The incubation was performed in a polystyrene tube (13 by 75 mm) (Soria Greiner, Barcelona, Spain), and the cells were then washed three times in PBS (at 100 × g and 4°C for 10 min) to remove noningested yeast cells. Phagocytosis was arrested, and the cells were fixed by addition of cold 2% paraformaldehyde.
Fluorescence was analyzed on an EPICS Elite ESP flow cytometer (Coulter) within 30 min of cell fixation. At least 5 × 103 events were measured for each sample. Scattergrams were generated by combining forward light scatter (FS) with 7AAD fluorescence (FL3), and phagocytic cells were drawn around clear-cut populations that had bright red fluorescence. Phagocytosis was determined by gating the cells and calculating the percentage of phagocyte-associated red fluorescent cells.
MHC class II surface expression.
To detect I-A major histocompatibility complex (MHC) class II molecules, adherent spleen cells were incubated for 30 min at 4°C with a MAb specific for mouse H-2 I-Ab class II molecules (clone 28-16-8S) that cross-reacts with the I-Ad class II antigen of BALB/c mice (Caltag Laboratories, Burlingame, Calif.). Cells were harvested by centrifugation, washed twice in cold PBS supplemented with 2% FCS and 0.1% sodium azide, and finally resuspended in 500 μl of a 2% paraformaldehyde solution. Samples were analyzed by flow cytometry on an EPICS Elite ESP flow cytometer. Flow cytometry histograms were generated by logarithmic amplification of fluorescence emitted by single viable cells. A total of 104 viable cells were analyzed in each sample.
RESULTS
Cytokine (IL-6, TNF-α, and IL-12) production profile in splenic macrophages from naïve BALB/c mice.
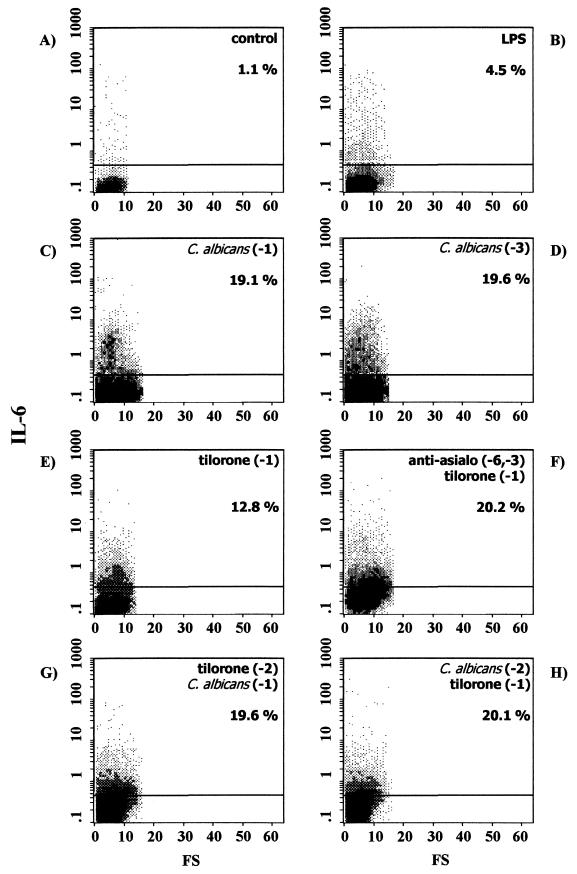
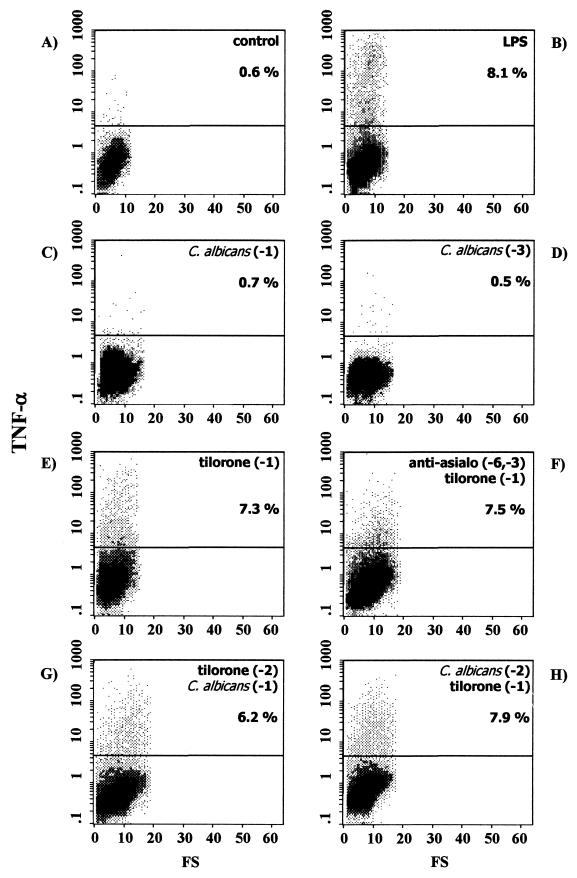
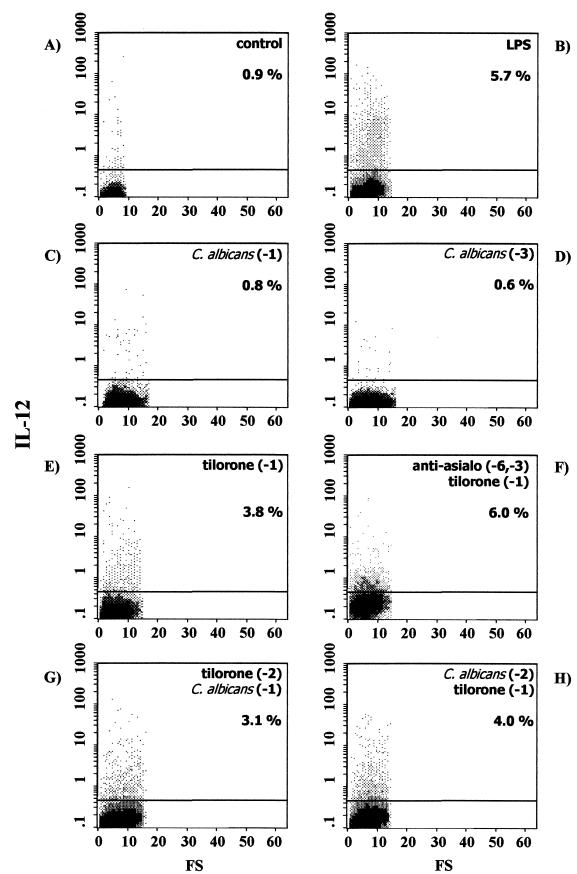
Flow cytometric determination of intracellular IL-6, TNF-α, and IL-12 production at the single-cell level was performed. In order to enhance the sensitivity of cytokine detection, cells were incubated for 6 h with brefeldin A, which prevents cytokine secretion and leads to intracellular accumulation. The profiles of IL-6, TNF-α, and IL-12 production in splenic macrophages from naïve BALB/c mice under these conditions are shown in Fig. 1A, 2A, and 3A, respectively. As shown, few, if any, splenic macrophages produced IL-6, TNF-α, or IL-12 (1.1, 0.6, and 0.9%, respectively).
FIG. 1.
Intracellular IL-6 expression in splenic macrophages from control naïve BALB/c mice (A) or BALB/c mice exposed to different stimuli, as follows: in vitro LPS stimulation (B), C. albicans infection 24 h before the assay (C), C. albicans infection 72 h before the assay (D), tilorone treatment 24 h before the assay (E), depletion of NK cells by intraperitoneal injection of anti-asialo-GM1 on the indicated days, followed by tilorone treatment 24 h before the assay (F), tilorone treatment 48 h before the assay, followed by i.v. challenge with live C. albicans 24 h before the assay (G), and i.v. C. albicans infection 48 h before the assay, followed by tilorone treatment 24 h before the assay (H). Percentages reflect cytokine-positive cells. Results are from one representative experiment out of three performed with cells from different mice.
FIG. 2.
Intracellular TNF-α expression in splenic macrophages from control naïve BALB/c mice (A) or BALB/c mice exposed to different stimuli, as follows: in vitro LPS stimulation (B), C. albicans infection 24 h before the assay (C), C. albicans infection 72 h before the assay (D), tilorone treatment 24 h before the assay (E), depletion of NK cells by intraperitoneal injection of anti-asialo-GM1 on the indicated days, followed by tilorone treatment 24 h before the assay (F), tilorone treatment 48 h before the assay, followed by i.v. challenge with live C. albicans 24 h before the assay (G), and i.v. C. albicans infection 48 h before the assay, followed by tilorone treatment 24 h before the assay (H). Percentages reflect cytokine-positive cells. Results are from one representative experiment out of three performed with cells from different mice.
FIG. 3.
Intracellular IL-12 expression in splenic macrophages from control naïve BALB/c mice (A) or BALB/c mice exposed to different stimuli, as follows: in vitro LPS stimulation (B), C. albicans infection 24 h before the assay (C), C. albicans infection 72 h before the assay (D), tilorone treatment 24 h before the assay (E), depletion of NK cells by intraperitoneal injection of anti-asialo-GM1 on the indicated days, followed by tilorone treatment 24 h before the assay (F), tilorone treatment 48 h before the assay, followed by i.v. challenge with live C. albicans 24 h before the assay (G), and i.v. C. albicans infection 48 h before the assay, followed by tilorone treatment 24 h before the assay (H). Percentages reflect cytokine-positive cells. Results are from one representative experiment out of three performed with cells from different mice.
In vitro effect of LPS on secretion of IL-6, TNF-α, and IL-12 by splenic macrophages from naïve BALB/c mice.
In order to obtain a positive control, adherent splenic macrophages obtained from naïve BALB/c mice were first cocultured for 24 h at 37°C with LPS from E. coli serotype O26:B6, and then cytokine production was determined by flow cytometry. Under these conditions, the proportions of splenic macrophages staining positive for intracellular cytokines were 4.5% for IL-6 (Fig. 1B), 8.1% for TNF-α (Fig. 2B), and 5.7% for IL-12 (Fig. 3B). Thus, in vitro stimulation of splenic macrophages with LPS increased production of IL-6 (4-fold), TNF-α (13-fold), and IL-12 (6-fold) over that by unstimulated splenic macrophages.
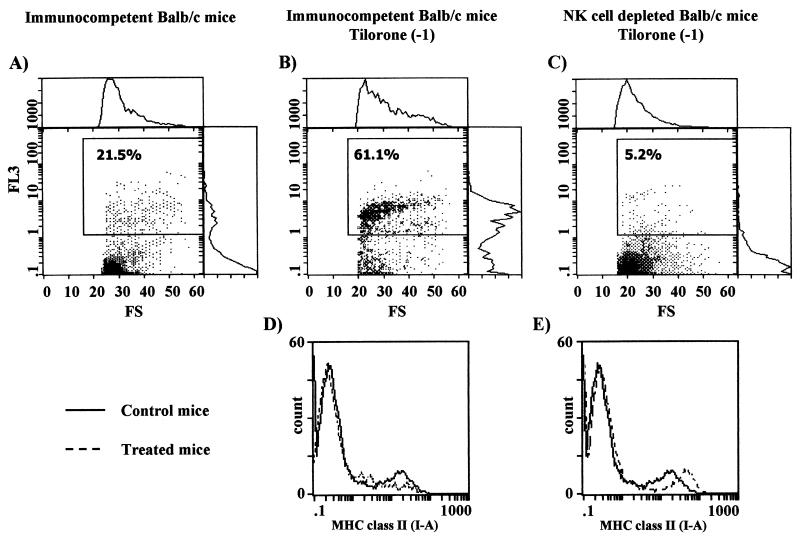
FIG. 5.
(A through C) C. albicans phagocytosis by splenic macrophages from immunocompetent BALB/c control mice (A), immunocompetent BALB/c mice treated with tilorone 24 h before the phagocytosis assay (B), or BALB/c mice previously depleted of NK cells and treated with tilorone 24 h before the phagocytosis assay (C). Percentages of phagocytic cells are given. (D and E) MHC class II expression on the surfaces of splenic macrophages from BALB/c mice. (D) Solid line, immunocompetent control mice; dashed line, immunocompetent mice treated with tilorone 24 h before the assay. (E) Solid line, immunocompetent control mice; dashed line, mice previously depleted of NK cells and treated with tilorone 24 h before the assay. Results are from one representative experiment out of three performed with cells from different mice.
In vivo increase in IL-6 secretion, but not in TNF-α or IL-12 secretion, by splenic macrophages from naïve BALB/c mice after i.v. challenge with live C. albicans.
We characterized the in vivo cytokine responses of the splenic macrophages during acute disseminated C. albicans infection in BALB/c mice. As shown in Fig. 1, i.v. challenge with live C. albicans on day −1 (Fig. 1C) or on day −3 (Fig. 1D) elicited splenic macrophage responses in immunocompetent BALB/c mice, as determined by intracellular cytokine staining of cells for IL-6 detection. The experimental systemic candidiasis induced an increase in IL-6 secretion by splenic macrophages assessed at 24 h (19.1% positive cells [Fig. 1C]) or at 72 h (19.6% positive cells [Fig. 1D]) over that observed for splenic macrophages from uninfected naïve BALB/c mice (1.1% positive cells [Fig. 1A]). Interestingly, under these conditions, no increase in TNF-α or IL-12 secretion was observed at any time point studied. Thus, 24 h after i.v. inoculation with C. albicans, 0.7% of splenic macrophages produced TNF-α (Fig. 2C), and 72 h after infection, 0.5% of cells produced the cytokine (Fig. 2D). In the same way, 24 h after C. albicans inoculation, 0.8% of splenic macrophages produced IL-12 (Fig. 3C), and 72 after infection, 0.6% of cells produced the cytokine (Fig. 3D). Thus, during acute disseminated C. albicans infection in BALB/c mice, an increase in IL-6 production by splenic macrophages was detected, whereas TNF-α and IL-12 production remained similar to those in uninfected control mice.
Tilorone increases the in vivo secretion of IL-6, TNF-α, and IL-12 by splenic macrophages from naïve BALB/c mice.
Preliminary studies with immunocompetent BALB/c mice treated with tilorone had shown enhanced resistance to experimental systemic candidiasis (22). Mice that were injected i.v. with a lethal suspension of C. albicans and treated with tilorone remained without evidence of disease, while untreated control mice died on the third day. Thus, we have further investigated intracellular cytokine (IL-6, TNF-α, and IL-12) production by splenic macrophages elicited by tilorone treatment of naïve BALB/c mice. The mice were treated orally with tilorone on day −1, and splenic macrophages adhering to the plastic culture flask were harvested 24 h post-tilorone administration. Under these conditions, the proportions of splenic macrophages staining positive for intracellular cytokines were 12.8% for IL-6 (Fig. 1E), 7.3% for TNF-α (Fig. 2E), and 3.8% for IL-12 (Fig. 3E). Thus, tilorone treatment increases the production by splenic macrophages of the three proinflammatory cytokines studied (IL-6 [11-fold], TNF-α [12-fold], and IL-12 [4-fold]) over that by cells isolated from untreated BALB/c mice.
Tilorone increases the in vivo secretion of IL-6, TNF-α, and IL-12 by splenic macrophages from BALB/c mice previously depleted of NK cells.
We had previously reported that there is a correlation between natural killer cell activation and resistance to systemic candidiasis (22). In those experiments we had used tilorone to boost NK cell activity in mice. Since it has been reported that the resistance of mice to systemic infections caused by C. albicans is associated with activated splenic macrophages, we now wanted to investigate in an in vivo experiment the effects of tilorone treatment on the secretion of IL-6, TNF-α, and IL-12 by splenic macrophages in BALB/c mice previously depleted of NK cells. Thus, we would be able to elucidate whether NK cells mediate the tilorone-related increase in cytokine production by splenic macrophages.
It had previously been demonstrated that there is a population of asialo-GM1-positive cells in tilorone-treated BALB/c mice that is responsible for in vitro NK cytotoxicity (3). In these experiments, in order to abolish NK activity, mice were treated intraperitoneally on days −6 and −3 with anti-asialo-GM1. The mice were further treated orally with tilorone on day −1. Plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration. Under these conditions, the proportions of splenic macrophages staining positive for intracellular cytokines were 20.2% for IL-6 (Fig. 1F), 7.5% for TNF-α (Fig. 2F), and 6.0% for IL-12 (Fig. 3F). Thus, tilorone treatment increased the secretion of IL-6 (18-fold), TNF-α (12-fold), and IL-12 (6-fold) by splenic macrophages from BALB/c mice previously depleted of NK cells over that by splenic macrophages from untreated BALB/c mice. These results suggest that neither NK cells nor any cytokine produced by them mediates the tilorone-related activation of splenic macrophages measured as increased secretions of IL-6, TNF-α, and IL-12.
FIG. 6.
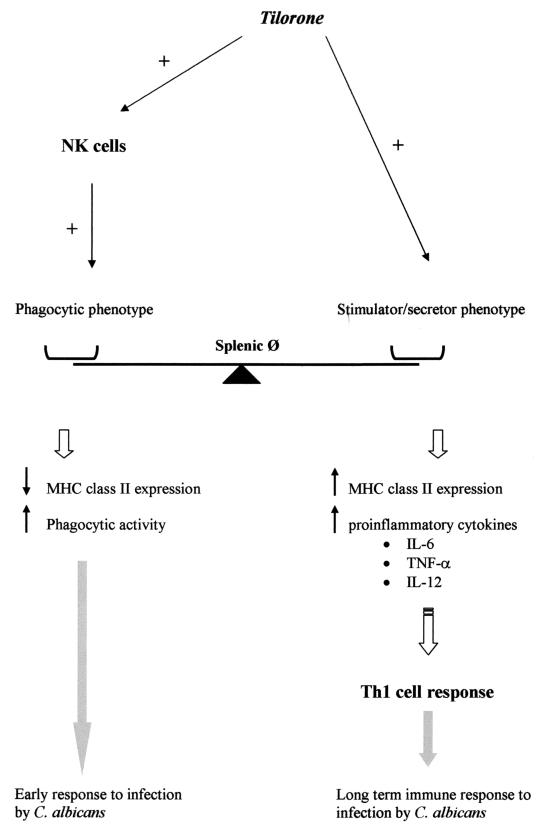
Proposed mechanism of tilorone-triggered response of splenic macrophages to C. albicans infection.
Tilorone increases the secretion of IL-6, TNF-α, and IL-12 by splenic macrophages during acute disseminated C. albicans infection in BALB/c mice.
We had previously reported that tilorone treatment prevents the death of mice injected i.v. with a lethal suspension of C. albicans (22). Thus, we performed flow cytometric analysis in order to investigate intracellular cytokine production by splenic macrophages isolated from mice with both acute disseminated candidiasis and tilorone treatment. Two sets of experiments were performed. (i) In the first set of experiments, in order to study the use of tilorone as an immunoprophylactic, mice were treated with tilorone on day −2, and i.v. challenge with live C. albicans was performed subsequently, on day −1. We observed increases in the proportions of splenic macrophages positive for IL-6 (Fig. 1G), TNF-α (Fig. 2G), and IL-12 (Fig. 3G) 24 h after C. albicans injection over those of splenic macrophages from naïve BALB/c mice, as follows: 19.6% versus 1.1% of cells were positive for IL-6, 6.2% versus 0.6% were positive for TNF-α, and 3.1% versus 0.9% were positive for IL-12. (ii) In the second set of experiments, in order to study the use of tilorone as an immunotherapeutic, acute disseminated candidiasis was induced in BALB/c mice by i.v. challenge with live C. albicans on day −2, and mice were treated with tilorone subsequently, on day −1. We observed increases in the proportions of splenic macrophages positive for IL-6 (Fig. 1H), TNF-α (Fig. 2H), and IL-12 (Fig. 3H) 24 h after tilorone treatment over those of splenic macrophages from naïve BALB/c mice, as follows: 20.1% versus 1.1% of cells were positive for IL-6, 7.9% versus 0.6% were positive for TNF-α, and 4.0% versus 0.9% were positive for IL-12. Thus, tilorone treatment induces increases in IL-6, TNF-α, and IL-12 synthesis by splenic macrophages during acute systemic candidiasis.
Tilorone increases the in vivo secretion of IL-6, TNF-α, and IL-12 by splenic macrophages from both control and NK cell-depleted athymic nude mice.
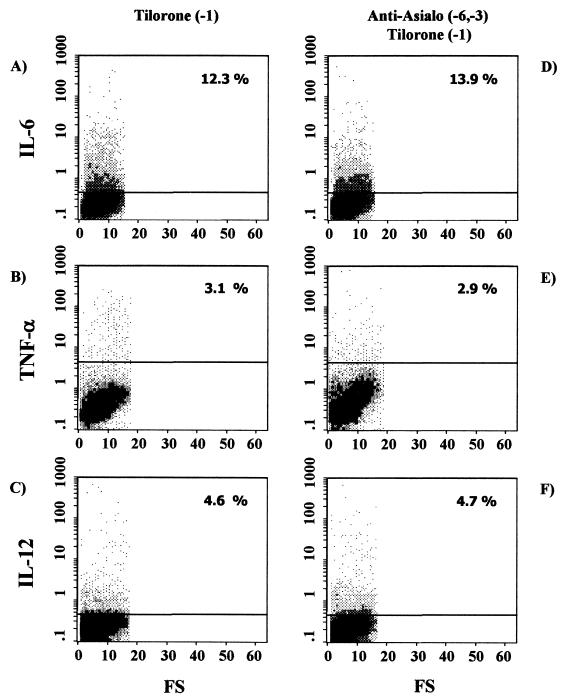
As described above, NK cells are not involved in the tilorone-related activation of splenic macrophages measured as an increase in the secretion of IL-6, TNF-α, or IL-12. We wanted to further investigate if T cells mediate the effect of tilorone on intracellular cytokine production by splenic macrophages. To this end, we first investigated intracellular cytokine (IL-6, TNF-α, and IL-12) production by splenic macrophages from tilorone-treated athymic nude mice (T-cell-deficient hosts) by means of flow cytometry. T-cell-deficient mice were treated orally with tilorone on day −1, and plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration. Under these conditions, the proportions of splenic macrophages staining positive for intracellular cytokines were 12.3% for IL-6 (Fig. 4A), 3.1% for TNF-α (Fig. 4B), and 4.6% for IL-12 (Fig. 4C). The results show increases in the production of the three proinflammatory cytokines studied over production by untreated athymic mice, which showed cytokine profiles similar to those of naïve BALB/c mice (data not shown). These results suggest that T cells are not involved in the tilorone-related activation of splenic macrophages measured as increased secretion of IL-6, TNF-α, or IL-12.
FIG. 4.
Intracellular cytokine expression in splenic macrophages from athymic nude mice under different stimuli. Expression of IL-6 (A and D), TNF-α (B and E), and IL-12 (C and F) was determined. (A through C) Cytokine production was induced by tilorone treatment 24 h before the assay. (D through F) Mice were depleted of NK cells by intraperitoneal injection of anti-asialo-GM1 on the indicated days and then treated with tilorone 24 h before the assay. Percentages reflect cytokine-positive cells. Results are from one representative experiment out of three performed with cells from different mice.
In the second set of experiments we used athymic nude mice previously depleted of NK cells in order to confirm that NK cells are not involved in this tilorone-related activation of splenic macrophages. In these experiments, in order to abolish NK activity, T-cell-deficient mice were treated intraperitoneally on days −6 and −3 with anti-asialo-GM1. The mice were further treated orally with tilorone on day −1. Plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration. Under these conditions, the proportions of splenic macrophages staining positive for intracellular cytokines were 13.9% for IL-6 (Fig. 4D), 2.9% for TNF-α (Fig. 4E), and 4.7% for IL-12 (Fig. 4F). The results obtained with T-cell- and NK-cell-deficient hosts confirm the hypothesis that neither NK cells nor T cells mediate the tilorone-related activation of splenic macrophages measured as increased secretions of the proinflammatory cytokines IL-6, TNF-α, and IL-12. Moreover, in vitro coincubation of splenic macrophages from naïve BALB/c mice with tilorone (100 μg/ml for 18 h) upregulates the production of IL-6 (6.3% positive cells), TNF-α (5.0% positive cells), and IL-12 (9.0% positive cells) by macrophages (data not shown). Thus, these data suggest that tilorone directly triggers the production of proinflammatory cytokines (IL-6, TNF-α, and IL-12) by splenic macrophages.
Tilorone increases C. albicans phagocytosis by splenic macrophages.
Macrophages do not act only as potent cytokine-secretory cells; they can also act as phagocytes. Thus, in this set of experiments, emphasis was placed on the effect of tilorone treatment on the capacity of splenic macrophages to phagocytize C. albicans. Naïve BALB/c mice were treated orally with tilorone on day −1, plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration, and then a flow cytometric phagocytosis assay was performed. The targets for these in vitro phagocytosis assays were heat-inactivated 7AAD-stained C. albicans cells. The results show that the percentage of phagocytic cells in tilorone-treated mice was significantly increased (Fig. 5B) over that in control untreated BALB/c mice (Fig. 5A) (61.1% versus 21.5%). Similar results were obtained when tilorone-treated and untreated naïve athymic nude mice were used for the experiments (2). These results suggest that tilorone treatment increases the capacity of splenic macrophages to phagocytize C. albicans but that this effect is not mediated by T cells. Nevertheless, it is not clear whether tilorone directly affects the phagocytic activity of splenic macrophages or induces its effects via accessory cell populations, i.e., NK cells.
Suppression of C. albicans phagocytosis by splenic macrophages in tilorone-treated mice previously depleted of NK cells.
In order to investigate the contribution of NK cells in relation to our previous observation, we performed the same experiments with NK cell-depleted mice. In these experiments, in order to abolish NK cell activity, naïve BALB/c mice were treated intraperitoneally on days −6 and −3 with anti-asialo-GM1. The mice were further treated orally with tilorone on day −1, plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration, and then a flow cytometric phagocytosis assay was performed. The targets for these in vitro phagocytosis assays were also heat-inactivated 7AAD-stained C. albicans cells. Surprisingly, we found that in spite of tilorone treatment, the percentage of phagocytic cells was lower in NK cell-depleted mice (Fig. 5C) than in control immunocompetent BALB/c mice (Fig. 5A) (5.2% versus 21.5%, respectively).
In another set of experiments, NK cell-depleted athymic nude mice were treated orally with tilorone on day −1, and then a flow cytometric phagocytosis assay was performed. The results also showed a decrease in the percentage of phagocytic cells in NK cell-depleted mice from that in control athymic nude mice (2).
These results demonstrate that (i) tilorone treatment increases the capacity of splenic macrophages to phagocytize C. albicans through activation of NK cells and (ii) the presence of NK cells is essential in order to maintain a basal level of phagocytic activity of splenic macrophages in untreated control mice.
Tilorone decreases MHC class II expression on the surfaces of splenic macrophages in immunocompetent BALB/c mice but increases expression in mice previously depleted of NK cells.
MHC class II molecules play a pivotal role in the induction and regulation of immune responses. Heterogeneity of macrophages is also evident in relation to surface expression of MHC class II molecules. In fact, macrophages stimulated for tumoricidal activity show decreased MHC class II gene transcription and surface expression, whereas macrophages involved in the regulation of the immune response express high levels of MHC class II molecules. Since we have already shown that tilorone can activate splenic macrophages, we next investigated whether tilorone could, in addition, modulate MHC class II expression on the surfaces of these immune cells.
First, we examined by flow cytometry the expression of MHC class II molecules (I-Ad) on the surfaces of splenic macrophages isolated from untreated naïve BALB/c mice. As shown in Fig. 5D and E, two distinct populations of macrophages were identified. One population of splenic macrophages was negative or low for MHC class II surface expression, while the other population expressed moderate or high levels of I-Ad molecules. The first population could represent resident macrophages that are quiescent immunologically, whereas the second population would be primed splenic macrophages. This hypothesis assumes a constitutive presence of primed splenic macrophages in untreated naïve mice. In fact, in these mice, 21.5% of splenic macrophages were found to be phagocytic cells (Fig. 5A), but in contrast, no proinflammatory cytokine secretion was observed (Fig. 1A, 2A, and 3A).
To determine whether tilorone can modulate MHC class II expression on the surfaces of splenic macrophages in vivo, immunocompetent BALB/c mice were treated with tilorone on day −1, and then isolated splenic macrophages were investigated by flow cytometry using a specific labeled MAb directed against I-Ad molecules. Plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration. We observed a decrease in MHC class II surface expression in the macrophage population (primed cells) which expressed moderate or high levels of I-Ad molecules in tilorone-treated immunocompetent BALB/c mice (Fig. 5D). It should be noted that under these conditions an increase in the capacity of splenic macrophages to phagocytize C. albicans was also observed (Fig. 5B).
We further investigated MHC class II expression on the surfaces of splenic macrophages isolated from NK cell-depleted BALB/c mice which had been treated with tilorone on day −1. Plastic-adherent splenic macrophages were harvested 24 h post-tilorone administration. We observed an upregulation of MHC class II surface expression in the macrophage population (primed cells) which expressed moderate or high levels of I-Ad molecules in NK cell-depleted, tilorone-treated BALB/c mice (Fig. 5E). It should be noted that under these conditions a suppression of the capacity of splenic macrophages to phagocytize C. albicans was also observed (Fig. 5C). These results suggest that splenic macrophages can regulate their functional capabilities during an immune response depending on both the stimuli and the host immune state. This implies an exquisite level of regulation of splenic macrophage function.
DISCUSSION
Macrophages are pivotal effector cells of the innate immune system, which is vital for recognizing and eliminating invasive pathogens. Previous studies have demonstrated that innate immunity, mediated by splenic macrophages, is crucial to containment and resolution of systemic infection caused by C. albicans (24, 37). Thus, we have selected splenic macrophages as the cell population of choice for study of the status of host resistance against C. albicans infection. Macrophages orchestrate innate immunity by phagocytosing pathogens and coordinating inflammatory responses through cytokine synthesis.
Early in C. albicans infection, production of some proinflammatory cytokines (IL-6, TNF-α, and IL-12) appears to be essential for the successful control of infection and the resulting protective Th1-dependent immunity (26). Previously, we had reported that i.v. challenge of naïve BALB/c mice with 106 CFU of C. albicans (ATCC 2091) led to death of the mice on the third day (22). The results of the present study show that acute systemic candidiasis induced in naïve BALB/c mice elicits an increase in the level of IL-6-producing splenic macrophages assessed at 24 or 72 h after yeast injection but does not increase levels of TNF-α- or IL-12-positive cells at any time point studied. IL-6 is a key cytokine mediator of the acute-phase response against severe infections caused by gram-negative and gram-positive bacteria (32). The precise mechanism of IL-6 action in C. albicans infections has not been elucidated yet, but it is known that IL-6-deficient mice are more susceptible to disseminated candidiasis than wild-type mice (27, 34). Moreover, severe impairment of the macrophage response to infection has been observed in IL-6-deficient mice. Taken together, these results indicate that IL-6 production by spleen macrophages is essential but not sufficient for the defense against disseminated candidiasis in mice.
Increased IL-6, TNF-α, or IL-12 production by macrophages has been reported in in vitro and in vivo experiments in response to C. albicans or to different cell wall extracts of C. albicans (7, 9, 15, 25, 30, 35, 36, 38, 40). Nevertheless, it should be remembered that stimulation of murine macrophages by C. albicans in vitro appears to be quite different from stimulation of murine cells in vivo. Moreover, the interaction between macrophages and C. albicans triggers differential secretory responses depending on the anatomical localization of the immune cells.
It has been reported that an imbalance in Th1-type and Th2-type responses may allow C. albicans to modify the host response so as to favor its own persistence (23); the general conclusion from several studies is that resistance to C. albicans infection in mice results from the development of Th1-specific cell responses (6, 26). However, we have demonstrated that i.v. lethal challenge of naïve BALB/c mice with C. albicans induces production of IL-6 by splenic macrophages but is unable to elicit production of TNF-α or IL-12, cytokines that seem to be essential for successful control of the infection and the resulting specific Th1 response.
The use of immunomodulators or biologic response modifiers as therapeutic agents in infectious diseases is a promising reality (for a review, see reference 33). These compounds have been characterized as molecules that are capable of interacting with the immune system to upregulate or downregulate specific aspects of the host response. The purpose of these therapeutic agents is to enhance the host's ability to resist microbial infection. Cytokines and LPS are well-known examples of such molecules.
Tilorone is a low-molecular-weight compound widely used to boost NK cell activity in mice, and it has been classically described as a potent in vivo inducer of IFN-γ by the oral route (1, 19, 31). Moreover, tilorone has been identified as a potent antiviral (19) and antitumor agent (3, 4) in animal models. We had previously reported that tilorone treatment induces resistance to experimental systemic candidiasis in BALB/c mice (22). Indeed, mice that were injected i.v. with a lethal suspension of C. albicans and treated with tilorone remained without evidence of disease, while untreated control mice died on the third day after challenge. As shown by the results of the present study, tilorone treatment elicits the production of IL-6, TNF-α, and IL-12 by a direct effect on splenic macrophages.
The protective effect of TNF-α with regard to systemic candidiasis is a well-documented event. TNF-α augments host resistance in systemic candidiasis and prolongs survival in murine models (5, 16). Moreover, mice deficient in TNF are highly susceptible to challenge with C. albicans (18). In the same way, previous studies have suggested that endogenous production of IL-12 is associated with protective immunity in mice during candidiasis (28, 29). In support of the important role played by this cytokine, Nau et al. (20) have recently reported that another intracellular pathogen, Mycobacterium tuberculosis, is able to inhibit macrophage IL-12 production, suggesting one means by which this organism survives host defenses. IL-12 acts by enhancing the activity of NK cells and is also the major cytokine responsible for the differentiation of Th1 cells, which are potent producers of IFN-γ. IFN-γ, in turn, has a powerful enhancing effect on the ability of phagocytes to produce IL-12, thus acting as a potent positive-feedback mechanism that leads to a strong defensive response against intracellular pathogens.
Thus, in our experimental murine model, resistance to systemic C. albicans infections is associated with increases in levels of IL-6, TNF-α, and IL-12 produced by splenic macrophages. Several studies clearly suggest that protection is associated with the development of a Th1 immune response (10, 26), and accumulating evidence indicates that TNF-α and IL-12 are crucial for the development of these protective Th1 responses. The results reported here show that tilorone is effective both as an immunoprophylactic (administered before i.v. challenge with C. albicans) and as an immunotherapeutic (administered after i.v. challenge with C. albicans) in systemic C. albicans infections, because it elicits the synthesis of the proinflammatory cytokines that promote a Th1 immune response during systemic infection by C. albicans. This could explain the in vivo effect exerted by tilorone treatment on mouse survival (22). These results also confirm the idea that enhancing the host's ability to resist yeast infection by promotion of fungus-specific Th1 responses may be a realistic objective in the development of therapeutic strategies against C. albicans (26, 23).
The phagocytic activity of splenic macrophages is also crucial to host defense against C. albicans, particularly during the early stages of infection. The results reported here clearly show that the percentage of phagocytic splenic macrophages in tilorone-treated BALB/c mice is significantly increased over that in untreated control BALB/c mice. It is known that tilorone activates NK cells and induces high levels of IFN in mice and that IFN titers are maximal 18 h after oral tilorone treatment. This suggests that spleen NK cells constitute the source of the IFN that induces the activation of splenic macrophage phagocytosis in tilorone-treated mice. Thus, the results strongly suggest that tilorone treatment activates splenic macrophage phagocytosis through NK cell activation. This hypothesis was confirmed by the results obtained with NK cell-depleted BALB/c mice. Indeed, phagocytosis was suppressed in tilorone-treated NK cell-depleted BALB/c mice relative to that in untreated control BALB/c mice. Similar results were obtained with T-cell-deficient nude mice (2). It has been reported that C. albicans causes an enhancement of splenic NK cell activity (17, 39), although NK cells do not directly kill C. albicans (41). It is also known that NK cells produce IFN-γ in response to C. albicans (30). These results, together with those reported here, suggest that NK cells mediate immune protection against C. albicans infections by enhancing the phagocytic activity of splenic macrophages. Based on our finding, we demonstrate that the presence of NK cells is essential to maintaining a basal phagocytic activity of splenic macrophages in untreated control mice. This is based on the assumption that NK cells are the source of a stimulus (perhaps IFN-γ) that primes splenic macrophages in the basal state. The primed macrophages are characterized as moderately phagocytic and as expressing moderate to high levels of MHC class II molecules on their surfaces. The result obtained by us is consistent with that of Gomez-Flores et al. (12), who demonstrated that treatment with morphine, a known NK cell activity inhibitor, significantly inhibited phagocytosis of C. albicans by splenic macrophages. Balish et al. (8) also reported that germ-free transgenic epsilon 26 mice, which present a defect in NK cells, are extremely susceptible to candidiasis. These findings have important clinical implications, as we can enhance the immune response against C. albicans infections through activation of NK cell activity. Indeed, we have recently reported that activation of NK cell activity by tilorone treatment enhances resistance to experimental systemic candidiasis in mice (22). Thus, these results help to clarify the role played by NK cells in resistance to candidiasis, and they open new perspectives for the treatment of C. albicans infections.
In order to understand the pathway of splenic macrophage activation by tilorone treatment, we examined the expression of MHC class II molecules (I-A) on the surfaces of these immune cells by flow cytometry. We investigated the expression of MHC class II molecules in adherent splenic macrophages from immunocompetent BALB/c mice, and we have identified two cell populations: (i) a cell population predominantly negative for, or low in, MHC class II molecules (quiescent or resting cells) and (ii) a cell population which expressed moderate or high levels of I-Ad molecules (primed cells).
Tilorone treatment of immunocompetent BALB/c mice resulted in downregulation of MHC class II expression in the cell population (primed cells) that expressed moderate to high levels of I-Ad molecules in untreated control mice. This is closely associated with a significant increase in C. albicans phagocytosis by splenic macrophages but not with an increase in cytokine secretion. These results demonstrate that after tilorone treatment of immunocompetent BALB/c mice, it is possible to identify a phenotypically and functionally peculiar cell population. This population could represent the “phagocytic phenotype,” characterized by expression of low levels of MHC class II molecules, high phagocytic activity, and lack of secretion of proinflammatory cytokines (Fig. 6).
On the other hand, tilorone treatment of NK cell-depleted BALB/c mice resulted in upregulation of MHC class II expression in the cell population (primed cells) that expressed moderate to high levels of I-Ad molecules in untreated control mice. This is closely associated with both a suppression of C. albicans phagocytosis by splenic macrophages and a high level of proinflammatory cytokine synthesis. Thus, under these conditions we identify another phenotypically and functionally peculiar cell population. This population could represent the “stimulator/secretor phenotype,” characterized by high levels of MHC class II surface expression, low phagocytic activity, and synthesis of the proinflammatory cytokines IL-6, TNF-α, and IL-12 (Fig. 6). These results strongly suggest that NK cells prime splenic macrophages to be moderately phagocytic in naïve BALB/c mice, probably mediated by IFN-γ production.
This functional transition demonstrates an exquisite level of regulation and allows splenic macrophages to regulate their functional capabilities during an immune response depending on the stimuli and the host immune state. These results are in agreement with those of Hamerman and Aderem (13), who have found two distinct phenotypes in peritoneal macrophages during in vivo infection with Mycobacterium bovis.
In conclusion, we have established a mechanism of action by splenic macrophages, triggered by tilorone treatment, which could explain their effect on mouse survival during acute disseminated C. albicans infection (see Fig. 6). We propose that tilorone could serve as a model for development of new biologic response modifiers that could be of particular interest for therapies inducing a specific immune resistance to C. albicans infections as well as for vaccine adjuvants.
Acknowledgments
This work was supported by the Plan Andaluz de Investigación (CTS 442). M. J. Serrano was supported by a fellowship from the University of Jaén.
REFERENCES
- 1.Algarra, I., A. Gonzalez, M. Pérez, J. J. Gaforio, and F. Garrido. 1996. Effect of in vivo activation of natural killer (NK) cells by a tilorone analogue on the survival of mice injected intravenously with different experimental murine tumours. Clin. Exp. Immunol. 103:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algarra, I., E. Ortega, M. J. Serrano, G. Alvarez de Cienfuegos, and J. J. Gaforio. 2002. Suppression of splenic macrophage Candida albicans-phagocytosis following in vivo depletion of natural killer cells in immunocompetent BALB/c mice and T-cell-deficient nude mice. FEMS Immunol. Med. Microbiol. 33:159-163. [DOI] [PubMed] [Google Scholar]
- 3.Algarra, I., M. Pérez, J. J. Gaforio, F. Gasca, and F. Garrido. 1994. In vivo activation of NK cells induces inhibition of lung colonization of H-2 positive and H-2 negative fibrosarcoma tumor clones. Clin. Exp. Metastasis 12:31-36. [DOI] [PubMed] [Google Scholar]
- 4.Algarra, I., M. Pérez, P. Hoglund, J. J. Gaforio, H. G. Ljunggren, and F. Garrido. 1993. Generation and control of metastasis in experimental tumor systems: inhibition of experimental metastases by a tilorone analogue. Int. J. Cancer 54:518-523. [DOI] [PubMed] [Google Scholar]
- 5.Allendoerfer, R., D. M. Magge, J. G. Smith, L. Bonewald, and J. R. Graybill. 1993. Induction of tumor necrosis factor-α in murine Candida albicans infection. J. Infect. Dis. 167:1168-1172. [DOI] [PubMed] [Google Scholar]
- 6.Ashman, R. B., and J. M. Papadimitriou. 1995. Production and function of cytokines in natural and acquired immunity to Candida albicans infection. Microbiol. Rev. 59:646-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aybay, C., and T. Imir. 1996. Tumor necrosis factor (TNF) induction from monocytes/macrophages by Candida species. Immunobiology 196:363-374. [DOI] [PubMed] [Google Scholar]
- 8.Balish, E., T. Warner, C. J. Pierson, D. M. Block, and R. D. Wagner. 2001. Oroesophageal candidiasis is lethal for transgenic mice with combined natural killer and T-cell defects. Med. Mycol. 39:261-268. [DOI] [PubMed] [Google Scholar]
- 9.Brieland, J., D. Essig, G. Jackson, D. Frank, D. Loebenberg, F. Menzel, B. Arnold, B. DiDomenico, and R. Hare. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 69:5046-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenci, E., A. Mencacci, R. Spaccapelo, L. Tonetti, P. Mosci, K. H. Enssle, P. Puccetti, L. Romani, and F. Bistoni. 1995. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis, but protective immunity is associated with Th1 development. J. Infect. Dis. 171:1279-1288. [DOI] [PubMed] [Google Scholar]
- 11.Cenci, E., L. Romani, A. Vecchiarelli, P. Puccetti, and F. Bistoni. 1989. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect. Immun. 57:3581-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Flores, R., J. L. Suo, and R. J. Weber. 1999. Suppression of splenic macrophage functions following acute morphine action in the rat mesencephalon periaqueductal gray. Brain Behav. Immun. 13:212-224. [DOI] [PubMed] [Google Scholar]
- 13.Hamerman, J. A., and A. Aderem. 2001. Functional transitions in macrophages during in vivo infection with Mycobacterium bovis bacillus Calmette-Guérin. J. Immunol. 167:2227-2233. [DOI] [PubMed] [Google Scholar]
- 14.Han, Y., S. Kelm, M. H. Riesselman, P. R. Crocker, and J. E. Culter. 1994. Mouse sialoadhesin is not responsible for Candida albicans yeast cell binding to splenic marginal zone macrophages. Infect. Immun. 62:2115-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouault, T., C. Fradin, P. A. Trinel, and D. Poulain. 2000. Candida albicans-derived β-1,2-linked mannooligosaccharides induce desensitization of macrophages. Infect. Immun. 68:965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie, A., A. L. Baltch, R. P. Smith, M. A. Franke, W. J. Ritz, J. K. Singh, and M. A. Gordon. 1994. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect. Immun. 62:2761-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marconi, P., L. Scaringi, L. Tissi, M. Boccanera, F. Bistoni, E. Bonmassar, and A. Casone. 1985. Induction of natural killer cell activity by inactivated Candida albicans in mice. Infect. Immun. 50:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer, G. D., and R. F. Krueger. 1980. Tilorone hydrochloride and related molecules, p. 187-221. In D. A. Stringfellow (ed.), Interferon and interferon inducers: clinical applications. Marcel Dekker, New York, N.Y.
- 20.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega, E., I. Algarra, M. J. Serrano, G. Alvarez de Cienfuegos, and J. J. Gaforio. 2001. The use of 7-amino-actinomycin D in the analysis of Candida albicans phagocytosis and opsonization. J. Immunol. Methods 253:189-193. [DOI] [PubMed] [Google Scholar]
- 22.Ortega, E., I. Algarra, M. J. Serrano, M. A. de Pablo, G. Alvarez de Cienfuegos, and J. J. Gaforio. 2000. Enhanced resistance to experimental systemic candidiasis in tilorone-treated mice. FEMS Immunol. Med. Microbiol. 28:283-289. [DOI] [PubMed] [Google Scholar]
- 23.Puccetti, P., L. Romani, and F. Bistoni. 1995. A Th1-Th2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol. 3:237-240. [DOI] [PubMed] [Google Scholar]
- 24.Qian, Q., M. A. Jutila, N. van Rooijen, and J. E. Cutler. 1994. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 152:5000-5008. [PubMed] [Google Scholar]
- 25.Riipi, L., and E. Carlson. 1990. Tumor necrosis factor (TNF) is induced in mice by Candida albicans: role of TNF in fibrinogen increase. Infect. Immun. 58:2750-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 27.Romani, L., A. Mencacci, E. Cenci, R. Spaccapelo, C. Toniatti, P. Puccetti, F. Bistoni, and V. Poli. 1996. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romani, L., A. Mencacci, L. Tonetti, R. Spaccapelo, E. Cenci, P. Puccetti, S. F. Wolf, and F. Bistoni. 1994. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J. Immunol. 153:5167-5175. [PubMed] [Google Scholar]
- 29.Romani, L., A. Mencacci, L. Tonetti, R. Spaccapelo, E. Cenci, S. F. Wolf, P. Puccetti, and F. Bistoni. 1994. Interleukin-12 but not IFN-γ production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur. J. Immunol. 24:909-915. [DOI] [PubMed] [Google Scholar]
- 30.Rosati, E., L. Scaringi, P. Cornacchione, K. Fettucciari, R. Sabatini, R. Rossi, and P. Marconi. 1995. Cytokine response to inactivated Candida albicans in mice. Cell. Immunol. 162:256-264. [DOI] [PubMed] [Google Scholar]
- 31.Salcedo, M., M. Andersson, S. Lemieux, L. van Kaer, B. J. Chambers, and H. G. Ljunggren. 1998. Fine tuning of natural killer cell specificity and maintenance of self tolerance in MHC class I-deficient mice. Eur. J. Immunol. 28:1315-1321. [DOI] [PubMed] [Google Scholar]
- 32.Tilg, H., C. A. Dinarello, and J. W. Mier. 1997. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol. Today 18:428-432. [DOI] [PubMed] [Google Scholar]
- 33.Tzianabos, A. O. 2000. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin. Microbiol. Rev. 13:523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Enckevort, F. H., M. G. Netea, A. R. Hermus, C. G. Sweep, J. F. Meis, J. W. Van der Meer, and B. J. Kullberg. 1999. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice. Med. Mycol. 37:419-426. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez, N., H. R. Buckley, and T. J. Rogers. 1996. Production of IL-6 and TNF-α by the macrophage-like cell line RAW 264.7 after treatment with a cell wall extract of Candida albicans. J. Interf. Cytok. Res. 16:465-470. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez, N., H. R. Buckley, D. M. Mosser, and T. J. Rogers. 1995. Activation of murine resident peritoneal macrophages by a cell wall extract of Candida albicans. J. Med. Vet. Mycol. 33:385-393. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecchiarelli, A., M. Puliti, A. Torasantucci, A. Cassone, and F. Bistoni. 1991. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell. Immunol. 134:65-76. [DOI] [PubMed] [Google Scholar]
- 39.Wojdani, A., and M. Ghoneum. 1987. In vivo augmentation of natural killer cell activity by Candida albicans. Int. J. Immunopharmacol. 9:827-832. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macro-phage colony-stimulating factor responses, but not in chemokine macro-phage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zunino, S. J., and D. Huding. 1988. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect. Immun. 56:564-569. [DOI] [PMC free article] [PubMed] [Google Scholar]