Abstract
A baculovirus carrying the SAG2 gene of Toxoplasma gondii was constructed, and recombinant SAG2 protein (S-rSAG2) was expressed in insect cells. S-rSAG2 was recognized by sera from cats and pigs infected with T. gondii. Mice immunized with S-rSAG2 produced high titers of specific immunoglobulin G2a (IgG2a) and IgG1 antibodies. In an indirect fluorescent antibody test, all mouse antisera against S-rSAG2 reacted strongly to the natural parasites, but those against rSAG2 expressed in Escherichia coli (E-rSAG2) only showed very weak reaction, although no markedly difference was found in the reaction to denatured antigen, T. gondii lysate, in Western blot analysis. The results suggest that S-rSAG2 is better than E-rSAG2 in both antigenicity and immunogenicity. Enzyme-linked immunosorbent assay (ELISA) with S-rSAG2 could differentiate clearly between sera from 30 specific-pathogen-free cats and 4 experimentally infected cats. Serum samples from domestic cats in Japan were tested by the ELISA and compared with a latex agglutination test (LAT) and ELISA with E-rSAG2. Of 187 samples, all 35 LAT-positive sera had strong reactions to S-rSAG2 and E-rSAG2. Of the 152 LAT-negative sera, 18 were positive in the ELISA with S-rSAG2, whereas only 2 were positive in the ELISA with E-rSAG2. Although there were significant correlations among the three methods, the ELISA with S-rSAG2 was more sensitive than the others, which could be attributed to the fact that S-rSAG2 shares some common conformational structure with the native antigen. The results suggest that S-rSAG2 would be a useful reagent for the detection of T. gondii infection in cats.
Toxoplasmosis is a worldwide zoonosis caused by Toxoplasma gondii. As many as one-third of the world's population shows serological evidence of infection (10). Although it is generally asymptomatic in healthy adults, it may cause abortion, neonatal death, severe sequelae in neonates, and life-threatening lesions in AIDS patients. Furthermore, it may complicate immunosuppressive therapy. Of all the encephalitis occurring in human immunodeficiency virus-positive patients, ca. 50% is due to T. gondii infection (4). In addition to being as major source of infection for humans, it is also of considerable importance in domestic animals and is responsible for abortion in sheep and swine (25). Therefore, there is an urgent need to develop an effective diagnostic kit and vaccine.
Surface antigen 2 (SAG2 and P22) of T. gondii is a major surface protein known as an attachment ligand (8) that also has good antigenicity and immunogenicity (1, 14, 21, 23). The recombinant SAG2 expressed in Escherichia coli (E-rSAG2) was effective in detecting the immunoglobulin G (IgG) antibody to T. gondii in human patients with acute toxoplasmosis (21, 23). In our previous study, E-rSAG2 was used as an antigen for ELISA to detect T. gondii infection in domestic cats and was shown to be a good reagent for the diagnosis of toxoplasmosis (9). Vaccination with E-rSAG2 provided partial protection against a lethal infection of T. gondii and inhibited cyst formation in the brains of infected mice (16). However, the protein expressed in bacterial cells may be in incorrect folding, which might influence its antigenicity and immunogenicity to a certain extent, as it was observed in the SAG1 of T. gondii (3, 15).
To express SAG2 in a conformation that is closer to that of the native molecule, the baculovirus-insect cell expression system was used in the present study. The baculovirus expression system is a popular method of expressing foreign genes, mainly for other viruses. Recently, it has been used to express foreign genes from protozoan parasites, and animals immunized with recombinant antigens expressed in insect cells developed protective immunity against virulent parasite infections.
We report here the construction of the recombinant baculovirus carrying the SAG2 gene and the expression of the gene as a recombinant protein (S-rSAG2) in insect cells. We then offer an evaluation of its diagnostic potential.
MATERIALS AND METHODS
Parasite.
Tachyzoites of the T. gondii RH strain (24) were cultured in Vero cell monolayers in a minimum essential medium (Sigma, St. Louis, Mo.) supplemented with 8% fetal bovine serum and kanamycin (100 μg/ml) at 37°C in a 5% CO2 environment.
Cell and virus.
The Autographa californica nuclear polyhedrosis virus (AcNPV) and its recombinant virus were grown in Spodoptera frugiperda (Sf9) cells in a TC-100 insect medium (Gibco-BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum and 0.26% Bacto tryptose broth (Difco, Detroit, Mich.).
Cloning of the SAG2 gene.
The template DNA for PCR was extracted from tachyzoites of the T. gondii RH strain as described previously (12). Two oligonucleotide primers, 5′-ACGAATTCAACTATGAGTTTCT-3′ and 5′-ACGAATTTCCTTTTACACAAAGG-3′, were used to amplify the SAG2 gene by PCR (23). The PCR product was digested with EcoRI and then inserted into an EcoRI site of the pUC19. The resulting plasmid was designated as pUC/SAG2. The SAG2 gene was recovered from pUC/SAG2 after digestion with EcoRI and ligated into the EcoRI site of the baculovirus transfer vector pBlueBac4.5/his-v5 (Invitrogen, Carlsbad, Calif.). The resulting plasmid was designated pBB/SAG2.
Construction of recombinant baculovirus.
Sf9 cells were cotransfected with the recombinant transfer vector, pBB/SAG2, and linear AcNPV viral DNA (Bac-N-Blue DNA; Invitrogen, Carlsbad, Calif.) by using the Cellfectin reagent (Invitrogen). After 4 days of incubation at 27°C, the culture supernatant containing the recombinant virus was harvested and subjected to plaque purification. After 3 cycles of purification, a recombinant baculovirus (AcSAG2) was obtained. A recombinant baculovirus carrying green fluorescent protein (GFP) gene (AcGFP) was obtained by the same method and used as a control virus.
IFAT.
Indirect fluorescent antibody test (IFAT) was performed as described previously (27). For the location of the expressed rSAG2 protein, the infected cells with or without an acetone fixation were used to determine whether the recombinant protein was transported to the cell surface or not. Sf9 cells infected with AcLacZ were used as a negative control (27).
Mouse antisera against S-rSAG2.
Female ddY mice (Clea Japan, Inc., Tokyo, Japan) were inoculated intraperitoneally once with S-rSAG2 or S-rGFP (recombinant GFP expressed in Sf9 cells by AcGFP) antigen mixed with an equal volume of complete Freund adjuvant. The antigens were prepared from AcSAG2 or AcGFP infected Sf9 cells by sonication after three cycles of freezing and thawing at a dose of 6 × 106 cells in 200 μl of sterile phosphate-buffered saline (PBS) per mouse. Two booster immunizations were performed at an interval of 14 days with the same antigens mixed with Freund incomplete adjuvant. The mice were bled before each booster immunization and 10 days after the second booster.
Cat serum samples.
Thirty normal serum samples were collected from 30 different specific-pathogen-free (SPF) cats (CSK, Shizuoka, Japan). Four cats were bled for serum before and after the experimental infection with T. gondii. Each of these experimental cats was infected orally with 10,000 bradyzoites of the T. gondii Beverley strain. A total of 187 field cat sera were collected from domestic cats, which had been brought to veterinary hospitals near Tokyo and Sapporo for treatment, as described previously (6, 7).
Antigens for ELISA.
A monolayer of Sf9 cells was grown in T75 flasks and infected with AcSAG2 or AcGFP. After 4 days of incubation, the cells were harvested and centrifuged at 1,700 × g for 10 min to remove the medium. They were resuspended in PBS (1 ml/flask) after being washed twice with PBS. After three cycles of freezing and thawing, 0.25% Triton X-100 was added. Subsequently, the cells were sonicated for 2 min and incubated at room temperature for 1 h. The supernatant containing the antigens was recovered after centrifugation at 12,000 × g for 30 min at 4°C.
ELISA.
ELISA was performed in 96-well microplates (Nunc, Roskilde, Denmark) as described previously (12). For the detection of cat serum samples, the dilutions of the optimal antigen (S-rSAG2 or S-rGFP), serum, and horseradish peroxidase-conjugated sheep anti-cat IgG (Bethyl, Montgomery, Tex.) were preliminarily determined as 1:500, 1:100, and 1:4,000, respectively, by checkerboard titration with the known positive and negative sera. For the detection of mouse antisera against S-rSAG2 or S-rGFP, the final concentration of antigen (E-rSAG2) was 2.5 μg/ml, and the dilutions of serum and horseradish peroxidase-conjugated goat anti-mouse IgG2a or IgG1 (Bethyl) were preliminarily determined as 1:100 and 1:2,000, respectively. The optical density (OD) at 415 nm was read by using an MTP-120 ELISA reader (Corona Electric, Tokyo, Japan). The ELISA result was determined for each sample by taking the mean OD value of two readings with S-rSAG2 minus the mean value of two readings with S-rGFP protein. A sample was considered positive if the calculated absorbance value was >0.1.
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 15% polyacrylamide gel as described by Laemmli in 1970 (13). Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Bedford, Mass.), and then the membranes were treated as described previously (2).
LAT.
The latex agglutination test (LAT) was performed according to the manufacturer's instructions (Toxocheck-MT; Eiken Chemical, Tokyo, Japan). It was considered positive when agglutination was observed at dilutions of 1:64 and greater.
Phospholipase C treatment.
The treatment was carried out as described previously (20). Sf9 cells (2 × 106) infected with AcSAG2 were harvested and washed three times with Sf-900 II medium (Gibco). The cells were treated with or without 1 U of phosphatidylinositol-specific phospholipase C (PI-PLC; Sigma) at 27°C for 2 h with gentle rotation. The reaction volume was 50 μl in Sf-900 II medium. After centrifuging at 1,700 × g for 5 min, the cells and supernatants were harvested separately and subjected to Western blot analysis.
Statistical method.
Correlation coefficient was calculated by the paste function of Microsoft Excel according to the following equation:
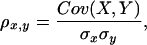 |
where 1 ≥ ρx,y ≥ −1 and
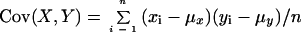 |
RESULTS
Expression of rSAG2 in Sf9 cells by recombinant baculovirus.
A recombinant baculovirus, AcSAG2, expressing the SAG2 of T. gondii was constructed and infected to Sf9 cells. After incubation for 4 days at 27°C, the cells were harvested and subjected to IFAT to determine whether the S-rSAG2 expressed by the recombinant virus was transported to the cell surface. Specific fluorescence was observed in whole fixed cells and on the surface of unfixed Sf9 cells that had been infected with the AcSAG2 virus; however, no specific fluorescence was found in the cells infected with the negative control virus, AcLacZ. The results indicated that the S-rSAG2 expressed in Sf9 cells by AcSAG2 was transported onto the cell surface.
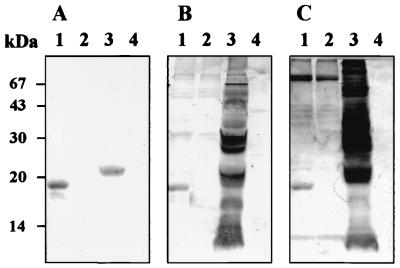
Sf9 cells infected with AcSAG2 were treated with or without PI-PLC, and then the cells and the medium were harvested separately and analyzed by Western blot analysis with antiserum samples from mice immunized with E-rSAG2. The result showed that the resulting protein was still retained in both cells treated with or without PI-PLC, which suggest the S-rSAG2 could not be cleaved from the cell surface by PI-PLC (Data not shown). The molecular mass of S-rSAG2 was identical to the theoretical one, 19 kDa (23), and smaller than the native one (Fig. 1).
FIG. 1.
Western blot analysis of S-rSAG2. Antigens: lane 1, AcSAG2-infected cell extract; lane 2, AcGFP-infected cell extract; lane 3, T. gondii lysate; lane 4, Vero cell lysate. Sera: A, from mouse immunized with E-rSAG2; B and C, from a cat and a pig, respectively, infected with T. gondii.
Antigenicity and immunogenicity of S-rSAG2.
The S-rSAG2 was recognized by sera from cats and pigs infected with T. gondii in Western blot analysis (Fig. 1). However, the control antigen S-rGFP did not react to any sera against T. gondii (Fig. 1).
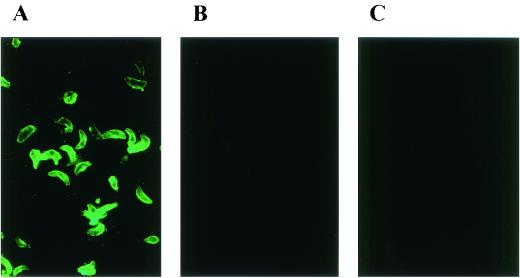
The immunogenicity of S-rSAG2 was compared with the E-rSAG2 by IFAT. The antisera against S-rSAG2 reacted strongly to T. gondii natural parasites, but the antisera to E-rSAG2 only showed very weak reaction (Fig. 2), although no significant difference was found in their reaction to the denatured antigen, the T. gondii lysate, by Western blot analysis (data not shown).
FIG. 2.
Comparison of the reaction to T. gondii parasites between mouse antiserum against S-rSAG2 (A) and mouse antiserum against E-rSAG2 (B). (C) Mouse antiserum against S-rGFP was used as negative control.
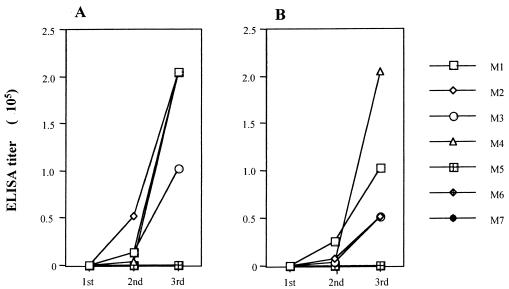
Specific IgG2a and IgG1 antibody responses in mice immunized with S-rSAG2 were detected by ELISA with E-rSAG2 antigen. As shown in Fig. 3, both antibodies were detectable before the third immunization, and their titers reached ca. 102,400 to 204,800 and ca. 51,200 to 204,800, respectively, 10 days after the third immunization. However, sequential sera from mice immunized with S-rGFP did not show any reaction to E-rSAG2.
FIG. 3.
Specific IgG2a and IgG1 antibody responses in S-rSAG2-immunized mice (M1 to M4) detected by ELISA with E-rSAG2. Sera from S-rGFP-immunized mice (M5 to M7) were used as negative controls. (A) IgG2a antibody; (B) IgG1 antibody. 1st, 2nd, and 3rd: before first booster immunization, before second booster immunization, and 10 days after the second booster immunization, respectively.
Diagnosis of T. gondii infection in cats by ELISA with S-rSAG2 as an antigen.
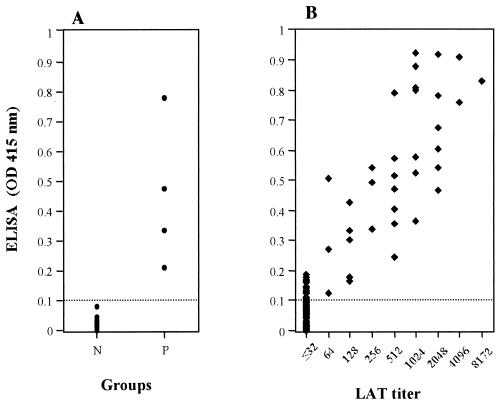
To evaluate the potential of S-rSAG2 for the diagnosis of T. gondii infection, serum samples collected from 30 SPF cats and 4 cats before and after experimental infection with T. gondii were tested by ELISA with S-rSAG2. The ELISA with S-rSAG2 as an antigen could very clearly differentiate between sera from infected and uninfected cats (Fig. 4). A total of 187 serum samples from domestic cats in Japan were investigated by ELISA, and the results were compared to those from the LAT. As shown in Table 1 and Fig. 4, of the 187 samples all 35 LAT-positive sera had strong reactions to S-rSAG2. Of the 152 LAT-negative sera, 18 were positive in the ELISA with S-rSAG2. There were significant correlations between the titers of the LAT and the ELISA with S-rSAG2 (ρ = 0.57, n = 53, P < 0.001). However, the ELISA titers were much higher than those of the LAT.
FIG. 4.
Results of the ELISA with S-rSAG2. (A) Values from the known negative (N) and positive (P) cat sera; (B) comparison between the ELISA and LAT in the serodiagnosis of T. gondii infection in domestic cats. The cutoff point in ELISA is shown by a dotted line.
TABLE 1.
Seroprevalence of T. gondii infection in domestic cats as determined by the LAT and ELISA with S-rSAG2
| LAT result | No. of samples (%)
|
||
|---|---|---|---|
| Tested by ELISA
|
Total | ||
| Positivea | Negative | ||
| Positiveb | 35 (18.72) | 0 (0) | 35 (18.72) |
| Negative | 18 (9.63) | 134 (71.66) | 152 (81.28) |
| Total | 53 (28.35) | 134 (71.66) | 187 (100) |
ELISA was considered positive when an OD at 415 nm of ≥0.1 was observed at dilutions of 1:100 and greater.
LAT was considered positive when agglutination was observed at dilutions of 1:64 and greater.
The E-rSAG2 antigen was used as an antigen in an ELISA to test the same cat serum samples in our previous study (9). Comparison of the results between the two ELISAs showed that there was a significant correlation either between the OD values (ρ = 0.92, n = 183, P < 0.001) or between the titers (ρ = 0.71, n = 53, P < 0.001). It seems that the ELISA with S-rSAG2 was more sensitive than that with E-rSAG2 (Table 2).
TABLE 2.
Comparison between the two ELISAs with different recombinant antigens in the serodiagnosis of T. gondii infection
| ELISA (E) result | No. of samples (%)
|
||
|---|---|---|---|
| Tested by ELISA (S)a
|
Total | ||
| Positive | Negative | ||
| Positiveb | 35 (19.13) | 1 (0.55) | 36 (19.67) |
| Negative | 17 (9.27) | 130 (71.04) | 147 (80.33) |
| Total | 52 (28.42) | 131 (71.58) | 183 (100) |
ELISA (S), ELISA with S-rSAG2 as antigen.
ELISA (E), ELISA with E-rSAG2 as antigen.
DISCUSSION
In this study, a recombinant baculovirus carrying the gene encoding SAG2 of T. gondii was constructed, and the recombinant SAG2 was expressed in Sf9 cells. The molecular mass of the S-rSAG2 protein is 19 kDa, which is identical to the size of its theoretical gene product but smaller than the native one of 22 kDa. The discrepancy in molecular mass might be attributed to the difference in posttranslational modification, such as glycosylphosphatidylinositol (GPI) linkage or glycosylation at other sites. A previous study suggested the addition of a glycolipid anchor in the mature native SAG2 (23). However, analysis by PI-PLC treatment showed that S-rSAG2 lacks GPI linkage. It is possible that the Sf9 cells do not recognize the GPI attachment site and degraded the full-length molecule (11). It has been shown that the GPI linkage attachment site in a protozoan is not efficiently used in mammalian cells (17) and that the site in the SAG1 gene of T. gondii is not efficiently used in insect cells (11). These previous researches and our present results may support the speculation that native SAG2 has a GPI anchor, but S-rSAG2 does not. As judged by IFAT, S-rSAG2 protein was transported onto the Sf9 cell surface, which might indicate that S-rSAG2 was expressed as a transmembrane protein. However, further work has to be done on subcellular localization and membrane attachment of the recombinant protein. Proper expression and folding of the molecule are independent of the addition of GPI linkage (18). Therefore, whether the recombinant protein lacks a GPI anchor or not, its antigenicity and immunogenicity might not be affected. Western blot analysis showed that S-rSAG2 was recognized by sera from mice, cats, and pigs infected with T. gondii, which indicated that it has good antigenicity.
It has been reported that an antibody produced during infection is biased toward a set of conformational epitopes present on the native protein and that the protein expressed in insect cells shares some conformational epitopes with the native one (11, 26). In the present study, antisera against S-rSAG2 reacted much more strongly to the natural antigen than the antisera against E-rSAG2; however, no significant difference was found between the reactivity of antisera against S-rSAG2 and antisera against E-rSAG2 to denatured antigen, which demonstrated that the conformational structure of S-rSAG2 might be one of the factors to improve antibody response to the natural antigen.
To evaluate the diagnostic potential of S-rSAG2, known positive and negative cat sera were tested by ELISA with S-rSAG2 as an antigen. The result showed that it could differentiate very clearly between sera from infected and uninfected cats, which demonstrated its high specificity. Furthermore, the results of detecting field domestic cat sera showed that there were significant correlations between the ELISA and the commercial LAT kit and between two ELISAs with different recombinant SAG2 antigens, S-rSAG2 or E-rSAG2. However, the ELISA with S-rSAG2 appeared to be more sensitive than the ELISA with E-rSAG2, which might be attributed to that S-rSAG2 shares some conformational structure with the natural antigen. The result suggested that S-rSAG2 would be a good reagent for the diagnosis of T. gondii infection in cats.
Natural infections with tachyzoites or tissue cysts induced a Th1-driven response with a high IgG2a production and immunization with rSAG1 expressed in E. coli mounted a Th2 response with a high IgG1 production (19, 22). In the present study, analysis on subclass of specific IgG antibodies showed that S-rSAG2 induced high levels of IgG2a and IgG1 antibodies in mice before the third immunization, which further rose to higher levels 10 days after the third immunization. IgG1 and IgG2a are good indicators of stimulated humoral and cellular immunity, respectively (5). Therefore, our results indicate that S-rSAG2 might stimulate both humoral and cellular immunity in immunized mice. In the present study, however, we have not examined cytokine responses to determine which Th1 and/or Th2 are important for the protection in immunized mice. Further study on cytokine responses such as gamma interferon and interleukin-2, -4, and -12 is required to examine the protective mechanism in mice immunized with S-rSAG2 against T. gondii infection.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Aubert, D., G. T. Maine, I. Villena, J. C. Hunt, L. Howard, M. Sheu, S. Brojanac, L. E. Chovan, S. F. Nowlan, and J. M. Pinon. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 38:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boldbaataar, D., X. Xuan, E. N. Kimbita, X. Huang, I. Igarashi, B. Byambaa, B. Battsetseg, B. Battur, G. Battsetseg, Z. Batsukh, H. Nagasawa, K. Fujisaki, and T. Mikami. 2001. Detection of antibodies to Hypoderma lineatum in cattle by Western blotting with recombinant hypodermin C antigen. Vet. Parasitol. 99:147-154. [DOI] [PubMed] [Google Scholar]
- 3.Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware, J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141:3584-3591. [PubMed] [Google Scholar]
- 4.Chen, X., X. Ma, K. Wu, H. Peng, S. Shen, G. Liu, and S. Chen. 1998. Expression of P30, The major surface antigen of Toxoplasma gondii in baculovirus-insect system and the evaluation of immune response induced by P30. J. Protozool. Res. 8:19-27. [Google Scholar]
- 5.Chuang, Y. H., B. L. Chiang, C. C. Chou, and K. H. Hsieh. 1997. Immune effector cells induced by complete Freund's adjuvant exert an inhibitory effect on antigen-specific type 2 T helper responses. Clin. Exp. Allergy 27:315-324. [PubMed] [Google Scholar]
- 6.Furuya, T., A. Hasegawa, T. Miyazawa, K. Miki, and T. Mikami. 1992. Detection of anti-gag antibodies of feline immunodeficiency virus in cat sera by enzyme-linked immunosorbent assay. Arch. Virol. 124:355-361. [DOI] [PubMed] [Google Scholar]
- 7.Furuya, T., Y. Kawaguchi, T. Miyazawa, Y. Fujikawa, Y. Tohya, M. Azetaka, E. Takahashi, and T. Mikami. 1990. Existence of feline immunodeficiency virus infection in Japanese cat population since 1968. Jpn. J. Vet. Sci. 52:891-893. [DOI] [PubMed] [Google Scholar]
- 8.Grimwood, J., and J. E. Smith. 1996. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int. J. Parasitol. 26:169-173. [DOI] [PubMed] [Google Scholar]
- 9.Huang, X., X. Xuan, E. N. Kimbita, T. Miyazawa, S. Fukumoto, M. Mishima, L. H. Makala, H. Suzuki, C. Sugimoto, H. Nagasawa, K. Fujisaki, T. Mikami, and I. Igarashi. 2002. Development and evaluation of an enzyme-linked immunosorbent assay with recombinant SAG2 for the diagnosis of Toxoplasma gondii infection in cats. J. Parasitol. 88:804-807. [DOI] [PubMed] [Google Scholar]
- 10.Hughes, H. P. A. 1985. Toxoplasmosis: the need for improved diagnostic techniques and accurate risk assessment. Curr. Top. Microbiol. Immunol. 120:105-111. [DOI] [PubMed] [Google Scholar]
- 11.Hunter, S., L. Ashbaugh, P. Hair, C. M. Bozic, and M. Milhausen. 1999. Baculovirus-directed expression and secretion of truncated version of Toxoplasma SAG1. Mol. Biochem. Parasitol. 103:267-272. [DOI] [PubMed] [Google Scholar]
- 12.Kimbita, E. N., X. Xuan, X. Huang, T. Miyazawa, S. Fukumoto, M. Mishima, H. Suzuki, C. Sugimoto, H. Nagasawa, K. Fujisaki, N. Suzuki, T. Mikami, and I. Igarashi. 2001. Serodiagnosis of Toxoplasma gondii infection in cats by enzyme-linked immunosorbent assay using recombinant SAG1. Vet. Parasitol. 102:35-44. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Li, S., G. Galvan, F. G. Araujo, Y. Suzuki, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7:781-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makioka, A., A. Kobayashi. 1991. Expression of the major surface antigen (P30) gene of Toxoplasma gondii as an insoluble glutathione S-transferase fusion protein. Jpn. J. Parasitol. 40:344-351. [Google Scholar]
- 16.Mishima, M., X. Xuan, A. Shioda, Y. Omata, K. Fujisaki, H. Nagasawa, and T. Mikami. 2001. Modified protection against Toxoplasma gondii lethal infection and brain cyst formation by vaccination with SAG2 and SRS1. J. Vet. Med. Sci. 63:433-438. [DOI] [PubMed] [Google Scholar]
- 17.Moran, P., and I. W. Caras. 1994. Requirements for glycosylphosphatidylinositol attachment are similar but not identical in mammalian cells and parasitic protozoa. J. Cell Biol. 125:333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagel, S. D., and J. C. Boothroyd. 1989. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J. Biol. Chem. 264:5569-5574. [PubMed] [Google Scholar]
- 19.Nguyen, T. D., G. Bigaignon, J. V. Broeck, M. Vercammen, T. N. Nguyen, M. Delmee, M. Turneer, S. F. Wolf, and J. P. Coutelier. 1998. Acute and chronic phases of Toxoplasma gondii infection in mice modulate the host immune responses. Infect. Immun. 66:2991-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikawa, Y., K. Tragoolpua, L. Makala, X. Xuan, and H. Nagasawa. Neospora caninum NcSRS2 is a transmembrane protein that contains a glycosylphoshatidylinositol anchor in insect cells. Vet. Parasitol., in press. [DOI] [PubMed]
- 21.Parmley, S. F., G. D. Sgarlato, J. Mark, J. B. Prince, and J. S. Remington. 1992. Expression, characterization, and serological reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J. Clin. Microbiol. 30:1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen, E., H. V. Nielsen, L. Christiansen, and J. Spenter. 1998. Immunization with E. coli produced recombinant T. gondii SAG1 with alum as adjuvant to protect mice against lethal infection with Toxoplasma gondii. Vaccine 16:1283-1289. [DOI] [PubMed] [Google Scholar]
- 23.Prince, J. B., K. L. Auer, J. Huskinson, S. F. Parmley, F. G. Araujo, and J. S. Remington. 1990. Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol. Biochem. Parasitol. 43:97-106. [DOI] [PubMed] [Google Scholar]
- 24.Sabin, A. B. 1941. Toxoplasmic encephalitis in children. JAMA 116:801-807. [Google Scholar]
- 25.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animal to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xuan, X., K. Maeda, T. Mikami, and H. Otsuka. 1996. Characterization of canine herpesvirus glycoprotein C expressed by a recombinant baculovirus in insect cells. Virus Res. 46:57-64. [DOI] [PubMed] [Google Scholar]
- 27.Xuan, X., T. Nakamura, I. Sato, K. Tuchiya, E. Nosetto, A. Ishihana, and S. Ueda. 1995. Characterization of pseudorabies virus glycoprotein gII expressed by recombinant baculovirus. Virus Res. 36:151-161. [DOI] [PubMed] [Google Scholar]