Abstract
This study focused on products of the bovine Mx1 gene as specific markers for acute viral infections. The rationale for this is the fact that viral infections are commonly paralleled by the synthesis, release, and remote action of alpha/beta interferons (IFN-α/β). Released IFN-α/β act through specific receptors present on nucleated cells to transduce signals for the transcription of numerous IFN-regulated genes, such as the ones for double-stranded-RNA-dependent protein kinase, 2′-5′-oligoadenylate synthetase, or the Mx proteins. In this study, cultured MDBK cells and bovine white blood cells (WBC) were treated with recombinant IFN-α or infected with either bovine herpesvirus 1 (BHV-1) or bovine rotavirus (BRV). Treatment of cultured cells with IFN-α was followed within 4 h by a time- and dose-dependent accumulation of intracytoplasmic Mx protein as revealed by immunostaining and Western blot immunoassay. This was preceded by a distinct rise of Mx mRNA in similarly treated cells, as revealed by a newly established quantitative TaqMan PCR technique. The two viruses displayed a cell-dependent in vitro ability to induce Mx proteins, which was limited to bovine WBC with BHV-1 and to MDBK cells with BRV. The established methods were successfully used to show that infection of calves with a noncytopathic strain of bovine viral diarrhea virus, a pestivirus, was followed within 2 days postinfection by strong expression of both Mx mRNA and Mx proteins in WBC.
The system of alpha/beta interferons (IFN-α/β) is a central part of the host innate immune defense system. This system is preferentially activated by viral pathogens and also by some nonviral, pathogen-associated molecules, such as lipopolysaccharide and unmethylated DNA (23, 51). Once produced by and released from virus-infected cells, the IFN-α/β transmit signals to the interior of remote cells via a specific receptor complex to induce an antiviral response by activating a number of genes. Among these are double-stranded RNA (dsRNA)-dependent protein kinase, 2′-5′-oligoadenylate synthetase, and the Mx proteins. dsRNA-dependent protein kinase and 2′-5′-oligoadenylate synthetase, produced as inactive precursor molecules, are activated by viral dsRNA intermediates and act in conjunction with RNase L to inhibit the host cell transcription machinery and ultimately contribute to the induction of apoptosis of virus-infected cells. In ruminants, the IFN-α/β system encompasses, besides IFN-α/β, IFN-τ, which is secreted by the early embryo (trophoblast) and functions as a local signal for maternal recognition of pregnancy (13, 19).
The Mx proteins are induced specifically by IFN-α/β (47, 48). They are large GTPases that belong to the dynamin superfamily (24, 54). Two or more corresponding genes have been identified in mammals (29), birds (3, 4), and fish (35). The Mx proteins were first discovered in A2G mice, where the Mx1 protein confers resistance to influenza viruses (30, 36, 37). Mx proteins are usually located in the cytoplasm, with the exception that in some rodents the proteins also display an intranuclear appearance (29). The Mx proteins are known to exert an antiviral effect by targeting specific yet poorly defined steps of the viral replication cycle (38, 39). Specific antiviral activities have been described during experimental infections with RNA viruses, such as influenza A viruses, thogotovirus, dhorivirus, vesicular stomatitis virus, measles virus, bunyavirus, and Semliki Forest virus (24, 26), parainfluenza 3 viruses (56), and hantavirus (20).
Efforts have been made not only to elucidate the basic antiviral mechanisms of the Mx proteins but also to use up-regulated expression of the Mx gene as a marker for an IFN-α/β-mediated antiviral state in clinical and paraclinical settings. In one of these settings an elevated expression of Mx protein in peripheral blood leukocytes indicated the possibility of discriminating between viral and bacterial infections (10, 12, 18, 25, 32). In a second setting it was shown that Mx gene expression after live yellow fever vaccination was a sensitive marker for endogenous IFN-α/β (40). Furthermore, the MxA protein, which is known to be specifically induced by IFN-α/β, is considered to be a useful marker in the management of IFN-α treatment in humans (11, 34). Finally, the detection of IFN-α or antiviral proteins, namely, Mx proteins, is likely to constitute an indirect approach for investigating the hypothesis of the role of viruses in chronic diseases with suspected infectious etiology (9). In the paraclinical setting it was ultimately recognized that Mx protein was a sensitive and selective substitute to measure an IFN-α/β-induced state (16, 19, 53).
Horisberger and Gunst first described two Mx proteins in bovine Madin-Darby bovine kidney (MDBK) cells that were intracytoplasmically apparent in IFN-α/β-stimulated cells but not in untreated control cells (27, 29). Those authors presented in vitro evidence that an effective Mx system possibly mediates the natural resistance of cattle to influenza viruses.
Ellinwood and coworkers (14, 15) isolated from an endometrial phage library two allelic cDNAs encoding Mx proteins with open reading frames predicting proteins of 654 (Mx1) and 648 (Mx1-a) amino acid residues and described the corresponding proteins of 74.7 and 75.5 kDa, respectively. The bovine amino acid sequences (99% identity) show 93% similarity to the ovine Mx protein, 73% similarity to the human MxA protein, and 63% similarity to the mouse Mx1 protein. Of eight cattle studied, two were found to express the two allelic forms of the Mx1 gene.
Bovine viral diarrhea virus (BVDV), a pestivirus within the Flaviviridae family, is an important cattle pathogen worldwide (31). Two biotypes of BVDV, cytopathic BVDV (cpBVDV) and noncytopathic BVDV (ncpBVDV), can be differentiated by their effect in cell culture (55). Infection of bovine fetuses with ncpBVDV during the first 120 days of pregnancy can result in the birth of persistently infected offspring that are immunotolerant to the virus. It has been shown that ncpBVDV isolates do not induce IFN-α/β in vitro and even block the induction of IFN-α/β by other activators, namely, dsRNA and viruses (43, 44, 46). These findings are in accordance with the detection of both IFN-α/β and Mx1 protein in bovine fetal spleens following intrauterine infection with cpBVDV only (8). In contrast to this, the same authors recently reported on the appearance of IFN-α/β in calves following infection with both cpBVDV and ncpBVDV (7).
This study aimed at establishing methods for the detection of bovine Mx1 proteins in vitro and in vivo by using Western blot immunoassays and immunostaining of cell monolayers. Furthermore, we established a quantitative assay for the detection of Mx1 mRNA in cultured cells as well as in white blood cells (WBC). With these tools we addressed the question of whether transient infection of calves with a strain of ncpBVDV leads to an induction of Mx1 mRNA and Mx1 proteins in WBC.
The results obtained show that both Mx1 mRNA and Mx1 proteins are up-regulated in bovine cells in a virus- and host-cell-specific manner. The findings are thought to represent a promising tool for monitoring animals with respect to the manifestation of viral infections.
MATERIALS AND METHODS
Cell cultures, IFN, and viruses.
MDBK cells at passage level 120 to 140 were grown at 37°C in HEPES-buffered Eagle's minimal essential medium that was supplemented with 5% fetal bovine serum and penicillin-streptomycin. Cells were routinely subcultured on a weekly basis and were used for the experiments at preconfluency in 24-well plates (250,000 cells/well) and cell culture flasks (2 × 106 cells/25cm2).
WBC from healthy-appearing cattle were obtained by ammonium chloride lysis of uncoagulated blood samples. Briefly, blood was collected in EDTA Vacutainer tubes and processed immediately. Up to 12 ml of blood was added to 40 ml of autoclaved lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 M EDTA, pH 7.2) and incubated for 10 min at room temperature. After centrifugation (400 × g, 10 min, 4°C), the pelleted WBC were resuspended in phosphate-buffered saline (PBS) and recentrifuged. After this washing step the WBC were either cultured at 37°C or frozen and used for Western blotting, or total RNA was isolated by using the RNeasy Mini Kit (Qiagen, Basel, Switzerland). For culture, the WBC from 12 ml of blood were routinely suspended in 12 ml of serum-free medium (QBSF-56; Sigma, Buchs, Switzerland), supplemented with either recombinant IFN-α or viruses, and partitioned in 0.5-ml aliquots into 24-well plates.
A recombinant IFN-α B/D hybrid (rIFN-α), known to be active in a broad host range (28, 29, 45), was kindly provided by M. A. Horisberger (Novartis AG, Basel, Switzerland) and used for in vitro stimulation experiments. rIFN-α was consistently used with serum-free medium and in concentrations ranging from 0 to 1,000 U/ml.
For the in vitro studies, the Jura strain of bovine herpesvirus 1 (BHV-1) and the NCDV strain of bovine rotavirus (BRV) were used throughout (6, 50). Virus stocks were produced and titrated under serum-free conditions in MDBK and MA-104 cells, respectively. Viral infections of WBC and MDBK cells were routinely effected by inoculating 1 50% tissue culture infective dose (TCID50)/cell.
Detection of Mx1 proteins.
MDBK monolayers in 24-well plates were either treated with rIFN-α, infected with viruses, or left as untreated controls. To assess up-regulation of Mx1 proteins, the cells were subjected to either Western blotting or immunostaining. For Western blotting, 106 cells were washed with PBS, resuspended in 100 μl of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer (250 mM Tris-HCl [pH 6.8], 5% SDS, 10% β-mercaptoethanol, 40% glycerol, 0.03% bromphenol blue), boiled for 5 min, and subjected to electrophoretic separation on SDS-10% polyacrylamide gels by using a III-Cell (Bio-Rad Laboratories AG, Reinach, Switzerland). Cultures of WBC were prepared for Western blotting accordingly, with the exception that 105 cells were resuspended in 100 μl of SDS-polyacrylamide gel electrophoresis sample buffer. Following electrophoretic separation, the proteins were electrotransferred in blotting buffer (25 mM Tris base, 192 mM glycine, 20% methanol) to a nitrocellulose membrane (Schleicher & Schuell, Riehen, Switzerland) for 1 h at 100 V. Blots were blocked overnight at 4°C in high-salt Tris-buffered saline, consisting of 50 mM Tris-HCl (pH 7.0), 500 mM NaCl, 0.2% (wt/vol) Tween 20, and 5% (wt/vol) skim milk. The blots were then washed three times (5 min each) with high-salt Tris-buffered saline, followed by incubation with the murine anti-Mx monoclonal antibody (MAb) M143 (17, 33) for 1 h at room temperature. This MAb, generated as a hybridoma culture supernatant and kindly provided by J. Pavlovic (Institute of Medical Virology, University of Zurich, Zurich, Switzerland), has been shown to react with Mx proteins of a broad host range (J. Pavlovic, unpublished data). After further washing steps, blots were incubated for 1 h at room temperature with appropriately diluted peroxidase-labeled rabbit anti-mouse immunoglobulin G (A9044; Sigma). After final washing steps, blots were developed by enhanced chemiluminescence (Amersham Pharmacia Biotech, Dübendorf, Switzerland) and exposed to X-ray films (Roche Pharma Schweiz AG, Rotkreuz, Switzerland).
MDBK monolayers treated overnight with various doses of rIFN-α were subjected to immunostaining for Mx proteins with MAb M143. Briefly, cells were washed with PBS and fixed with ice-cold methanol-acetone (2:1) for 30 min at −20°C. Monolayers were then washed with PBS containing 0.25% (wt/vol) bovine gelatin (G9391; Sigma). Following incubation for 1 h at 37°C with the anti-Mx antibody (MAb M143) and further washing steps, the cells were successively incubated with a biotinylated rabbit anti-mouse antibody (B8520; Sigma) and a biotinylated horseradish peroxidase complex (Amersham Pharmacia Biotech). After a final wash step with distilled water, the preparations were developed with an appropriate substrate (AEC-101 staining kit; Sigma) according to the manufacturer's instructions.
RNA extraction and reverse transcription.
Bovine WBC derived from 1 ml of blood or MDBK cells (106 cells) were processed for RNA isolation with the RNeasy kit (Qiagen) according to the manufacturer's instructions. Purified RNA, diluted in 50 μl of diethyl pyrocarbonate (DEPC)-treated water, was digested with 1 U of DNase (RQ1 RNase-free DNase; Promega, Madison, Wis.) for 1 h at 37°C, followed by an inactivation step (95°C, 10 min). Reverse transcription was according to the instructions supplied with a commercial kit (A3500; Promega). Briefly, 9.75 μl of RNA was denatured at 70°C for 10 min, cooled on ice, and mixed with 10.25 μl of master mix containing 4 μl of MgCl2 (25 mM), 2 μl of 10× buffer, 2 μl of a deoxynucleoside triphosphate mixture (10 mM), 0.5 μl of RNasin (40 U/μl), 0.75 μl of avian myeloblastosis virus reverse transcriptase (20 U/μl), and 1 μl of random primers (0.5 μg/μl). After incubation for 10 min at ambient temperature, the samples were subjected to reverse transcription at 42°C for 45 min followed by inactivation of the reverse transcriptase (99°C, 5 min), and the resulting cDNA was stored at −80°C.
Detection of Mx1 mRNA by use of TaqMan technology.
PCRs were performed on an ABI PRISM 7700 sequence detection system (Applied Biosystems, Rotkreuz, Switzerland). Primer and probe sequences (Table 1) were designed by using Primer Express software (version 1.0; Applied Biosystems, Foster City, Calif.) to amplify a 78-bp sequence within the published sequence (accession number AF047692) of bovine Mx1 (15). The primers and probe were selected to be suitable to amplify the corresponding fragment from the published sequence of Mx1-a (accession number U88329) as well. The oligonucleotide primers and probe were synthesized by Microsynth GmbH (Balgach, Switzerland).
TABLE 1.
Primers and probe for the amplification of a 78-bp sequence within the bovine Mx1 gene (Genbank accession number AF047692) by TaqMan PCR
| Primer or probe | Length (nucleotide) | Start nucleotide | Sequence (5′-3′) and fluorogenic labelsa | Melting point (°C) |
|---|---|---|---|---|
| Forward primer | 19 | 1714 | AAC ACC TGA CCG CGT ACC A | 59 |
| Reverse primer | 22 | 1791 | GCA CGA AGA ACT GGA TGA TCA A | 59 |
| Probe | 24 | 1734 | FAM-CAG GAA GTC AGC ACC CGC ATC TCC-TAMRA | 69 |
FAM, 6-carboxy-fluorescein; TAMRA, 6-carboxy-tetramethyl-rhodamine.
PCRs were carried out in 25-μl volumes by mixing 5 μl (1:100 diluted) of cDNA with 20 μl of master mix composed of 12.5 μl of TaqMan Master Mix (Applied Biosystems, Rotkreuz, Switzerland), 0.4 μl of upstream primer (final concentration, 240 nM), 0.4 μl of downstream primer (final concentration, 240 nM), 0.16 μl of probe (final concentration, 160 nM), and 6.54 μl of DEPC-treated water. PCR conditions were set as follows: 2 min at 50°C, 10 min at 95°C, and then 40 cycles consisting of 15 s at 95°C (denaturation) and 1 min at 60°C (annealing-elongation). Recorded data were analyzed with the appropriate sequence detector software (version 1.6).
To standardize the amount of input Mx1 cDNA used for TaqMan analysis, the amplification of 18S rRNA was determined in parallel for each sample and the results were used as an internal control (22; ABI Prism 7700 Sequence Detection System User Bulletin [P/N 4303859], Perkin-Elmer Corporation, Foster City, Calif., 1997). For this a separate reaction mixture was set up with each sample, containing 5 μl (1:100 diluted) of cDNA and 20 μl of master mix consisting of 12.5 μl of PCR buffer, 1.25 μl of predeveloped 18S rRNA assay reagent (4310893E; Applied Biosystems), and 6.25 μl of DEPC-treated water.
Detection of Mx1 gene products in WBC of calves during transient infection with ncpBVDV.
Six conventionally reared calves, age 3 to 6 months and seronegative for BVDV, were each orally infected with 5 × 107 TCID50 of a cell culture-propagated (nasal epithelial cells) field isolate (110/39) of ncpBVDV. This noncytopathogenic virus was isolated in 1995 from a 22-month-old persistently infected Swiss cow in the absence of clinically manifest disease (P. Schaller [University of Berne], unpublished data). The rationale for this experiment was that in the context of BVDV eradication programs, the recognition of persistently infected individuals seems to be possible by demonstrating BVDV antigens in skin biopsies (52). This experimental study aimed to prove that a transient infection of immunocompetent animals with ncpBVDV was not accompanied by the appearance of viral antigens as seen with persistently infected animals. Virus inoculation was invariably followed by a transient phase of clinical distress, including nasal discharge, occasional coughing, and elevated body temperature; by a phase of viremia and virus shedding with nasal discharges (4 to 10 days postinfection [p.i.]); and by a virus-specific seroconversion towards the end of the experiment. Complete recovery was seen in each case within 3 weeks (A. Arquint et al., unpublished data). By using the previously established methods for detection of bovine Mx1 gene products, WBC from the infected animals were evaluated for elevated expression of Mx1 gene products. Uncoagulated (EDTA) blood samples were taken at different times in the experiment, and WBC pellets were prepared and analyzed by Western blotting (Mx1 proteins) and TaqMan technology (Mx1 mRNA and 18S rRNA). The total number of cells used for Western blotting experiments was standardized using the 18S rRNA signal obtained by TaqMan PCR.
RESULTS
Induction of Mx1 protein in cultured bovine cells following in vitro stimulation with rIFN-α.
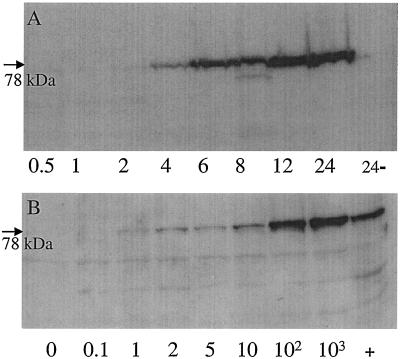
Preconfluent monolayers of MDBK cells were treated with various concentrations of rIFN-α and analyzed at specified time points for up-regulation of Mx1 proteins as revealed by Western blotting with a specific MAb. In MDBK cells stimulated with 1,000 U of rIFN-α/ml, a distinct appearance of Mx1 proteins was evident after 4 h, and the intensity of the signal was observed to increase with the duration of the experiment (Fig. 1A). The dominant band had an apparent molecular mass of 78 kDa which is in good agreement with the predicted size of Mx1 and Mx1-a. The likely presence of two Mx1 proteins with similar apparent molecular masses was obvious from most Western blots (Fig. 1 and 2). When incubated in the absence of rIFN-α, the cells were most consistently observed to exhibit minute amounts of Mx1 proteins, indicating a low-level constitutive expression of this gene product in MDBK cells. On occasion some lower-molecular-mass proteins were observed to slightly react with the MAb. Incubation with different doses of rIFN-α overnight showed that Mx1 expression was induced even in the presence of 1 U/ml and that the amount of Mx1 protein detected reflected the amount of interferon used for stimulation (Fig. 1B). MDBK monolayers were treated with various doses of rIFN-α and immunostained with MAb M143 following incubation for 16 h. From this it was clearly discernible that Mx1 proteins were up-regulated in dependence on the rIFN-α concentration used (data not shown). It additionally became apparent that the proteins were virtually restricted to the cell cytoplasm. In accordance with the previous findings, nonstimulated cells were found to consistently express low levels of intracytoplasmic Mx1 proteins. Together, these results show that Mx1 proteins are up-regulated following stimulation of MDBK cells with rIFN-α and that the induction is time and dose dependent. A further finding was the fact that MDBK cells exhibit low levels of (possibly constitutively) expressed Mx1 proteins.
FIG. 1.
Expression of Mx proteins in MDBK cells following stimulation with rIFN-α is time and dose dependent. (A) Cells were stimulated in the presence of 1,000 U of rIFN-α/ml, harvested at different time points (0.5 to 24 h), and analyzed by Western blotting. Cells incubated for 24 h in the absence of rIFN-α were used as a negative control (24−). (B) MDBK cells were stimulated for 16 h in the absence (0) or presence (0.1 to 103 U/ml) of rIFN-α and analyzed by Western blotting. +, positive control.
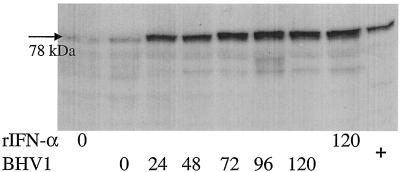
FIG. 2.
In vitro expression of Mx proteins in bovine WBC infected with BHV-1 (multiplicity of infection = 1 TCID50) or stimulated in vitro with rIFN-α (1,000 U/ml). Cells were harvested and analyzed by Western blotting at different time points (0 to 120 h). +, positive control.
Induction of Mx1 proteins in cultured bovine cells during infection with viruses.
MDBK and bovine WBC were infected in vitro with either BHV-1 or BRV and incubated for up to 24 h p.i. The two viruses led to an up-regulation of Mx1 proteins, albeit in a cell-specific way. Whereas BHV-1 strongly induced Mx1 proteins in bovine WBC (Fig. 2) but did not do so in MDBK cells, BRV behaved inversely (data not shown). From the data presented in Fig. 2 it is apparent that freshly prepared WBC from healthy-appearing calves are associated with a weak yet distinct expression of Mx1 proteins, as seen with MDBK cells.
Evaluation of primers and probe used for TaqMan PCR.
Detection of Mx1 proteins by Western blotting was possible as early as 4 h after stimulation with rIFN-α. It was therefore of interest to determine the time point of the up-regulation of the Mx1 mRNA signal in stimulated cells. In view of this it was first necessary to show that real-time PCR, using the designed primers and probe, was suitable to detect Mx1 mRNA in bovine cells. For this purpose MDBK cells were incubated overnight in the presence (1,000 U/ml) or absence of rIFN-α, harvested, counted, and subjected to TaqMan reverse transcription-PCR. Performing PCR with cDNA from 10,000 treated cells resulted in a threshold cycle (CT value) of 19, whereas the same number of untreated cells gave a CT value of 25. Assuming that a ΔCT of 3 indicates a 10-fold difference of RNA present, this would mean that treated cells contained 100 times more Mx1 mRNA than the untreated controls. The same experiment was done with bovine WBC, and essentially identical results were obtained, i.e., 10,000 stimulated and nonstimulated WBC resulted in CT values of 24 and 29.5, respectively. With both WBC and MDBK cells it became apparent that freshly prepared, nonstimulated cells displayed a basic level of Mx1 mRNA expression (data not shown). This was consistent with the earlier findings for protein.
Sensitivity of Mx1 TaqMan PCR.
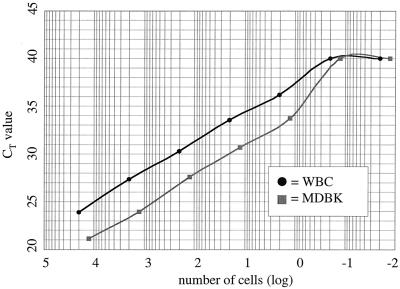
To determine the sensitivity of the established assay, MDBK cells and bovine WBC were stimulated overnight with 1,000 U/ml of rIFN-α, harvested, and counted, and cell-associated RNA was purified. Reverse transcription was performed as described in Materials and Methods, and a 10-fold dilution series of the resulting cDNA was subjected to TaqMan PCR. From the data presented in Fig. 3, it is apparent that the procedure detected Mx1 mRNA in as little as a single MDBK cell. Considering the likelihood that the various WBC types responded with varied efficiency to the IFN stimulus, the detection limit of Mx mRNA in this cell mixture could be expected to be less than a single cell. By extrapolating from the linear range of each of the two curves, it could finally be confirmed that a 1-log-unit difference of input RNA resulted in a ΔCT of approximately 3.
FIG. 3.
Sensitivity of TaqMan PCR for Mx mRNA. Cultured MDBK cells or bovine WBC were stimulated over night with 1,000 U of rIFN-α/ml, harvested, counted, and prepared for TaqMan PCR. After reverse transcription, the cDNAs were serially diluted 10-fold and subjected to TaqMan PCR. With each cell type, Mx mRNA could be detected in as little as one cell.
Time course of Mx1 mRNA expression in cultured cells.
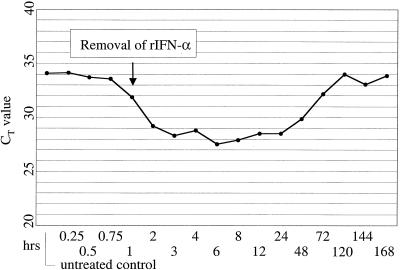
To determine the time point at which levels of Mx1 mRNA started to rise following rIFN-α stimulation, MDBK cells were treated for 1 h with 1,000 U of rIFN-α/ml and analyzed by TaqMan PCR at different times after exposure to the cytokine (Fig. 4). A clear increase of the Mx1 mRNA level was seen after 1 h, and a plateau was reached at between 3 and 6 h. Interestingly, stimulation with rIFN-α for 1 h was sufficient to maintain Mx1 mRNA at maximal levels for 24 h. Pretreatment Mx1 mRNA levels were not seen until 5 days posttreatment. The fact that untreated cells were not clearly negative by TaqMan PCR (CT value of 34) again indicated a basic low-level expression of Mx1 mRNA in MDBK cells.
FIG. 4.
Sustained up-regulation of the Mx1 gene in MDBK cells following stimulation with rIFN-α. The cells were exposed for 1 h to 1,000 U of rIFN-α/ml, washed, incubated with maintenance medium (Eagle's minimal essential medium), and harvested for TaqMan PCR at the time points indicated. The Mx mRNA signal reached baseline levels after 5 days only.
Establishment of a reference for standardization of Mx1 TaqMan PCR.
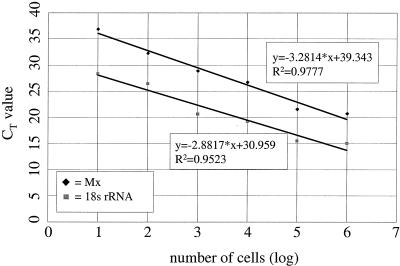
Upon analyzing blood specimens, the samples are usually obtained without reference to the actual number of WBC present. To accurately evaluate such samples for Mx1 gene products, it was thus necessary to establish a baseline that would allow comparison of Western blot signals and CT values on the basis of a comparable cell number. Standard curves for 18S rRNA and Mx1 mRNA were determined as follows. Bovine WBC were stimulated overnight with 1,000 U of rIFN-α/ml, harvested, and counted, and purified RNA was reverse transcribed. A 10-fold dilution series of cDNA was then tested by TaqMan PCR for both 18S rRNA and Mx1 mRNA. The results obtained were used to calculate the equations of the corresponding regression curves (Fig. 5). The slope of each of these curves is regarded as a measure of the efficiency of a fluorogenic PCR system; hence, the smaller the value of the slope, the more efficient the system. Two independent PCRs are considered to have the same efficiency if the difference between the slopes of their regression curves is less than 0.1 (ABI Prism 7700 Sequence Detection System User Bulletin [P/N 4303859]). In our case the two PCRs were not of the same efficiency (Δa = 0.3997), and consequently the CT values alone could not be used for relative quantification. However, since the slopes of Mx1 mRNA curves always have the same value (only the intercept will vary according to the input Mx1 mRNA), a curve for each individual sample with the equation y = −3.2814 × x + intercept can be determined (where x stands for the cell number obtained from the 18S rRNA standard curve). Using the newly designed equation, a standardized CT value for any given number of input cells can be calculated.
FIG. 5.
Establishment of standard curves to correlate CT values from samples of WBC with a specified cell number. A 10-fold dilution series of cDNA from rIFN-α-stimulated WBC (1,000 U/ml, 24 h) was tested by TaqMan PCR for both 18S rRNA and Mx1 mRNA, and the equations of the respective regression curves were calculated. The slopes of the two curves are not the same (−3.2814 versus −2.8817), indicating that the efficiencies of the two PCR systems were not the same. The actual cell number in a given sample can be calculated by using the equation for 18S rRNA. Using the actual cell number, the CT value for Mx mRNA, and the slope for the Mx standard curve, a normalized CT value for any cell number (within the linear range of the curve) can be calculated.
Detection of Mx1 gene products in WBC from calves transiently infected with ncpBVDV.
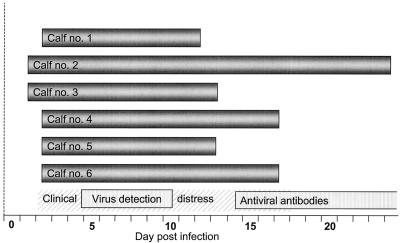
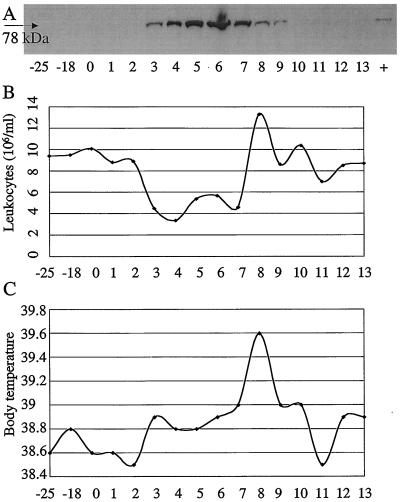
Six calves, age 3 to 6 months, were infected with a strain of ncpBVDV and sequentially tested for up-regulation of the Mx1 gene in WBC by using Western blotting and TaqMan PCR. In addition, the animals were scored with respect to clinical, hematological, and virological parameters. For Western blotting, similar WBC numbers of consecutive samples were evaluated based on predetermined 18S rRNA values obtained with TaqMan PCR. A distinct rise of the Mx protein level in WBC was consistently apparent within 3 days p.i. (Fig. 6), and maximum levels were recorded between days 4 and 8. Representative data for one of the six calves are shown in Fig. 7A. A strong up-regulation of the Mx1 gene was first observed on day 3 p.i., elevated signals were distinct up to day 9 p.i., and preinoculation levels were reached by day 13 p.i. During the course of the experiment, this animal, like the five others, showed clinical, hematological, and virological signs that were consistent with bovine viral diarrhea, namely, leukopenia (3 to 7 days p.i.), mucopurulent nasal discharge (4 to 14 days p.i.), elevated body temperature (7 to 10 days p.i.), viremia, and virus shedding, as well as seroconversion (Fig. 6 and 7B and C and unpublished data). Together the results indicated that transient infection with ncpBVDV does provoke an up-regulation of Mx1 proteins in WBC and that this signal paralleled or even preceded clinical, hematological, and virological parameters of infection.
FIG. 6.
WBC of calves experimentally inoculated with BVDV transiently express elevated levels of the IFN-α/β-induced Mx proteins from 1 to 23 days p.i. The bars on the bottom of the schematic indicate phases of clinical distress in the animal group, viremia and virus shedding, and the appearance of virus-specific serum antibodies.
FIG. 7.
Up-regulation of the Mx gene in a calf during transient infection with a noncytopathic strain of BVDV and comparison with hematological and clinical data. (A) Blood samples were obtained at the time points indicated, and WBC were analyzed for Mx1 proteins by Western blotting. (B and C) Corresponding hematological and clinical data, respectively. Note that the first appearance of Mx1 proteins correlates with the manifestation of leukopenia and the development of elevated body temperature.
In summary, IFN-α/β is preferentially synthesized by virus-infected cells to provide a signal for the generation of an antiviral state in remote cells, which is reflected by the up-regulation of the Mx1 gene. Using cultured bovine cells, this study showed up-regulated Mx1 gene expression following exposure to rIFN-α as revealed by newly adapted techniques. Upon using a herpesvirus and a rotavirus to trigger the IFN-α/β response in vitro, it was observed that synthesis of Mx1 proteins was dictated by both the virus and the host cell. During infection of immunocompetent calves with a strain of ncpBVDV, an early innate immune response was invariably discernible as revealed by the transient appearance of elevated Mx1 protein levels in WBC.
DISCUSSION
This study showed that bovine cells can be stimulated with rIFN-α, leading to an up-regulation of the Mx1 gene as revealed by Western blotting or immunostaining with a MAb and by TaqMan PCR with a newly designed set of primers and probe. The last technique was found to be reasonably sensitive, allowing recording of an Mx1 mRNA signal in as little as one rIFN-α-stimulated cell. Following stimulation with rIFN-α, a rise of Mx1 mRNAs above background levels could be detected as early as after 1 h in MDBK cells, and maximal levels were reached within 3 to 6 h (Fig. 4). Stimulation of bovine WBC with rIFN-α gave essentially similar results (data not shown). It was of interest that stimulation of MDBK cells for 1 h led to an up-regulated Mx1 mRNA level that lasted up to 5 days. In view of the reported short half-life of human MxA transcripts (40), our data would indicate that a short activation of the Mx1 gene would lead to prolonged gene expression in MDBK cells. This is in remarkable contrast to findings obtained with human and murine cells (21, 41, 42, 49), where the steady-state Mx mRNA levels in the presence of IFN-α were highest at 4 to 6 h, with a return to basal levels within 24 to 48 h. It seems possible that transcription of the bovine Mx gene may be regulated differently than that of the respective human and murine genes and/or that the bovine Mx1 transcripts may possess sustained stability. These possibilities are in line with findings of others (51) showing a significant Mx1 gene expression in IFN-α/β-stimulated cells beyond 24 h postinduction. It was additionally noted that untreated MDBK cells consistently expressed low levels of Mx1 mRNA. This could be due to constitutive low-level expression of the IFN-α/β gene, as reported by others (51).
Detection of Mx1 proteins by Western blotting was greatly facilitated by the availability of a MAb active in a broad range of different species. Using this antibody, it was observed that the major target was a pair of proteins with similar apparent molecular masses (78 kDa). It seems likely that these two proteins were the products of the reported allelic forms of the bovine Mx1 gene, Mx1 and Mx1-a (15). Sometimes the MAb used was observed to react also with some low-molecular-mass proteins (Fig. 1 and 2). These proteins are possibly related to the postulated third allele of the bovine Mx1 gene or to differentially spliced Mx1 transcripts (15), or they could represent proteolytic cleavage products.
For quantitative detection of human Mx proteins, several enzyme-linked immunosorbent assay (ELISA) formats have been described (10, 12, 16, 53). These procedures are easily adopted to evaluate large sample numbers, yet they depend on two antibodies targeting different epitopes on the same antigen. In order to determine bovine Mx1 proteins with ELISA tests, such antibodies still need to be generated. In addition to conventional ELISA tests for detection of bovine IFN-α/β, an MDBK cell-based Mx-chloramphenicol acetyltransferase reporter gene assay has been developed and validated (19).
Upon infection of MDBK cells and bovine WBC with a herpesvirus and a rotavirus, the activation of the IFN-α/β system, followed by an up-regulation of the Mx1 gene, was anticipated. However, these viruses were found to activate this trait of the innate immune system in a restricted, cell-specific manner only. Whereas BHV-1 led to a strong Mx1 protein induction in bovine WBC, confirming the findings of others (46), the inverse was the case with BRV. Owing to the facts that rotaviruses possess a segmented dsRNA genome and that dsRNA is established to be essential for IFN-α/β induction, this finding was of considerable interest. During productive infection of MDBK cells, BRV was found to trigger the synthesis of Mx1 proteins, whereas BHV-1 did not. Clearly, elucidation of these in vitro phenotypes needs a more detailed study with special emphasis on the possible manifestations of host cell-directed impairment of virus-induced IFN-α/β induction and signaling (45).
Monitoring animal health in livestock is, from the point of view of veterinary public health, of considerable interest. Not only do healthy farm animals have to provide farmers with a net income, but at the same time there is a continuously growing demand for safe animal-derived food. The possible use of Mx1 proteins as a marker for ongoing virus infection in livestock would be beneficial in various aspects. Early knowledge about ongoing viral infections could, among other benefits, facilitate decision making with respect to quarantine management, vaccination programs, and preslaughter controls. However, in order for Mx1 gene products to be of practical value as a meaningful indicator for ongoing viral infections, there is a need to identify those viruses that either induce or circumvent the innate IFN-α/β system (23). Based on this, it was of interest to use the newly established tests with calves experimentally infected with BVDV. Bovine viral diarrhea, a pestivirus infection, represents an economically important cattle disease with worldwide prevalence (31). Two biotypes of the virus, cytopathic and noncytopathic, are identifiable based on their lytic activities under in vitro conditions. Strains of ncpBVDV can infect the bovine fetus and induce lifelong immunotolerance. Previous studies with infected fetuses have suggested that ncpBVDVs do not activate the IFN-α/β system (8), thus confirming earlier findings obtained with cell cultures. However, recent findings indicate that in young calves ncpBVDV strains do provoke the synthesis of IFN-α/β (7). By monitoring the course of a transient infection of 3- to 6-month-old calves with ncpBVDV, this study revealed an early and strong up-regulation of the Mx1 gene (mRNA and proteins) in each of the six animals (Fig. 6 and 7 and unpublished data). This finding indirectly confirms generation and activity of endogenously produced IFN-α/β, even though it cannot be ruled out that ncpBVDV could directly activate the Mx1 gene, as seen with other viruses (21).
In the past, different approaches have been used to record the kinetics of Mx1 mRNA expression in cells derived from clinical settings, namely, hybridization, semiquantitative reverse transcription-PCR, and quantitative reverse transcription-competitive PCR (1, 2, 5, 40). The TaqMan PCR established in this study adds to these possibilities. As seen during previous clinical studies, a low-level, baseline expression of Mx1 mRNA was consistently discernible in untreated WBC from healthy cattle and in MDBK cells as well. The present in vivo study was consistent with the earlier findings, showing that the WBC of each of the animals (apart from the time period of infection-induced strong up-regulation of the Mx1 gene) consistently displayed low levels of cell-associated Mx1 proteins. This could be explained by two possibly synergistically acting mechanisms. First, it seems likely that a basic level of Mx1 mRNA is expressed in bovine WBC. On the other hand, and of clinical importance, it was possible that concurrent yet unrecognized viral infections could have triggered, either through a direct virus effect or indirectly through the induction of IFN-α/β, a weak expression of the Mx1 gene. In any case, following infection with ncpBVDV a strong and transient expression of large quantities of Mx1 proteins was seen with each animal. This indicated that monitoring of Mx1 levels in WBC was indeed a valuable tool to record the manifestation of this viral pathogen.
In summary, aiming to use the innate immune response as an indicator for the manifestation of viruses in farm animals, tests were established that allow detection of an IFN-α/β-induced state by demonstrating up-regulation of the bovine Mx1 gene. In vitro studies conducted with a herpesvirus and a rotavirus indicated that the ultimate induction of Mx1 proteins was dictated by both the virus and the host. Using the established techniques to record Mx1 gene products, it was shown in an in vivo experiment that a strain of ncpBVDV invariably did provoke an up-regulation of the bovine Mx1 gene. In the future, monitoring of Mx1 gene products may help to disclose the actual virus infection state of livestock.
Acknowledgments
We express our gratitude to Adrian Arquint and Felix Ehrensperger (Institute of Veterinary Pathology, University of Zurich, Zurich, Switzerland) and Patrick Schaller (Institute of Veterinary Virology, University of Berne, Berne, Switzerland) for providing blood samples and relevant clinical data from ncpBVDV-infected animals, to Rita Castro for excellent technical support, to Jovan Pavlovic for kindly providing the MAb, to Michel Horisberger for kindly providing the rIFN-α, and to Hans-Peter Hefti for valuable discussions and careful reading of the manuscript.
REFERENCES
- 1.Aebi, M., J. Fah, N. Hurt, C. E. Samuel, D. Thomis, L. Bazzigher, J. Pavlovic, O. Haller, and P. Staeheli. 1989. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Biol. 9:5062-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonelli, G., E. Simeoni, O. Turriziani, R. Tesoro, A. Redaelli, L. Roffi, L. Antonelli, M. Pistello, and F. Dianzani. 1999. Correlation of interferon-induced expression of MxA mRNA in peripheral blood mononuclear cells with the response of patients with chronic active hepatitis C to IFN-alpha therapy. J. Interferon Cytokine Res. 19:243-251. [DOI] [PubMed] [Google Scholar]
- 3.Bazzigher, L., A. Schwarz, and P. Staeheli. 1993. No enhanced influenza virus resistance of murine and avian cells expressing cloned duck Mx protein. Virology 195:100-112. [DOI] [PubMed] [Google Scholar]
- 4.Bernasconi, D., U. Schultz, and P. Staeheli. 1995. The interferon-induced Mx protein of chickens lacks antiviral activity. J. Interferon Cytokine Res. 15:47-53. [DOI] [PubMed] [Google Scholar]
- 5.Brod, S. A., L. Nelson, R. Jin, and J. S. Wolinsky. 1999. Ingested interferon alpha induces Mx mRNA. Cytokine 11:492-499. [DOI] [PubMed] [Google Scholar]
- 6.Brussow, H., W. Eichhorn, A. Rohwedder, D. Snodgrass, and J. Sidoti. 1991. Cattle develop neutralizing antibodies to rotavirus serotypes which could not be isolated from faeces of symptomatic calves. J. Gen. Virol. 72:1559-1567. [DOI] [PubMed] [Google Scholar]
- 7.Charleston, B., L. S. Brackenbury, B. V. Carr, M. D. Fray, J. C. Hope, C. J. Howard, and W. I. Morrison. 2002. Alpha/beta and gamma interferons are induced by infection with noncytopathic bovine viral diarrhea virus in vivo. J. Virol. 76:923-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charleston, B., M. D. Fray, S. Baigent, B. V. Carr, and W. I. Morrison. 2001. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J. Gen. Virol. 82:1893-1897. [DOI] [PubMed] [Google Scholar]
- 9.Chieux, V., W. Chehadeh, P. Hautecoeur, J. Harvey, P. Wattre, and D. Hober. 2001. Increased levels of antiviral MxA protein in peripheral blood of patients with a chronic disease of unknown etiology. J. Med. Virol. 65:301-308. [DOI] [PubMed] [Google Scholar]
- 10.Chieux, V., D. Hober, W. Chehadeh, J. Harvey, G. Alm, J. Cousin, H. Ducoulombier, and P. Wattre. 1999. MxA protein in capillary blood of children with viral infections. J. Med. Virol. 59:547-551. [PubMed] [Google Scholar]
- 11.Chieux, V., D. Hober, W. Chehadeh, and P. Wattre. 1999. Interferon alpha, proteines antivirales et applications medicales. Ann. Biol. Clin. (Paris) 57:659-666. [PubMed] [Google Scholar]
- 12.Chieux, V., D. Hober, J. Harvey, G. Lion, D. Lucidarme, G. Forzy, M. Duhamel, J. Cousin, H. Ducoulombier, and P. Wattre. 1998. The MxA protein levels in whole blood lysates of patients with various viral infections. J. Virol. Methods 70:183-191. [DOI] [PubMed] [Google Scholar]
- 13.Demmers, K. J., K. Derecka, and A. Flint. 2001. Trophoblast interferon and pregnancy. Reproduction 121:41-49. [DOI] [PubMed] [Google Scholar]
- 14.Ellinwood, N. M., T. G. Berryere, B. P. Fournier, R. A. Bowen, F. C. Buchanan, and S. M. Schmutz. 1999. MX1 maps to cattle chromosome 1. Anim. Genet. 30:164-165. [DOI] [PubMed] [Google Scholar]
- 15.Ellinwood, N. M., J. M. McCue, P. W. Gordy, and R. A. Bowen. 1998. Cloning and characterization of cDNAs for a bovine (Bos taurus) Mx protein. J. Interferon Cytokine Res. 18:745-755. [DOI] [PubMed] [Google Scholar]
- 16.Files, J. G., J. L. Gray, L. T. Do, W. P. Foley, J. D. Gabe, E. Nestaas, and E. Pungor, Jr. 1998. A novel sensitive and selective bioassay for human type I interferons. J. Interferon Cytokine Res. 18:1019-1024. [DOI] [PubMed] [Google Scholar]
- 17.Flohr, F., S. Schneider-Schaulies, O. Haller, and G. Kochs. 1999. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 463:24-28. [DOI] [PubMed] [Google Scholar]
- 18.Forster, J., M. Schweizer, R. F. Schumacher, K. Kaufmehl, and S. Lob. 1996. MxA protein in infants and children with respiratory tract infection. Acta Paediatr. 85:163-167. [DOI] [PubMed] [Google Scholar]
- 19.Fray, M., G. Mann, and B. Charleston. 2001. Validation of an Mx/CAT reporter gene assay for the quantification of bovine type-I interferon. J. Immunol. Methods 249:235-244. [DOI] [PubMed] [Google Scholar]
- 20.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 70:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetschy, J. F., H. Zeller, J. Content, and M. A. Horisberger. 1989. Regulation of the interferon-inducible IFI-78K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J. Virol. 63:2616-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goidin, D., A. Mamessier, M. J. Staquet, D. Schmitt, and O. Berthier-Vergnes. 2001. Ribosomal 18S RNA prevails over glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as internal standard for quantitative comparison of mRNA levels in invasive and noninvasive human melanoma cell subpopulations. Anal. Biochem. 295:17-21. [DOI] [PubMed] [Google Scholar]
- 23.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 24.Haller, O., M. Frese, and G. Kochs. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Technol. 17:220-230. [DOI] [PubMed] [Google Scholar]
- 25.Halminen, M., J. Ilonen, I. Julkunen, O. Ruuskanen, O. Simell, and M. J. Makela. 1997. Expression of MxA protein in blood lymphocytes discriminates between viral and bacterial infections in febrile children. Pediatr. Res. 41:647-650. [DOI] [PubMed] [Google Scholar]
- 26.Hefti, H. P., M. Frese, H. Landis, C. Di Paolo, A. Aguzzi, O. Haller, and J. Pavlovic. 1999. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections. J. Virol. 73:6984-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horisberger, M. A. 1988. The action of recombinant bovine interferons on influenza virus replication correlates with the induction of two Mx-related proteins in bovine cells. Virology 162:181-186. [DOI] [PubMed] [Google Scholar]
- 28.Horisberger, M. A., and K. de Staritzky. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J. Gen. Virol. 68:945-948. [DOI] [PubMed] [Google Scholar]
- 29.Horisberger, M. A., and M. C. Gunst. 1991. Interferon-induced proteins: identification of Mx proteins in various mammalian species. Virology 180:185-190. [DOI] [PubMed] [Google Scholar]
- 30.Horisberger, M. A., P. Staeheli, and O. Haller. 1983. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc. Natl. Acad. Sci. USA 80:1910-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houe, H. 1999. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet. Microbiol. 64:89-107. [DOI] [PubMed] [Google Scholar]
- 32.Jakschies, D., R. Zachoval, R. Muller, M. Manns, K. U. Nolte, H. K. Hochkeppel, M. A. Horisberger, H. Deicher, and P. Von Wussow. 1994. Strong transient expression of the type I interferon-induced MxA protein in hepatitis A but not in acute hepatitis B and C. Hepatology 19:857-865. [PubMed] [Google Scholar]
- 33.Kochs, G., C. Janzen, H. Hohenberg, and O. Haller. 2002. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl. Acad. Sci. USA 99:3153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kracke, A., P. von Wussow, A. N. Al-Masri, G. Dalley, A. Windhagen, and F. Heidenreich. 2000. Mx proteins in blood leukocytes for monitoring interferon beta-1b therapy in patients with MS. Neurology 54:193-199. [DOI] [PubMed] [Google Scholar]
- 35.Leong, J. C., G. D. Trobridge, C. H. Kim, M. Johnson, and B. Simon. 1998. Interferon-inducible Mx proteins in fish. Immunol. Rev. 166:349-363. [DOI] [PubMed] [Google Scholar]
- 36.Lindenmann, J. 1962. Resistance of mice to mouse-adapted influenza A-virus. Virology 16:203-204. [DOI] [PubMed] [Google Scholar]
- 37.Lindenmann, J., E. Deuel, S. Fanconi, and O. Haller. 1978. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J. Exp. Med. 147:531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlovic, J., O. Haller, and P. Staeheli. 1992. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 66:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavlovic, J., and P. Staeheli. 1991. The antiviral potentials of Mx proteins. J. Interferon Res. 11:215-219. [DOI] [PubMed] [Google Scholar]
- 40.Roers, A., H. K. Hochkeppel, M. A. Horisberger, A. Hovanessian, and O. Haller. 1994. MxA gene expression after live virus vaccination: a sensitive marker for endogenous type I interferon. J. Infect. Dis. 169:807-813. [DOI] [PubMed] [Google Scholar]
- 41.Ronni, T., S. Matikainen, T. Sareneva, K. Melen, J. Pirhonen, P. Keskinen, and I. Julkunen. 1997. Regulation of IFN-alpha/beta, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J. Immunol. 158:2363-2674. [PubMed] [Google Scholar]
- 42.Ronni, T., K. Melen, A. Malygin, and I. Julkunen. 1993. Control of IFN-inducible MxA gene expression in human cells. J. Immunol. 150:1715-1726. [PubMed] [Google Scholar]
- 43.Rossi, C. R., and G. K. Kiesel. 1980. Factors affecting the production of bovine type I interferon on bovine embryonic lung cells by polyriboinosinic-polyribocytidylic acid. Am. J. Vet. Res. 41:557-560. [PubMed] [Google Scholar]
- 44.Rossi, C. R., G. K. Kiesel, and E. J. Hoff. 1980. Factors affecting the assay of bovine type I interferon on bovine embryonic lung cells. Am. J. Vet. Res. 41:552-556. [PubMed] [Google Scholar]
- 45.Schlender, J., B. Bossert, U. Buchholz, and K. K. Conzelmann. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234-8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon, A., J. Fah, O. Haller, and P. Staeheli. 1991. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J. Virol. 65:968-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staeheli, P. 1990. Interferon-induced proteins and the antiviral state. Adv. Virus Res. 38:147-200. [DOI] [PubMed] [Google Scholar]
- 49.Staeheli, P., P. Danielson, O. Haller, and J. G. Sutcliffe. 1986. Transcriptional activation of the mouse Mx gene by type I interferon. Mol. Cell. Biol. 6:4770-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suarez Heinlein, A., A. E. Metzler, R. Weiblen, P. Berrios, A. A. Schudel, and M. Rodriguez. 1993. Molecular characterization of South American bovine herpesvirus-1 isolates with monoclonal antibodies and SDS-PAGE. Zentralbl. Vet. B 40:125-130. [DOI] [PubMed] [Google Scholar]
- 51.Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111-116. [DOI] [PubMed] [Google Scholar]
- 52.Thur, B., K. Zlinszky, and F. Ehrensperger. 1996. Immunohistochemical detection of bovine viral diarrhea virus in skin biopsies: a reliable and fast diagnostic tool. Zentralbl. Vet. B 43:163-166. [DOI] [PubMed] [Google Scholar]
- 53.Towbin, H., A. Schmitz, D. Jakschies, P. Von Wussow, and M. A. Horisberger. 1992. A whole blood immunoassay for the interferon-inducible human Mx protein. J. Interferon Res. 12:67-74. [DOI] [PubMed] [Google Scholar]
- 54.van der Bliek, A. M. 1999. Functional diversity in the dynamin family. Trends Cell. Biol. 9:96-102. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, G., S. Aldridge, M. C. Clarke, and J. W. McCauley. 1996. Cell death induced by cytopathic bovine viral diarrhoea virus is mediated by apoptosis. J. Gen. Virol. 77:1677-1681. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, H., B. P. De, T. Das, and A. K. Banerjee. 1996. Inhibition of human parainfluenza virus-3 replication by interferon and human MxA. Virology 220:330-338. [DOI] [PubMed] [Google Scholar]