Abstract
We investigated the expression of membrane-bound CD14 (mCD14) on monocytes and soluble CD14 (sCD14) released into the culture supernatants of peripheral blood lymphocytes (PBMC) from human immunodeficiency virus (HIV)-infected individuals. Monocytes from HIV-positive individuals exhibited both enhanced mCD14 expression and sCD14 production in the PBMC culture supernatants compared to the levels of mCD14 and sCD14 in HIV-negative individuals. This enhanced mCD14 expression and sCD14 production in HIV-infected individuals may be due to the effects of cytokines, the bacterial product lipopolysaccharide (LPS), and/or the HIV regulatory antigens Tat and Nef. Interleukin-10 (IL-10), an immunoregulatory cytokine, as well as LPS enhanced mCD14 expression and the release of sCD14 in the culture supernatants. HIV-Nef, unlike Tat, enhanced mCD14 expression on monocytes but did not induce the release of sCD14 into the culture supernatants. Studies conducted to investigate the mechanism underlying HIV-Nef-induced mCD14 expression revealed that HIV-Nef upregulated mCD14 expression via a mechanism that does not involve endogenously produced IL-10. In contrast, LPS upregulated the expression of mCD14 and increased the release of sCD14 via a mechanism that involves, at least in part, endogenously produced IL-10. Furthermore, dexamethasone, an anti-inflammatory and immunosuppressive agent, inhibited HIV-Nef-induced CD14 expression in an IL-10-independent manner. In contrast, dexamethasone inhibited IL-10-dependent LPS-induced CD14 expression by interfering with IL-10-induced signals but not by blocking IL-10 production. These results suggest that HIV-Nef and IL-10 constitute biologically important modulators of CD14 expression which may influence immunobiological responses to bacterial infections in HIV disease.
The complex glycolipid lipopolysaccharide (LPS), a cell membrane component of gram-negative bacteria, is a potent immune stimulant that is responsible for many of the cellular responses to gram-negative septic shock. These responses may be induced after the association of LPS with the LPS-binding protein (LBP) (49), and the binding of this complex with the CD14/Toll-like receptor (TLR-4) complex (53, 54). CD14, the primary LPS receptor, exists in both cell surface membrane-bound and -soluble forms (27, 49). Membrane-bound CD14 (mCD14) is a 53-kDa GPI-linked cell surface antigen expressed on cells of the monocyte/macrophage lineage and to a lesser extent on neutrophils (58). Soluble CD14 (sCD14) likely represents cell surface CD14 that has been shed in response to either monocyte activation or differentiation (34). sCD14 can bind to circulating LPS-LBP complexes and can activate cells that do not normally express mCD14, such as CD14-negative monocytes and endothelial cells (24, 50).
CD14 expression is regulated by a number of factors, including the state of cell activation and the cytokines present in the microenvironment (8, 26, 30, 39, 52). Gamma interferon (IFN-γ) has been shown to induce CD14 expression in immature cell lines such as the myelomonocytic and monoblastic cell lines HL60 and U937 (20), although it has variable effects in more mature cell lines of the myelomonocytic lineage. Other cytokines that upregulate CD14 expression on monocytes include tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6, whereas IL-4 strongly reduces monocyte CD14 expression (48, 58).
The role of mCD14 and sCD14 in various disease states has been studied. Elevated levels of circulating sCD14 have been identified in patients with inflammatory conditions such as systemic lupus erythematosus (42), chronic active hepatitis (43), and septic shock (31). Immune suppression via administration of glucocorticoids has been shown to decrease mCD14 and sCD14 levels (41). In human immunodeficiency virus (HIV) infection, upregulated expression of mCD14, and increased levels of circulating sCD14 on monocytes (32, 40) and alveolar macrophages (56) have been reported. Elevated levels of sCD14 have been shown to correlate with disease progression in cases of HIV/AIDS (32, 40). Circulating LPS may have direct clinical significance in HIV disease, since LPS has been found to increase HIV production in latently infected cell lines via a CD14-dependent mechanism (4).
The molecular mechanism through which monocyte CD14 expression is upregulated in HIV infection remains unclear. Bacterial endotoxins (LPS), immunoregulatory cytokines (IFN-γ, TNF-α, IL-1, IL-4, IL-6, and IL-10) whose expression is upregulated in HIV infection (9, 10, 16, 18, 29, 36, 37), and circulating levels of HIV-regulatory antigens such as Tat, Nef, or gp120 may modulate CD14 expression on monocytes. HIV gp120 has been shown to modulate the expression of mCD14 expression on monocytes (57). In the present study, we investigated the effect of the HIV-regulatory proteins Tat and Nef, as well as immunoregulatory cytokines such as IL-10, on the expression of mCD14 on monocytic cells and the release of sCD14. The results suggest that mCD14 and sCD14 expression is enhanced in HIV-positive individuals and that this may be mediated in part through the effects of HIV-Nef and/or IL-10. We further investigated the mechanism underlying HIV-Nef-induced mCD14 expression on normal human monocytes. Our results suggest that HIV-Nef upregulates mCD14 expression via a mechanism that does not involve endogenously produced IL-10. In contrast, LPS upregulated the expression of mCD14 and increased the release of sCD14 via a mechanism that involves, at least in part, endogenously produced IL-10, suggesting that LPS and HIV-Nef antigen may induce CD14 expression on monocytes by distinct mechanisms. Furthermore, dexamethasone, an anti-inflammatory and immunosuppressive agent, inhibited HIV-Nef-induced CD14 expression in an IL-10-independent manner. In contrast, dexamethasone inhibited IL-10-dependent LPS-induced CD14 expression in monocytes by interfering with IL-10-induced signals but not by blocking IL-10 production.
MATERIALS AND METHODS
Reagents.
HIV-Nef was purchased from two sources, namely, Research Diagnostics, Inc. (Flanders, N.J.), and ImmunoDiagnostics, Inc. (Bedford, Mass.). The HIV-Nef obtained from Research Diagnostics was a 140-kDa recombinant protein fused at the N terminus with β-galactosidase. The HIV-Nef obtained from ImmunoDiagnostics, Inc., was produced as a glutathione S-transferase fusion protein in Escherichia coli. The recombinant protein was purified by glutathione-affinity chromatography, and the purified fusion protein was cleaved by thrombin to produce a 27-kDa HIV-Nef protein. Endotoxin content was determined by BioWhittaker QCL-1000 Kit (Walkersville, Md.) essentially as described by the manufacturer. Both the HIV-Nef preparations were found to contain endotoxin. Endotoxin was removed by passing both HIV-Nef preparations through a column of polymyxin B agarose beads (Sigma) by using standard methods (38). The HIV-Nef preparations thus obtained contained <0.01 U of endotoxin per mg of protein. Endotoxin-free HIV-Tat was also purchased from ImmunoDiagnostics, Inc. Human recombinant IL-10 was purchased from R&D Systems (Minneapolis, Minn.). Neutralizing anti-IL-10 antibodies and isotype-matched control antibodies were purchased from Pharmingen (Mississauga, Ontario, Canada). Dexamethasone was purchased from Sabex (Boucherville, Quebec, Canada).
Isolation and culture of PBMC.
Blood was obtained for isolation of peripheral blood mononuclear cells (PBMC) from healthy adult volunteers and HIV-positive individuals with CD4 counts ranging from 100 to 600 cells/μl after approval of the protocol by the Ethics Review Committee of the Ottawa General Hospital, University of Ottawa, Ottawa, Ontario, Canada. All patients were receiving reverse transcriptase inhibitors, and none had clinical evidence of acute infection at the time of specimen collection. PBMC were isolated by density gradient centrifugation over Ficoll-Hypaque (Pharmacia, Baie d'Urfe 130, Quebec, Canada) as previously described (14, 16). The cell layer, consisting mainly of mononuclear cells, was collected and washed three times in phosphate-buffered saline (PBS). PBMC were resuspended in Iscove modified Dulbecco medium (Sigma, St. Louis, Mo.) supplemented with 10% fetal bovine serum (FBS; Gibco Laboratories, Grand Island, N.Y.), 100 U of penicillin/ml, 100 μg of gentamicin/ml, and 10 mM HEPES.
Isolation of monocytes from PBMC.
Purified nonactivated monocytes were isolated by Optiprep density gradient medium (Nycomed Pharma AS, Oslo, Norway) (25). Briefly, Optiprep medium was mixed with blood and overlaid sequentially with lymphocyte specific density medium (1.078 g/ml), a solution with a density of 1.068 g/ml, followed by Hanks' balanced salt solution. The tubes were centrifuged at 600 × g for 25 min. The cell preparation obtained in the first fraction contained highly enriched (88 to 96%) CD14+ monocytes as determined by flow cytometric analysis. Contaminating T and B cells were eliminated by anti-CD2 and anti-CD19 antibody-conjugated immunomagnetic beads (Dynal AS, Oslo, Norway), respectively, as described earlier (16). These highly purified monocytes were not activated as determined by the comparable levels of HLA-DR expression on monocytes before and after isolation.
Cell stimulation and collection of culture supernatants.
PBMC from HIV-infected and uninfected individuals (2 × 106 cells/ml) or monocytes from HIV-seronegative individuals (1.0 × 106 cells/ml) were stimulated with HIV-Nef (0.5 to 4 μg/ml), LPS (0.1 to 1 μg/ml), or IL-10 (1 ng/ml) in 24-well tissue culture plates (Falcon, Becton Dickinson, Lincoln Park, N.J.). Cells were harvested after 48 h and analyzed for CD14 expression by flow cytometry as described below. The supernatants were harvested after 48 h and frozen at −70°C. Supernatants were thawed at the time of analysis for measurement of soluble CD14 production by a commercially available enzyme-linked immunosorbent assay (ELISA) kit. The cell supernatants were also analyzed for IL-10 and IL-12p40 production by ELISA. All experiments were done at least three times and figures show representative experiments.
Measurement of sCD14 in the culture supernatants.
Soluble CD14 was measured in the culture supernatant by ELISA by using a commercially available kit (IBL, Hamburg, Germany) as described by the manufacturer. Briefly, microtiter strips coated with oligoclonal antibody to human sCD14 were incubated with 50 μl of supernatant or 50 μl of the standards provided in the kit. The secondary biotinylated anti-CD14 antibody and the sample or supernatant were added simultaneously and incubated at room temperature for 2 h on an orbital shaker. The plates were washed three times after which peroxidase-labeled streptavidin was added for one h at room temperature on an orbital shaker. The plates were washed again three times. Soluble CD14 was detected with the tetramethyl benzidine (TMB) substrate solution. The color reaction was stopped with 1 M sulfuric acid, and the absorbance was read at 450 nm by using an ELISA reader (Bio-Rad Laboratories). The sensitivity of this assay was 2.2 ng/ml. Curve fitting and concentration calculations were performed by using the Microplate Manager 4.0 Software (Bio-Rad Laboratories).
Measurement of IL-10 and IL-12p40 in the culture supernatants by ELISA.
IL-10 and IL-12p40 were measured in the culture supernatant by ELISA by using two different monoclonal antibodies (MAb) that recognize distinct epitopes as described previously (16, 28, 33, 35). Briefly, the plates (Nunc Immunomodules, Roskilde, Denmark) were coated overnight at 4°C with the primary antibody (anti-IL-10 antibody 18551D from Pharmingen at a final concentration of 5 μg/ml; anti-IL-12p40 antibody MAb 609 from R&D Systems at a final concentration of 4 μg/ml) in coating buffer (0.04 M Na2CO3, 0.06 M NaHCO3; pH 9.6). The plates were washed with PBS-Tween 20 and blocked with PBS-10% FBS. The cytokines were detected by employing a second biotinylated MAb in PBS-10% FBS (anti-IL-10 antibody 18562D from Pharmingen at a final concentration of 4 μg/ml; anti-IL-12p40 antibody BAF219 from R&D Systems at a final concentration of 350 ng/ml). Streptavidin-peroxidase was used at a final dilution of 1:1,000 (Jackson Immunoresearch, West Grove, Pa.). The color reaction was developed by using OPD (Sigma) and hydrogen peroxide and then read at 450 nm. Recombinant IL-10 and IL-12 were used as standards.
RNA isolation and semiquantitative reverse transcription-PCR (RT-PCR) for IL-10 and IL-12p40.
Total cellular RNA was extracted from cells by using a monophase solution containing guanidine isothiocyanate and phenol (Tri-Reagent solution; Molecular Research Center, Inc., Cincinnati, Ohio) as described by the manufacturer (33, 35). Briefly, 1 μg of total RNA was reverse transcribed to generate cDNA. IL-10 and IL-12 expression was quantified by measuring their relative expression to that of β-actin gene, since β-actin is constitutively expressed in monocytic cells. Equal aliquots (5 μl) of cDNA equivalent to 100 ng of RNA were subsequently amplified for IL-10, IL-12p40, and β-actin by using 1.25 U of AmpliTaq DNA polymerase, 1 μM concentrations of each of the appropriate 5′ and 3′ primers, 0.5 mM concentrations of each deoxynucleoside triphosphates, and 2 mM MgCl2 in a total volume of 50 μl. The oligonucleotide primer sequences for IL-10 and β-actin (Stratagene, La Jolla, Calif.) are as follows: IL-10, sense (5′-GCC TAA CAT GCT TCG AGA TC-3′); IL-10, antisense (5′-TGA TGT CTG GGT CTT GGT TC-3′); β-actin, sense (5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′); and β-actin, antisense (5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3′) (14, 35). The primers for IL-12 p40 were purchased from Stratagene (La Jolla, Calif.). The conditions for amplification for IL-10 and β-actin have been described (14). For IL-12, the first cycle consisted of denaturing at 94°C for 5 min and annealed at 60°C for 5 min, followed by 35 cycles as follows: 72°C for 1.5 min, 94°C for 45 s, and 60°C for 45 s. The last cycle was an extension at 72°C for 10 min. The amplified products for IL-10 (204 bp), IL-12 (373 bp), and β-actin (610 bp) were resolved by electrophoresis on 1.2% agarose gels and visualized by ethidium bromide staining. To ensure that equal quantities of cDNA were used in the PCRs, we performed densitometric analysis of the PCR results and normalized it to the β-actin gene expression.
Flow cytometric analysis.
Cells were subjected to flow cytometric analysis, after 48 h of culture as described earlier (33). Briefly, cells were washed once at the time of harvesting with PBS-0.1% sodium azide and distributed into flow cytometry tubes (Falcon, Lincoln Park, N.J.). Cells were stained with 3 μl of fluorescein isothiocyanate (FITC)-labeled anti-CD14 MAbs (Becton Dickinson). Autofluorescence and isotype-matched control antibodies, including immunoglobulin G2b (Becton Dickinson), were included. For PBMC, two distinct populations representing the lymphocytes and monocytes could be visualized based on forward scatter versus side scatter dot plot diagrams. Analysis of CD14 expression was performed on CD14+ cells in the monocyte gate. The gates were set in accordance with the gates obtained with the isotype-matched control antibodies. Mean channel fluorescence (MCF) was obtained from the CD14+ expressing clusters of monocytes. Data were acquired on a Becton Dickinson FACScan flow cytometer. Validity of comparisons in the expression levels of CD14 between different patient populations was ensured through the use of Calibrite Beads (Becton Dickinson). The data were analyzed by using the WinMDI software package (J. Trotter, The Scripps Research Institute, San Diego, Calif.).
Statistical analysis.
As small sample sizes precluded an assumption of a normal distribution, the data were analyzed by using Fisher Exact test. The results are expressed as means ± the standard errors of the means.
RESULTS
HIV infection is associated with elevated monocyte cell surface CD14 expression and elevated levels of soluble CD14 production in PBMC.
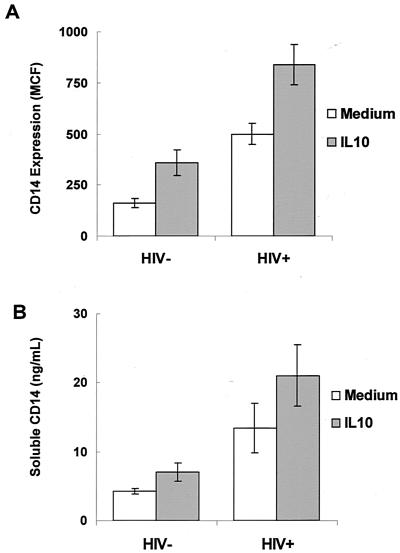
PBMC from eight HIV-infected individuals and five uninfected individuals were cultured without stimulation, and monocytes were analyzed for the levels of CD14 expression by flow cytometry. Monocytes from HIV-infected individuals expressed significantly higher levels of mCD14 compared to monocytes from uninfected controls (P < 0.05; Fig. 1A). Increased expression of mCD14 on monocytes may lead to increased shedding of sCD14 into the extracellular medium. To determine whether increased expression of mCD14 on monocytes from HIV-infected individuals correlates with higher levels of sCD14 in the culture medium, PBMC from eight HIV-infected individuals and five healthy controls were cultured in the absence of any mitogenic stimuli. PBMC from HIV-infected individuals released higher levels of sCD14 into the culture supernatants compared to that of the unstimulated PBMC from uninfected controls (P < 0.05; Fig. 1B). There were no differences in the amount of sCD14 secreted by PBMC from HIV-infected or HIV-negative individuals after stimulation with either phytohemagglutinin (PHA) or anti-CD3 antibodies (data not shown).
FIG. 1.
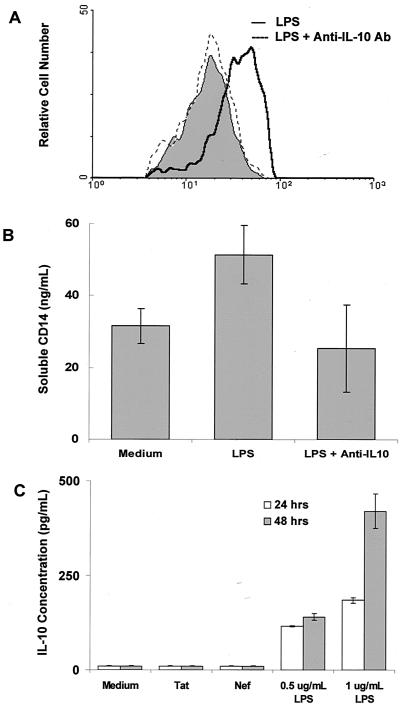
IL-10 upregulates mCD14 expression on monocytes and enhances the production of sCD14 in HIV-infected and uninfected individuals. PBMC (2 × 106 per ml) from HIV-infected (n = 8) and uninfected controls (n = 5) were cultured in the presence or in the absence of IL-10 (1 ng/ml) for 48 h. (A) PBMC were stained with FITC-labeled anti-CD14 antibodies and subjected to flow cytometric analysis after 48 h of culture. (B) Culture supernatants were removed after 48 h of culture, and sCD14 was measured by ELISA.
IL-10 upregulates mCD14 expression on monocytes in HIV-positive and HIV-negative individuals.
IL-10 expression is altered in HIV infection (9, 10, 16, 18, 29, 36, 37). To determine whether IL-10 can modulate CD14 expression or sCD14 secretion, PBMC from HIV-positive (n = 8) and HIV-negative individuals (n = 5) were cultured in the presence or in the absence of IL-10. IL-10 induced the expression of mCD14 on monocytes from both HIV-positive and HIV-negative individuals (P < 0.05; Fig. 1A). A representative histogram showing upregulation of CD14 by IL-10 on CD14+ monocytes from an HIV-negative individual is shown in Fig. 3C. Although not reaching statistical significance, IL-10 enhanced sCD14 production by PBMC of both HIV-positive and HIV-negative individuals (Fig. 1B). Addition of PHA or anti-CD3 antibodies in addition to IL-10 did not cause further alterations in sCD14 production (data not shown).
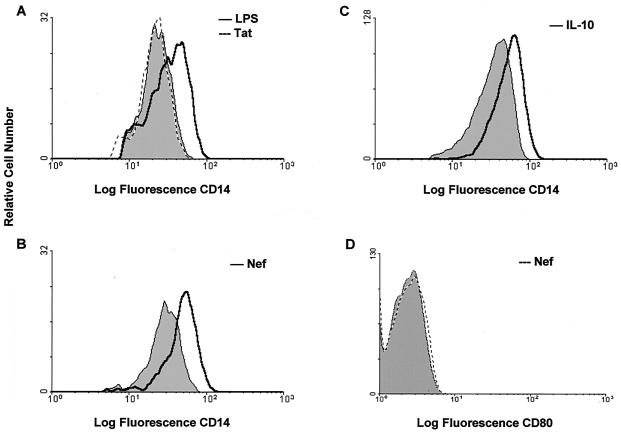
FIG. 3.
Flow cytometric analysis of mCD14 expression on monocytes after stimulation with HIV-Tat and LPS (A), HIV-Nef (B), and IL-10 (C). Monocytes (1.0 × 106 cells/ml) from healthy controls were cultured in the presence or absence of HIV-Tat or -Nef proteins (2 μg/ml), IL-10 (1 ng/ml), or LPS (1 μg/ml) for 48 h. The monocytes were stained with FITC-labeled anti-CD14 antibodies and subjected to flow cytometric analysis. (D) HIV-Nef-stimulated cells were stained for the expression of B7.1 (CD80) by using FITC-labeled anti-B7.1 antibodies. The shaded area indicates CD14 expression on cells cultured with isotype-matched control antibodies. The histograms shown are of one representative experiment from a total of six experiments.
Effect of HIV-Nef protein and LPS on CD14 expression on monocytes and the production of sCD14.
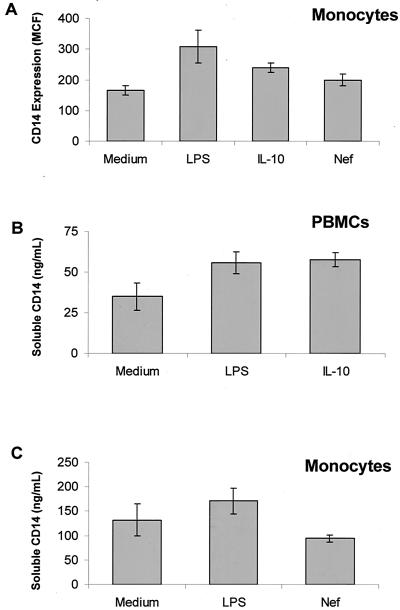
Enhanced levels of mCD14 and sCD14 in HIV-infected individuals could be attributed to several factors, such as prior bacterial infection, HIV regulatory antigens such as Tat or Nef antigens, or altered expression of immunoregulatory cytokines such as IL-10. To determine that increased expression of mCD14 and the release of sCD14 in culture supernatants of PBMC from HIV-positive individuals is mediated by bacterial products such as LPS, PBMC or monocytes from HIV-negative healthy individuals (n = 5) were exposed to LPS for 48 h. Expression of mCD14 on monocytes and release of sCD14 was upregulated by LPS to similar levels as by IL-10 (P <0.05; Fig. 2A and B). Figure 2B shows the release of sCD14 by LPS- or IL-10-stimulated PBMCs whereas Fig. 2C shows the sCD14 released from LPS-stimulated purified monocytes. A representative histogram showing upregulation of CD14 by LPS is shown in Fig. 3A.
FIG. 2.
Effect of HIV-1 Nef protein, LPS, and IL-10 on mCD14 expression and the production of sCD14. PBMC (2 × 106 cells/ml) or monocytes (1.0 × 106 cells/ml) from healthy individuals (n = 6) were cultured with LPS (1 μg/ml), HIV-Nef protein (2 μg/ml), and IL-10 (1 ng/ml) for 48 h. (A) Cells were stained with FITC-labeled anti-CD14 antibodies and subjected to flow cytometric analysis. Culture supernatants were removed after 48 h of culture, and sCD14 was measured by ELISA. (B) Release of sCD14 from PBMC (2 × 106 cells/ml) stimulated with LPS and IL-10. (C) Release of sCD14 from monocytes (1.0 × 106 cells/ml) stimulated with LPS and HIV-Nef.
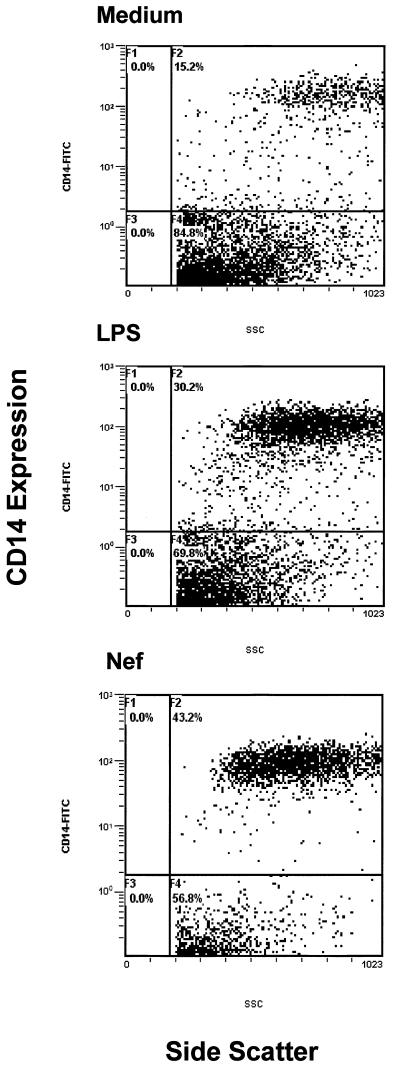
To investigate the effect of HIV regulatory proteins Tat and Nef on CD14 expression, we cultured monocytes from healthy controls (n = 5) in the presence or absence of LPS-free HIV-Tat and -Nef proteins and subjected the cells to flow cytometric analysis. The HIV-Tat protein did not affect mCD14 expression (Fig. 3A) or sCD14 production (data not shown). The HIV-Nef antigen in the soluble form enhanced CD14 expression to levels comparable to IL-10 (P < 0.05; Fig. 2A). Similar results were obtained by using HIV-Nef in the form of a fusion protein (data not shown). A representative histogram showing upregulation of CD14 by HIV-Nef and IL-10 on purified human monocytes is shown in Fig. 3B and C, respectively. HIV-Nef did not influence the expression of B7.1 (Fig. 3D) or CD44 (data not shown) on monocytes, indicating that the HIV-Nef-induced CD14 expression was specific. However, HIV-Nef did not induce the production of sCD14 by monocytes (Fig. 2C). Since significant differences were not found in HIV-Nef-induced CD14 expression on purified monocytes and on CD14+ monocytic cells within the bulk PBMC populations, and because it was not feasible to obtain large quantities of blood for monocytes isolation, we analyzed CD14 expression on monocytic cells within the PBMC of HIV-infected individuals, following stimulation with HIV-Nef. PBMC from HIV-infected individuals were stimulated with the HIV-Nef antigen for 48 h. CD14 was expressed on ca. 15% of unstimulated monocytic cells. HIV-Nef enhanced CD14 expression on 43% monocytes of HIV-infected individuals to levels similar to that observed after LPS stimulation on 30% monocytes (Fig. 4).
FIG. 4.
HIV-Nef upregulates mCD14 expression on monocytes from HIV-infected individuals. PBMC (2 × 106 per ml) from HIV-infected (n = 4) were cultured with medium alone, LPS (15 pg/ml), or HIV-Nef (2 μg/ml). PBMC were stained with FITC-labeled anti-CD14 antibodies and then subjected to flow cytometric analysis after 48 h of culture.
Differential utilization of endogenously produced IL-10 in LPS- and HIV-Nef-induced mCD14 expression on monocytes.
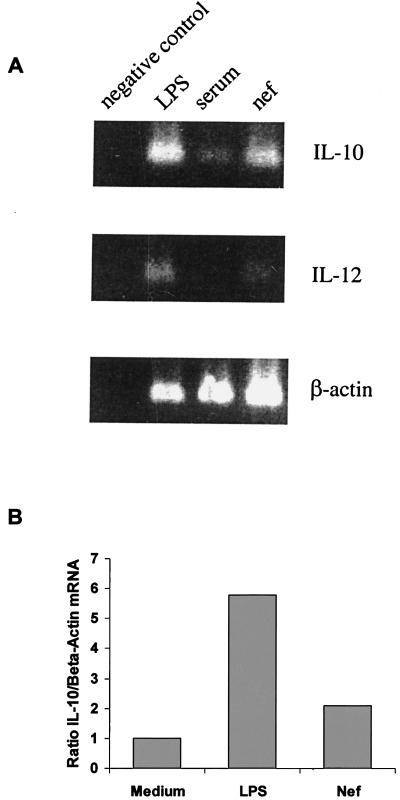
Stimulation of monocytes with LPS and HIV-Nef has been shown to produce IL-10 (5, 21). To determine the role of endogenously produced IL-10 in LPS- and HIV-Nef-induced CD14 expression, monocytes from healthy controls were cultured with LPS or HIV-Nef antigens in the presence or absence of neutralizing concentrations of anti-IL-10 antibodies or isotype-matched control antibodies. Addition of anti-IL-10 antibodies blocked the LPS-induced upregulation of cell surface CD14 expression. Histograms from one representative experiment from a total of five experiments performed are shown in Fig. 5A. Likewise, increased production of sCD14 induced by LPS was blocked by the addition of neutralizing concentrations of anti-IL-10 antibodies (Fig. 5B). Addition of anti-IL-10 antibodies did not neutralize HIV-Nef-induced expression of mCD14 (data not shown). This was attributed to the lack of IL-10 production induced by HIV-Nef antigens as determined by ELISA (Fig. 5C). However, HIV-Nef induced low levels of IL-10 mRNA expression in monocytes as determined by semiquantitative RT-PCR analysis (Fig. 6A). Densitometric analysis revealed that HIV-Nef induced a twofold increase in IL-10 mRNA compared to the sixfold increase observed after LPS stimulation (Fig. 6B). To rule out the possibility that HIV-Nef-induced IL-10 mRNA expression was due to the trace amounts of endotoxin contamination, we compared HIV-Nef-induced IL-12 expression with IL-12 production induced by various concentrations of LPS. LPS induced IL-10 (120 pg/ml) and IL-12 (2,200 pg/ml) production in monocytes at concentrations as low as 15 pg/ml, as determined by ELISA and RT-PCR analysis (Fig. 6A). However, HIV-Nef, unlike LPS, did not induce the production of IL-10 and IL-12 as determined by ELISA (Fig. 5C and data not shown). HIV-Nef also failed to induce IL-12 mRNA expression in monocytes as determined by RT-PCR analysis (Fig. 6A), indicating that HIV-Nef preparations were endotoxin free. Taken together, these results suggest that HIV-Nef upregulates the expression of cell surface CD14 via a mechanism that does not involve endogenously produced IL-10. In contrast, LPS upregulates the expression of cell surface CD14 and increases the release of sCD14 via a mechanism that involves, at least in part, endogenously produced IL-10, suggesting that LPS and HIV-Nef antigen may induce CD14 expression on monocytes by distinct mechanisms.
FIG. 5.
Differential effect of endogenously produced IL-10 on CD14 expression and release of sCD14 by LPS and HIV-Nef. Monocytes (1.0 × 106 cells/ml) from healthy controls (n = 5) were stimulated with LPS or Nef antigens in the presence or in the absence of neutralizing anti-IL-10 antibodies (20 μg/ml) or isotype-matched control antibodies (20 μg/ml). Monocytes were stained with FITC-labeled anti-CD14 antibodies and subjected to flow cytometric analysis after 48 h of culture. (A) Histograms from one representative experiment of the total five performed are shown. The shaded area indicates CD14 expression on monocytes cultured with isotype-matched control antibodies. The dark line and the broken line indicate CD14 expression on monocytes stimulated with LPS in the presence of control antibodies and anti-IL-10 antibodies, respectively. (B) Supernatants harvested after 48 h of culture were analyzed for the production of sCD14 by ELISA as described in Materials and Methods. (C) Supernatants harvested after 48 h were also analyzed for the production of IL-10 by ELISA.
FIG. 6.
Effect of HIV-Nef and LPS on IL-10 and IL-12 expression by RT-PCR. Monocytes (1.0 × 106 cells/ml) were stimulated with HIV-Nef (2 μg/ml) or LPS (15 pg/ml) for 8 h. mRNA was isolated from cells, reverse transcribed and amplified for IL-10 and IL-12 by RT-PCR analysis as described in Materials and Methods. The amplified products were subjected to gel electrophoresis. IL-10 and IL-12 bands were visualized by ethidium bromide staining. The results shown are representative of three experiments performed. (B) Densitometric ratios of IL-10 mRNA to β-actin induced by LPS and HIV-Nef (medium = 1).
Dexamethasone inhibits HIV-Nef- and LPS-induced CD14 expression in an IL-10-independent and IL-10-dependent manner, respectively.
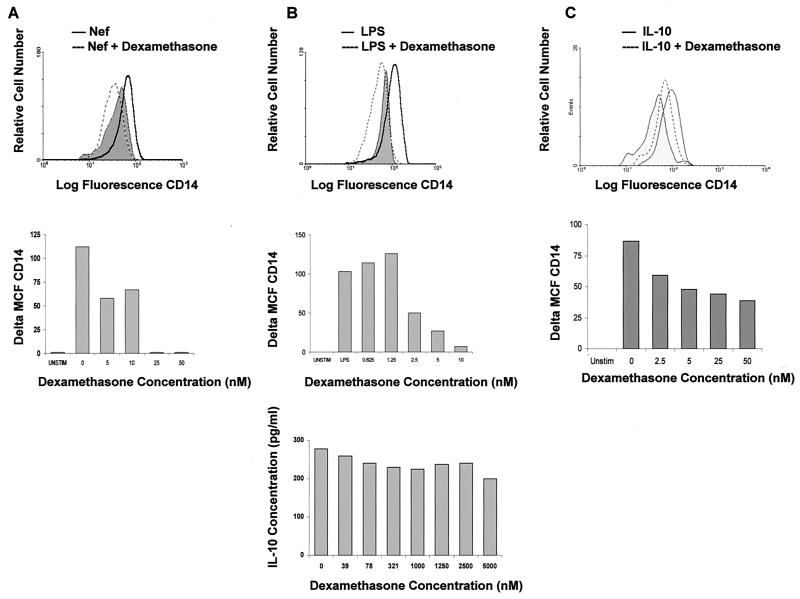
To further investigate that LPS and HIV-Nef induce mCD14 expression by distinct mechanisms, we studied the effects of a glucocorticoid, dexamethasone, on the LPS- and HIV-Nef-induced mCD14 expression. Dexamethasone, an anti-inflammatory and immunosuppressive agent, has been shown to inhibit the expression of several cell surface molecules, including B7 (23, 33) and cytokines such as IL-12 (55). To determine the effect of dexamethasone on CD14, purified monocytes obtained from healthy individuals were treated with dexamethasone for 2 h prior to stimulation with either LPS or HIV-Nef. Very low concentrations of dexamethasone (25 nM) completely inhibited HIV-Nef-induced CD14 expression (Fig. 7A). Similarly, LPS-induced CD14 expression was inhibited by dexamethasone in a dose-dependent manner (Fig. 7B, top and middle panel).
FIG. 7.
Effect of dexamethasone on IL-10- and HIV-Nef-induced mCD14 upregulation. Purified monocytes (1.0 × 106 cells/ml) were treated with 5 to 50 nM dexamethasone for 2 h prior to stimulation with HIV-Nef (2 μg/ml) (A), LPS (1 μg/ml) (B), or IL-10 (1 ng/ml) (C). CD14 expression was analyzed 48 h after stimulation by flow cytometry. The results shown are representative of three experiments performed. A change in CD14 expression (Delta MCF CD14) is represented by a difference in MCF between stimulated cells and the cells treated with dexamethasone prior to stimulation. A representative histogram is shown in the upper portion of panels A, B, and C in which a single concentration of 50 nM dexamethasone has been used. Supernatants harvested from dexamethasone- and LPS-stimulated cells were analyzed for IL-10 production by ELISA. The results are shown at the bottom of panel B.
Since LPS-induced CD14 expression is mediated by endogenously produced IL-10, dexamethasone may inhibit LPS-induced CD14 expression by blocking either the production of IL-10 or by interfering with the signals delivered after the interaction of IL-10 with its receptor. To determine the effect of dexamethasone on LPS-induced IL-10 production, monocytes were treated with dexamethasone for 2 h prior to stimulation with LPS. Treatment of monocytes with dexamethasone even at very high concentrations of 5000 nM failed to alter IL-10 production (Fig. 7B, lower panel). These results indicate that dexamethasone inhibits LPS-induced CD14 expression by a mechanism independent of endogenously produced IL-10. To determine the effect of dexamethasone on IL-10-induced signals, leading to CD14 expression, monocytes were treated with dexamethasone for 2 h prior to stimulation with IL-10. Dexamethasone treatment of monocytes inhibited IL-10-induced CD14 upregulation in a dose-dependent manner (Fig. 7C), indicating that dexamethasone may modulate IL-10-mediated signals, at least with respect to CD14 expression. Taken together, the results suggest that dexamethasone inhibited HIV-Nef-induced CD14 expression in an IL-10-independent manner. In contrast, LPS-induced CD14 expression was found to be dependent, at least in part, on endogenously produced IL-10, and dexamethasone inhibited LPS-induced CD14 expression in monocytes by interfering with IL-10-induced signals but not by blocking IL-10 production.
DISCUSSION
Monocyte/macrophages play important roles in HIV/AIDS pathogenesis by acting as reservoirs for HIV through dysregulated production of immunoregulatory cytokines and through their altered performance as antigen-presenting cells (11). In the present study, we investigated the regulation of monocyte mCD14 expression and the release of sCD14 as a potential mechanism by which alterations in monocyte function may contribute to HIV/AIDS immunopathogenesis. Monocytes from HIV-infected individuals expressed higher levels of mCD14 and released significantly higher levels of sCD14 into the culture supernatants than did those of HIV-negative individuals. Monocytic CD14 may be modulated by bacterial endotoxins, immunoregulatory cytokines (IFN-γ, TNF-α, IL-1, IL-4, IL-6, and IL-10) (9, 10, 16, 18, 29, 36, 37), and circulating levels of HIV-regulatory antigens such as Tat or Nef. In the present study, we show that HIV-Nef enhanced mCD14 expression on human monocytes derived from HIV-negative and HIV-infected individuals that was comparable to the levels induced by the bacterial product LPS. However, HIV-Nef, unlike LPS, did not alter the release of sCD14 into the culture supernatants. Studies designed to understand the molecular mechanism underlying HIV-Nef-induced CD14 expression suggest that HIV-Nef upregulates mCD14 expression via a mechanism that does not involve endogenously produced IL-10. In contrast, LPS upregulates the expression of cell surface CD14 and increases the release of sCD14 via a mechanism that involves, at least in part, endogenously produced IL-10. We also show that dexamethasone, an anti-inflammatory/immunosuppressive agent, inhibited HIV-Nef- and LPS-induced mCD14 expression in an IL-10-independent and IL-10-dependent manner, respectively. Our results further suggest that dexamethasone inhibited LPS-induced CD14 expression by interfering with IL-10-mediated signals.
The findings of increased mCD14 expression and increased sCD14 release in HIV-positive individuals are in agreement with a previous publication reporting similar increases in subjects with HIV infection at all stages of disease (40). We did not observe an increase in mCD14 expression on monocytes infected in vitro with HIV (data not shown). This may be attributed to differences in the microenvironment in which the cells become infected in vitro as opposed to in vivo. The functional significance of the enhanced expression of mCD14 on monocytic cells is not clear at present. Higher levels of mCD14 and circulating sCD14 in HIV-infected individuals may reflect their chronic state of inflammation and may be involved in enhanced susceptibility to gram-negative infections, as well as the production of cytokines such as IL-1, IL-6, and TNF-α (19). Increased levels of sCD14 released in culture supernatants would be expected to be associated with decreased mCD14 expression if sCD14 represents mCD14 which has been shed into the extracellular environment (58). However, concomitant increases in the levels of sCD14 and mCD14 expression suggest that mCD14 upregulation could be mediated through increased transcription/translation or through the release of intracellular stores.
We show for the first time that IL-10 enhances the expression of mCD14 and the release of sCD14 in culture supernatants of PBMC of both HIV-positive and uninfected individuals, and LPS-induced CD14 expression is mediated, at least in part, by endogenously produced IL-10. In general, IL-10 downregulates the expression of cell surface receptors and synthesis of cytokines (51). Our results suggest that CD14, along with CD16 (7), is one of the few monocyte surface receptors whose expression is enhanced by IL-10. We and others have shown altered IL-10 production by PBMC of HIV-positive individuals (10, 13, 16, 36) which has been correlated with impaired ability to secrete IL-2 (10) and reduced T-cell proliferation (13). IL-10 has also been shown to modulate HIV replication in cells of the monocyte/macrophage lineage (1, 3). Our results raise questions as to whether enhanced CD14 expression induced by IL-10 also translates into increased susceptibility of the host to bacterial infections.
HIV-Nef has been identified as playing a central role in HIV/AIDS immunopathogenesis (45). HIV-Nef is produced early in the HIV life cycle and features a highly immunogenic extracellular carboxyl terminus (44). Among other activities, HIV-Nef aids in the maintenance of a high viral load and protects virally infected cells from immune clearance, in part through downregulation of surface expression of the viral coreceptor CD4 via accelerated endocytosis in clathrin-coated pits (17) and decreased surface expression of MHC class I molecules (12). HIV-Nef specific antibodies are present in the circulation of two-thirds of HIV-seropositive individuals (2, 47). Exogenously added HIV-Nef inhibits the proliferation of CD4+ cells and reduces the responsiveness of T cells and PBMC to mitogenic stimulation by PHA (22).
Our results also show for the first time that HIV-Nef upregulates mCD14 expression but fails to induce the release of sCD14 into the culture supernatants. The HIV-Nef-induced CD14 expression was specific, as other cell surface antigens such as B7 or CD44 were not affected. It may be noted that HIV-Nef-induced CD14 expression levels (increase of 100 to 120 MCF) were similar to the levels observed following LPS and IL-10 stimulation. The HIV-Nef preparations used in these studies were endotoxin free that was supported by the lack of IL-10 or IL-12 production after HIV-Nef stimulation. Furthermore, in contrast to LPS-induced CD14 induction, HIV-Nef-induced CD14 induction was not mediated by the endogenously produced IL-10. Our HIV-Nef preparations were derived from prokaryotic cells and hence lacked myristylation in the N-terminal region. The possibility that HIV-Nef derived from eukaryotic cell lines may have yielded different results cannot be ruled out.
HIV-Nef has been shown to induce IL-10 production by human monocytes in a study reported by Brigino et al. (5). However, our studies failed to show enhanced IL-10 production in HIV-Nef-stimulated normal human monocytes as determined by ELISA, although HIV-Nef did induce low-level expression of IL-10 mRNA, as revealed by semiquantitative RT-PCR analysis. HIV-Nef induced an ∼2-fold increase in IL-10 mRNA expression compared to a >8-fold increase induced by LPS. This low-level increase in mRNA may not be sufficient to translate into a detectable levels of secreted IL-10. We were unable to detect IL-10 production in monocytic cells stimulated with HIV-Nef for up to 4 days. It is likely that very low levels of IL-10 may be produced by monocytes stimulated with our HIV-Nef preparations, but these levels may be below the detection limit of our assay systems. The reasons for the differences in these two studies are not clear but may be attributed to the specific HIV-Nef preparations used.
In the present study, we attempted to elucidate the molecular mechanism underlying the HIV-Nef-induced CD14 expression in normal human monocytic cells. Our results suggest that the HIV-Nef-induced CD14 expression may be mediated via mechanisms independent of endogenously produced IL-10. In contrast, our results reveal that LPS-induced CD14 expression in monocytic cells may be mediated, at least in part, through endogenous production of IL-10. To further understand the molecular mechanism in the regulation of CD14 expression, we employed an immunosuppressive and anti-inflammatory glucocorticoid, dexamethasone. Dexamethasone has been suggested to favor the development of T helper type 2 responses potentially through the inhibition of IFN-γ and IL-2 production and/or the stimulation of IL-4 and IL-10 production by primed human T cells (6, 15, 46). Since dexamethasone has been shown to inhibit mCD14 and sCD14 production when administered to patients with acute inflammatory diseases (41), and it alters the synthesis of cytokines which modulate CD14 expression (6, 15, 46), we hypothesized that dexamethasone may differentially inhibit IL-10-, LPS-, and HIV-Nef-induced CD14 expression. In the present study we show that dexamethasone inhibited HIV-Nef-induced CD14 expression. Since HIV-Nef did not induce IL-10 production, our results suggest that dexamethasone inhibited HIV-Nef-induced CD14 expression independent of endogenously produced IL-10. Like HIV-Nef, dexamethasone downregulated LPS-induced CD14 expression. Since dexamethasone did not inhibit LPS-induced IL-10 production but inhibited IL-10-induced CD14 upregulation, the results suggest that dexamethasone interferes with the IL-10 receptor-mediated signaling pathway, at least with respect to CD14 expression. It is likely that dexamethasone inhibits CD14 expression by interfering with the signals delivered through the CD14-mediated pathway. Whether dexamethasone inhibits LPS-mediated CD14 induction through TOLL receptors needs to be investigated. Nonetheless, these results suggest that LPS- and HIV-Nef-induced CD14 expression are mediated by distinct mechanisms, although the exact mechanism by which dexamethasone regulates LPS-, IL-10-, and HIV-Nef-induced CD14 expression remains unknown.
In summary, our results suggest that the HIV-Nef antigen and IL-10 constitute important modulators of CD14 expression on monocytes. The biological significance of CD14 upregulation by LPS, IL-10, and HIV-Nef is not clear at present. CD14 has been suggested to be a potential target for therapeutic strategies because of its ability to interact with LPS-LBP complexes, allowing for their removal from the circulation. Enhanced expression of mCD14 and sCD14 by LPS, HIV-Nef, and IL-10 may serve a negative regulatory role to help combat invading organisms. Although the regulation of CD14 expression and the release of sCD14 is a very complex issue requiring extensive future investigation, our results point toward an important mechanism by which HIV may alter immune responses and may have implications with respect to immunobiological responses to bacterial infections and bacterial products in HIV/AIDS.
Acknowledgments
This work was supported by grants from the Ministry of Health, Ontario, Canada, the Research Institute, Children's Hospital of Eastern Ontario, and the Canadian Foundation for AIDS Research (A.K.).
REFERENCES
- 1.Akridge, R. E., L. K. Oyafuso, and S. G. Reed. 1994. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J. Immunol. 153:5782-5789. [PubMed] [Google Scholar]
- 2.Ameisen, J. C., B. Guy, S. Chamaret, M. Loche, B. Mach, A. Tartar, Y. Mouton, and A. Capron. 1989. Persistent antibody response to the HIV-1-negative regulatory factor in HIV-1-infected seronegative persons. N. Engl. J. Med. 320:251-252. [DOI] [PubMed] [Google Scholar]
- 3.Angel, J. B., B. M. Saget, M. Z. Wang, A. Wang, C. A. Dinarello, and P. R. Skolnik. 1995. Interleukin-10 enhances human immunodeficiency virus type 1 expression in a chronically infected promonocytic cell line (U1) by a tumor necrosis factor alpha-independent mechanism. J. Interferon Cytokine Res. 15:575-584. [PubMed] [Google Scholar]
- 4.Bagasra, O., S. D. Wright, T. Seshamma, J. W. Oakes, and R. J. Pomerantz. 1992. CD14 is involved in control of human immunodeficiency virus type 1 expression in latently infected cells by lipopolysaccharide. Proc. Natl. Acad. Sci. USA 89:6285-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigino, E., S. Haraguchi, A. Koutsonikolis, G. J. Cianciolo, U. Owens, R. A. Good, and N. K. Day. 1997. Interleukin-10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc. Natl. Acad. Sci. USA 94:3178-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann, V., and C. Kristofic. 1995. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO− and CD45RO+ subsets. J. Immunol. 155:3322-3328. [PubMed] [Google Scholar]
- 7.Calzada-Wack, J. C., M. Frankenberger, and H. W. Ziegler-Heitbrock. 1996. Interleukin-10 drives human monocytes to CD16 positive macrophages. J. Inflamm. 46:78-85. [PubMed] [Google Scholar]
- 8.Canque, B., S. Camus, M. Yagello, and J. C. Gluckman. 1998. IL-4 and CD40 ligation affect differently the differentiation, maturation, and function of human CD34+ cell-derived CD1a+ CD14− and CD1a− CD14+ dendritic cell precursors in vitro. J. Leukoc. Biol. 64:235-244. [DOI] [PubMed] [Google Scholar]
- 9.Clerici, M., and G. M. Shearer. 1994. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol. Today 15:575-581. [DOI] [PubMed] [Google Scholar]
- 10.Clerici, M., T. A. Wynn, J. A. Berzofsky, S. P. Blatt, C. W. Hendrix, A. Sher, R. L. Coffman, and G. M. Shearer. 1994. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Investig. 93:768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, O. J., A. Kinter, and A. S. Fauci. 1997. Host factors in the pathogenesis of HIV disease. Immunol. Rev. 159:31-48. [DOI] [PubMed] [Google Scholar]
- 12.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 13.Daftarian, M. P., F. Diaz-Mitoma, W. D. Creery, W. Cameron, and A. Kumar. 1995. Dysregulated production of interleukin-10 (IL-10) and IL-12 by peripheral blood lymphocytes from human immunodeficiency virus-infected individuals is associated with altered proliferative responses to recall antigens. Clin. Diagn. Lab. Immunol. 2:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daftarian, P. M., A. Kumar, M. Kryworuchko, and F. Diaz-Mitoma. 1996. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J. Immunol. 157:12-20. [PubMed] [Google Scholar]
- 15.Daynes, R. A., and B. A. Araneo. 1989. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur. J. Immunol. 19:2319-2325. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Mitoma, F., A. Kumar, S. Karimi, M. Kryworuchko, M. P. Daftarian, W. D. Creery, L. G. Filion, and W. Cameron. 1995. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin. Exp. Immunol. 102:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerman, M., and M. H. Malim. 1998. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280:1880-1884. [DOI] [PubMed] [Google Scholar]
- 18.Emilie, D., R. Fior, L. Llorente, A. Marfaing-Koka, M. Peuchmaur, O. Devergne, B. Jarrousse, J. Wijdenes, F. Boue, and P. Galanaud. 1994. Cytokines from lymphoid organs of HIV-infected patients: production and role in the immune disequilibrium of the disease and in the development of B lymphomas. Immunol. Rev. 140:5-34. [DOI] [PubMed] [Google Scholar]
- 19.Esser, R., W. Glienke, R. Andreesen, R. E. Unger, M. Kreutz, H. Rubsamen-Waigmann, and H. von Briesen. 1998. Individual cell analysis of the cytokine repertoire in human immunodeficiency virus-1-infected monocytes/macrophages by a combination of immunocytochemistry and in situ hybridization. Blood 91:4752-4760. [PubMed] [Google Scholar]
- 20.Firestein, G. S., and N. J. Zvaifler. 1987. Down regulation of human monocyte differentiation antigens by interferon gamma. Cell. Immunol. 104:343-354. [DOI] [PubMed] [Google Scholar]
- 21.Foey, A. D., S. L. Parry, L. M. Williams, M. Feldmann, B. M. Foxwell, and F. M. Brennan. 1998. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. 160:920-928. [PubMed] [Google Scholar]
- 22.Fujii, Y., M. Ito, and K. Ikuta. 1993. Evidence for the role of human immunodeficiency virus type 1 Nef protein as a growth inhibitor to CD4+ T lymphocytes and for the blocking of the Nef function by anti-Nef antibodies. Vaccine 11:837-847. [DOI] [PubMed] [Google Scholar]
- 23.Girndt, M., U. Sester, H. Kaul, F. Hunger, and H. Kohler. 1998. Glucocorticoids inhibit activation-dependent expression of costimulatory molecule B7-1 in human monocytes. Transplantation 66:370-375. [DOI] [PubMed] [Google Scholar]
- 24.Golenbock, D. T., R. R. Bach, H. Lichenstein, T. S. Juan, A. Tadavarthy, and C. F. Moldow. 1995. Soluble CD14 promotes LPS activation of CD14-deficient PNH monocytes and endothelial cells. J. Lab. Clin. Med. 125:662-671. [PubMed] [Google Scholar]
- 25.Graziani-Bowering, G. M., J. M. Graham, and L. G. Filion. 1997. A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes. J. Immunol. Methods 207:157-168. [DOI] [PubMed] [Google Scholar]
- 26.Imai, K., A. Takeshita, and S. Hanazawa. 2000. Transforming growth factor-beta inhibits lipopolysaccharide-stimulated expression of inflammatory cytokines in mouse macrophages through downregulation of activation protein 1 and CD14 receptor expression. Infect. Immun. 68:2418-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchens, R. L. 2000. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem. Immunol. 74:61-82. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, A., J. B. Angel, S. Aucoin, W. D. Creery, M. P. Daftarian, D. W. Cameron, L. Filion, and F. Diaz-Mitoma. 1999. Dysregulation of B7.2 (CD86) expression on monocytes of HIV-infected individuals is associated with altered production of IL-2. Clin. Exp. Immunol. 117:84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, A., J. B. Angel, M. P. Daftarian, K. Parato, W. D. Cameron, L. Filion, and F. Diaz-Mitoma. 1998. Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: association of IL-10 production with CD28-mediated immune responsiveness. Clin. Exp. Immunol. 114:78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landmann, R., H. P. Knopf, S. Link, S. Sansano, R. Schumann, and W. Zimmerli. 1996. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect. Immun. 64:1762-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landmann, R., A. M. Reber, S. Sansano, and W. Zimmerli. 1996. Function of soluble CD14 in serum from patients with septic shock. J. Infect. Dis. 173:661-668. [DOI] [PubMed] [Google Scholar]
- 32.Lien, E., P. Aukrust, A. Sundan, F. Muller, S. S. Froland, and T. Espevik. 1998. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood 92:2084-2092. [PubMed] [Google Scholar]
- 33.Lim, W., W. Ma, K. Gee, S. Aucoin, D. Nandan, F. Diaz-Mitoma, M. Kozlowski, and A. Kumar. 2002. Distinct role of p38 and c-Jun N-terminal kinases in IL-10-dependent and IL-10-independent regulation of the costimulatory molecule B7.2 in lipopolysaccharide-stimulated human monocytic cells. J. Immunol. 168:1759-1769. [DOI] [PubMed] [Google Scholar]
- 34.Lynn, W. A., and D. T. Golenbock. 1992. Lipopolysaccharide antagonists. Immunol. Today 13:271-276. [DOI] [PubMed] [Google Scholar]
- 35.Ma, W., W. Lim, K. Gee, S. Aucoin, D. Nandan, M. Kozlowski, F. Diaz-Mitoma, and A. Kumar. 2001. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276:13664-13674. [DOI] [PubMed] [Google Scholar]
- 36.Maggi, E., M. Mazzetti, A. Ravina, F. Annunziato, M. de Carli, M. P. Piccinni, R. Manetti, M. Carbonari, A. M. Pesce, and G. del Prete. 1994. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 265:244-248. [DOI] [PubMed] [Google Scholar]
- 37.Meyaard, L., E. Hovenkamp, I. P. Keet, B. Hooibrink, I. H. de Jong, S. A. Otto, and F. Miedema. 1996. Single cell analysis of IL-4 and IFN-gamma production by T cells from HIV-infected individuals: decreased IFN-gamma in the presence of preserved IL-4 production. J. Immunol. 157:2712-2718. [PubMed] [Google Scholar]
- 38.Molvig, J., and L. Baek. 1987. Removal of endotoxin from culture media by a polymyxin B-Sepharose column: the activity of contaminating endotoxin in culture media measured by the interleukin 1 inducing effect on human monocyte cultures and by the Limulus test. Scand. J. Immunol. 26:611-619. [DOI] [PubMed] [Google Scholar]
- 39.Nadeau, S., and S. Rivest. 2000. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J. Neurosci. 20:3456-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nockher, W. A., L. Bergmann, and J. E. Scherberich. 1994. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin. Exp. Immunol. 98:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nockher, W. A., and J. E. Scherberich. 1997. Expression and release of the monocyte lipopolysaccharide receptor antigen CD14 are suppressed by glucocorticoids in vivo and in vitro. J. Immunol. 158:1345-1352. [PubMed] [Google Scholar]
- 42.Nockher, W. A., R. Wigand, W. Schoeppe, and J. E. Scherberich. 1994. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin. Exp. Immunol. 96:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oesterreicher, C., F. Pfeffel, D. Petermann, and C. Muller. 1995. Increased in vitro production and serum levels of the soluble lipopolysaccharide receptor sCD14 in liver disease. J. Hepatol. 23:396-402. [DOI] [PubMed] [Google Scholar]
- 44.Otake, K., Y. Fujii, T. Nakaya, Y. Nishino, Q. Zhong, K. Fujinaga, M. Kameoka, K. Ohki, and K. Ikuta. 1994. The carboxyl-terminal region of HIV-1 Nef protein is a cell surface domain that can interact with CD4+ T cells. J. Immunol. 153:5826-5837. [PubMed] [Google Scholar]
- 45.Peter, F. 1998. HIV Nef: the mother of all evil? Immunity 9:433-437. [DOI] [PubMed] [Google Scholar]
- 46.Ramierz, F., D. J. Fowell, M. Puklavec, S. Simmonds, and D. Mason. 1996. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J. Immunol. 156:2406-2412. [PubMed] [Google Scholar]
- 47.Reiss, P., A. de Ronde, J. M. Lange, F. de Wolf, J. Dekker, C. Debouck, and J. Goudsmit. 1989. Antibody response to the viral negative factor (Nef) in HIV-1 infection: a correlate of levels of HIV-1 expression. AIDS 3:227-233. [DOI] [PubMed] [Google Scholar]
- 48.Ruppert, J., D. Friedrichs, H. Xu, and J. H. Peters. 1991. IL-4 decreases the expression of the monocyte differentiation marker CD14, paralleled by an increasing accessory potency. Immunobiology 182:449-464. [DOI] [PubMed] [Google Scholar]
- 49.Schutt, C. 1999. Fighting infection: the role of lipopolysaccharide binding proteins CD14 and LBP. Pathobiology 67:227-229. [DOI] [PubMed] [Google Scholar]
- 50.Schutt, C., T. Schilling, U. Grunwald, F. Stelter, S. Witt, C. Kruger, and R. S. Jack. 1995. Human monocytes lacking the membrane-bound form of the bacterial lipopolysaccharide (LPS) receptor CD14 can mount an LPS-induced oxidative burst response mediated by a soluble form of CD14. Res. Immunol. 146:339-350. [DOI] [PubMed] [Google Scholar]
- 51.Stordeur, P., and M. Goldman. 1998. Interleukin-10 as a regulatory cytokine induced by cellular stress: molecular aspects. Int. Rev. Immunol. 16:501-522. [DOI] [PubMed] [Google Scholar]
- 52.Takeshita, S., K. Nakatani, Y. Takata, H. Kawase, I. Sekine, and S. Yoshioka. 1998. Interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) enhance lipopolysaccharide binding to neutrophils via CD14. Inflamm. Res. 47:101-103. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 55.Visser, J., A. Boxel-Dezaire, D. Methorst, T. Brunt, E. R. de Kloet, and L. Nagelkerken. 1998. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 91:4255-4264. [PubMed] [Google Scholar]
- 56.Wasserman, K., M. Subklewe, G. Pothoff, N. Banik, and E. Schell-Frederick. 1994. Expression of surface markers on alveolar macrophages from symptomatic patients with HIV infection as detected by flow cytometry. Chest 105:1324-1334. [DOI] [PubMed] [Google Scholar]
- 57.Zembala, M., S. Bach, A. Szczepanek, G. Mancino, and V. Colizzi. 1997. Phenotypic changes of monocytes induced by HIV-1 gp120 molecule and its fragments. Immunobiology 197:110-121. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler-Heitbrock, H. W., and R. J. Ulevitch. 1993. CD14: cell surface receptor and differentiation marker. Immunol. Today 14:121-125. [DOI] [PubMed] [Google Scholar]