Sepsis remains a significant cause of morbidity and mortality in the intensive care unit (68), affecting more than 500,000 patients per year in the United States (39). The incidence is increasing despite the major advances in the development of antimicrobial agents and other supportive treatments (56). Septic patients often succumb to a systemic inflammatory response, of which multiple organ failure is a main complication. There is no definitive treatment for multiple organ failure other than supportive treatment, such as assisted ventilation or renal dialysis. Research efforts now heavily focus on the mechanisms of multiple organ failure, with the hope that treatment options can be identified through a better understanding of these mechanisms. One current theory implicates an inappropriate cytokine response as the main protagonist in the development of multiple organ failure following an inflammatory stimulus (35, 48).
Cytokines are low-molecular-weight polypeptides or glycoproteins that regulate numerous cellular functions and allow both autocrine and paracrine signaling. Cytokines regulate many of the pathways involved in the host inflammatory response to sepsis. They influence cell differentiation, proliferation, and activation and modulate proinflammatory and anti-inflammatory responses to allow the host to react appropriately to pathogens.
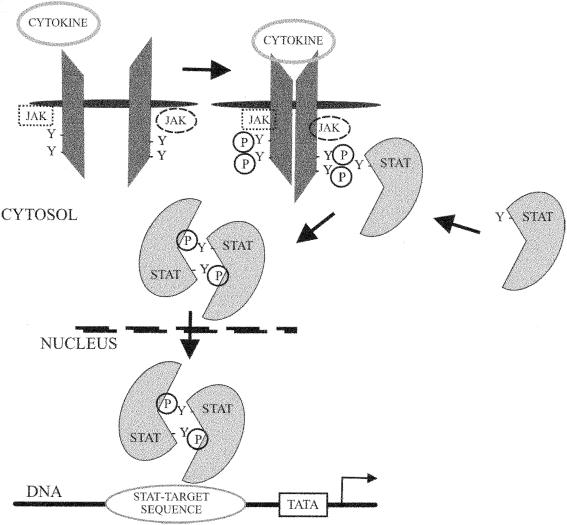
The binding of a cytokine to a corresponding receptor initiates intracellular signal cascades, ultimately leading to changes in gene expression. These signals must rapidly transduce an extracellular signal to the nucleus. The Janus-kinase/signal transducers and activators of transcription (Jak/STAT) pathway is employed in the signaling of many cytokines. Cytokine receptors do not, in general, have intrinsic tyrosine kinase activity and therefore require association with receptor-associated kinases in order to propagate a phosphorylation cascade. Jaks fulfill this function by their ability to associate with many of the cytokine receptors that are involved in regulatory pathways during a sepsis response. Cytokines bind to their receptors and cause receptor dimerization. This enables Jaks to phosphorylate each other as well as the cytokine receptors. Cytokine receptor phosphorylation allows binding of STATs via a specific binding domain. Once bound to the receptor, STATs are also phosphorylated by Jaks. STATs then dissociate from the receptors and dimerize. Dimerization enables translocation of STAT into the nucleus and binding to DNA in order to alter transcription (Fig. 1). Four Jaks and seven STATs have been identified to date in mammalian cells. Multiple Jaks and STATs, as well as the ability of STATs to homo- or hetero-dimerize, allow for cytokine-specific cellular responses (47).
FIG. 1.
Pathway of signal transduction from cytokine binding to a receptor to the nucleus via the Jak/STAT pathway. Y, tyrosine residue able to be phosphorylated.
This minireview describes a number of important cytokines involved in regulating the inflammatory response to sepsis, the importance of the Jak/STAT pathways, and how these pathways are regulated.
CYTOKINE STRUCTURE
Cytokines can be classified according to structure as well as function (19). Type I cytokines form the largest group and are classified mainly according to their structure, which includes four α-helices, and also according to the fact that they signal via type I cytokine receptors. Type I cytokines include many of the interleukins (ILs), such as IL-2, IL-4, IL-5, IL-7, IL-9, IL-11, IL-13, and IL-15. These cytokines are often referred to as hematopoietins because they tend to induce growth and differentiation of red blood cells and leukocytes, especially B and T lymphocytes (19). Type I cytokine receptors also have many conserved structural features. There are conserved extracellular domains, together with a proline-rich intracellular domain, that allow association with Jaks (27).
Type II cytokines include alpha interferon (IFN-α), IFN-β, and IFN-γ. Interferons enhance cell-mediated immune responses, especially to viral infection, as well as increase activation of macrophages (19). Two other cytokine families have been described based on similarities in their receptor structure. These are the tumor necrosis factor (TNF) family, which includes TNF-α, TNF-β, CD40 ligand, and Fas ligand, and the immunoglobulin superfamily, which includes B7.1 and B7.2 that are responsible for costimulation of T cells. TNF family receptors are trimers and provide signal transduction through pathways leading to B- and T-cell activation, as well as important regulatory signals for apoptosis.
A number of cytokines cannot be directly included in any of the four cytokine families described above, although several have been categorized as belonging to these families in the past. These cytokines remain unclassified at present. They include transforming growth factor β, IL-1, IL-10, IL-12, and IL-18, all of which have been shown to be important in either the pro- or anti-inflammatory immune response to sepsis.
TH1 AND TH2 CYTOKINES
Cytokines may also be classified in terms of their production by T-helper (TH) lymphocytes (5). TH1 cytokines are primarily involved in promoting a cell-mediated immune response. These cytokines are generally proinflammatory and include primarily IFN-γ and IL-2 (37). TH2 cytokines are those primarily involved in humoral immune responses and responses to disseminated infection. These cytokines are generally anti-inflammatory and include IL-4, IL-10, and IL-13. Both TH1 and TH2 cytokines are required for an appropriate immune response to a septic challenge (37).
CYTOKINE REGULATION
There is considerable antagonism and regulation between the TH1 and TH2 cytokines (30, 37, 51). This regulation is required for an appropriate immune response to sepsis. If either proinflammatory or anti-inflammatory cytokines are allowed to predominate without appropriate regulation, there is great potential for tissue and organ damage. In this way, it has been proposed that an overexuberant proinflammatory response, or an equally overexuberant anti-inflammatory response, can lead to early and late onset of multiple organ failure, respectively (35, 48). Patients with severe sepsis produce predominantly a TH2 response, and this correlates with a poor outcome, possibly secondary to the resulting immunosuppression (10).
There are, therefore, many regulatory systems that have evolved in an attempt to maintain the cytokine balance during sepsis. The regulation and mechanism of TH1 and TH2 differentiation remains a major source of immunological investigation, and these mechanisms are far from being fully understood. Differentiation is, to some degree, reliant on correct Jak/STAT activation. Other signaling pathways are also involved. Differential regulation of TH1 and TH2 responses in a sepsis model was found to be regulated in part by p38 mitogen-activated protein kinase (53). p38 mitogen-activated protein kinase also appears to be important in IL-12-induced STAT4 phosphorylation and transcriptional activation (65).
CYTOKINES IN INFECTION
Cytokine responses are, of course, a necessary part of the host response. There are many studies investigating the effects of individual cytokines during sepsis. Those experiments and studies described below relate to cytokines that have been shown to involve one of the Jak/STAT pathways, either directly via signaling or via regulatory control of these pathways.
IL-12.
The importance of IL-12 in sepsis was highlighted in a recent study of surgical patients (67). This study showed that decreased IL-12 levels before surgery were a positive predictor of lethal outcome if subsequent postoperative sepsis developed. This confirms many studies performed in mice. IL-12-deficient mice are unable to produce adequate IFN-γ responses and therefore are unable to produce good TH1 differentiation (31). In other studies, blocking antibodies to IL-12 were administered to mice at the time of peritonitis induced either by cecal ligation and puncture (CLP) (54) or by intraperitoneal Escherichia coli injection (72). Both studies resulted in decreased clearance of bacteria in the treated animals compared with controls and had a detrimental effect on survival. However, in an endotoxin model of sepsis, anti-IL-12 antibodies were protective (72). Treatment of mice with IL-12 also improved outcome after CLP (42).
IFN-γ.
Another important proinflammatory cytokine during sepsis is IFN-γ. This cytokine increases TH1 differentiation and cell-mediated immune responses (44). Treatment of human monocytes with IFN-γ increases monocyte human leukocyte antigen DR (HLA-DR) expression (16). Trauma patients also express lower levels of HLA-DR on their blood monocytes compared with a control population, and lower HLA-DR expression is related to worsened outcome (17) as well as an increasing incidence of sepsis (4). A clinical trial for treatment with IFN-γ in trauma patients was undertaken in the early 1990s based on this and other research, with the hope that there would be increased antigen presentation by monocytes and augmentation of the proinflammatory immune response. Treatment did improve HLA-DR levels in these patients, but no overall improvement in outcome was shown by this study (43).
IL-4.
This is another important cytokine that serves to increase TH2 differentiation and promote humoral immunity (37). DiPiro et al. (8) found that IL-4 levels correlated directly with increased severity of trauma as well as with subsequent development of sepsis. The same study, however, showed that low IL-4 levels in these patients at the time of admission correlated with an increased incidence of nosocomial pneumonia. Administration of anti-IL-4 antibodies restored suppressed TH1 cytokine production in splenocytes of mice after CLP, and this was associated with decreased STAT6 phosphorylation (52). In a Staphylococcus aureus infection model, IL-4-deficient mice had conflicting survival results, depending on the original background strain of the mice (18). Survival was improved in IL-4-deficient mice with 129SV background, which tend to produce predominantly TH2 responses, but decreased in IL-4-deficient mice with C57BL/6 background, which produce mainly a TH1 response. Treatment with IL-4 decreased survival in a Pseudomonas aeruginosa infection model when administered 24 h prior to infection but increased survival when administered at the time of infection (12). These studies emphasize the importance of the natural tendency of some inbred mice to produce a predominant either TH1 or TH2 response, as well as the importance of temporal production of cytokines in order to achieve desirable responses.
IL-10.
This potent anti-inflammatory cytokine suppresses macrophage inflammatory functions during sepsis (36). Increased IL-10 in patients with sepsis is related to an adverse outcome (40). In mice, administration of IL-10 decreases the inflammatory response to sepsis and is also detrimental (40). A separate study (23) confirmed the importance of IL-10 in burn injury. In this study, the decreased TH1 function commonly found after burns was reversed by the administration of anti-IL-10 antibodies in mice. Neutralizing anti-IL-10 antibodies also increased survival in a Klebsiella pneumoniae model of lung infection (15).
Jak STRUCTURE AND FUNCTION
As stated above, the Jak/STAT signaling pathway is one of the major pathways for many cytokine signals. Four Jaks have been identified in mammals (28): Jak1, Jak2, Jak3, and Tyk2. They have molecular masses ranging from 120 to 140 kDa and a conserved structure with distinct regions. Two of these regions possess kinase-like activity; one is a functional catalytic kinase domain, and the other is a pseudokinase domain. From these two domains Jaks were named after the Roman two-faced god, Janus. The function of the pseudokinase domain is still under investigation, but it is thought that it may be a potential regulatory site (64) or a docking site for STATs (11). Initial experiments showed that Jak1 and Tyk2 were important in IFN-α/β signaling. Subsequently, it was shown that kinase activity could be induced in Jak family members by a wide range of cytokines. Two Jaks of the same or different class must be in close proximity for phosphorylation to occur. If one Jak is absent, then no signal will be propagated. However, Jak kinases are not solely responsible for specificity of signaling, as many cytokines activate the same Jak (Table 1).
TABLE 1.
STAT activation and function in response to infection
| Protein | Cytokine activator | Jaks activated | Response to infection |
|---|---|---|---|
| STAT1 | IFN-γ | Jak1, Jak2 | TH1 response, monocyte/macrophage activation |
| IFN-α/β | Jak1, Tyk2 | ||
| STAT2 | IFN-α/β | Jak1, Tyk2 | Viral neutralization |
| STAT3 | IL-6 | Jak1/2, Tyk2 | Anti-inflammatory regulatory actions, monocyte/macrophage suppression |
| IL-10 | Jak1, Tyk2 | ||
| STAT4 | IL-12 | Jak2, Tyk2 | TH1 response, NK cell function, intracellular pathogen neutralization |
| STAT6 | IL-4 | Jak1, Jak3 | TH2 response, immunoglobulin E class switching, parasite neutralization |
| IL-13 | Jak1/2, Tyk2 |
STAT STRUCTURE AND FUNCTION
Seven STAT proteins have currently been identified in mammalian cells (28): STAT1, -2, -3, -4, -5a, -5b, and -6, with molecular masses between 750 and 950 kDa (Fig. 2). Their arrangement on three separate chromosomes suggests that there was duplication of a common ancestral gene (7). STATs also share functional structural domains. An SH2 (Src homology) domain allows the STAT to associate with an appropriately phosphorylated cytokine receptor; it is this domain that may contribute to selectivity of response to cytokines (47). There is also a conserved tyrosine residue approximately 700 amino acids from the N terminus. This tyrosine residue is phosphorylated by Jaks to allow the STAT dimerization that is essential for its ability to enter the nucleus and achieve its effects. STATs are also able to homo- or heterodimerize, thus likely contributing to specificity of action, by perhaps binding to different transcription regulation sites. The inherent redundancy of cytokines and the fact that cytokines can be grouped into functionally related subclasses must surely play a role in the diverse response observed despite only a limited combination of signaling molecules.
FIG. 2.
Generalized structure of STAT proteins. Conserved tyrosine (Y) and serine (S) amino acids are indicated. These provide potential sites for phosphorylation and regulation of STAT activity.
EFFECTS OF STAT DEFICIENCY IN MICE
The function of each STAT has been elucidated mainly through the use of genetic knockout mice deficient in these proteins. These studies have revealed their critical role, particularly in host defense against many forms of sepsis. STAT1-deficient mice have reduced IFN-α/β/γ signaling as well as reduced amounts of growth factors such as IL-6, IL-10, and growth hormone. Lack of STAT1 leaves these mice susceptible to viruses and many microbial pathogens (34). STAT2-deficient mice also have reduced responses to IFN-γ as well as IFN-α and IFN-β signaling and therefore have similar susceptibilities to multiple bacterial and viral pathogens. STAT3-deficient mice die at an early fetal stage, although there is indirect evidence to show the importance of STAT3 in T-cell proliferation (58) and responsiveness to IL-10 (57) and IL-6 (1), all of which are important during sepsis. IL-18 has also been shown to activate STAT3 to produce IFN-γ in natural killer (NK) cells (20). STAT4 appears to be acted on almost exclusively by IL-12, one of the major regulators of TH1-lymphocyte development; however, IL-2 has been shown to activate STAT4 in NK cells but not in T cells (66). Mice lacking STAT4 show decreased TH1 cytokine production, other deficiencies within the innate immune system, and increased susceptibilities to intracellular pathogens (21, 62). STAT5a- and -5b-deficient mice show closely related defects, reflecting the closely related primary structure of the two proteins. In terms of responses to infection, there are major decreases in NK cell number and function, together with marked IL-2 hyporesponsiveness in these deficient mice (38). Finally, STAT6 deficiency results in severely decreased IL-4 and IL-13 responses (59). There is a defect in TH2 development as well as a lack of immunoglobulin E class switching (49). Therefore, mice with STAT6 deficiency are susceptible to parasites as well as many bacterial pathogens.
REGULATION OF Jak/STAT SIGNALING
Regulation of Jak/STAT signaling is important in controlling the immune response to sepsis. Pathways can be regulated in a number of ways. STATs themselves can be dephosphorylated by tyrosine phosphatases or degraded by a ubiquitin-proteasome pathway. One example of this is the degradation of STAT1 in order to regulate the actions of IFN-γ during sepsis (24). There are also a number of dominant negative forms of the STAT proteins that are formed by alternative splicing of mRNA. Another major pathway of regulation is through the suppressors of cytokine signaling (SOCS) proteins. These proteins are a relatively recent discovery but have already undergone several changes in nomenclature based on their original discovery and different actions. Eight separate SOCS family proteins have been identified and are most easily classified as SOCS 1 through 7 and CIS 1 (6, 70). The structure of SOCS proteins includes a kinase inhibitory region and an extended SH2 subdomain, which allows SOCS members to act in a number of ways to suppress Jak/STAT signaling. SOCS 1 and 3 directly bind to Jaks to inhibit their function, whereas others act on the receptor to prevent STAT binding or receptor phosphorylation. SOCS 3 is induced by IL-10; this is one of the mechanisms responsible for IL-10-mediated inhibition of proinflammatory macrophage function (2). The production of SOCS proteins is regulated in a classical feedback loop, as SOCS gene expression is induced by STATs themselves and their respective cytokines.
Jak AND STAT IN SEPSIS
As stated earlier, the balance of TH1 and TH2 cytokines is crucial to the host response to infection. Many different models of sepsis have been used to investigate the role of STATs and Jaks in response to infection and the subsequent TH1/TH2 balance. These models range from clinically relevant models, such as polymicrobial peritonitis after CLP (50) and K. pneumoniae lung infection (15), to intraperitoneal injection of pathogens or their infectious components, such as endotoxin or lipopolysaccharide (72). The results from studies using these models of infection have all contributed to current knowledge on the mechanism and function of Jak/STAT signaling in sepsis (Table 1).
STAT4 and STAT6.
Two of the most important STAT pathways in the immune response to sepsis are STAT4 and STAT6 (69), primarily because of their effect on TH-cell differentiation. They are also the most widely investigated in sepsis models, due in part to the availability of the genetic knockout mice. In one study, survival of both STAT4-deficient and STAT6-deficient mice was improved after CLP, compared with that of wild-type controls, and survival was greater in STAT4-deficient mice compared with STAT6-deficient mice (32). Bacterial levels in STAT6-deficient mice after CLP were also lower than those in wild-type mice, which was proposed to be associated with increased IL-12 levels in these mice from activated macrophages (32). However, paradoxically, STAT4-deficient mice had higher bacterial levels after CLP, despite their significantly improved survival (13). The survival benefit with STAT4 deficiency may result from their blunted IL-12 levels in peritoneal washout as well as serum, which may prevent an overwhelming proinflammatory response to the bacterial challenge (13). Interestingly, STAT4-deficient mice had increased levels of hepatic IL-10 and IL-13, combined with decreased indices for hepatic injury after CLP (32). Matsukawa et al. speculate that this finding links STAT4 with multiple organ failure, as the liver is the organ most commonly affected.
IL-4-activated STAT6 is essential for TH2 differentiation (21, 49, 60). Negative regulation of STAT6 can be achieved by the TH1-inducing cytokine IFN-γ (69). STAT4 is also extremely important for TH1 responses, although there are STAT-independent pathways (3, 22). A recent study provided evidence that levels and activation of STAT4 are partly responsible for the TH1-shifted host responses found in some inbred strains of mice, such as C57BL/6, and for the TH2-shifted immune response exhibited by BALB/c mice (26). This supports other studies that investigated the role of STAT4 and STAT6 in resolution of infection with intracellular pathogens such as Toxoplasma gondii (3) and Trypanosoma cruzi (61). BALB/c mice are more susceptible to infection by these intracellular pathogens, as they are unable to mount an appropriate TH1-cell-mediated immune response required for effective immune control of organisms. TH2 responses develop later in the course of infection to combat organism persistence. Similarly, STAT4-deficient patients tend to develop atypical mycobacterial and staphylococcal infections (14). STAT6, but not STAT4, plays a role in TH1 and TH2 differential chemokine responses (71). Chemokines are, of course, also extremely important during sepsis for appropriate recruitment of leukocytes to sites of infection. Different chemokines regulate the specificity of leukocyte recruitment (33), and it seems likely that TH2 cells produce chemokines to attract other TH2 lymphocytes, rather than TH1 lymphocytes (71).
STAT3 and STAT1.
In a CLP model in rats, increased hepatic STAT3 activation correlated with a survival advantage (1). A concurrent loss of serum and intrahepatic IL-6 activity, which signals via STAT3, correlated with increased mortality. One possibility for this is that during sepsis hepatocytes can become hyporesponsive to IL-6, therefore decreasing STAT3 phosphorylation. The ability to produce adequate amounts of IL-6 during sepsis is important, and levels of this cytokine have been shown to be a predictor of final outcome both in murine models of sepsis and in trauma patients (45).
STAT1 forms part of the major signaling pathway for IFN-γ, which is critical in the activation of macrophages and monocytes. STAT1 plays a role in combining IFN-γ signals with other stimulatory signals, usually derived from microbial products such as LPS, in order to fully activate macrophages. This effect is achieved by alteration of phosphorylation of both the serine and tyrosine phosphorylation sites on STAT1 (25). STAT1 is also required for LPS-induced gene expression (41). Mycobacterium tuberculosis is able to prevent its phagocytosis by IFN-γ-activated human macrophages by specifically targeting the interaction of STAT1 with transcriptional activators responsible for upregulating IFN-γ-dependent gene expression (63). Many viruses are similarly able to inhibit STAT1 (29). Clinically, it has also been demonstrated that mutations of STAT1 or IFN-γ receptors affect outcome after mycobacterial infection (9). Other pathogens, such as Listeria monocytogenes, are able to decrease STAT1 phosphorylation in an attempt to limit the host immune response (55). Listeria is also able to increase mRNA expression of SOCS 3 (55). There is further evidence that STAT1 may be an important immunoregulatory protein, as it also appears to be involved in the induction of apoptosis (46). These findings are currently under further investigation.
CONCLUSION
STAT proteins and Jak/STAT intracellular signaling form some of the most important pathways involved in producing both cell-mediated and acquired immune responses. Many important cytokines that are required to combat a wide range of infectious agents exert their effects through them. They are also integral to the differentiation of T lymphocytes to produce TH1 or TH2 cytokines in response to infection. This review focused on the role of Jak/STAT signaling in sepsis, but these pathways are equally important in many other immune processes, such as allergic and autoimmune responses. It is, therefore, not surprising that these molecules are currently under intense experimental scrutiny. Recognition of their importance to the understanding of many different immunological mechanisms is likely to increase as more studies are published. This recognition has the potential to provide new therapies for a wide range of diseases and clinical problems in the future.
Acknowledgments
We thank D. Godshall for help with the figures and J. C. Peyton and J. J. Hoth for assistance in preparing the manuscript.
This work was funded by a VA Merit Review Award.
REFERENCES
- 1.Andrejko, K. M., J. Chen, and C. S. Deutschman. 1998. Intrahepatic STAT-3 activation and acute phase gene expression predict outcome after CLP sepsis in the rat. Am. J. Physiol. 275:G1423-G1429. [DOI] [PubMed] [Google Scholar]
- 2.Berlato, C., M. A. Cassatella, I. Kinjyo, L. Gatto, A. Yoshimura, and F. Bazzoni. 2002. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J. Immunol. 168:6404-6411. [DOI] [PubMed] [Google Scholar]
- 3.Cai, G., T. Radzanowski, E. N. Villegas, R. Kastelein, and C. A. Hunter. 2000. Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii. J. Immunol. 165:2619-2627. [DOI] [PubMed] [Google Scholar]
- 4.Cheadle, W. G., M. J. Hershman, S. R. Wellhausen, and H. C. Polk, Jr. 1991. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am. J. Surg. 161:639-645. [DOI] [PubMed] [Google Scholar]
- 5.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 6.Cooney, R. N. 2002. Suppressors of cytokine signaling (SOCS): inhibitors of the JAK/STAT pathway. Shock 17:83-90. [DOI] [PubMed] [Google Scholar]
- 7.Copeland, N. G., D. J. Gilbert, C. Schindler, Z. Zhong, Z. Wen, J. E. Darnell, Jr., A. L. Mui, A. Miyajima, F. W. Quelle, and J. N. Ihle. 1995. Distribution of the mammalian Stat gene family in mouse chromosomes. Genomics 29:225-228. [DOI] [PubMed] [Google Scholar]
- 8.DiPiro, J. T., T. R. Howdieshell, J. K. Goddard, D. B. Callaway, R. G. Hamilton, and A. R. Mansberger, Jr. 1995. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch. Surg. 130:1159-1162. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis, S., R. Doffinger, C. Picard, C. Fieschi, F. Altare, E. Jouanguy, L. Abel, and J. L. Casanova. 2000. Human interferon-gamma-mediated immunity is a genetically controlled continuous trait that determines the outcome of mycobacterial invasion. Immunol. Rev. 178:129-137. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, N. R., H. F. Galley, and N. R. Webster. 1999. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 25:106-109. [DOI] [PubMed] [Google Scholar]
- 11.Fujitani, Y., M. Hibi, T. Fukada, M. Takahashi-Tezuka, H. Yoshida, T. Yamaguchi, K. Sugiyama, Y. Yamanaka, K. Nakajima, and T. Hirano. 1997. An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene 14:751-761. [DOI] [PubMed] [Google Scholar]
- 12.Giampietri, A., U. Grohmann, C. Vacca, M. C. Fioretti, P. Puccetti, and F. Campanile. 2000. Dual effect of IL-4 on resistance to systemic gram-negative infection and production of TNF-alpha. Cytokine 12:417-421. [DOI] [PubMed] [Google Scholar]
- 13.Godshall, C. J., A. B. Lentsch, J. C. Peyton, M. J. Scott, and W. G. Cheadle. 2001. STAT4 is required for antibacterial defense but enhances mortality during polymicrobial sepsis. Clin. Diagn. Lab. Immunol. 8:1044-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollob, J. A., K. G. Veenstra, H. Jyonouchi, A. M. Kelly, P. Ferrieri, D. J. Panka, F. Altare, C. Fieschi, J. L. Casanova, D. A. Frank, and J. W. Mier. 2000. Impairment of STAT activation by IL-12 in a patient with atypical mycobacterial and staphylococcal infections. J. Immunol. 165:4120-4126. [DOI] [PubMed] [Google Scholar]
- 15.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, R. E. Goodman, and T. J. Standiford. 1995. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 155:722-729. [PubMed] [Google Scholar]
- 16.Hershman, M. J., S. H. Appel, S. R. Wellhausen, G. Sonnenfeld, and H. C. Polk, Jr. 1989. Interferon-gamma treatment increases HLA-DR expression on monocytes in severely injured patients. Clin. Exp. Immunol. 77:67-70. [PMC free article] [PubMed] [Google Scholar]
- 17.Hershman, M. J., W. G. Cheadle, S. R. Wellhausen, P. F. Davidson, and H. C. Polk, Jr. 1990. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br. J. Surg. 77:204-207. [DOI] [PubMed] [Google Scholar]
- 18.Hultgren, O., M. Kopf, and A. Tarkowski. 1999. Outcome of Staphylococcus aureus-triggered sepsis and arthritis in IL-4-deficient mice depends on the genetic background of the host. Eur. J. Immunol. 29:2400-2405. [DOI] [PubMed] [Google Scholar]
- 19.Janeway, C. A., Jr. 2001. Appendix III, p. 677-679. In C. A. Janeway Jr., P. Travers, M. Walport, and M. J. Shlomchick (ed.), Immunobiology, 5th ed. Garland Publishing, New York, N.Y.
- 20.Kalina, U., D. Kauschat, N. Koyama, H. Nuernberger, K. Ballas, S. Koschmieder, G. Bug, W. K. Hofmann, D. Hoelzer, and O. G. Ottmann. 2000. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J. Immunol. 165:1307-1313. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, M. H., Y. L. Sun, T. Hoey, and M. J. Grusby. 1996. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382:174-177. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, M. H., A. L. Wurster, and M. J. Grusby. 1998. A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J. Exp. Med. 188:1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly, J. L., A. Lyons, C. C. Soberg, J. A. Mannick, and J. A. Lederer. 1997. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery 122:146-152. [DOI] [PubMed] [Google Scholar]
- 24.Kim, T. K., and T. Maniatis. 1996. Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science 273:1717-1719. [DOI] [PubMed] [Google Scholar]
- 25.Kovarik, P., D. Stoiber, M. Novy, and T. Decker. 1998. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 17:3660-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda, E., T. Kito, and U. Yamashita. 2002. Reduced expression of STAT4 and IFN-gamma in macrophages from BALB/c mice. J. Immunol. 168:5477-5482. [DOI] [PubMed] [Google Scholar]
- 27.Leonard, W. J., and J. X. Lin. 2000. Cytokine receptor signaling pathways. J. Allergy Clin. Immunol. 105:877-888. [DOI] [PubMed] [Google Scholar]
- 28.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16:293-322. [DOI] [PubMed] [Google Scholar]
- 29.Look, D. C., W. T. Roswit, A. G. Frick, Y. Gris-Alevy, D. M. Dickhaus, M. J. Walter, and M. J. Holtzman. 1998. Direct suppression of Stat1 function during adenoviral infection. Immunity 9:871-880. [DOI] [PubMed] [Google Scholar]
- 30.Maggi, E., P. Parronchi, R. Manetti, C. Simonelli, M. P. Piccinni, F. S. Rugiu, M. De Carli, M. Ricci, and S. Romagnani. 1992. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J. Immunol. 148:2142-2147. [PubMed] [Google Scholar]
- 31.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 32.Matsukawa, A., M. H. Kaplan, C. M. Hogaboam, N. W. Lukacs, and S. L. Kunkel. 2001. Pivotal role of signal transducer and activator of transcription (Stat)4 and Stat6 in the innate immune response during sepsis. J. Exp. Med. 193:679-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellado, M., J. M. Rodriguez-Frade, S. Manes, and A. Martinez. 2001. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu. Rev. Immunol. 19:397-421. [DOI] [PubMed] [Google Scholar]
- 34.Meraz, M. A., J. M. White, K. C. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riley, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 35.Moore, F. A., A. Sauaia, E. E. Moore, J. B. Haenel, J. M. Burch, and D. C. Lezotte. 1996. Postinjury multiple organ failure: a bimodal phenomenon. J. Trauma 40:501-510. [DOI] [PubMed] [Google Scholar]
- 36.Moore, K. W., M. R. de Waal, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 37.Morel, P. A., and T. B. Oriss. 1998. Crossregulation between Th1 and Th2 cells. Crit. Rev. Immunol. 18:275-303. [DOI] [PubMed] [Google Scholar]
- 38.Mui, A. L., H. Wakao, N. Harada, A. M. O'Farrell, and A. Miyajima. 1995. Interleukin-3, granulocyte-macrophage colony-stimulating factor, and interleukin-5 transduce signals through two forms of STAT5. J. Leukoc. Biol. 57:799-803. [DOI] [PubMed] [Google Scholar]
- 39.Oberholzer, A., C. Oberholzer, and L. L. Moldawer. 2000. Cytokine signaling—regulation of the immune response in normal and critically ill states. Crit. Care Med. 28:N3-12. [DOI] [PubMed] [Google Scholar]
- 40.Oberholzer, A., C. Oberholzer, and L. L. Moldawer. 2002. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit. Care Med. 30:S58-S63. [PubMed] [Google Scholar]
- 41.Ohmori, Y., and T. A. Hamilton. 2001. Requirement for STAT1 in LPS-induced gene expression in macrophages. J. Leukoc. Biol. 69:598-604. [PubMed] [Google Scholar]
- 42.Ono, S., C. Ueno, S. Aosasa, H. Tsujimoto, S. Seki, and H. Mochizuki. 2001. Severe sepsis induces deficient interferon-gamma and interleukin-12 production, but interleukin-12 therapy improves survival in peritonitis. Am. J. Surg. 182:491-497. [DOI] [PubMed] [Google Scholar]
- 43.Polk, H. C., Jr., W. G. Cheadle, D. H. Livingston, J. L. Rodriguez, K. M. Starko, A. E. Izu, H. S. Jaffe, and G. Sonnenfeld. 1992. A randomized prospective clinical trial to determine the efficacy of interferon-gamma in severely injured patients. Am. J. Surg. 163:191-196. [DOI] [PubMed] [Google Scholar]
- 44.Qiu, G., C. Wang, R. Smith, K. Harrison, and K. Yin. 2001. Role of IFN-gamma in bacterial containment in a model of intra-abdominal sepsis. Shock 16:425-429. [DOI] [PubMed] [Google Scholar]
- 45.Remick, D. G., G. R. Bolgos, J. Siddiqui, J. Shin, and J. A. Nemzek. 2002. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463-467. [DOI] [PubMed] [Google Scholar]
- 46.Schindler, C. 1998. STATs as activators of apoptosis. Trends Cell Biol. 8:97-98. [DOI] [PubMed] [Google Scholar]
- 47.Schindler, C. 1999. Cytokines and JAK-STAT signaling. Exp. Cell Res. 253:7-14. [DOI] [PubMed] [Google Scholar]
- 48.Scott, M. J., C. J. Godshall, and H. C. Polk, Jr. 2001. Current concepts in immunosuppression and multiple organ dysfunction following trauma. Curr. Orthop. 15:192-198. [Google Scholar]
- 49.Shimoda, K., J. van Deursen, M. Y. Sangster, S. R. Sarawar, R. T. Carson, R. A. Tripp, C. Chu, F. W. Quelle, T. Nosaka, D. A. Vignali, P. C. Doherty, G. Grosveld, W. E. Paul, and J. N. Ihle. 1996. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380:630-633. [DOI] [PubMed] [Google Scholar]
- 50.Shrotri, M. S., J. C. Peyton, and W. G. Cheadle. 1999. Mouse peritonitis model using cecal ligation and puncture, p. 173-181. In M. A. Sande, C. Carbon, B. Fantin, R. Kaminsky, E. R. Kern, and T. O'Reilly (ed.), Handbook of animal models of infection. Academic Press, San Diego, Calif.
- 51.Smeltz, R. B., J. Chen, J. Hu-Li, and E. M. Shevach. 2001. Regulation of interleukin (IL)-18 receptor alpha chain expression on CD4(+) T cells during T helper (Th)1/Th2 differentiation. Critical downregulatory role of IL-4. J. Exp. Med. 194:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song, G. Y., C. S. Chung, I. H. Chaudry, and A. Ayala. 2000. IL-4-induced activation of the Stat6 pathway contributes to the suppression of cell-mediated immunity and death in sepsis. Surgery 128:133-138. [DOI] [PubMed] [Google Scholar]
- 53.Song, G. Y., C. S. Chung, I. H. Chaudry, and A. Ayala. 2000. Immune suppression in polymicrobial sepsis: differential regulation of Th1 and Th2 responses by p38 MAPK. J. Surg. Res. 91:141-146. [DOI] [PubMed] [Google Scholar]
- 54.Steinhauser, M. L., C. M. Hogaboam, N. W. Lukacs, R. M. Strieter, and S. L. Kunkel. 1999. Multiple roles for IL-12 in a model of acute septic peritonitis. J. Immunol. 162:5437-5443. [PubMed] [Google Scholar]
- 55.Stoiber, D., S. Stockinger, P. Steinlein, J. Kovarik, and T. Decker. 2001. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J. Immunol. 166:466-472. [DOI] [PubMed] [Google Scholar]
- 56.Stone, R. 1994. Search for sepsis drugs goes on despite past failures. Science 264:365-367. [DOI] [PubMed] [Google Scholar]
- 57.Takeda, K., B. E. Clausen, T. Kaisho, T. Tsujimura, N. Terada, I. Forster, and S. Akira. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10:39-49. [DOI] [PubMed] [Google Scholar]
- 58.Takeda, K., T. Kaisho, N. Yoshida, J. Takeda, T. Kishimoto, and S. Akira. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161:4652-4660. [PubMed] [Google Scholar]
- 59.Takeda, K., M. Kamanaka, T. Tanaka, T. Kishimoto, and S. Akira. 1996. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J. Immunol. 157:3220-3222. [PubMed] [Google Scholar]
- 60.Takeda, K., T. Tanaka, W. Shi, M. Matsumoto, M. Minami, S. Kashiwamura, K. Nakanishi, N. Yoshida, T. Kishimoto, and S. Akira. 1996. Essential role of Stat6 in IL-4 signalling. Nature 380:627-630. [DOI] [PubMed] [Google Scholar]
- 61.Tarleton, R. L., M. J. Grusby, and L. Zhang. 2000. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J. Immunol. 165:1520-1525. [DOI] [PubMed] [Google Scholar]
- 62.Thierfelder, W. E., J. M. van Deursen, K. Yamamoto, R. A. Tripp, S. R. Sarawar, R. T. Carson, M. Y. Sangster, D. A. Vignali, P. C. Doherty, G. C. Grosveld, and J. N. Ihle. 1996. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382:171-174. [DOI] [PubMed] [Google Scholar]
- 63.Ting, L. M., A. C. Kim, A. Cattamanchi, and J. D. Ernst. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898-3906. [PubMed] [Google Scholar]
- 64.Velazquez, L., K. E. Mogensen, G. Barbieri, M. Fellous, G. Uze, and S. Pellegrini. 1995. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-alpha/beta and for signal transduction. J. Biol. Chem. 270:3327-3334. [DOI] [PubMed] [Google Scholar]
- 65.Visconti, R., M. Gadina, M. Chiariello, E. H. Chen, L. F. Stancato, J. S. Gutkind, and J. J. O'Shea. 2000. Importance of the MKK6/p38 pathway for interleukin-12-induced STAT4 serine phosphorylation and transcriptional activity. Blood 96:1844-1852. [PubMed] [Google Scholar]
- 66.Wang, K. S., J. Ritz, and D. A. Frank. 1999. IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J. Immunol. 162:299-304. [PubMed] [Google Scholar]
- 67.Weighardt, H., C. D. Heidecke, A. Westerholt, K. Emmanuilidis, S. Maier, M. Veit, K. Gerauer, E. Matevossian, K. Ulm, J. R. Siewert, and B. Holzmann. 2002. Impaired monocyte IL-12 production before surgery as a predictive factor for the lethal outcome of postoperative sepsis. Ann. Surg. 235:560-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wheeler, A. P., and G. R. Bernard. 1999. Treating patients with severe sepsis. N. Engl. J. Med. 340:207-214. [DOI] [PubMed] [Google Scholar]
- 69.Wurster, A. L., T. Tanaka, and M. J. Grusby. 2000. The biology of Stat4 and Stat6. Oncogene 19:2577-2584. [DOI] [PubMed] [Google Scholar]
- 70.Yasukawa, H., A. Sasaki, and A. Yoshimura. 2000. Negative regulation of cytokine signaling pathways. Annu. Rev. Immunol. 18:143-164. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, S., N. W. Lukacs, V. A. Lawless, S. L. Kunkel, and M. H. Kaplan. 2000. Cutting edge: differential expression of chemokines in Th1 and Th2 cells is dependent on Stat6 but not Stat4. J. Immunol. 165:10-14. [DOI] [PubMed] [Google Scholar]
- 72.Zisman, D. A., S. L. Kunkel, R. M. Strieter, J. Gauldie, W. C. Tsai, J. Bramson, J. M. Wilkowski, K. A. Bucknell, and T. J. Standiford. 1997. Anti-interleukin-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock 8:349-356. [DOI] [PubMed] [Google Scholar]