Abstract
We studied the mechanism by which external acidification from pH 7.3 to 6.8 reduced current magnitude in the Kv1.5 potassium channel. At physiological external [K+], a shift in the voltage-dependence of activation was entirely responsible for the acidification-induced decrease in Kv1.5 current magnitude (pK = 7.15). Elevation of external [Ca2+] or [Mg2+] identically shifted activation curves to the right and identically shifted the pH-sensitivity of the activation curves to more acidic values. Similar observations were made with the Kv2.1 K+ channel, except that the pK for the activation shift was out of the physiological range. These data are consistent with a mechanism by which acidification shifted activation via modification of a local surface potential. Elimination of eight positive charges within the outer vestibule of the conduction pathway had no effect on the voltage-dependence of activation at pH 7.3 or higher, which suggested that sites exposed to the conduction pathway within the outer vestibule did not directly contribute to the relevant local surface potential. However, mutations at position 487 (within the conduction pathway) displaced the pK of the pH-sensitive shift in activation, such that the sensitivity of Kv1.5 current to physiologically relevant changes in pH was reduced or eliminated. These results suggest that, among voltage-gated K+ channels, activation in Kv1.5 is uniquely sensitive to physiologically relevant changes in pH because the pK for the sites that contribute to the local surface potential effect is near pH 7. Moreover, the pK for the activation shift depends not only on the nature of the sites involved but also on structural orientation conferred, in part, by at least one residue within the conduction pathway.
INTRODUCTION
The Kv1.5 potassium channel is a rapidly activating delayed rectifier that helps shape the cardiac action potential (Fedida et al., 1993; Barry et al., 1995; Bou-Abboud and Nerbonne, 1999). It is exquisitely sensitive to changes in extracellular pH in the physiological range, such that acidification by several tenths of a pH unit can substantially reduce current magnitude (Steidl and Yool, 1999; Trapani et al., 2001; Kehl et al., 2002). Given the many conditions that can produce acidification in the heart, these effects may be of substantial physiological and pathological significance.
There appear to be at least two mechanisms, both poorly understood, by which acidification reduces Kv1.5 current magnitude. Several lines of evidence suggest that one mechanism involves an action of protons within the conduction pathway (Steidl and Yool, 1999; Jager and Grissmer, 2001; Kehl et al., 2002). Mutation of a histidine in the outer vestibule of the conduction pathway (position 463 in the human Kv1.5 channel) to a glutamine essentially abolishes the acidification-induced decrease in current magnitude by this mechanism (Steidl and Yool, 1999; Kehl et al., 2002). The pH-mediated current reduction associated with this histidine is also abolished by elevation of external [K+] (Jager and Grissmer, 2001; Kehl et al., 2002). Finally, this pH-dependent mechanism of current reduction is abolished by mutation of an arginine located within the conduction pathway at the position equivalent to the site associated with external TEA binding in other voltage-gated K+ channels (Jager and Grissmer, 2001; Kehl et al., 2002; Trapani et al., 2002). It appears that current is reduced by this mechanism because acidification makes channels unavailable for opening. However, the actual mechanism by which this occurs is still unclear.
The second mechanism of current reduction involves an acidification-induced shift in activation (Steidl and Yool, 1999; Kehl et al., 2002; Trapani et al., 2002). Acidification from pH 7.4 to 6.4 produces approximately an 8 mV shift to the right in the voltage-dependence of hKv1.5 activation (Kehl et al., 2002). This shift is also observed in the voltage-dependence of both the on- and off-gating current (Kehl et al., 2002). Mutation of both the histidine and the arginine in the conduction pathway does not abolish this acidification-induced shift in activation (Kehl et al., 2002), which has led to the conclusion that protons influence activation by acting at a site outside the conduction pathway. However, the mechanism by which protons produce this gating shift has remained obscure.
A long-known mechanism by which changes in external pH can shift activation in voltage-gated channels is via effects on local surface potentials (Hille, 2001). These local surface potentials may be partly associated with negatively charged sites on phospholipid headgroups (Bell and Miller, 1984; Moczydlowski et al., 1985) or with sialic acid residues on glycosylated proteins (Bennett et al., 1997). However, the ion channel protein itself appears to be a major source of negatively charged sites that contribute to local surface potentials (MacKinnon et al., 1989; Laver and Fairley-Grenot, 1994; Elinder and Arhem, 1999). Indeed, Elinder and Arhem (1999) have proposed that a region of conserved negative charge near the S4 domain contributes to a local surface potential. Shifts in activation consistent with this type of mechanism have been observed in voltage-gated K+, Na+, and Ca2+ channels (Campbell and Hahin, 1984; Deutsch and Lee, 1989; Zhang and Siegelbaum, 1991; Klockner and Isenberg, 1994; Ishii et al., 2001). In voltage-gated K+ channels other than Kv1.5, these acidification-induced gating shifts typically occur outside of the physiological range of pH (Deutsch and Lee, 1989; Anumonwo et al., 1999; Steidl and Yool, 1999; Ishii et al., 2001). However, the apparent pK of the gating shift, especially when sites are located on the channel protein itself, will depend on a variety of factors, including the specific residues involved and the local protein environment.
In this paper, we present evidence that the pH-sensitive shift in activation of Kv1.5 is due to a proton-mediated change in local surface potential. Whereas this effect is also observed in other voltage-gated K+ channels, it becomes important in Kv1.5 because the pK for this effect is within a range traversed in both physiological and pathological conditions. Our data also suggest that the local surface potential is not created by sites within the outer vestibule of the conduction pathway. However, a residue within the conduction pathway is at least partly responsible for structural characteristics that place the pK for the activation shift into the physiological range of pH. Some of these data have been presented in abstract form (Trapani et al., 2001; 2002).
METHODS
Molecular biology and channel expression
Experiments were done on several channels derived from hKv1.5 and Kv2.1. Point mutations were made with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). For all mutations, silent restriction sites were inserted within three bases of the mutation site. Mutagenesis success and specificity were confirmed by restriction digest and subsequent DNA sequencing. K+ channel cDNA was subcloned into the pcDNA3 expression vector and channels were expressed in the human embryonic kidney cell line, HEK293 (American Type Culture Collection, Rockville, MD). Cells were maintained in DMEM plus 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) with 1% penicillin/streptomycin. Cells (2 × 106 cells/ml) were cotransfected by electroporation (Bio-Rad Gene Pulser II at 220V, 350 μF) with K+ channel expression plasmid (1 μg/0.2 ml) and CD8 expression plasmid (0.5 μg/0.2 ml). After electroporation, cells were plated on glass coverslips submerged in maintenance media. Electrophysiological recordings were made 18–28 h later. On the day of recording, cells were washed with fresh media and incubated with Dynabeads M450 conjugated with antibody to CD8 (0.5 μl/ml; Dynal, Lake Success, NY). Cells that expressed CD8 became coated with beads, which allowed visualization of transfected cells (Jurman et al., 1994).
Electrophysiology
Currents were recorded at room temperature in the whole-cell patch-clamp configuration. Patch pipets were fabricated from N51A glass (Garner Glass, Claremont, CA), coated with Sylgard and firepolished. Currents were collected with an Axopatch 200B amplifier, pClamp 8 software and a Digidata 1322A A/D board (Axon Instruments, Foster City, CA). Currents were filtered at 2 KHz and sampled at 10–200 μs/pt. Series resistance ranged from 0.5 to 2.5 MΩ and was compensated 80–90%. The holding potential was −80 mV, and depolarizing stimuli were presented once every 8–10 s. Activation curves were constructed as follows. Cells were depolarized from a holding potential of −80 mV in 5 mV increments. Channel activation was measured from the peak of the tail current upon repolarization to −90 mV in experiments with 10 mM external K+ and −50 mV in experiments with 0 external K+. Currents were digitally leak subtracted during analysis based on the average current measured at the holding potential. To minimize the influence of inactivation on tail current measurements, the duration of the voltage step that activated channels was adjusted for each step potential so that tail currents were evoked at the peak of the step current. To ensure that the step current was fully activated and displayed no decline associated with inactivation, currents activated by steps to a single potential were evoked by stimuli of different duration. Activation curves were fit using a modified Boltzmann equation, with both the half activation potential (V1/2) and the slope of the curve determined by best fit calculations. Only V1/2 values are reported in the legends; with a few exceptions, slopes were between 4 and 6. Raw data were analyzed with Clampfit (Axon Instruments); curve fitting and significance testing (paired or unpaired Student's t-test, as appropriate) were done with SigmaPlot 8.0. All plotted data are represented as mean ± SE, with the number of data points denoted by n. When means were compared, the error of the difference was calculated as the quadratic sum of the errors of the means (Taylor, 1997).
Electrophysiological solutions
Currents were recorded in a constantly flowing gravity fed bath. Solutions were placed in one of six reservoirs, each of which fed via plastic tubing into a single quartz tip (∼100 μm diameter; ALA Scientific Instruments, Westbury, NY). The tip was placed within 20 μm of the cell being recorded before the start of the experiment. One solution was always flowing, and solutions were changed by manual switching (solution exchange was complete within 5–10 s). Control internal solutions contained (in mM): 100 KCl, 35 NMG-Cl, 10 HEPES, 10 EGTA, 1 CaCl2, and 4 MgCl2; pH 7.3, osmolality 295. Control external solutions contained (in mM): 160 NMG-Cl, 10 HEPES, 10 glucose, 2 CaCl2, and 1 MgCl2; pH 7.3, osmolality 335. In some experiments, external solutions contained 3–10 mM K+ or elevated divalent cation concentrations. These changes are reported in the legends. In each case, osmotic balance was maintained by removal of NMG+.
RESULTS
Separation of two mechanisms of acidification-induced current reduction
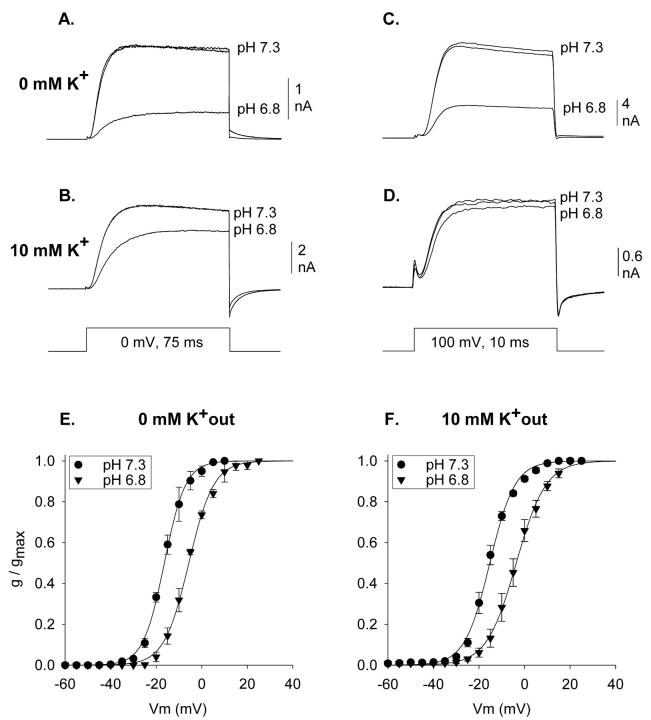
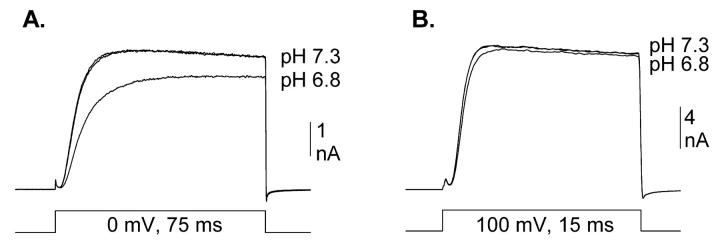
Acidification reduces the magnitude of Kv1.5 potassium channel currents by two independent mechanisms. Fig. 1 illustrates that these two mechanisms can be separated by manipulation of either membrane potential or external [K+]. Fig. 1 A illustrates outward K+ currents recorded at 0 mV in the absence of external K+. Acidification from pH 7.3 to 6.8 reduced current magnitude by 67.0 ± 1.7% (n = 8). The currents in Fig. 1 B were recorded identically, except that 10 mM K+ was included in the external solution. Under these conditions, acidification from pH 7.3 to 6.8 reduced current magnitude by 25.5 ± 1.9% (n = 8). This reduction is quantitatively accounted for by a 10 mV shift to the right in the activation curve (Fig. 1 F). Fig. 1, C and D, illustrate currents recorded similarly, except at +100 mV. In the absence of external K+, acidification from pH 7.3 to 6.8 reduced currents by 62.0 ± 1.5% (Fig. 1 C; n = 3). Given the hypothesis that two independent effects are involved, a 62% reduction by one mechanism (Fig. 1 C) combined with a 25% reduction by a second mechanism (Fig. 1 B) predicts the observed 67% reduction when both mechanisms are combined (Fig. 1 A; see figure legend for calculation). At +100 mV, where the acidification-induced shift in activation becomes irrelevant, 10 mM K+ essentially abolished the acidification-mediated current reduction (n = 5; Fig. 1 D).
FIGURE 1.
Isolation of acidification-induced shift in activation in Kv1.5. (A–D) Three superimposed currents are shown, two at pH 7.3 and one at pH 6.8, recorded between the two at pH 7.3. Data shown are from four different cells. Currents were recorded in 0 mM external K+ at 0 mV (A) and +100 mV (C), and in 10 mM external K+ at 0 mV (B) and +100 mV (D). At +100 mV in the presence of 10 mM K+, both mechanisms of acidification-induced current reduction were abolished (D). At 0 mV, in the presence of 10 mM K+, acidification to 6.8 reduced current to 74.5 ± 1.9% of that at pH 7.3 (n = 8; B). At +100 mV in the absence of K+, acidification to pH 6.8 reduced current to 38.0 ± 1.5% of that at pH 7.3 (n = 3; C). If these two conditions isolate two independent mechanisms of pore reduction, the predicted current reduction by acidification from pH 7.3 to 6.8 for currents recorded at 0 mV in 0 mM K+ was 71.7 ± 1.3% (100% − [38% of 74.5%]). Experimentally, currents recorded at 0 mV in 0 mM external K+ were reduced by 67.0 ± 1.7% (n = 8; A), which was statistically identical to the predicted value for the two independent effects. (E) Activation curves recorded in 0 mM K+. V1/2 values: −16.2 ± 0.9 (pH 7.3, n = 3) and −5.7 ± 0.9 (pH 6.8, n = 3). (F) Activation curves recorded in 10 mM K+. V1/2 values: −15.3 ± 0.8 (pH 7.3, n = 5) and −3.6 ± 2.4 (pH 6.8, n = 3).
External K+ had no effect on the acidification-induced shift in activation. This is illustrated in the comparison between Fig. 1, E and F. The V1/2 values for activation of Kv1.5 currents at pH 7.3 (filled circles) and pH 6.8 (filled triangles) were identical in 0 and 10 mM K+. Consequently, we were able to examine the influence of pH on the voltage-dependence of activation, in the absence of the pore-mediated mechanism of proton action, by recording currents in the presence of 10 mM external K+.
Potential role of a histidine in the conduction pathway
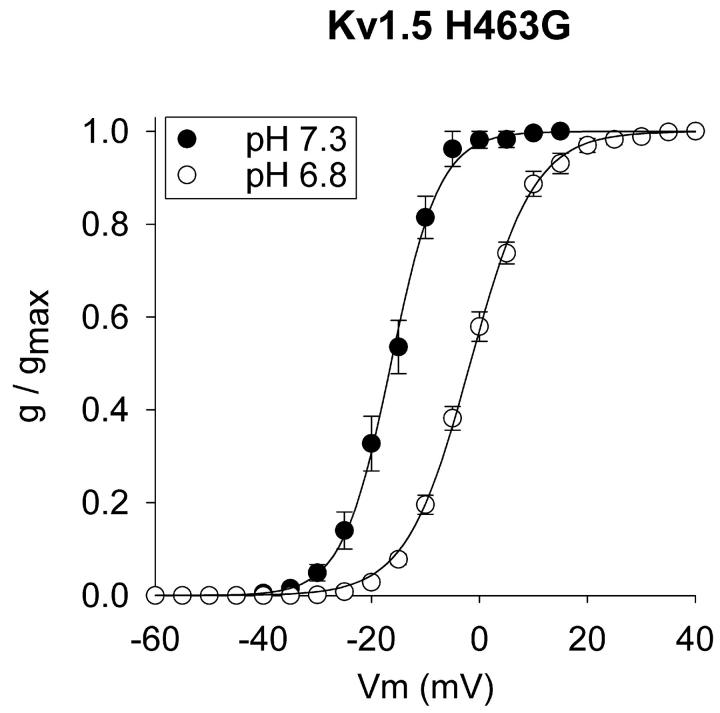
Kv1.5 has a histidine at amino acid position 463. This histidine is located in the outer vestibule of the conduction pathway and appears to be the only channel histidine exposed to the external solution. To determine whether this histidine contributed to the pH sensitivity of activation, we examined the voltage dependence of activation of Kv1.5 H463G, in the presence of 5 mM external K+ (Fig. 2). Mutation of this residue had no effect on the voltage dependence of activation at either pH 7.3 or 6.8 (compare Fig. 2 to Fig. 1 F). Thus, this residue did not influence the voltage sensitivity of the channel nor was it involved in the acidification-induced shift in the activation curve.
FIGURE 2.
Acidification-induced shift in activation in Kv1.5 H463G. Currents were recorded in 5 mM external K+. V1/2 values: −16.3 ± 1.4 (pH 7.3, n = 5) and −1.8 ± 0.9 (pH 6.8, n = 8).
The role of local surface charges
It has long been known that activation of voltage-gated ion channels is sensitive to the presence of negative charges on the cell surface (Hille, 2001). These negative charges, which are probably primarily associated with the channel itself (Elinder and Arhem, 1999), influence the voltage-dependence of activation due to the creation of a negative surface potential near the gating apparatus. A key test of whether negative surface charges are contributing to the effects of acidification on ion channel activation is to examine the influence of divalent cations on the acidification-induced shift. If a local surface potential is involved, three predictions can be made. First, elevation of divalent cation concentration should shift the activation curve to the right. This occurs as a result of neutralization of the surface charges, either due to a charge screening effect or a weak binding interaction between divalents and negatively charged sites (see Hille, 2001). Second, divalent cations would be expected to compete with protons for access to these sites. Thus, at higher divalent cation concentrations, greater acidification would be required to shift the activation curve. Third, if a relatively nonspecific local surface charge mechanism is involved, different divalent cations should produce similar effects. An alternative possibility would be that protons shifted the activation curve via an interaction with a specific Ca2+ binding site on the channel. This possibility predicts that elevation of [Ca2+], but not [Mg2+], would interfere with the acidification-induced shift in activation. (We do not distinguish here between a highly localized set of negatively charged sites that may predominantly influence activation versus a more globally located set of sites that might influence multiple voltage-dependent phenomena.).
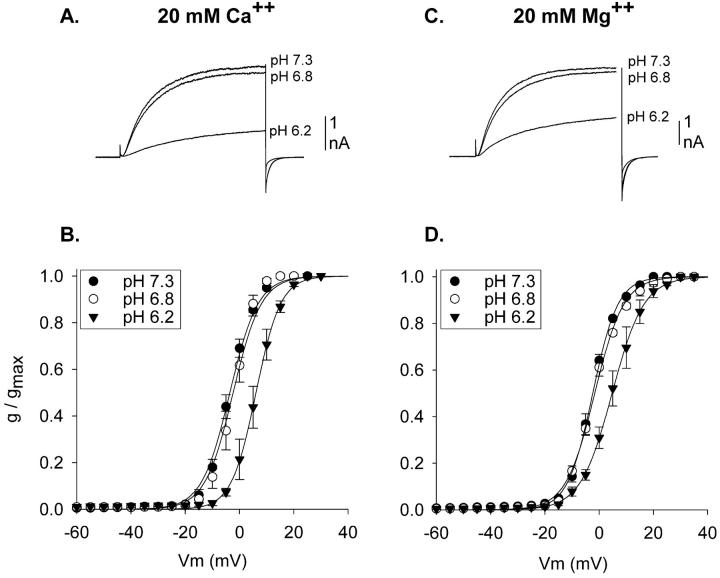
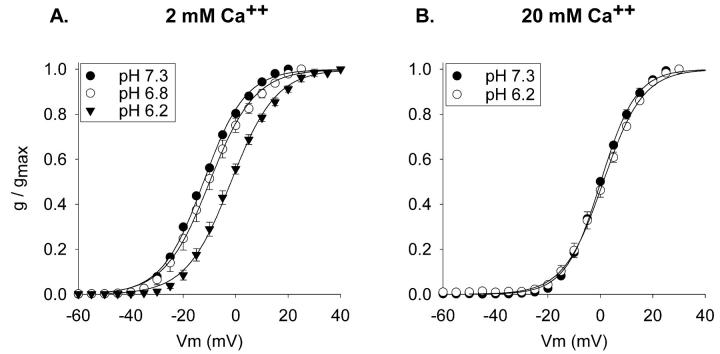
The experiments in Fig. 3 tested these predictions. The voltage-dependence of activation of Kv1.5 currents was examined in external solutions in which external Ca2+ (Fig. 3, A and B) or Mg2+ (Fig. 3, C and D) were elevated to 20 mM. As expected for a charge screening activity of divalent cations, activation curves at pH 7.3 in both high [Ca2+] and high [Mg2+] were shifted to the right (filled circles; compare to Fig. 1 F). Moreover, elevation of [Ca2+] and [Mg2+] shifted the activation curves identically. In contrast to the results obtained at lower divalent cation concentrations (Fig. 1 F), acidification from pH 7.3 to 6.8 had no effect on the voltage-dependence of activation when the external solution contained either high [Ca2+] or high [Mg2+] (Fig. 3, open circles). Additional acidification, to pH 6.2, shifted the activation curves in both high [Ca2+] and high [Mg2+] (Fig. 3, filled triangles). Moreover, the shifts produced by acidification to pH 6.2 were statistically identical (see figure legend 3). These results are consistent with the hypothesis that acidification shifted the activation curves due to the interaction of protons with negative surface charges that influence the channel gating apparatus.
FIGURE 3.
Acidification-induced activation shifts in elevated external [divalent cation] in Kv1.5. (A) Currents recorded in normal external solution except that [Ca2+] was raised to 20 mM. Three superimposed currents are shown, one each at pH 7.3, 6.8, and 6.2, evoked by 200 ms steps to 0 mV. (B) Activation curves produced in 20 mM external Ca2+ and 1 mM Mg2+. V1/2 values: −3.6 ± 0.9 (pH 7.3, n = 3), −2.5 ± 1.8 (pH 6.8, n = 4), 6.0 ± 1.6 (pH 6.2, n = 4). (C) Currents recorded in normal external solution except that [Mg2+] was raised to 20 mM. Three superimposed currents are shown, one each at pH 7.3, 6.8, and 6.2, evoked by steps to 0 mV. At the end of the voltage step, before repolarization, 200 ms worth of data were blanked out so that currents could be displayed on the same timescale as in A. (D) Activation curves produced in 2 mM Ca2+ and 20 mM Mg2+. V1/2 values: −2.4 ± 0.6 (pH 7.3, n = 3), −1.6 ± 0.3 (pH 6.8, n = 3), 4.9 ± 1.6 (pH 6.2, n = 4). In paired recordings, acidification from pH 7.3 to 6.2 produced average shifts of 7.5 ± 0.5 mV (n = 3) in high [Ca2+] and 7.4 ± 1.8 mV (n = 3) in high [Mg2+]. All external solutions contained 10 mM K+. In both A and B, osmolality was maintained in high [divalent cation] solutions by removal of 30 mM NMG-Cl.
Amino acids in the conduction pathway influence the pK of activation shifts
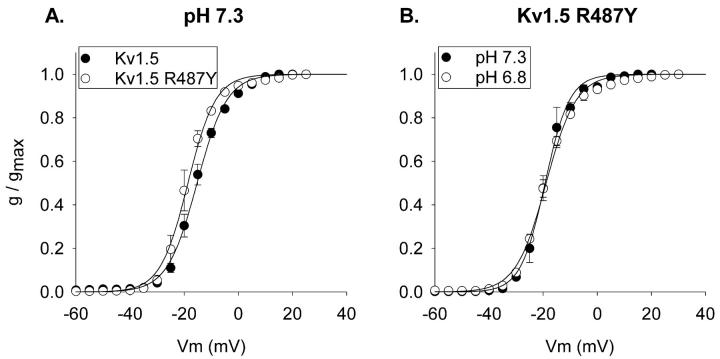
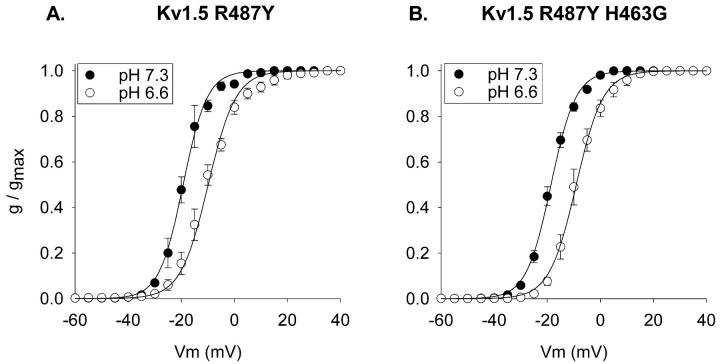
During the course of another study, we mutated the arginine at position 487 to a variety of other amino acids. This arginine occupies the site equivalent to the presumed TEA binding site in Shaker (T449) and Kv2.1 (Y380), and is located just external to the selectivity filter. Mutation of position 487 from the positively charged arginine to the aromatic tyrosine produced a slight (but statistically significant, p < 0.03) leftward shift in activation at pH 7.3 (Fig. 4 A). More importantly, however, this mutation dramatically shifted the pK for the acidification-induced shift in activation. Fig. 4 B illustrates that, in contrast to the wild-type channel, acidification from pH 7.3 to 6.8 had no effect on the voltage-dependence of activation of Kv1.5 R487Y. However, additional acidification, to pH 6.6, produced a 10-mV shift in the voltage-dependence of activation (Fig. 5 A). Mutation of the histidine at position 463 to a glycine in the Kv1.5 R487Y channel did not affect either the voltage-dependence of activation at pH 7.3 or the shift in activation produced by acidification to pH 6.6 (Fig. 5 B). Thus, as in the wild-type channel, the histidine at position 463 in the outer vestibule had no impact on activation.
FIGURE 4.
pH-sensitivity of activation in the Kv1.5 R487Y channel. (A) Comparison of activation curves at pH 7.3 for wild-type Kv1.5 (filled circles) and Kv1.5 R487Y mutant (open circles). Currents were recorded in 10 mM external K+. V1/2 values: −15.3 ± 0.8, (Kv1.5, n = 5) and −18.7 ± 0.8 mV (Kv1.5 R487Y, n = 3). In Kv1.5 R487Y, activation curves were identical in 0 and 10 mM external K+ (see B). (B) Activation curves for the R487Y channel at pH 7.3 and pH 6.8. Currents were recorded in 0 mM external K+. V1/2 values for the R487Y channel: −19.1 ± 1.0 (pH 7.3, n = 4), −18.9 ± 0.2 (pH 6.8, n = 3).
FIGURE 5.
H463G mutation does not affect acidification-induced activation shift in the Kv1.5 R487Y channel. (A) Activation at pH 7.3 and 6.6 in Kv1.5 R487Y. V1/2 values: −19.1 ± 1.0 (pH 7.3, n = 4) and −9.5 ± 1.5 (pH 6.6, n = 5). (B) Activation at pH 7.3 and 6.6 in Kv1.5 H463G R487Y. V1/2 values: −18.7 ± 0.7 (pH 7.3, n = 3) and −9.0 ± 1.5 (pH 6.6, n = 3). Currents were recorded in 0 external K+.
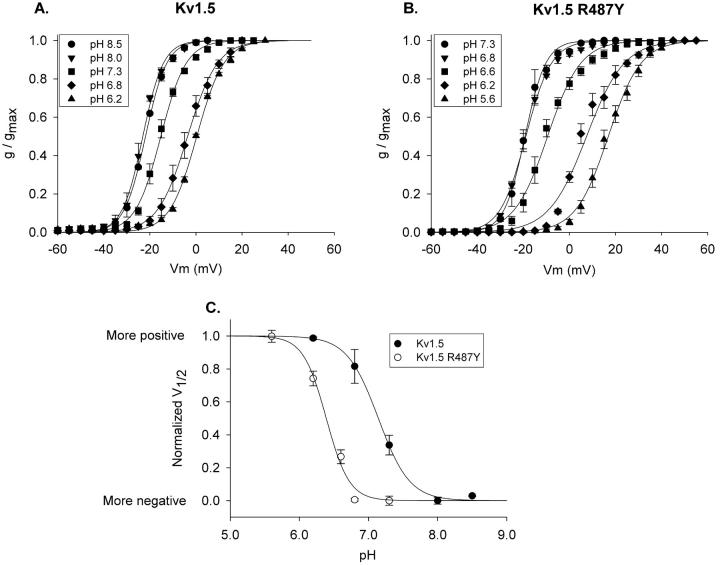
Fig. 6, A and B, illustrate activation curves for a range of extracellular pH values. For wild-type Kv1.5, activation shifted at pH values more acidic than pH 8.0 (Fig. 6 A). In contrast, the Kv1.5 R487Y channel became sensitive to changes in pH only at values more acidic than pH 6.8 (Fig. 6 B). Fig. 6 C illustrates the normalized V1/2 values, taken from data in Fig. 6, A and B, plotted as a function of pH. The R487Y mutation shifted the pK almost a full pH unit, from 7.15 for wild-type to 6.39 for the R487Y mutant.
FIGURE 6.
Shift in pK for pH-dependent activation shift with R487Y mutation. (A and B) Activation curves were constructed at the pH values indicated for Kv1.5 and Kv1.5 R487Y, respectively. (C) Plot of membrane potential shift as a function of pH. Activation curves at the two most alkaline pH values for each channel superimposed (see A and B), and were therefore considered to be maximally shifted in the negative direction. To fit the data with a Law of Mass Action function, each V1/2 value, for each channel, was offset identically in the positive direction, so that all V1/2 values were greater than or equal to 0 (the most negative V1/2 value became 0). Values for V1/2max and n were then found by best fit to the equation, F = (V1/2max* xn)/pKn + xn), where V1/2max is the maximum positive shift (in mV) in the curve, n is the slope of the curve, and pK is the pH at which the V1/2 was shifted by 50% of maximum. Data were then normalized to the best fit V1/2max values for each individual plot. Calculated pK values: 7.15 (Kv1.5) and 6.39 (Kv1.5 R487Y). Best fit values for n were 2 and 2.6, respectively. However, reasonably good fits could be obtained with n values ranging from 2 to 4. The calculated pK values were essentially unaffected when fits were constrained to n = 4 [pK = 7.22 (Kv1.5) and 6.39 (Kv1.5 R487Y)].
The mutation of position 487 from the positively charged arginine to the aromatic tyrosine had a small but significant effect on the voltage-dependence of activation (recall Fig. 4 A). This was consistent with two possibilities. First, the residue at this position may have made a small contribution to the local surface potential that influenced activation. This possibility is supported by the leftward shift in activation upon removal of positive charge. Alternatively, this mutation may have indirectly altered the influence of surface charge on activation via a small structural perturbation (i.e., an allosteric effect) in the channel region bearing the sites associated with the surface potential. The R487Y mutation had a more dramatic impact on the pK of acidification-induced activation shifts. However, the direction of this effect was opposite to that predicted for a direct interaction between positively charged arginines and negative charges that would contribute to local surface potential. That is, a direct interaction would predict that removal of a local positive charge would facilitate, not interfere with, protonation of a nearby negative site. To examine these issues further, we tested channels with two other mutations (an alanine and a phenylalanine) to position 487, to determine 1), whether the residue at position 487 directly contributed to the relevant local surface potential, and 2), whether the removal of positive charge was a relevant factor for the mutation-induced change in pK.
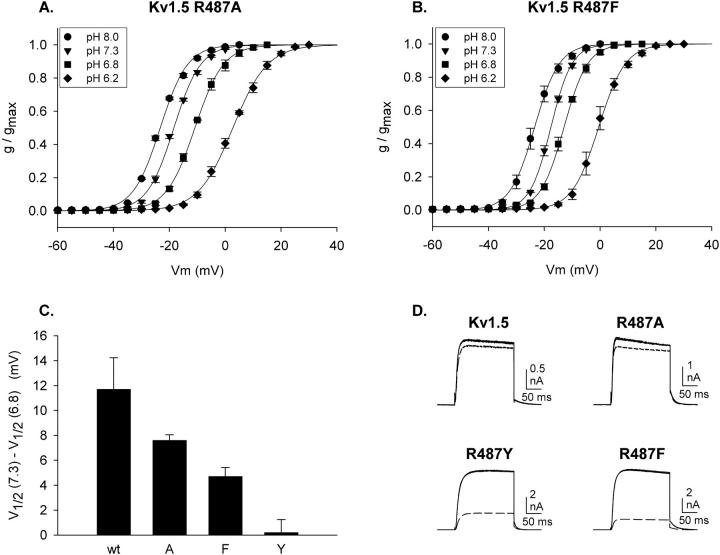
Fig. 7, A and B, illustrate the acidification-induced shifts in activation in Kv1.5 R487A and Kv1.5 R487F. Two important results emerge from these experiments. In both wild-type and Kv1.5 R487Y, activation curves were fully shifted to the left at pH 8 (Fig. 6), which suggests that the functionally relevant sites were fully unprotonated at pH 8.0. In the alanine and phenylalanine mutants, the V1/2 values for activation at pH 8 were identical to that of the wild-type channel, which contained the arginine at position 487. These data support the conclusion that the charge associated with the 487 residue itself did not contribute significantly to the functionally relevant local surface potential. However, the shift in activation produced by changing pH from 7.3 to 6.8 was significantly different in all four channels (Fig. 7 C). This supports the conclusion that the mutations to position 487 influenced the pK of sites associated with the local surface potential allosterically, rather than electrostatically via the removal of positive charge.
FIGURE 7.
Acidification-induced shifts in activation for other position 487 mutants. (A and B) Activation curves were constructed at the pH values indicated for Kv1.5 R487A and Kv1.5 R487F. Each curve was constructed from three cells, with all four pH values tested on each cell. (C) The shift in V1/2 between pH 7.3 and 6.8 is plotted for Kv1.5 channels with four different residues (arginine [wt], alanine, phenylalanine, and tyrosine) at position 487. (D) Block of currents through channels with different residues at position 487 by TEA. Three superimposed currents are illustrated in each panel, two recorded in the absence of external TEA (recorded before and after TEA application), and one recorded in the presence of TEA (30 mM for Kv1.5 and R487A and 1 mM for R487Y and R487F). Currents were recorded with 100 mM internal K+ and 150 mM external Na+ (no K+) to demonstrate the integrity of ionic selectivity (note the outward tail currents). Currents were evoked by depolarization for 200 ms to 0 mV. Repolarization was to −80 mV.
Fig. 7 D illustrates currents from each of the mutant channels, as well as wild-type Kv1.5. These currents were recorded with 100 mM internal K+ and 150 mM external Na+. As indicated by the outward tail currents recorded at −80 mV, each of these mutant channels retained their high selectivity for K+. In addition, they displayed relatively similar activation, deactivation, and inactivation kinetics. Finally, they were blocked by TEA with differences in potency predicted for the particular residue at position 487 (Heginbotham and MacKinnon, 1992; Snyders et al., 1993). These observations, combined with the identical voltage-dependence of activation at pH 8.0, suggest that the structural and functional integrity of the pore and gating apparatus was maintained in the mutant channels. Consequently, we conclude from these data that very subtle structural alterations near the selectivity filter had a marked impact on the sensitivity of activation to pH. Moreover, even though the residue at position 487 made little or no direct contribution to the local surface potential, the subtle structural alterations produced by mutation of this residue could perturb the proton sensitivity of charged sites that did contribute to the local surface potential.
Surface charge and the Kv2.1 potassium channel
Other voltage-gated K+ channels display activation shifts upon reduction of external pH that are consistent with an effect of local surface potential on activation (cf. Deutsch and Lee, 1989; Anumonwo et al., 1999; Steidl and Yool, 1999; Ishii et al., 2001). However, these activation shifts occur outside of the physiological pH range. Elinder and Arhem (1999) have proposed that the S5-P linker regions of voltage-gated K+ channels contain the charged sites associated with the surface potential effect on activation in K+ channels. In accordance with their hypothesis, they calculated that, although this region contains different amino acids in Kv1.5 and Kv2.1, the local surface potential in these two channels is the same (Elinder and Arhem, 1999). However, activation in Kv2.1 is insensitive to changes in pH within the physiological range (unpublished observations). Our hypothesis proposes that the primary difference between Kv1.5 and Kv2.1 with respect to surface charge and activation is that the pK for this effect is inside the physiological range for Kv1.5 and outside the physiological range for Kv2.1. Consequently, our hypothesis predicts that, 1), activation in Kv2.1 should shift with acidification but with a pK outside of the physiological range, and 2), that elevation of Ca2+ will competitively inhibit the pH-dependent activation shift in Kv2.1. Moreover, according to the hypothesis proposed by Elinder and Arhem (1999), elevation of external [Ca2+] will shift activation identically in the two channels.
To test these predictions, we studied the pH sensitivity of activation of the Kv2.1 K+ channel, expressed in the same environment as Kv1.5. Acidification from pH 7.3 to 6.8 had no effect on activation of Kv2.1 (Fig. 8 A). However, additional acidification, to pH 6.2, produced a significant shift in the voltage-dependence of Kv2.1 channel activation (Fig. 8 A). To test whether this sensitivity to pH represented an interaction of protons with local negative surface charges, we asked whether the shift in activation upon acidification to pH 6.2 was reduced in high external [Ca2+]. Elevation of external [Ca2+] to 20 mM shifted activation of Kv2.1 by 12 mV (Fig. 8 B), which was identical to the shift produced by elevated [Ca2+] on Kv1.5 activation. As predicted by our hypothesis, elevation of external Ca2+ abolished the shift in activation produced by acidification to pH 6.2. These data indicate that Kv2.1 channels and Kv1.5 channels are influenced similarly by local negative surface charges. However, the functionally relevant pK for the interaction of protons with these negative charges is within the physiological range for Kv1.5 and is out of the physiological range for Kv2.1.
FIGURE 8.
Acidification-induced activation shifts in Kv2.1. (A) Activation curves produced in 2 mM Ca2+. V1/2 values: −12.2 ± 0.1 (pH 7.3, n = 3), −10.0 ± 1.7 (pH 6.8, n = 3), −1.8 ± 1.0 (pH 6.2, n = 3). V1/2 values at pH 7.3 and 6.8 were not statistically different (p < 0.29). (B) Activation curves produced in 20 mM Ca2+. V1/2 values: 0.1 ± 0.4 mV (pH 7.3, n = 4), 1.1 ± 0.8 (pH 6.2, n = 4). All recordings were made in 0 mM external K+.
Predominance of the activation shift as a physiologically relevant mechanism of current modulation by external pH
Fig. 9 illustrates the influence of acidification from pH 7.3 to 6.8 on Kv1.5 currents evoked by stimulation to 0 mV and +100 mV in the presence of 3 mM external K+. As described in Fig. 1, stimulation to +100 mV avoids the pH-sensitive reduction in current associated with the activation shift. In the presence of 3 mM external K+, acidification to pH 6.8 had no impact on currents recorded at +100 mV (Fig. 9 B). As demonstrated previously for other external [K+], depolarization to 0 mV in the presence of 3 mM external K+ resulted in current block associated with a slowing of activation (Fig. 9 A). These results indicate that the pore-mediated influence on current magnitude is abolished by 3 mM external K+. Thus, with external [K+] that are likely to be encountered under physiological conditions, the acidification-induced shift in activation appears to be exclusively responsible for changes in current magnitude that occur with changes in pH between 7.3 and 6.8.
FIGURE 9.
Acidification-mediated reduction of Kv1.5 current magnitude in the presence of 3 mM K+. In both A and B, three superimposed currents are shown, one recorded at pH 6.8 and two recorded at pH 7.3, one before and one after the current recorded at pH 6.8. (A) Currents recorded at 0 mV. (B) Currents recorded at +100 mV. Acidification from pH 7.3 to 6.8 reduced currents by 2.3 ± 0.3% (n = 3).
DISCUSSION
External acidification reduces current magnitude in Kv1.5 via two independent effects. One effect appears to reduce channel availability, and involves the interaction of protons with residues within the outer vestibule of the conduction pathway (Steidl and Yool, 1999; Jager and Grissmer, 2001; Trapani et al., 2001; Kehl et al., 2002). Consistent with results obtained by Jager and Grissmer (2001), we show here that this effect is probably irrelevant at [K+] that occur in either physiological or pathological conditions. In addition, acidification also decreases current magnitude due to a shift to the right in the voltage-dependence of activation (Steidl and Yool, 1999; Kehl et al., 2002; Trapani et al., 2002). The results presented in this paper indicate that the mechanism by which acidification shifts activation in Kv1.5 involves a modification of local surface potential. This mechanism apparently occurs in all potassium channels. However, Kv1.5 is unique because, in Kv1.5, the pK for this effect is within the physiological range. Moreover, our data indicate that the overall pK of the surface charges that influence activation can be allosterically modified by at least one residue within the conduction pathway.
Divalent cation and pH-dependent activation shifts consistent with the presence of local surface potentials have been observed in many voltage-gated ion channels, including the K+ channels Kv1.1, Kv1.2, Kv1.3, Kv1.4, Kv1.5, Kv1.6, Kv2.1, and Kv3.4, and human ether-a-go-go-related gene (Deutsch and Lee, 1989; Elinder et al., 1996; Anumonwo et al., 1999; Ishii et al., 2001). Our data suggest that, indeed, the pH-dependent activation shift in most or all of these channels is due to the influence of surface charges on activation. Perhaps surprisingly, even though the two K+ channels, Kv1.5 and Kv2.1, have a significantly different primary sequence, elevation of external [Ca2+] produced an identical activation shift in the two channels (Figs. 3 and 8). This is consistent with the conclusion that a similar local surface potential exists for each channel type (Elinder and Arhem, 1999). However, the pK for the activation shift is significantly different in these two channels. The different pKs could result from the different complement of charged residues that create the local surface potential. Whereas this possibility has been postulated for the Kv1.5 and Kv2.1 channels (Elinder and Arhem, 1999), our data suggest that the pK can be markedly altered by subtle changes in the orientation of a set of negative sites, or in the environment surrounding the negative sites. Our data further demonstrate that amino acids inside the conduction pathway, even though they do not directly contribute to the relevant surface potential, can markedly affect the pK of residues that do directly contribute.
How did mutation of the position 487 residue alter the pH-sensitivity of activation?
Activation was fully shifted to the left at pH 8.0 (Fig. 6), which suggests that the negative sites associated with surface potential were mostly, or perhaps fully, unprotonated at this pH. Because changing the charge on residue 487 (mutation from an arginine to an alanine or phenylalanine) did not change the V1/2 of activation at the most alkaline pH values, we conclude that this residue did not directly contribute to the local surface potential, or directly interact with residues that did. However, mutation of the 487 residue, which lies within the conduction pathway of Kv1.5, dramatically influenced the pK of the acidification-induced activation shift. Thus, although these mutations didn't appear to alter the surface potential, they altered the interaction of protons with the negative sites (presumably amino acids) that created the surface potential. This could occur if the mutation created a subtle perturbation of the channel region that contained the negative sites, or the region immediately surrounding the negative sites.
Mutation of the residue at position 487 to a tyrosine had two effects. First, similar to the other mutations at position 487, the R487Y mutation changed the pK for the activation shift. This effect was somewhat greater for the R487Y mutant than for the other amino acid substitutions. Second, it also significantly shifted, albeit slightly, the activation curve at the most alkaline pH values, where the negative sites are fully unprotonated. Because the mutations to alanine and phenylalanine did not produce this shift, we conclude that removal of the positive charge from this position was not the cause of the shift. Rather, it appears that the R487Y mutation caused a reorientation of the charged residues that contributed to the local surface potential relative to the voltage sensor. Our data suggest that the mutations to alanine or phenylalanine also shifted the pK via a similar but more subtle reorientation.
Several observations indicate that the structural change was quite minor. First, the different 487 mutations produced incrementally different effects on the pH-dependence of activation (Fig. 7 C), with some changes in pH sensitivity being fairly small. Second, mutation of the 487 residue had little impact on the kinetics of activation, inactivation, or deactivation at pH 7.3 (Fig. 7). This suggests that the mutations had little impact on the kinetics of structural dynamics of either the S4 region or in the outer vestibule. Third, even though the 487 residue is located adjacent to the selectivity filter, high selectivity of the channel for K+ over Na+ was maintained (Fig. 7). Fourth, channels with position 487 mutations were blocked by TEA, with potencies that were similar to those in other channels with these or similar residues at the equivalent position (Fig. 7; Heginbotham and MacKinnon, 1992; Immke et al., 1999). Finally, two of the position 487 mutations (alanine and phenylalanine) did not alter the functional relationship between residues that contributed to the local surface potential and the gating apparatus (there was no shift in activation at the most alkaline pH values).
Where is the site of proton action?
The molecular site(s) at which protons influence gating remains unknown. However, several experiments suggest that residues in the outer vestibule of the conduction pathway do not directly contribute to the local surface potential involved in gating. Neutralization of both a histidine and an arginine within the conduction pathway had little or no influence on the voltage-dependence of activation at pH 7.3. This argues not only that these positively charged residues do not directly contribute to surface charge, but that these residues do not interact electrostatically with other residues that are involved. Similarly, neutralization of two lysines (removal of eight positive charges) in the Kv2.1 potassium channel outer vestibule had no impact on the voltage-dependence of activation at pH 7.3 (unpublished data).
Elinder and Arhem (1999) suggested that a series of eight residues located in the S5-P linker (part of the outer vestibule turret) are major contributors to the surface charge that influences activation in voltage-gated K+ channels. One of these residues, position 8 of the Elinder and Arhem (1999) sequence, is the position 463 histidine of Kv1.5 (the position 356 lysine in Kv2.1). In our experiments, neutralization of this residue had no impact on the V1/2 of activation or the pH-sensitive shift in activation. This suggests that this residue neither contributed to nor interacted with the sites involved in creating the local surface potential that affected activation.
However, the contribution of these eight residues to surface potential appears to reflect a twisted arrangement (Elinder and Arhem, 1999), consistent with the structural information obtained from the KcsA channel (Doyle et al., 1998). The side chain of the residue at position 8 of Elinder and Arhem (1999) (Kv1.5 H463 and Kv2.1 K356) is functionally exposed to the conduction pathway (Gross et al., 1994; Kurz et al., 1995; Immke et al., 1999). Thus, charges from other residues within this sequence would be expected to face away from the pore, and perhaps have a functional impact on the S4 channel segment. Indeed, amino acids in the S4 segment have been shown to interact with residues in the S5-P linker sequence (Gandhi et al., 2000; Loots and Isacoff, 2000; Elinder et al., 2001). Our experiments suggest that mutations at position 487 in Kv1.5 (the pore residue associated with TEA binding in other channels) can subtly change the spatial orientation of this sequence, or the environment immediately surrounding the side chains of these residues. In the case of the R487Y mutation, this reorientation was apparently substantial enough to also alter the influence of local surface potential on activation (Fig. 4 A).
The lack of effect of mutations at position 463 (or position 356 in Kv2.1) on local surface potential also has important implications for other studies. Mutations at this position in Kv2.1 have a dramatic impact on channel function (Immke et al., 1999; Wood and Korn, 2000). Mutations at this position (residue 425) in Shaker have been made to facilitate measurements of dynamic structural changes during gating (Cha and Bezanilla, 1997; Cha et al., 1999; Gandhi et al., 2000). Interpretations in these studies suppose that this mutation does not disturb the more global structure of this region, as it relates to function. If, indeed, the residues in the S5-P linker region contribute to the local surface potential that influences activation (Elinder and Arhem, 1999), our data support the conclusion that mutation of the residue at position equivalent to 463 does not disturb the structure of the S5-P linker region, or its relationship to the S4 region.
Finally, the observed effects of position 487 mutations have a practical impact on the search for the site of proton action. Although the amino acid at this position did not directly contribute to the local surface potential, electrostatically interact with residues that contributed to the local surface potential, or functionally change the relationship between the gating apparatus and residues that contributed to surface potential, mutation at this position dramatically influenced the pH-sensitivity of the residues that contributed to local surface potential. One could envision a similar result when studying gating shifts produced by divalent cations. That is, mutagenesis might alter the [divalent cation]-dependence of gating even if the residues mutated are not directly involved in the creation of local surface potential. These results suggest that it might be difficult to unequivocally identify the sites, using site-directed mutagenesis, that contribute to local surface potential.
Physiological relevance
Kv1.5 has two mechanisms by which changes in external pH within the physiological range influence current magnitude, one based on an action of protons within the outer vestibule (pore-mediated) and one associated with an acidification-induced shift in activation. The pore-mediated effect of protons is abolished in the presence of 3 mM external K+ (Fig. 9). However, because of the pH-sensitive activation shift, current magnitude in Kv1.5 remains quite sensitive to changes in external pH in the presence of physiological [K+] (in contrast to the suggestion of Jager and Grissmer; 2001). Our data indicate that the acidification-induced shift in activation represents the sole mechanism by which the Kv1.5 channel responds to changes in extracellular pH under physiological and pathological conditions.
Acknowledgments
Supported by the National Science Foundation and National Institutes of Health grant NS41090.
References
- Anumonwo, J. M., J. Horta, M. Delmar, S. M. Taffet, and J. Jalife. 1999. Proton and zinc effects on HERG currents. Biophys. J. 77:282–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, D. M., J. S. Trimmer, J. P. Merlie, and J. M. Nerbonne. 1995. Differential expression of voltage-gated potassium channel subunits in adult rat heart: relation to functional potassium channels. Circ. Res. 77:361–369. [DOI] [PubMed] [Google Scholar]
- Bell, J. E., and C. Miller. 1984. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys. J. 45:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, E., M. S. Urcan, S. S. Tinkle, A. G. Koszowski, and S. R. Levinson. 1997. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J. Gen. Physiol. 109:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Abboud, E., and J. M. Nerbonne. 1999. Molecular correlates of the calcium-independent, depolarization-activated K+ currents in rat atrial myocytes. J. Physiol. 517:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, D. T., and R. Hahin. 1984. Altered sodium and gating current kinetics in frog skeletal muscle caused by low external pH. J. Gen. Physiol. 84:771–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, A., and F. Bezanilla. 1997. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 19:1127–1140. [DOI] [PubMed] [Google Scholar]
- Cha, A., G. E. Snyder, P. R. Selvin, and F. Bezanilla. 1999. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 402:809–813. [DOI] [PubMed] [Google Scholar]
- Deutsch, C., and S. C. Lee. 1989. Modulation of K+ currents in human lymphocytes by pH. J. Physiol. 413:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D. A., J. M. Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Elinder, F., M. Madeja, and P. Arhem. 1996. Surface charges of K channels. Effects of strontium on five cloned channels expressed in Xenopus oocytes. J. Gen. Physiol. 108:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder, F., and P. Arhem. 1999. Role of individual surface charges of voltage gated K channels. Biophys. J. 77:1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder, F., R. Mannikko, and H. P. Larsson. 2001. S4 charges move close to residues in the pore domain during activation in a K channel. J. Gen. Physiol. 118:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida, D., B. Wimble, Z. Wang, B. Ferninin, F. Faust, S. Nattel, and A. M. Brown. 1993. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ. Res. 73:210–216. [DOI] [PubMed] [Google Scholar]
- Gandhi, C. S., E. Loots, and E. Y. Isacoff. 2000. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron. 27:585–595. [DOI] [PubMed] [Google Scholar]
- Gross, A., R. Abramson, and R. MacKinnon. 1994. Transfer of the scorpion toxin receptor to an insensitive potassium channel. Neuron. 13:961–966. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., and R. MacKinnon. 1992. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 8:483–491. [DOI] [PubMed] [Google Scholar]
- Hille, B. 2001. Ion Channels of Excitable Membranes, 3rd ed. Sinauer Associates, Sunderland, MA.
- Immke, D., M. Wood, L. Kiss, and S. J. Korn. 1999. Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J. Gen. Physiol. 113:819–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, K., K. Nunoki, T. Yamagishi, H. Okada, and N. Taira. 2001. Differential sensitivity of Kv1.4, Kv1.2 and their tandem channel to acidic pH: Involvement of a histidine residue in high sensitivity to acidic pH. J. Pharm. Exp. Therap. 296:405–411. [PubMed] [Google Scholar]
- Jager, H., and S. Grissmer. 2001. Regulation of a mammalian Shaker-related potassium channel, hKv1.5, by extracellular potassium and pH. FEBS Lett. 488:45–50. [DOI] [PubMed] [Google Scholar]
- Jurman, M. E., L. M. Boland, Y. Liu, and G. Yellen. 1994. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 17:876–881. [PubMed] [Google Scholar]
- Kehl, S. J., C. Eduljee, D. C. H. Kwan, S. Zhang, and D. Fedida. 2002. Molecular determinants of the inhibition of human Kv1.5 potassium channel currents by external protons and Zn2+. J. Physiol. 541:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockner, U., and G. Isenberg. 1994. Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J. Gen. Physiol. 103:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz, L. L., R. D. Zuhlke, H. J. Zhang, and R. H. Joho. 1995. Side-chain accessibilities in the pore of a K+ channel probed by sulfhydryl-specific reagents after cysteine-scanning mutagenesis. Biophys. J. 68:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver, D. R., and K. A. Fairley-Grenot. 1994. Surface potentials near the mouth of the large-conductance K+ channel from Chara australis: a new method of testing for diffusion-limited ion flow. J. Membr. Biol. 139:149–165. [DOI] [PubMed] [Google Scholar]
- Loots, E., and E. Y. Isacoff. 2000. Molecular coupling of S4 to a K+ channel's slow inactivation gate. J. Gen. Physiol. 116:623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, R., R. Latorre, and C. Miller. 1989. Role of surface electrostatics in the operation of a high conductance Ca2+-activated K+ channel. Biochemistry. 28:8092–8099. [DOI] [PubMed] [Google Scholar]
- Moczydlowski, E., O. Alvarez, C. Vergara, and R. Latorre. 1985. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J. Membr. Biol. 83:273–282. [DOI] [PubMed] [Google Scholar]
- Snyders, D. J., M. M. Tamkun, and P. B. Bennett. 1993. A rapidly activating and slowly inactivating potassium channel cloned from human heart. J. Gen. Physiol. 101:513–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl, J. V., and A. J. Yool. 1999. Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Mol. Pharm. 55:812–820. [PubMed] [Google Scholar]
- Taylor, J. R. 1997. An Introduction to Error Analysis, 2nd ed. University Science Books, Sausalito, CA.
- Trapani, J., P. Andalib, and S. J. Korn. 2001. K+-dependent potentiation of currents in the Kv1.5 K+ channel: dependence on external pH and internal channel blockers.Biophys. J. 79:442a (Abstr.) [Google Scholar]
- Trapani, J., P. Andalib, and S. J. Korn. 2002. Influence of pore residues on the pH-dependent shift in activation in the Kv1.5 potassium channel. Biophys. J. 82:235a. (Abstr.) [Google Scholar]
- Wood, M. J., and S. J. Korn. 2000. Two mechanisms of K+-dependent potentiation in Kv2.1 potassium channels. Biophys. J. 79:2535–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. F., and S. A. Siegelbaum. 1991. Effects of external protons on single cardiac sodium channels from guinea pig ventricular myocytes. J. Gen. Physiol. 98:1065–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]