Abstract
The highly selective sodium channel blocker, tetrodotoxin (TTX) has been instrumental in characterization of voltage-gated sodium channels. TTX occludes the ion-permeation pathway at the outer vestibule of the channel. In addition to a critical guanidinium group, TTX possesses six hydroxyl groups, which appear to be important for toxin block. The nature of their interactions with the outer vestibule remains debatable, however. The C-11 hydroxyl (C-11 OH) has been proposed to interact with the channel through a hydrogen bond to a carboxyl group, possibly from domain IV. On the other hand, previous experiments suggest that TTX interacts most strongly with pore loops of domains I and II. Energetic localization of the C-11 OH was undertaken by thermodynamic mutant cycle analysis assessing the dependence of the effects of mutations of the adult rat skeletal muscle Na+ channel (rNav1.4) and the presence of C-11 OH on toxin IC50. Xenopus oocytes were injected with the mutant or native Na+ channel mRNA, and currents were measured by two-electrode voltage clamp. Toxin blocking efficacy was determined by recording the reduction in current upon toxin exposure. Mutant cycle analysis revealed that the maximum interaction of the C-11 OH was with domain IV residue D1532 (ΔΔG: 1.0 kcal/mol). Furthermore, C-11 OH had significantly less interaction with several domain I, II, and III residues. The pattern of interactions suggested that C-11 was closest to domain IV, probably involved in a hydrogen bond with the domain IV carboxyl group. Incorporating this data, a new molecular model of TTX binding is proposed.
INTRODUCTION
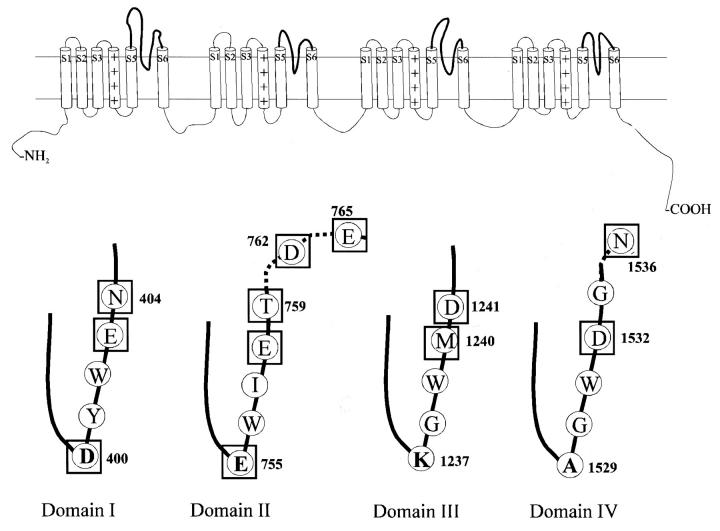
Tetrodotoxin, a naturally occurring site 1 guanidinium toxin, is a highly selective sodium channel blocker that has been instrumental in identification, isolation, purification, and characterization of voltage-gated sodium channels (Narahashi et al., 1967; Kao, 1986; Hille, 1992). Voltage-gated sodium channels are found in most excitable tissues like nerve, heart, and muscle. The ion-conducting pore is formed from a single α-subunit that consists of four homologous domains each with six transmembranous segments. The peptide chains between the fifth and sixth segments, known as P-loops, fold back into the membrane plane and line the ion-permeation path and outer vestibule. At the base of the P-loop structures from each of the four domains are amino acids that constitute the selectivity filter (Heinemann et al., 1992; Sun et al., 1997) (Fig. 1).
FIGURE 1.
(Top) Secondary structure of α-subunit of the voltage-gated sodium channel. The α-subunit is made of four homologous domains each with six transmembrane α-helices. (Bottom) The segments between the fifth and sixth helices loop down into the membrane to form the outer portion of the ion-permeation path, the outer vestibule. At the base of the pore-forming loops (P-loops) are the residues constituting the selectivity filter. The primary sequence of rat skeletal muscle sodium channel (Nav1.4) in the region of the P-loops is also shown. The selectivity filter residues are shown in bold. The residues tested are boxed.
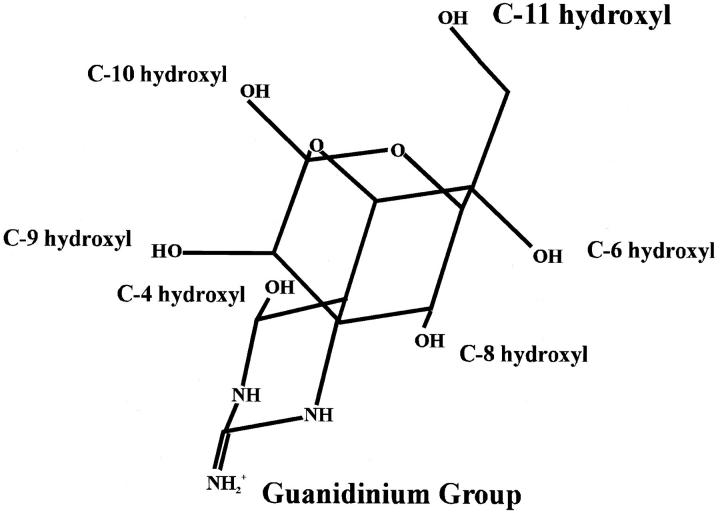
TTX is a rigid heterocyclic molecule consisting of a critical guanidinium group, positively charged at physiological pH, along with six hydroxyl groups (Fig. 2). TTX is believed to block the sodium current by occluding the ion-permeation pathway at the outer vestibule (Hille, 1992). Isolation of TTX analogs (Nakamura and Yasumoto, 1985; Yasumoto et al., 1988; Khora and Yasumoto, 1989) coupled with electrophysiological experiments (Kao, 1986; Kao and Yasumoto, 1985; Yang et al., 1992; Yang and Kao, 1992; Wu et al., 1996; Yotsu-Yamashita et al., 1999) identified the C-4, C-6, C-8, C-9, C-10, and C-11 hydroxyls as making significant contributions to TTX/channel interactions. Based on the facts that C-11 was important for binding and a C-11 carboxyl substitution dramatically reduced toxin block, the hydroxyl group at this location was proposed to interact with a carboxyl group in the outer vestibule (Yotsu-Yamashita et al., 1999). The most likely carboxyl was thought to be from domain IV because neutralization of this carboxyl had a similar effect on binding to the elimination of the C-11 OH.
FIGURE 2.
The structure of tetrodotoxin (Mosher, 1986). The molecule consists of a critical guanidinium group along with six hydroxyl groups. The guanidinium group is essential for blocking Na+ channels, and the hydroxyls, including the C-11 OH, have been shown to be important for binding. The C-11 OH group and the guanidinium group are at opposite ends of the molecule. 11-DeoxyTTX possesses a methyl group as the C6-equatorial substitution, instead of the hydroxymethyl group in TTX.
The view regarding TTX interactions has been formulated mostly on similarities with saxitoxin, another guanidinium toxin, and studies involving mutations of single residues on the channel or modification of toxin groups. No direct experimental evidence exists revealing specific interactions between the TTX groups and channel residues. This has led to variable proposals regarding the docking orientation of TTX in the pore wherein TTX is asymmetrically localized close to domains I and II or is tilted across the outer vestibule, interacting with domains II and IV (Penzotti et al., 1998; Yotsu-Yamashita et al., 1999). In this study, we provide evidence regarding the role and nature of the TTX C-11 OH in channel binding using thermodynamic mutant cycle analysis. We experimentally determined interactions of the C-11 OH with residues from all four domains to energetically localize and characterize the C-11 OH interactions in the outer vestibule. A molecular model of TTX/channel interactions explaining this and previous data on toxin binding is discussed.
MATERIALS AND METHODS
Preparation and expression of Nav1.4 channel
Most methods have been described previously in detail (Sunami et al., 1997; Penzotti et al., 2001). A brief description is provided. The Nav1.4 cDNA flanked by the Xenopus globulin 5′ and 3′ untranslated regions (provided by J.R. Moorman, Univ. of Virginia, Charlottesville, VA) was subcloned into either the Bluescript SK vector or pAlter vector (Promega, Madison, WI). Oligonucleotide-directed point mutations were introduced into the adult rat skeletal muscle Na+ channel (rNav1.4 or SCN4a) by one of the following methods: mutation D400A by the Unique Site Elimination Mutagenesis kit (Pharmacia Biotech, Piscataway, NJ), following the manufacturer's instructions; mutations E403Q, E755A, E758Q, T759D, T759I, T759K, D762N, E765Q, D1241A, and D1532N by four primer PCR and N404R, N404A, T759A, D1532A, D1532K, and N1536A by two primer PCR (Higuchi, 1990). Oligonucleotides were designed with silent restriction site changes for rapid identification of mutants. Except for D400A, which was sequenced in entirety, DNA sequencing of the polymerized regions subcloned back into the wild-type vector insured that only the intended mutations were present. The vectors were linearized and transcribed with a DNA-dependent RNA polymerase. Stage V and VI Xenopus oocytes from female frogs (NASCO, Ft. Atkinson, WI or Xenopus 1, Ann Arbor, MI) were injected with ∼50–100 ng of cRNA. Oocytes were incubated at 16°C for 12–72 h before examination.
Electrophysiology
Recordings were made in the two-electrode voltage clamp configuration. Data were collected using Axograph 4.4 software (Axon Instruments, Foster City, CA) at room temperature (20–22°C). All determinations of blocking efficacy of TTX and 11-deoxyTTX for channel mutants were performed over the same time period and with oocytes injected simultaneously. Affinity measurements for wild-type channels were reproducible over the experimental period.
A static bath was used to record affinity measurements because of high doses of toxin required to calculate the IC50 values (Stephan et al., 1994). The bath chamber was filled with 250 μl of bath solution, and after achieving a baseline current, toxin was added to the solution to achieve a known final toxin concentration in the bath. The affinity measurements by this method were comparable with the flowing bath measurements for some of the channel mutants, validating the method.
The standard bath solution consisted of (in mM): 90 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, and 5 HEPES titrated to pH 7.2 with 1 N NaOH. TTX was obtained from Sigma (St. Louis, MO) and purity confirmed by high-pressure liquid chromatography analysis. 11-DeoxyTTX was isolated from the newt Cynopus ensicauda (Yasumoto et al., 1988) and was quantified by 1H NMR spectroscopy using TTX as the standard, as described in Yotsu-Yamashita et al. (1999). Stocks were stored at −20°C and showed no degradation over the course of these experiments.
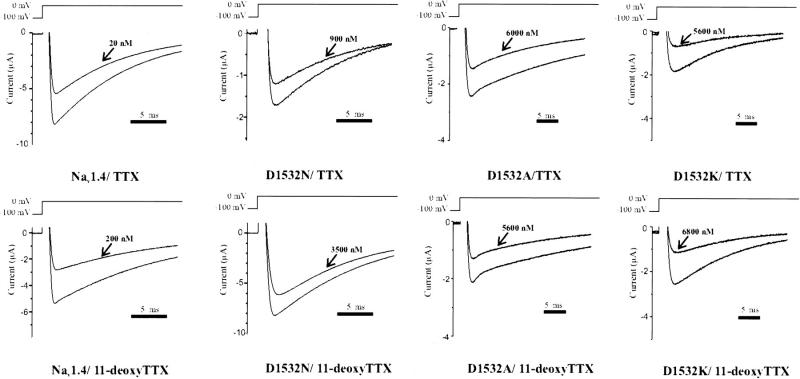
The effect of toxin addition was monitored by recording the peak current elicited every 20 s upon step pulses to 0 mV of 70 ms duration from a holding potential of −100 mV (Fig. 3). This protocol allowed the observation of toxin blocking, insured equilibrium was reached, and avoided the development of use-dependent toxin block. There was no accumulation of inactivated channels with this stimulus rate for the wild-type or mutant channels studied. The IC50 for toxin binding was calculated from the ratio of peak currents in the absence and presence of toxin based on a single site Langmuir adsorption isotherm. All IC50 values for a particular channel/toxin combination were tested for internal consistency by regression analysis involving various toxin concentrations used.
FIGURE 3.
Representative current tracings from the native channel and mutants upon exposure to TTX and 11-deoxyTTX. Sodium channels were expressed in Xenopus oocytes and studied by two-electrode voltage clamp. Only oocytes expressing currents <10 μA were studied to ensure adequate voltage control. The effect of toxin addition was monitored by recording the peak current elicited every 20 s upon step pulses to 0 mV of 70 ms duration from a holding potential of −100 mV. Control traces and those at the equilibrium bound state are shown.
Mutant cycle analysis
We defined ΔΔG as the difference of the ΔG values for TTX and 11-deoxyTTX, (ΔΔG = (ΔGwild type, TTX − ΔGwild type, 11-deoxyTTX) − (ΔGmutant, TTX − ΔGmutant, 11-deoxyTTX)), where the first subscript position refers to the channel. ΔG was calculated as: ΔG = −RTln (IC50). The standard error of ΔΔG was reported as the square root of the sum of the variances of the four RTln (IC50) averages, i.e., SQRT [Var1(ΔGwild type, TTX) + Var2(ΔGwild type, 11-deoxyTTX) + Var3(ΔGmutant, TTX) + Var4(ΔGmutant, 11-deoxyTTX)], divided by the square root of the sum of the total number of observations in all four combinations minus four (i.e., SQRT [n1(ΔGwild type, TTX) + n2(ΔGwild type, 11-deoxyTTX) + n3(ΔGmutant, TTX) + n4(ΔGmutant, 11-deoxyTTX) − 4]) (Bevington, 1969). Data are presented as means ± SE. The number of observations (n) was greater than or equal to four for all reported data. Statistical comparisons were performed using two-tailed Student's t-tests assuming unequal variances (Excel 2000, Microsoft Corp., Seattle, WA).
RESULTS
C-11 OH is important for toxin binding
The experimental goal was to determine the interactions of C-11 OH group with channel residues in the outer vestibule to localize the C-11 OH interactions. To test the hydrogen bond hypothesis, mutations of residues in the outer vestibule region known to be involved in site 1 toxin binding (Terlau et al., 1991) and whose side chains might bond with the C-11 OH were used. Additionally, extra-pore residues from domain II, D762 and E765, that have been shown recently to affect μ-conotoxin binding (Li et al., 2001a), and from domain IV, N1536, were evaluated. Domain I mutations D400A and E403Q and domain II mutations E755A and E758Q demonstrated no reduction in current when exposed to 3 μM, 100 μM, 100 μM, and 8 μM toxin, respectively, (Terlau et al., 1991; Penzotti et al., 1998). Therefore, the native toxin IC50 values for these mutations could not be calculated and to conserve the toxin, the IC50 values of 11-deoxyTTX were not determined. To increase the specificity of the results, multiple mutations were evaluated at selected locations.
Tetrodotoxin blocked the native channel with an IC50 of 48.6 ± 4.3 nM, similar to the previously reported value (Penzotti et al., 1998). Elimination of the –OH group at C-11 position increased the IC50 by sixfold to 294.0 ± 82.7 nM. The affinity decrease corresponded to a loss of ∼1 kcal/mol of binding energy, suggesting that the C-11 group played a significant role in the interaction of the toxin with the outer vestibule. To further define the interactions and energetically localize the C-11 group, we measured the affinity of the toxins with outer vestibule mutations.
Effect of outer vestibule mutations on toxin binding
Except for residues D762, E765, and N1536, all residues tested affected toxin binding. The effects of mutations were domain and site specific (Table 1). Based on these results, D762, E765, and N1536 would seem to lie beyond the TTX binding site.
TABLE 1.
IC50 (nM) and ΔΔG (kcal/mol) values
| TTX (nM) | 11-deoxyTTX (nM) | ΔΔG (kcal/mol) | |
|---|---|---|---|
| Nav1.4 | 48.6 ± 4.3 | 294.0 ± 82.7 | |
| (6) | (7) | ||
| D400A | >3000 | ND | ND |
| (6) | |||
| E403Q | >100,000 | ND | ND |
| (4) | |||
| N404A | 78.5 ± 9.9 | 664.0 ± 125.0 | 0.2 ± 0.1 |
| (9) | (10) | ||
| N404R | 132.0 ± 8.3 | 1190.0 ± 133.0 | 0.2 ± 0.1 |
| (10) | (6) | ||
| E758Q | >8000 | ND | ND |
| (8) | |||
| T759A | >100,000 | ND | ND |
| (4) | |||
| T759I | 89.7 ± 3.7 | 889.0 ± 50.9 | 0.3 ± 0.1 |
| (6) | (15) | ||
| T759K | 104.0 ± 10.6 | 694.0 ± 64.0 | 0.1 ± 0.1 |
| (18) | (13) | ||
| T759D | 387.0 ± 15.0 | 880.0 ± 43.1 | −0.6 ± 0.1 |
| (6) | (5) | ||
| D762N | 56.6 ± 9.5 | 441.0 ± 28.0 | 0.2 ± 0.1 |
| (22) | (20) | ||
| E765Q | 60.4 ± 4.7 | 422.0 ± 38.8 | 0.1 ± 0.1 |
| (4) | (4) | ||
| M1240A | 181.0 ± 15.0 | 2180.0 ± 43.8 | 0.4 ± 0.1 |
| (8) | (5) | ||
| D1241A | 29.7 ± 4.6 | 98.2 ± 8.2 | −0.3 ± 0.1 |
| (5) | (6) | ||
| D1532A | 7620.0 ± 691.0 | 8100.0 ± 868.0 | −1.0 ± 0.1 |
| (21) | (25) | ||
| D1532K | 3190.0 ± 173.0 | 6190.0 ± 855.0 | −0.7 ± 0.1 |
| (15) | (9) | ||
| D1532N | 1480.0 ± 185.0 | 8830.0 ± 721.0 | 0.0 ± 0.1 |
| (6) | (8) | ||
| N1536A | 71.8 ± 17.5 | 551.0 ± 81.5 | 0.1 ± 0.1 |
| (7) | (25) |
ND—Not Determined
Confirming the importance of domain I in overall toxin binding, both residues D400A and E403Q eliminated binding and could not be evaluated further. Domain I residue N404 was mutated to positively charged Arg, the native residue in cardiac channels, and neutral Ala, to evaluate possible domain I interactions with the toxins. Both mutations led to limited decreases in binding affinity. N404R worsening TTX binding by two- to threefold and 11-deoxyTTX by three- to fourfold, and N404A worsening binding of both toxins by twofold compared to the native channel (p < 0.05).
In domain II, mutations at T759 had a complex influence on toxin binding whereas mutations at D762 and E765 had minimal effects. Four mutations were tested at the T759 position. Three had constrained effects on TTX binding. T759D decreased TTX affinity the most, by eightfold, whereas T759I and T759K had similar, smaller effects (T759D: 387.0 ± 15.0 nM, T759I: 89.7 ± 3.7 nM, T759K: 104.0 ± 10.6 nM; p < 0.01) compared to Nav1.4 (48.6 ± 4.3 nM). All three mutations worsened 11-deoxyTTX affinity by a modest two- to threefold (Nav1.4: 294.0 ± 82.7 nM, T759D: 880.0 ± 43.1 nM, T759I: 889.0 ± 50.9 nM, T759K: 694.0 ± 64.0 nM; p < 0.01). Unlike other mutations at T759 position, T759A completely abolished toxin binding (T759A: IC50 > 100,000 nM). T759A did not differ from other mutations or the native channel in kinetics or extrapolated reversal potential, making major allosteric changes in the outer vestibule as a result of the mutation less likely. Because toxin binding effects did not correlate with amino acid side-chain functionality at this site, the effect of T759A on TTX binding was most likely because of a regional allosteric effect on E758, a known critical residue in toxin binding (Penzotti et al., 1998). The IC50 values for TTX with D762N and E765Q (D762N: 56.6 ± 9.5 nM, p = NS; E765Q: 60.4 ± 4.7 nM, p = NS) and 11-deoxyTTX with E765Q (E765Q: 422.0 ± 38.8 nM, p = NS) were not significantly different from the respective affinities with the native channel.
Paradoxically, neutralization of the domain III carboxyl group improved toxin binding slightly, but the opposite was true for the domain III Met. Domain III mutation D1241A improved affinities for both the toxins (TTX: 29.7 ± 4.6, p = 0.01; 11-deoxyTTX: 98.2 ± 8.2 nM, p < 0.01). 11-DeoxyTTX had worse binding compared to TTX, however, a trend similar to the native channel. Mutation of domain III M1240 to Ala led to decrease in affinity of TTX and 11-deoxyTTX (TTX: 181.0 ± 15.0 nM, p < 0.01; 11-deoxyTTX: 2,180.0 ± 43.8 nM, p < 0.01).
In domain IV, mutations of D1532 and N1536 were tested. Whereas N1536A had limited effects on binding, mutations at D1532 had larger effects. Multiple mutations were tested at the domain IV carboxyl group. In decreasing order, affinities of TTX for the channels were Nav1.4 > D1532N > D1532K > D1532A (D1532N: 1480.0 ± 185.0 nM, D1532K: 3190.0 ± 173.00 nM, D1532A: 7620.0 ± 691.0 nM). The IC50s of 11-deoxyTTX with D1532 mutations were in a tighter range, Nav1.4 > D1532K = D1532A > D1532N (D1532K: 6190.0 ± 855.0 nM, D1532A: 8100.0 ± 868.0 nM, D1532N: 8830.0 ± 721.0 nM). The mutation D1532A had equal affinity for TTX and 11-deoxyTTX. D1532N, like the native channel, had a sixfold worsening in binding with 11-deoxyTTX compared to TTX.
Interaction energies of C-11 OH with domain residues
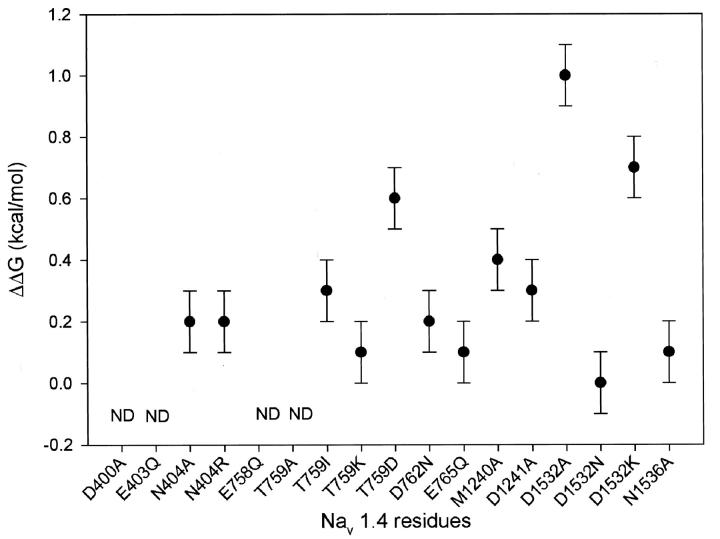
To evaluate specific interactions between the C-11 OH group and individual channel residues, we performed mutant cycle analysis (Fig. 4). Notably, residues outside the traditional outer vestibule showed no significant interactions with C-11 OH (ΔΔG: D762: 0.2 ± 0.1 kcal/mol; E765: 0.1 ± 0.1 kcal/mol; N1536: 0.1 ± 0.1 kcal/mol). In domains I, II, and III, interactions between the C-11 OH and the residues tested were limited. In the case of T759, the calculated interaction energies varied with the side chain substituted but not in a manner predictable from side-chain properties. (ΔΔGs: N404R: 0.2 ± 0.1 kcal/mol; N404A: 0.2 ± 0.1 kcal/mol; T759I: 0.3 ± 0.1 kcal/mol; T759K: 0.1 ± 0.1 kcal/mol; T759D: −0.6 ± 0.1 kcal/mol; M1240A: 0.4 ± 0.1 kcal/mol; D1241: −0.3 ± 0.1 kcal/mol). The domain IV D1532 interaction with C-11 OH was the largest identified and varied in a way that could be explained by the nature of side chain introduced at D1532. D1532N did not disrupt the interaction but D1532K and D1532A did (ΔΔGs: D1532N: 0.0 ± 0.1 kcal/mol; D1532K: 0.7 ± 0.1 kcal/mol; D1532A: 1.0 ± 0.1 kcal/mol), suggesting that D1532N with its free, nonbonded electron pair continues to participate in a hydrogen bond with the C-11 OH (see below). The interaction energy of D1532A with the C-11 was significantly different from the highest interaction energy in domain II, that of T759D (p < 0.001 by two-tailed Student's t-test).
FIGURE 4.
Coupling energies (ΔΔGs) for channel mutations with the 11-hydroxyl group on TTX. The C-11 OH has the strongest couplings with a domain IV carboxyl and the pattern is consistent with a C-11 OH interaction with domain IV. The error bars represent mean ±SE. ΔΔGs for D400, E403, E755, E758, and T759A could not be determined secondary to low native toxin binding affinity.
DISCUSSION
The docking orientation of TTX in the outer vestibule of voltage-gated sodium channel has been a matter of debate for some time (Yotsu-Yamashita et al., 1999; Yang et al., 1992; Penzotti et al., 1998; Kao, 1986). Most models rely on analogy to STX, but there is evidence that STX and TTX do not bind in an identical manner (Penzotti et al., 1998; Choudhary et al., 2002). The nature of TTX interactions with the outer vestibule residues could provide insight into the mechanism and biochemistry of this highly specific interaction. Though mutant cycle analysis has been used in defining STX and μ-conotoxin GIIIA interactions (Penzotti et al., 2001; Choudhary et al., 2002; Li et al., 2001b; Dudley et al., 2000), identification of specific interactions between the TTX molecule groups and channel residues has not been shown previously. The availability of 11-deoxyTTX provided a unique opportunity to evaluate the interactions of the C-11 OH group on TTX with the outer vestibule and the ability to test two proposed binding orientations.
The TTX C-11 OH is important for binding
Yang and his colleagues (Yang et al., 1992) reported the relative potency of 11-deoxyTTX in reducing INa in voltage-clamped frog muscle fiber as 0.04 compared to TTX. A similar decrease in potency was reported by Yotsu-Yamashita et al. in a rat brain synaptic membrane competitive binding assay with [3H]saxitoxin. (Yotsu-Yamashita et al., 1999; Yang et al., 1992). We found the relative potency to be 0.2 compared to TTX. This discrepancy may have resulted from differences in the channel isoform or the method of measurement (Ritchie and Rogart, 1977). Our results with the native toxin and shared channel mutations reproduced previously observed IC50 values using same method and preparation (Penzotti et al., 1998). Moreover, all results support the importance of C-11 OH for toxin binding.
The C-11 OH appears to interact with D1532 of domain IV
In 1998, Penzotti et al. proposed an asymmetric docking orientation for TTX in the outer vestibule based on comparing the effects of outer vestibule point mutations on TTX and STX affinities. Based on analogous reductions of TTX and STX binding with mutations in the selectivity filter and the similar actions of the two toxins, they concluded that the 1,2,3 guanidinium group of TTX and 7,8,9 guanidinium group of STX share a common binding site, the selectivity filter (Penzotti et al., 1998). On the other hand, differences in effect were noted at domain I Y401, domain II E758, and domain IV D1532. In the case of Y401, mutations had a much bigger impact on TTX and suggested that Y401 was closely interacting with TTX. In a molecular model, they suggested that TTX was more vertically oriented and closest to domains I and II, with the guanidinium group pointing toward the selectivity filter carboxyl groups. In this proposal, C-11 OH was closer to E403 and E758 and distant from D1532.
Using 11-deoxyTTX with native channels and observing the amount of binding energy lost upon removal of the –OH, Yang et al. (1992) and Yotsu-Yamashita et al. (1999) proposed that this hydroxyl is involved in a hydrogen bond and that the H-bond acceptor group may be D1532 because the ΔG upon mutation of this residue was almost equal to the ΔG for the TTX/11-deoxyTTX pair with native channel. Additionally, TTX-11-carboxylic acid showed a dramatic reduction in binding as if the new toxin carboxyl was being repelled by channel carboxyl. Because the guanidinium group is thought to interact with domain I and II carboxyl groups at the selectivity filter, this would mean that a tilted TTX molecule would span the outer vestibule so that the C-11 OH could interact near the domain IV D1532.
Our data suggest that the C-11 OH of TTX is most likely to interact with D1532, favoring the second hypothesis. This interaction is favored over the domain II for several reasons. First, the D1532/C-11OH interaction was the strongest identified. Second, the variation in the D1532/C-11 OH interaction was explicable by introduced D1532 side-chain properties. Third, we saw a similar sixfold change to Yang et al. (1992) and Yotsu-Yamashita et al. (1999) testing TTX and 11-deoxyTTX against native channels, suggesting an interaction energy of 1.1 kcal/mol contributed by the C-11 OH. This number is remarkably consistent with the C-11 OH/D1532 coupling energy calculated using D1532A. Finally, a molecular model with C-11 OH interacting with D1532 better explains all experimental results.
As predicted (Faiman and Horovitz, 1996), the calculated ΔΔGs are dependent on the introduced mutation. At D1532, the effect could be most easily explained if this residue was involved in a hydrogen bond with the C-11 OH. If mutation of the Asp to Asn were able to maintain the hydrogen bond between 1532 and the C-11 OH, this would explain the observed ΔΔG of 0.0 kcal/mol with D1532N. If this is true, elimination of the C-11 OH should have a similar effect on toxin affinity for D1532N as that seen with the native channel, and the same sixfold change was seen in both cases. The consistent ΔΔGs seen with mutation of the Asp to Ala and Lys suggest that both introduced residues eliminated the hydrogen bond between the C-11 OH with the D1532 position. Furthermore, the affinity of D1532A with TTX was similar to the affinity of D1532N with 11-deoxyTTX, suggesting equivalent effects of removal of the hydrogen bond participant on the channel and the toxin, respectively. It should be noted that while mutant cycle analysis allows isolation of specific interactions, mutations in D1532 position also have an effect on toxin binding that is independent of the presence of C-11 OH. The effect of D1532N on toxin affinity could be consistent with the loss of a through space electrostatic interaction of the carboxyl negative charge with the guanidinium group of TTX. Obviously, the explanation for the overall effect of D1532K on toxin binding must be more complex and awaits further experimentation.
Implications for TTX binding
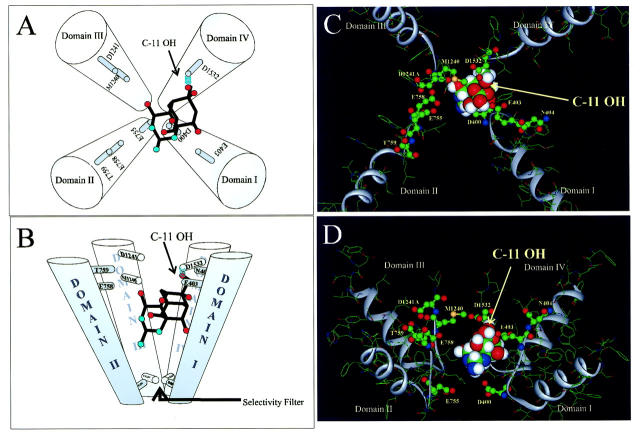
Based on the interaction of the C-11 OH with domain IV D1532 and the likelihood that the guanidinium group is pointing toward the selectivity filter, we propose a revised docking orientation of TTX with respect to the P-loops (Fig. 5) that explains our results, those of Yotsu-Yamashita et al. (1999), and those of Penzotti et al (1998). Using the Lipkind-Fozzard model of the outer vestibule (Lipkind and Fozzard, 2000), TTX was docked with the guanidinium group interacting with the selectivity filter and the C-11 OH involved in a hydrogen bond with D1532. The pore model accommodates this docking orientation well. This toxin docking orientation supports the large effect of Y401 and E403 residues on TTX binding affinity (Penzotti et al., 1998). In this orientation, the C-8 hydroxyl lies ∼3.5 Å from the aromatic ring of Trp. This distance and orientation is consistent with the formation of an atypical H-bond involving the π-electrons of the aromatic ring of Trp and the C-8 hydroxyl group (Nanda et al., 2000a; Nanda et al. 2000b). Also, in this docking orientation, C-10 hydroxyl lies within 2.5 Å of E403, enabling an H-bond between these residues. The close approximation TTX and domain I and a TTX-specific Y401 and C-8 hydroxyl interaction could explain the results noted by Penzotti et al. (1998) concerning the effect of mutations at the Y401 site and Kirsch et al. (1994) concerning the accessibility of the Y401 site in the presence of STX or TTX (Kirsch et al., 1994; Penzotti et al., 1998). Also, this arrangement could explain the differences in affinity seen between STX and TTX with channel mutations at E758. In the model, the closest TTX hydroxyls to E758 are C-4 OH and C-9 OH, at ∼7 Å each. This distance is much larger than those proposed for STX (Choudhary et al., 2002), suggesting an explanation of the larger effects on STX binding with mutations at this site. Finally, the docking orientation explains the loss of binding observed by Yotsu-Yamashita (1999) with TTX-11-carboxylic acid. When substituted for the –OH , the C-11 carboxyl group of the toxin lies within 2–3 Å of the carboxyl at D1532, allowing for a strong electrostatic repulsion between the two negatively charged groups.
FIGURE 5.
(A and B) Schematic emphasizing the orientation of TTX in the outer vestibule as viewed from top and side, respectively. The molecule is tilted with the guanidinium group pointing toward the selectivity filter and C-11 OH forming a hydrogen bond with D1532 of domain IV. (C and D) TTX docked in the outer vestibule model proposed by Lipkind and Fozzard (Lipkind and Fozzard, 2000). The docking arrangement is consistent with outer vestibule dimensions and explains several lines of experimental data. The ribbons indicate the P-loop backbone. Channel amino acids tested are in ball and stick format. Carbon (shown as green); nitrogen (blue); sulfur (yellow); oxygen (red); and hydrogen (white).
In summary, we show for the first time direct energetic interactions between a group on the TTX molecule and outer vestibule residues of the sodium channel. This puts spatial constraints on the TTX docking orientation. Contrary to earlier proposals of an asymmetrically docking close to domain II, the results favor a model where TTX is tilted across the outer vestibule. The identification of more TTX/channel interactions will give further clarity regarding the TTX binding site and mechanism of block.
Acknowledgments
Dr. Samuel C. Dudley, Jr. is supported by a Scientist Development Award from the American Heart Association, Grant-In-Aid from the Southeast Affiliate of the American Heart Association, a Proctor and Gamble University Research Exploratory Award, and the National Institutes of Health (HL64828). Dr. Mari Yotsu-Yamashita is supported by Grants-In-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 13024210).
References
- Bevington, P. R. 1969. Propagation of errors. In Data Reduction and Error Analysis for the Physical Sciences. McGraw-Hill Book Company, New York. 56–65.
- Choudhary, G., L. Shang, X. Li, and S. C. Dudley, Jr. 2002. Energetic localization of saxitoxin in the sodium channel outer vestibule. Biophys. J. 83:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, S. C., N. Chang, J. Hall, G. Lipkind, H. A. Fozzard, and R. J. French. 2000. μ-conotoxin GIIIA interactions with the voltage-gated Na+ channel predict a clockwise arrangement of the domains. J. Gen. Physiol. 116:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman, G. A., and A. Horovitz. 1996. On the choice of reference mutant states in the application of the double-mutant cycle method. Protein Eng. 9:315–316. [DOI] [PubMed] [Google Scholar]
- Heinemann, S. H., H. Terlau, W. Stühmer, K. Imoto, and S. Numa. 1992. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 356:441–443. [DOI] [PubMed] [Google Scholar]
- Higuchi, R. 1990. Recombinant PCR. In PCR Protocols: a Guide to Methods and Applications. M. A. Innis, editor. Academic Press, New York. 177–83.
- Hille, B. 1992. Ion Channels of Excitable Membranes. Sinauer Associates, Inc., Sunderland, MA.
- Kao, C. Y. 1986. Structure-activity relations of tetrodotoxin, saxitoxin, and analogues. Ann. N. Y. Acad. Sci. 479:52–67. [DOI] [PubMed] [Google Scholar]
- Kao, C. Y., and T. Yasumoto. 1985. Actions of 4-epitetrodotoxin and anhydrotetrodotoxin on the squid axon. Toxicon. 23:725–729. [DOI] [PubMed] [Google Scholar]
- Khora, S. S., and T. Yasumoto. 1989. Isolation of 11-oxotetrodotoxin from the puffer Arothorn nigropunctatus. Tetrahedron Lett. 30:4393–4394. [Google Scholar]
- Kirsch, G. E., M. Alam, and H. A. Hartmann. 1994. Differential effects of sulfhydryl reagents on saxitoxin and tetrodotoxin block of voltage-dependent Na channels. Biophys. J. 67:2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. A., I. I. Ennis, R. J. French, S. C. Dudley, Jr., G. F. Tomaselli, and E. Marban. 2001b. Clockwise domain arrangement of the sodium channel revealed by μ-conotoxin (GIIIA) docking orientation. J. Biol. Chem. 276:11072–11077. [DOI] [PubMed] [Google Scholar]
- Li, R. A., I. L. Ennis, G. F. Tomaselli, R. J. French, and E. Marban. 2001a. Latent specificity of molecular recognition in sodium channels engineered to discriminate between two “indistinguishable” μ- conotoxins. Biochemistry. 40:6002–6008. [DOI] [PubMed] [Google Scholar]
- Lipkind, G. M., and H. A. Fozzard. 2000. KcsA crystal structure as framework for a molecular model of the Na+ channel pore. Biochemistry. 39:8161–8170. [DOI] [PubMed] [Google Scholar]
- Mosher, H. S. 1986. The chemistry of tetrodotoxin. Ann. N. Y. Acad. Sci. 479:32–43. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., and T. Yasumoto. 1985. Tetrodotoxin derivatives in puffer fish. Toxicon. 23:271–276. [DOI] [PubMed] [Google Scholar]
- Nanda, V., and L. Brand. 2000a. Aromatic interactions in homeodomains contribute to the low quantum yield of a conserved, buried tryptophan. Proteins. 40:112–125. [DOI] [PubMed] [Google Scholar]
- Nanda, V., S. M. Liang, and L. Brand. 2000b. Hydrophobic clustering in acid-denatured IL-2 and fluorescence of a Trp NH-pi H-bond. Biochem. Biophys. Res. Commun. 279:770–778. [DOI] [PubMed] [Google Scholar]
- Narahashi, T., J. W. Moore, and R. N. Poston. 1967. Tetrodotoxin derivatives: chemical structure and blockage of nerve membrane conductance. Science. 156:976–979. [DOI] [PubMed] [Google Scholar]
- Penzotti, J. L., H. A. Fozzard, G. M. Lipkind, and S. C. Dudley, Jr. 1998. Differences in saxitoxin and tetrodotoxin binding revealed by mutagenesis of the Na+ channel outer vestibule. Biophys. J. 75:2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzotti, J. L., G. Lipkind, H. A. Fozzard, and S. C. Dudley, Jr. 2001. Specific neosaxitoxin interactions with the Na+ channel outer vestibule determined by mutant cycle analysis. Biophys. J. 80:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, J. M., and R. B. Rogart. 1977. Characterization of exchange-labeled saxitoxin and the origin of linear uptake by excitable tissue. Mol. Pharmacol. 13:1136–1146. [PubMed] [Google Scholar]
- Stephan, M. M., J. F. Potts, and W. S. Agnew. 1994. The μI skeletal muscle sodium channel: mutation E403Q eliminates sensitivity to tetrodotoxin but not to μ-conotoxins GIIIA and GIIIB. J. Membr. Biol. 137:1–8. [DOI] [PubMed] [Google Scholar]
- Sun, Y. M., I. Favre, L. Schild, and E. Moczydlowski. 1997. On the structural basis for size-selective permeation of organic cations through the voltage-gated sodium channel. Effect of alanine mutations at the DEKA locus on selectivity, inhibition by Ca2+ and H+, and molecular sieving. J. Gen. Physiol. 110:693–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami, A., S. C. Dudley, Jr., and H. A. Fozzard. 1997. Sodium channel selectivity filter regulates antiarrhythmic drug binding. Proc. Natl. Acad. Sci. USA. 94:14126–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau, H., S. H. Heinemann, W. Stühmer, M. Pusch, F. Conti, K. Imoto, and S. Numa. 1991. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 293:93–96. [DOI] [PubMed] [Google Scholar]
- Wu, B. Q., L. Yang, C. Y. Kao, S. R. Levinson, M. Yotsu-Yamashita, and T. Yasumoto. 1996. 11-Oxo-tetrodotoxin and a specifically labeled 3H-tetrodotoxin. Toxicon. 34:407–416. [DOI] [PubMed] [Google Scholar]
- Yang, L., and C. Y. Kao. 1992. Actions of chiriquitoxin on frog skeletal muscle fibers and implications for the tetrodotoxin/saxitoxin receptor. J. Gen. Physiol. 100:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L., C. Y. Kao, and T. Yasumoto. 1992. Actions of 6-epitetrodotoxin and 11-deoxytetrodotoxin on the frog skeletal muscle fiber. Toxicon. 30:635–643. [DOI] [PubMed] [Google Scholar]
- Yasumoto, T., M. Yotsu, M. Murata, and H. Naoki. 1988. New tetrodotoxin analogues from the newt Cynops ensicauda. J. Am. Chem. Soc. 110:2344–2345. [Google Scholar]
- Yotsu-Yamashita, M., A. Sugimoto, A. Takai, and T. Yasumoto. 1999. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 289:1688–1696. [PubMed] [Google Scholar]