Abstract
TRPM7 channels are nonselective cation channels that possess a functional α-kinase domain. It has been proposed that heterologously expressed TRPM7 channels are activated (Runnels et al., 2001) or inhibited (Nadler et al., 2001) by dialyzing the cell with millimolar levels of ATP. The endogenous correlate of TRPM7 has been identified in T-lymphocytes and RBL (rat basophilic leukemia) cells and named MagNuM (for Mg2+-nucleotide-inhibited metal) or MIC (for Mg2+-inhibited cation). Here, we report that internal Mg2+ rather than MgATP inhibits this current. Cytoplasmic MgATP, supplied by dialysis at millimolar concentrations, effectively inhibits only when a weak Mg2+ chelator is present in the pipette solution. Thus, MgATP acts as a source of Mg2+ rather than a source of ATP. Using an externally accessible site within the pore of the MIC channel itself as a bioassay, we show that equimolar MgCl2 and MgATP solutions contain similar amounts of free Mg2+, explaining the fact that numeric values of Mg2+ and MgATP concentrations necessary for complete inhibition are the same. Furthermore, we demonstrate that Mg2+ is not unique in its inhibitory action, as Ba2+, Sr2+, Zn2+, and Mn2+ can substitute for Mg2+, causing complete inhibition. We conclude that MIC current inhibition occurs simply by divalent cations.
INTRODUCTION
A member of the long TRP family of ion channels, TRPM7 (also known as ChaK1, Trp-PLIK, melanoma kinase and LTRPC7) has been recently cloned and characterized by several groups (Runnels et al., 2001; Ryazanova et al., 2001; Yamaguchi et al., 2001; Nadler et al., 2001). It contains a functional α-kinase domain at the C terminus (Runnels et al., 2001; Ryazanova et al., 2001), the structure of which has been determined by x-ray crystallography (Yamaguchi et al., 2001). Heterologously expressed TRPM7 forms a functional nonselective cation channel that conducts divalent ions in the inward direction and monovalent ions outwardly with a pronounced outwardly rectifying I/V relation (Runnels et al., 2001; Nadler et al., 2001). Upon removal of external divalent ions, both Mg2+ and Ca2+, the current drastically changes its I/V shape, becoming linear and conducting monovalent cations inwardly. The channel requires PIP2 for its function (Runnels et al., 2002), but the activation mechanism of this current remains unknown.
The presence of the α-kinase domain in the protein sequence led Runnels and co-workers to test whether inclusion of ATP in the internal solution would promote channel activity during whole-cell recording (Runnels et al., 2001). Dialysis of millimolar concentrations of NaATP led to activation of outwardly rectifying cation current, and ATP-dependent phosphorylation by the kinase domain was suggested to be a requirement for channel activity (Runnels et al., 2001). Subsequently, Nadler et al. showed that internal Mg2+ by itself (without ATP) inhibited TRPM7 currents (Nadler et al., 2001). This result provided an alternative explanation for the apparent channel activation by ATP; addition of NaATP simply reduced the level of free Mg2+ in the pipette solution and allowed the channel to conduct by reducing cytoplasmic Mg2+ during whole-cell dialysis and recording. In addition, MgATP and MgGTP were reported to inhibit the channel in a similar fashion, but more potently than Mg2+ alone (Nadler et al., 2001). It was suggested that MgATP requires micromolar free Mg2+ as a “cofactor” to exert its inhibitory effect. Lack of the “Mg2+ cofactor” was taken as the explanation for why NaATP did not inhibit on its own.
Northern analysis has shown that TRPM7 has a widespread tissue distribution and is highly expressed in lymph nodes, thymus, and bone marrow, but not in brain (Ryazanova et al., 2001). A native conductance with properties similar to expressed TRPM7 was identified and characterized in Jurkat T lymphocytes and RBL cells (Nadler et al., 2001; Hermosura et al., 2002; Prakriya and Lewis, 2002; Kozak et al., 2002). The channel was named MagNuM (for magnesium-nucleotide-inhibited metal) to emphasize a role for magnesium nucleotides in regulating the channel. An alternative nomenclature was also proposed: MIC for magnesium-inhibited cation (Prakriya and Lewis, 2002). Regardless of the nomenclature issue, the mechanism by which Mg2+ or MgATP inhibits the channel is uncertain.
Here we present evidence that the native MIC (MagNuM) current in RBL cells is inhibited by internal free Mg2+ and not by MgATP. We compare the maximal current inhibition with varying Mg2+ and ATP levels in the presence of weak and strong Mg2+ chelators. To test computed levels of free Mg2+, we made use of the fact that monovalent current through MIC channels can be blocked in a voltage-dependent manner by micromolar to millimolar levels of Mg2+ from the outside. We also used cells with preactivated MIC current in RBL cells and a rat T-lymphocyte cell line to assay and compare changes in current as dialysis with varying levels of Mg2+ (and ATP) progressed. Furthermore, internal Mg2+ appears not to be unique in its inhibitory action, as millimolar amounts of Ba2+, Sr2+, Mn2+, and Zn2+ also completely eliminated the current.
MATERIALS AND METHODS
Cell culture
Rat basophilic leukemia cells (RBL-2H3) (Siraganian et al., 1982) were cultured in Eagle's MEM supplemented with 10% fetal bovine serum, in 5% CO2-humidified atmosphere at 37°C. Cells were passaged twice weekly and plated on glass cover slips for recording. Rat PAS T cells were cultured as previously described (Beeton et al., 2001).
Patch-clamp recording
Whole-cell patch-clamp recordings were performed as previously described (Kozak et al., 2002). Briefly, patch pipettes (1.5–4 MΩ resistance) were manufactured from soda lime glass capillaries (Beckton-Dickinson, Parsippany, NJ and Kimble, Vineland, NJ). Voltage ramps (−120 to +85 mV, 211 ms duration) were delivered at 0.5 Hz frequency and current/voltage relations obtained. The cells were held at 0 mV between the ramps. Data were analyzed using Pulse/Pulsefit, v. 8.11 (HEKA Elektronik, Lambrecht, Germany), Igor Pro (v. 3.1.2) (WaveMetrics, Lake Oswego, OR), and Microcal Origin (v. 6) (Microcal Software, Northampton, MA) software.
Pipette solutions were designed to vary free Mg2+ and MgATP levels independently. Maxchelator (v. 1.78) software, written by Chris Patton (Stanford University), was used to calculate free divalent concentrations. The low-Mg2 internal solution, with free Mg2+ of 230 nM, consisted of (mM): 128 Cs+ glutamate, 8 NaCl, 10 EDTA, 1 mM MgCl2, 10 HEPES, pH 7.3. The intermediate-Mg2+ internal solution, with free Mg2+ of ∼270 μM, contained (mM): 130 Cs+ glutamate, 8 NaCl, 12 EGTA, 0.5 mM MgCl2, 10 HEPES, pH 7.3. The intermediate-Mg2+ solution with MgATP had approximately the same level (∼270 μM) of free Mg2+ and contained (mM): 128 Cs+ glutamate, 8 NaCl, 3 EGTA, 2.5 HEDTA, 5 mM MgATP, 10 HEPES, pH 7.3. Solutions with the same Mg2+ and chelator concentrations were used as external solutions to test the calculated level of free Mg2+ experimentally. The divalent-containing external solution contained (mM): 2 CaCl2, 10 HEPES, 167 Na+ aspartate, 2 mM Cs+ methanesulfonate, pH 7.3. The divalent-free external solution contained (mM): 154 Cs+ aspartate, 10 HEDTA, 10 HEPES, and 5 CsCl, pH 7.3. A total of 5 mM BaCl2, SrCl2, MnCl2, or ZnCl2 were added to an internal solution containing Cs+ glutamate, 1 mM EGTA, 10 mM HEPES, pH 7.3. The free Ba2+, Sr2+, Mn2+, Zn2+ concentrations were estimated at ∼4 mM.
MgATP (from a bacterial source) stock was stored at −20°C and diluted in the recording solution before the experiment. MgATP and salts were purchased from Sigma (St Louis, MO). The MgATP used in this study contained 1.3 mmol Mg2+ per mmol of ATP.
RESULTS
Mg2+ chelators distinguish the effect of internal Mg2+ and ATP on MIC current amplitude
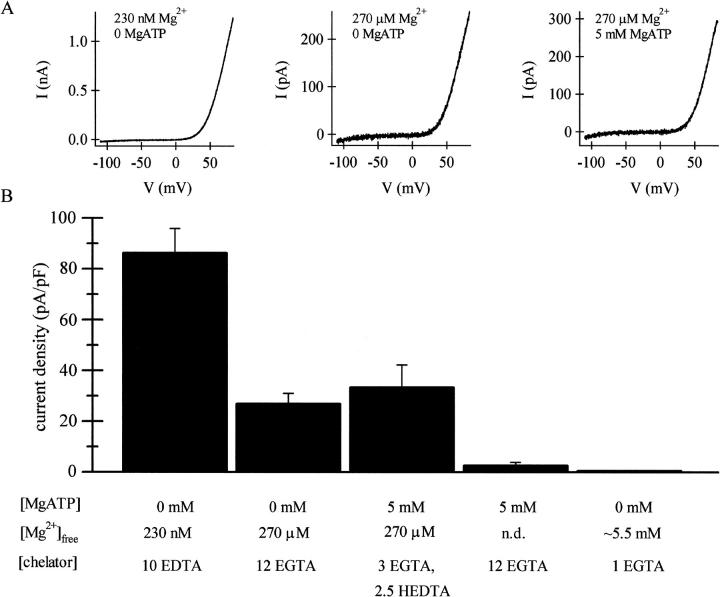
To test whether millimolar concentrations of MgATP inhibit MIC channels, we included 5 mM MgATP in the pipette and measured the current magnitude with different amounts of Mg2+ chelators present. Fig. 1 shows I/V curves and a comparison of MIC current amplitudes that develop with varying internal Mg2+ and MgATP concentrations. The characteristic outwardly rectifying current was allowed to develop to its maximal value, typically achieved 10–15 min after break-in and followed by a slow run-down of the current (Kozak et al., 2002). The largest MIC currents developed with low-Mg2+ internal solution. In agreement with Nadler et al. (2001), we saw a drastic reduction of MIC current with 5 mM MgATP compared to the current recorded when the pipette contained intermediate levels of Mg2+ (0.5 mM MgCl2 with a calculated free [Mg2+] of 270 μM; Fig. 1). MgATP did not change the I/V shape of MIC current. Also in agreement with Nadler et al. (2001), the inclusion of 6 mM MgCl2 in the pipette (with 1 mM EGTA) completely inhibited MIC current and served as a positive control. Thus, dialysis of 5 mM MgATP mimics the effect of millimolar internal Mg2+ in inhibiting the MIC current development.
FIGURE 1.
Inhibition of MIC current by internal MgATP is dependent on the type and amount of chelator. (A) The I/V relations of MIC current activated by dialysis with internal solutions containing 1 mM Mg2+ (10 mM EDTA), 0.5 mM Mg2+ (12 mM EGTA), and 5 mM MgATP (3 mM EGTA/2.5 mM HEDTA). The I/V shape did not vary with the composition of the internal solution. Traces are leak subtracted. (B) Summary of MIC current amplitudes. RBL cells were dialyzed with Mg2+- and MgATP-containing solutions; maximally activated MIC current amplitudes were measured at +80 mV. Maximal MIC current amplitudes were obtained by strongly chelating free Mg2+ with EDTA to an estimated 230 nM. An amount of 5 mM MgATP in the presence of 12 EGTA markedly inhibited the current, whereas the same amount of MgATP did not significantly inhibit when the internal chelator was 3 mM EGTA and 2.5 mM HEDTA. For comparison, ∼5.5 mM free [Mg2+] and no added MgATP are shown.
When 5 mM MgATP was included in a different intermediate-Mg2+ internal solution, containing 3 mM EGTA and 2.5 mM HEDTA (yielding a calculated free [Mg2+] of 270 μM), the size of the current was not different from that achieved with the solution that contained 270 μM free Mg2+ without MgATP. MgATP inhibition, therefore, depends on the nature of the Mg2+ chelator present. Inhibition is robust when a poor Mg2+ chelator is present (12 EGTA) but absent when a stronger (×100) Mg2+ chelator is employed (2.5 HEDTA). These results suggest strongly that MgATP inhibition is mediated by free Mg2+ ions rather than by ATP.
Comparison of free Mg2+ concentrations using the MIC current as a bioassay
Removal of external divalent ions enables inward monovalent currents in both expressed TRPM7 and native MIC channels, linearizing the I/V relationship. The monovalent inward current is highly sensitive to block by external Mg2+ in the micromolar range (Nadler et al., 2001; Kozak et al., 2002). Specifically, external free Mg2+ blocks the channel in a characteristic concentration- and voltage-dependent manner. We therefore decided to use the external Mg2+ block of the MIC channel as a bioassay to estimate and compare free Mg2+ content in various pipette and other test solutions simply by applying them from the outside and measuring the amount of monovalent current block. Since the common intracellular free Mg2+ concentrations used are 1–5 mM MgCl2 and 1–5 mM MgATP, we compared the extent of block of the monovalent MIC current by 1, 2, and 3 mM MgCl2 to 1, 2, and 3 mM MgATP. As seen in Fig. 2, the block caused by 1 or 2 mM MgCl2 was equal to that caused by corresponding concentrations of MgATP (Fig. 2, A and B). The same was true for 3 mM MgATP and MgCl2 solutions (data not shown). Since the MIC channel was not affected by external ATP, the voltage-dependent block can be attributed only to free Mg2+ acting from the outside. When the internal solutions containing no MgATP (with 0.5 mM MgCl2) or 5 mM MgATP (with EGTA/HEDTA) were applied externally, the block was equal in magnitude, confirming the calculated free Mg2+ concentration of ∼270 μM in both solutions (Fig. 2 C). Therefore, we conclude that intracellular solutions with weak Mg2+ chelators (such as BAPTA or EGTA) have close amounts of free Mg2+ whether MgCl2 or MgATP is used.
FIGURE 2.
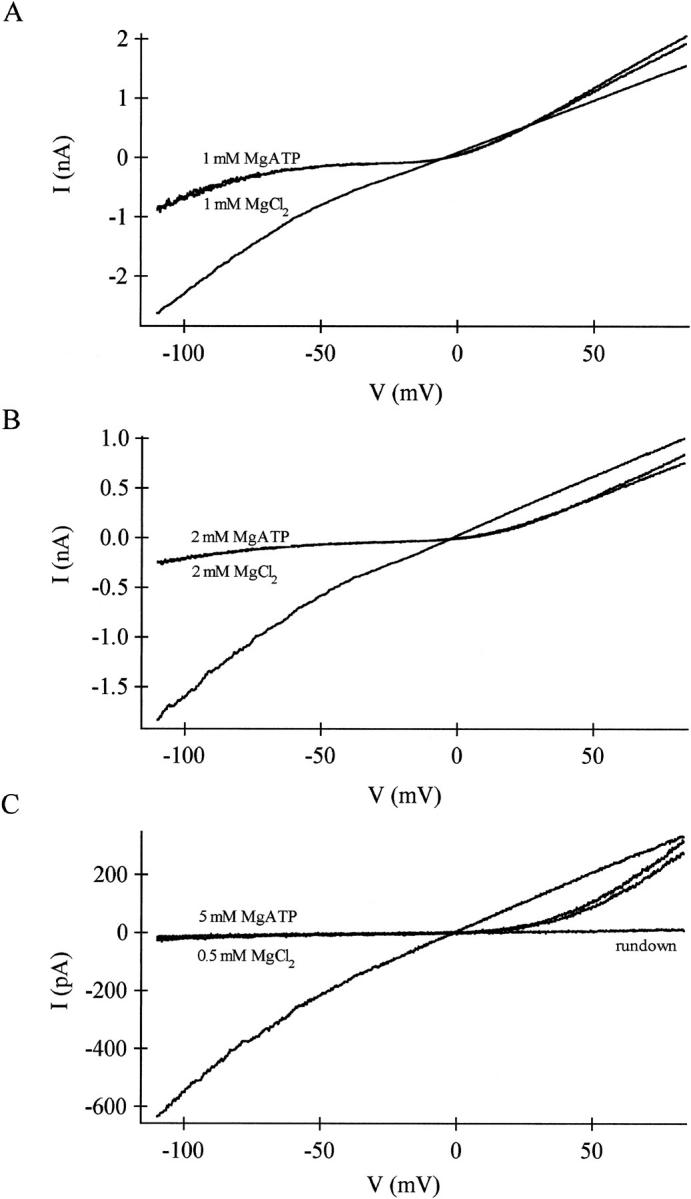
Monovalent MIC current as a bioassay for determining free Mg2+ concentrations. Solutions containing various amounts of MgCl2 and MgATP were applied externally and the I/V relations compared. (A) MIC monovalent current with external Cs+-HEDTA solution was blocked to the same extent by 1 mM MgCl2 (8 mM HEDTA) or by 1 mM MgATP (8 mM HEDTA). The calculated free [Mg2+] concentrations were 6.7 μM and 8.2 μM, respectively. (B) 2 mM MgCl2 and MgATP (in the presence of 8 mM HEDTA) blocked the monovalent MIC current to the same extent. The calculated free [Mg2+] concentrations were 15.8 μM and 17.5 μM, respectively. (C) The internal solutions from the experiment described in Fig. 1 were applied externally to compare the degree of block: 0.5 mM MgCl2 and 12 mM EGTA (calculated free [Mg2+] = 270 μM); compared with 5 mM MgATP and 3 mM EGTA/2.5 mM HEDTA (calculated free [Mg2+] = 270 μM). The current was allowed to run down completely to show the extent of block by 270 μM Mg2+.
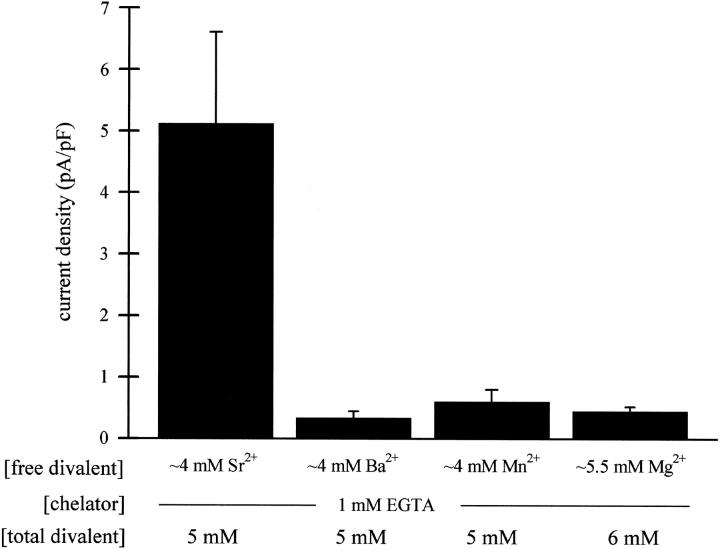
The divalent metal cations Ba2+, Sr2+, and Mn2+ mimic Mg2+
We further tested whether the inhibitory effect of internal Mg2+ is unique for that ion. It was previously demonstrated that MIC current develops in RBL cells with 1 mM EGTA and no divalents in the pipette (Kozak et al., 2002). As shown in Fig. 3, inclusion of 5 mM Ba2+ or Mn2+ (no ATP) in the pipette caused complete inhibition of MIC current (<1pA/pF remaining), comparable to 6 mM Mg2+. For comparison, the control levels of MIC current without divalent cations were in the range of 30–50 pA/pF (data not shown). Sr2+ (5 mM) also inhibited the current substantially, but not completely; 6 mM was necessary for complete inhibition (n = 4 cells). Zn2+ was also tested for its ability to prevent MIC current development. However, prolonged recordings with internal Zn2+ solutions proved difficult as the cells became leaky. Internal Zn2+ was tested on the preactivated current (see below) and was shown to be inhibitory. These results suggest that the channel is regulated by a metal-binding site with specificity for a high density of charge.
FIGURE 3.
Internal Sr2+, Ba2+, and Mn2+ can substitute for Mg2+ in inhibiting the MIC current in RBL cells. The maximal normalized current amplitudes (mean ± SE) during dialysis with ∼4 mM Sr2+ (n = 6 cells), Ba2+ (n = 5) and Mn2+ (n = 3) (1 mM EGTA), obtained as in Fig. 1 B, are compared to current inhibition by 5.5 mM Mg2+ (n = 3).
Testing inhibition in cells with preactivated MIC channels
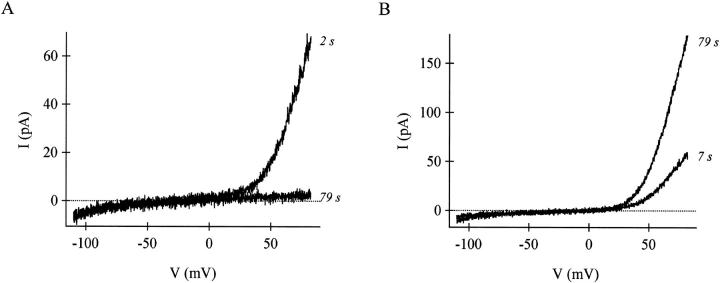
In the rat PAS T cell line, substantial endogenous MIC current is preactivated in the intact cell, as judged by current levels immediately after break-in to achieve whole-cell recording. These cells can be used to assess internal inhibition without waiting minutes for complete channel activation during dialysis. In the small fraction of RBL cells (∼5%) that also show preactivated MIC current, dialysis with millimolar free Mg2+ causes a gradual reduction of the current, leading to its disappearance within a few minutes (Kozak et al., 2002). Importantly, Mg2+ inhibition is voltage-independent, reducing the amplitude of the whole-cell current but leaving the I/V shape intact (Kozak et al., 2002). We used PAS T cells to test whether internal Ba2+ could inhibit the preactivated MIC current in the same fashion as Mg2+. Fig. 4 A shows an I/V of MIC current immediately after breaking in with an internal solution containing ∼4 mM [Ba2+]free (no ATP). Ba2+, like Mg2+, caused a gradual reduction of MIC current without affecting its I/V shape. The inhibition was complete by 79 s. Inclusion of ∼4 mM ZnCl2 also inhibited the preactivated MIC current in RBL cells (n = 3 cells, data not shown), without influencing the I/V shape before inhibition was complete. In contrast, when the pipette solution contained no divalents (12 mM EGTA), the current gradually increased in magnitude, as shown in Fig. 4 B; after 79 s of dialysis, MIC current was much larger than immediately after break-in. These experiments with pre-activated MIC current reinforce the conclusion that divalent metal cations inhibit nonspecifically.
FIGURE 4.
Millimolar concentrations of internal Ba2+ inhibit preactivated MIC current in rat PAS cells. (A) The MIC current I/V obtained 2 and 79 s after break-in with a pipette solution containing 5 mM BaCl2 and 1 mM EGTA. The inhibition is complete at 79 s. (B) The MIC current I/V obtained 7 and 79 s after break-in with a pipette solution containing no internal divalents (12 mM EGTA). The current was substantially increased after 79 s.
DISCUSSION
The TRPM subfamily of mammalian TRP cation channels has been identified recently by cloning and heterologous expression studies. Some TRPM members, TRPM2, TRPM6, and TRPM7, are remarkable in that they are “chanzymes” consisting of a channel domain and a C-terminal enzyme domain (Montell et al., 2002). In the case of TRPM2, the enzyme is an ADP-ribose pyrophosphatase (Perraud et al., 2001; Sano et al., 2001), and in TRPM6 and TRPM7 it is an α-kinase (reviewed in Ryazanov (2002)). The enzyme domains of TRPM2 and TRPM7 are functional in biochemical assays. When expressed by transfection, TRPM2 forms a Ca2+-permeable channel that is activated by ADP-ribose, but not by ATP or other nucleotides, suggesting a direct involvement of its enzyme domain in channel gating (Perraud et al., 2001; Sano et al., 2001). In the case of TRPM6 and TRPM7, however, the situation may be more complex regarding a possible role of the kinase domain. The initial study by Runnels and colleagues suggested that ATP might activate TRPM7 and concluded that the α-kinase domain was likely to be involved in channel gating (Runnels et al., 2001). Nadler et al. challenged this view and showed that Mg2+ and MgATP inhibit the current (Nadler et al., 2001). They also demonstrated that phosphorylation did not underlie MgATP action as other nucleotides, including nonhydrolyzable analogs, had similar effects in conjunction with Mg2+. ATP hydrolysis would be unlikely in any case given that the inhibitory effect of MgATP was observed at millimolar concentrations, well above amounts used in biochemical reactions (Hilgemann, 1997). Thus, a possible physiological role for the α-kinase domain of TRPM7 remains to be discovered.
In this study, we demonstrate that the inhibitory effect of millimolar MgATP concentrations on the native MIC current is dependent on the nature and amount of the Mg2+ chelator in the solution. MgATP (5 mM) indeed inhibits in the presence of EGTA (a weak Mg2+ chelator), but has no additional inhibitory effect when HEDTA (a strong Mg2+ chelator) is also present, reducing the free Mg2+ concentration. At the same concentration of free Mg2+ there is no additional inhibition by MgATP. Our results lead to the conclusion that TRPM7/MIC channels are not dependent on ATP levels within the cell. We also show that the mechanism of inhibition is not unique to internal Mg2+, as millimolar Ba2+ or Sr2+ exert similar effects.
The mechanism for internal divalent action on the MIC channel is not clear but may not involve direct channel blockade. It was previously shown that Mg2+ inhibition is voltage-independent and rather slow compared to dialysis of a blocker with a direct effect on the channel (Kozak et al., 2002). Prakriya and Lewis (2002) showed that micromolar and millimolar free Mg2+ concentrations were able to inhibit the single MIC channel in an inside-out patch, suggesting a membrane-delimited action. Interestingly, Mg2+ inhibited the MIC channel reversibly at 100 μM but irreversibly at 2 mM. It is important to note that in the inside-out patch, MIC channels were already activated before exposure to Mg2+, whereas in whole-cell recordings the number of functional channels is increased during dialysis and at the same time Mg2+ starts exerting its inhibitory effect. In an inside-out patch, the activation process has already occurred, and only inhibition is observed. In the case of macroscopic current on the other hand, Mg2+ may exert effects on both the activation process (addition of new functional channels) and on channels that are already opened. This experimental distinction, or possible factors lost during patch excision, may reconcile the observation that MgATP inhibition of TRPM7 was reversible even at millimolar concentrations (Nadler et al., 2001), whereas in the inside-out patch millimolar Mg2+ inhibited irreversibly (Prakriya and Lewis, 2002). In PAS T cells with MIC channels that are preactivated at break-in, nanomolar to micromolar concentrations of free internal Mg2+ did not inhibit the current, whereas 4–5 mM Mg2+ blocked both preactivated current and development of MIC current in RBL cells (Kozak et al., 2002). Preactivated MIC current provides a convenient assay for inhibitory ions at millimolar concentrations (Fig. 4) and may provide clues to physiological regulation of channel gating.
Most TRP channels have been investigated when the protein is overexpressed in a heterologous system. TRPM7 channels are unique among other TRP family members in that the native counterpart of TRPM7 (MagNuM or MIC) is functionally expressed in T-lymphocytes and RBL cells, systems that have been described in great detail over the past 10 years. TRPM7 and native MIC (in fibroblasts) were shown recently to be inhibited by PIP2 depletion (Runnels et al., 2002). Consistent with this finding, MIC current in RBL cells runs down together with the endogenous PIP2-sensitive IRK1 current (Huang et al., 1998; Kozak et al., 2002). It is likely that Mg2+ (or other divalent cations) prevents the electrostatic interaction between PIP2 and the channel by screening the negative charge on the lipid head group. Consistent with this idea, Fan and Makielski (1997) have demonstrated that polyvalent cations such as La3+ are able to abolish K-ATP channel activation by anionic phospholipids. Although divalent cations were not tested in that study, there may be a common mechanism through which polyvalent cations mediate inhibition by screening PIP2. This interpretation is consistent with the common mode of action of Mg2+ and Ba2+, divalent cations that exhibit very different binding characteristics and widely disparate biochemical activities as a result of differing charge coordination. Another possibility is the involvement of a low affinity Mg2+-binding site either within the α-kinase domain (Yamaguchi et al., 2001) or on another protein that can inhibit MIC current after binding divalent metal cations. Additional experiments are needed to understand the physiological and mechanistic basis for channel gating.
Acknowledgments
We thank Lu Forrest for technical assistance and Fran Jurnak and Jim Hall for valuable comments and discussion. We also thank George Chandy, Heike Wulff, and Christine Beeton for encouraging us to record MIC currents in the PAS T cell line. We are grateful to Dr. Michel Vivaudou for advice with free Mg2+ and ATP estimation.
This project was supported by National Institutes of Health grant NS14609.
References
- Beeton, C., H. Wulff, J. Barbaria, O. Clot-Faybesse, M. Pennington, D. Bernard, M. D. Cahalan, K. G. Chandy, and E. Beraud. 2001. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc. Natl. Acad. Sci. USA. 98:13942–13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Z., and J. C. Makielski. 1997. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272:5388–5395. [DOI] [PubMed] [Google Scholar]
- Hermosura, M. C., M. K. Monteilh-Zoller, A. M. Scharenberg, R. Penner, and A. Fleig. 2002. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J. Physiol. 539:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann, D. W. 1997. Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annu. Rev. Physiol. 59:193–220. [DOI] [PubMed] [Google Scholar]
- Huang, C. L., S. Feng, and D. W. Hilgemann. 1998. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 391:803–806. [DOI] [PubMed] [Google Scholar]
- Kozak, J. A., H. H. Kerschbaum, and M. D. Cahalan. 2002. Distinct properties of CRAC and MIC Channels in RBL Cells. J. Gen. Physiol. 120:221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell, C., L. Birnbaumer, and V. Flockerzi. 2002. The TRP channels, a remarkably functional family. Cell. 108:595–598. [DOI] [PubMed] [Google Scholar]
- Nadler, M. J., M. C. Hermosura, K. Inabe, A. L. Perraud, Q. Zhu, A. J. Stokes, T. Kurosaki, J. P. Kinet, R. Penner, A. M. Scharenberg, and A. Fleig. 2001. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 411:590–595. [DOI] [PubMed] [Google Scholar]
- Perraud, A. L., A. Fleig, C. A. Dunn, L. A. Bagley, P. Launay, C. Schmitz, A. J. Stokes, Q. Zhu, M. J. Bessman, R. Penner, J. P. Kinet, and A. M. Scharenberg. 2001. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 411:595–599. [DOI] [PubMed] [Google Scholar]
- Prakriya, M., and R. S. Lewis. 2002. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J. Gen. Physiol. 119:487–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels, L. W., L. Yue, and D. E. Clapham. 2001. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 291:1043–1047. [DOI] [PubMed] [Google Scholar]
- Runnels, L. W., L. Yue, and D. E. Clapham. 2002. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 4:329–336. [DOI] [PubMed] [Google Scholar]
- Ryazanov, A. G. 2002. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 514:26–29. [DOI] [PubMed] [Google Scholar]
- Ryazanova, L. V., K. S. Pavur, A. N. Petrov, M. V. Dorovkov, and A. G. Ryazanov. 2001. Novel type of signaling molecules: protein kinases covalently linked to ion channels. Mol. Biol. (Mosk). 35:321–332. [PubMed] [Google Scholar]
- Sano, Y., K. Inamura, A. Miyake, S. Mochizuki, H. Yokoi, H. Matsushime, and K. Furuichi. 2001. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 293:1327–1330. [DOI] [PubMed] [Google Scholar]
- Siraganian, R. P., A. McGivney, E. L. Barsumian, F. T. Crews, F. Hirata, and J. Axelrod. 1982. Variants of the rat basophilic leukemia cell line for the study of histamine release. Fed. Proc. 41:30–34. [PubMed] [Google Scholar]
- Yamaguchi, H., M. Matsushita, A. C. Nairn, and J. Kuriyan. 2001. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol. Cell. 7:1047–1057. [DOI] [PubMed] [Google Scholar]