Abstract
The association of water (D2O) with phospholipid membranes was studied by using pulsed-electron spin resonance techniques. We measured the deuterium electron spin echo modulation of spin-labeled phospholipids by D2O in membranes of dipalmitoyl phosphatidylcholine with and without 50 mol% of cholesterol. The Fourier transform of the relaxation-corrected two-pulse echo decay curve reveals peaks, at one and two times the deuterium NMR frequency, that arise from the dipolar hyperfine interaction of the deuterium nucleus with the unpaired electron spin of the nitroxide-labeled lipid. For phosphatidylcholine spin-labeled at different positions down the sn-2 chain, the amplitude of the deuterium signal decreases toward the center of the membrane, and is reduced to zero from the C-12 atom position onward. At chain positions C-5 and C-7 closer to the phospholipid headgroups, the amplitude of the deuterium signal is greater in the presence of cholesterol than in its absence. These results are in good agreement with more indirect measurements of the transmembrane polarity profile that are based on the 14N-hyperfine splittings in the conventional continuous-wave electron spin resonance spectrum.
INTRODUCTION
The penetration of water into lipid membranes and the resultant transmembrane polarity profile have important consequences not only for membrane permeability but also for the energetics of protein insertion into the membrane and the stability of transmembrane helices in integral proteins. Recently, the polarity profile of lipid membranes was mapped by using the isotropic 14N-hyperfine splittings in the continuous-wave (CW) electron spin resonance (ESR) spectra of phospholipids that were spin-labeled in the sn-2 chain (Marsh, 2001). A sigmoidal, trough-like profile was obtained in which a transition from the high polarity region at chain positions closer to the phospholipid polar headgroups occurs, in the range C-8 to C-9, to a low polarity region in the center of the membrane. Addition of cholesterol to the membranes was found to increase the polarity in the outer regions, and to decrease it almost to zero in the inner regions of the membrane. This increases the gradient of the polarity transition and shifts it by 1–2 C-atom positions at 50 mol% cholesterol content.
From the known dependence of nitroxide isotropic hyperfine splittings on hydrogen bonding (Gagua et al., 1978; Marsh, 2002a), the polarity profiles obtained in the above study (Marsh, 2001) were related to penetration of water into the membrane. Nevertheless, a more direct approach to the detection of membrane-associated water is desirable, not least to substantiate the interpretation given to the polarity dependence of the spin-label hyperfine splittings in membranes. This is done here by using pulse-Fourier transform ESR techniques with deuterium-labeled water.
Kevan and co-workers (Szajdzinska-Pietek et al., 1984) have used modulation of the electron spin echo decays of spin-labeled fatty acids by the deuterium hyperfine interactions with D2O to investigate the association of water with detergent micelles. Here we use this approach, specifically the Fourier transform of the relaxation-corrected echo decay, to probe the direct interactions of water with spin-labeled lipid chains in phospholipid bilayer membranes. Both the transmembrane profile and the effect of cholesterol on water association with the phospholipids is found to parallel the rather more indirect results based on nitroxide hyperfine splittings in CW-ESR spectra (Griffith et al., 1974; Marsh, 2001; Subczynski et al., 1994).
MATERIALS AND METHODS
Materials
Dipalmitoyl phosphatidylcholine (DPPC), cholesterol, and heavy water (D2O) were from Sigma/Aldrich (St. Louis, MO). Spin-labeled phosphatidylcholines (1-acyl-2(n-doxyl-stearoyl)-sn-glycero-phosphocholine, n-PCSL) were from Avanti Polar lipids (Birmingham, AL) or synthesized according to Marsh and Watts (1982).
Sample preparation
DPPC with 1 mol% of n-PCSL, with and without 50 mol% cholesterol, were codissolved in chloroform. Solvent was evaporated with a nitrogen gas stream and residual traces removed by drying under vacuum overnight. The lipid (15 mg) was dispersed either in H2O (phosphate-buffered saline, pH 7.5) or in D2O at a concentration of ∼100 mg/ml by vortex mixing with heating to 60°C, i.e., above the chain-melting phase transition. The sample was then transferred to a standard 4 mm-diameter, quartz ESR tube, concentrated by pelleting in a bench-top centrifuge (the sample in D2O floats) and the excess water removed.
Pulsed EPR spectroscopy
Data were collected on a ELEXSYS E 580 9 GHz FT-EPR spectrometer (Bruker, Karlsruhe, Germany) equipped with a Flexline MD5, variable-Q, dielectric resonator and a nitrogen gas flow temperature control unit. Two-pulse, Hahn-Echo decays were obtained by using microwave pulse widths of 12 ns and 24 ns, with the microwave power adjusted to give π/2 and π-pulses, or less, respectively. The interpulse spacing was incremented from τ = 88 ns in 4-ns steps. A simple exponential decay of the maximum echo amplitude (at 2τ), which is characterized by the phase memory time, T2M, was subtracted. Hamming-apodization was then applied, with one level of zero filling, followed by Fourier transformation. Where necessary, phase correction of the real part of the nuclear modulation spectrum was then applied. Echo-detected absorption ESR spectra were obtained by recording the echo maximum, with an interpulse spacing of τ = 88 ns, while sweeping the magnetic field.
RESULTS
Electron spin echo envelope modulation
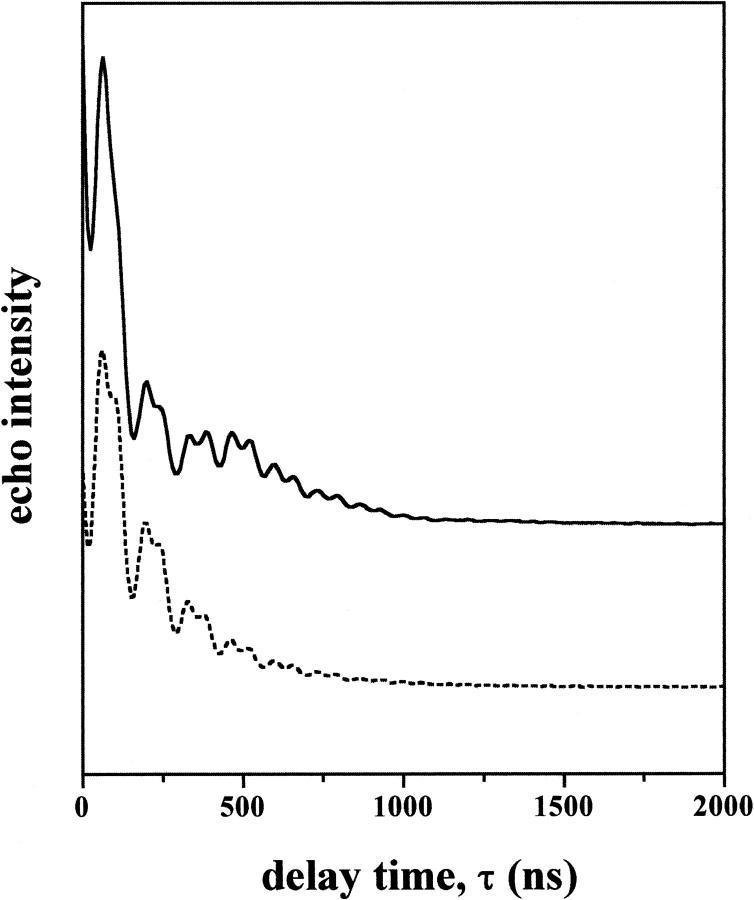
Fig. 1 gives typical electron spin echo decays of 7-PCSL spin-labeled phosphatidylcholine in membranes of dipalmitoyl phosphatidylcholine plus 50 mol% cholesterol that are hydrated in excess D2O or H2O. Both decay curves contain a high-frequency modulation, as a function of the interpulse spacing τ, that arises from interactions of the electron spin with nearby protons. In D2O, there is an additional low frequency modulation of appreciable amplitude that is not present for samples in H2O. This comes specifically from dipolar interactions of the spin label with the 2H-nuclear spins of the D2O molecules. The amplitude of the low-frequency modulation is proportional to the number of D2O molecules neighboring the spin label. Visualization and quantitation of the 2H-modulation is better achieved after Fourier transformation, which yields the spectrum in the nuclear frequency domain.
FIGURE 1.
Dependence of the electron spin echo amplitudes on interpulse spacing, τ, for spin-labeled phosphatidylcholine (7-PCSL) in membranes of dipalmitoyl phosphatidylcholine plus 50 mol% cholesterol at 150 K. The upper curve is for membranes dispersed in D2O and the lower curve for membranes in H2O.
ESEEM spectra in D2O and H2O
Fig. 2 gives the electron spin echo envelope modulation (ESEEM) spectra of phosphatidylcholine (n-PCSL) spin-labeled at different positions, n, in the sn-2 chain in bilayer membranes of dipalmitoyl phosphatidylcholine at 150 K. Spectra are given for membranes dispersed in D2O (solid lines) and for membranes dispersed in H2O (dashed lines). Proton modulations are observed at NMR frequencies of νH = 14.4 MHz and 2νH ≈ 29.5 MHz, as expected for two-pulse ESEEM spectra (Mims, 1972a; Mims, 1972b), in both cases. For membranes in D2O, additional peaks appear at deuterium NMR frequencies of νD = 2.7 MHz and 2νD ≈ 4.6 MHz. These arise from dipolar interactions of the deuterium nuclear spin in D2O with the electron spin in the nitroxide labeling the lipid chains. The amplitude of this deuterium signal decreases steadily with position, n, of the spin label down the lipid chain. It is completely absent at the C-12 and C-14 positions, close to the middle of the membrane. Unfortunately, the ESEEM spectrum of 10-PCSL in DPPC alone is distorted and the signal/noise ratio is low, because spectra at this position of labeling are strongly spin-spin broadened in gel-phase DPPC membranes (Fajer et al., 1992).
FIGURE 2.
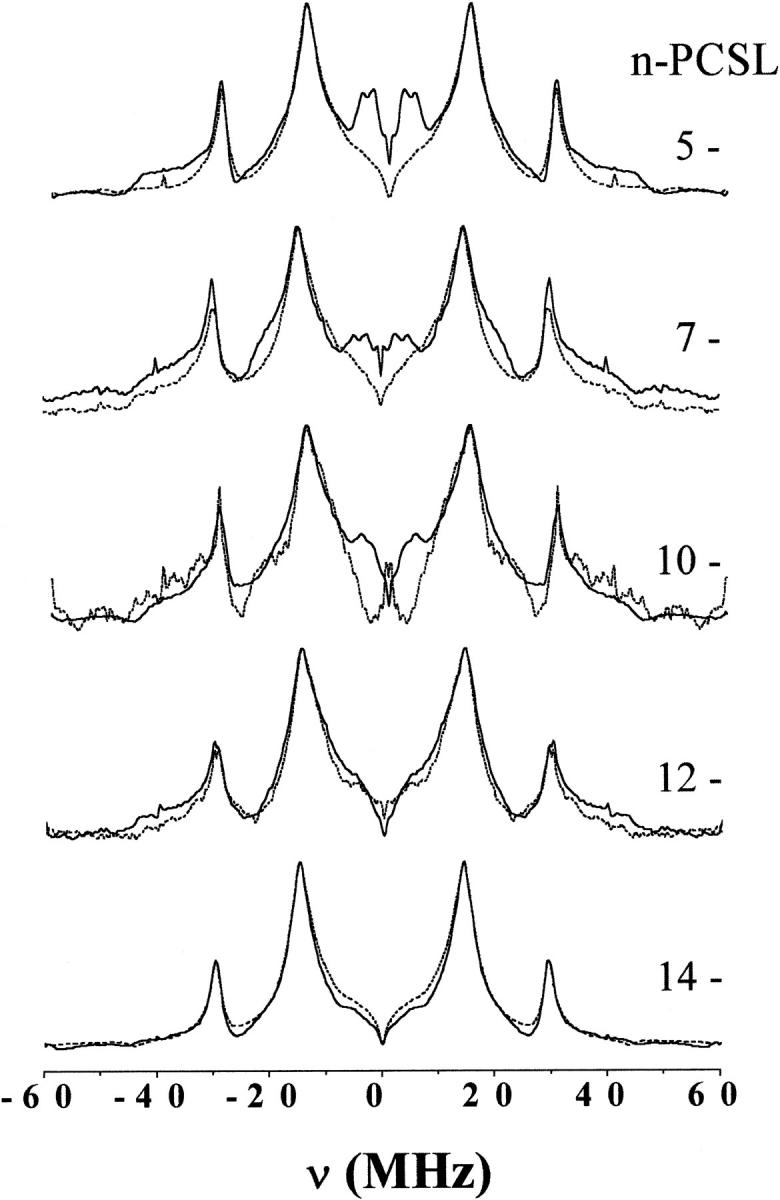
Fourier transform of the relaxation-corrected echo decays from n-PCSL spin-labeled phosphatidylcholines in bilayer membranes of dipalmitoyl phosphatidylcholine alone. Solid lines are for membranes dispersed in D2O and dashed lines are for membranes dispersed in H2O. The position, n, of spin-labeling in the sn-2 chain is indicated on the figure. Spectra are normalized to the maximum peak height.
Fig. 3 gives corresponding ESEEM spectra of the n-PCSL spin labels in membranes of dipalmitoyl phosphatidylcholine containing 50 mol% cholesterol. Again, spectra are given for membranes dispersed in H2O and in D2O. The normalized amplitudes of the deuterium modulation signals from 5-PCSL and from 7-PCSL are greater than those for membranes that do not contain cholesterol (Fig. 2). No deuterium modulation signal is seen at the C-12 and C-14 positions, just as in the absence of cholesterol.
FIGURE 3.
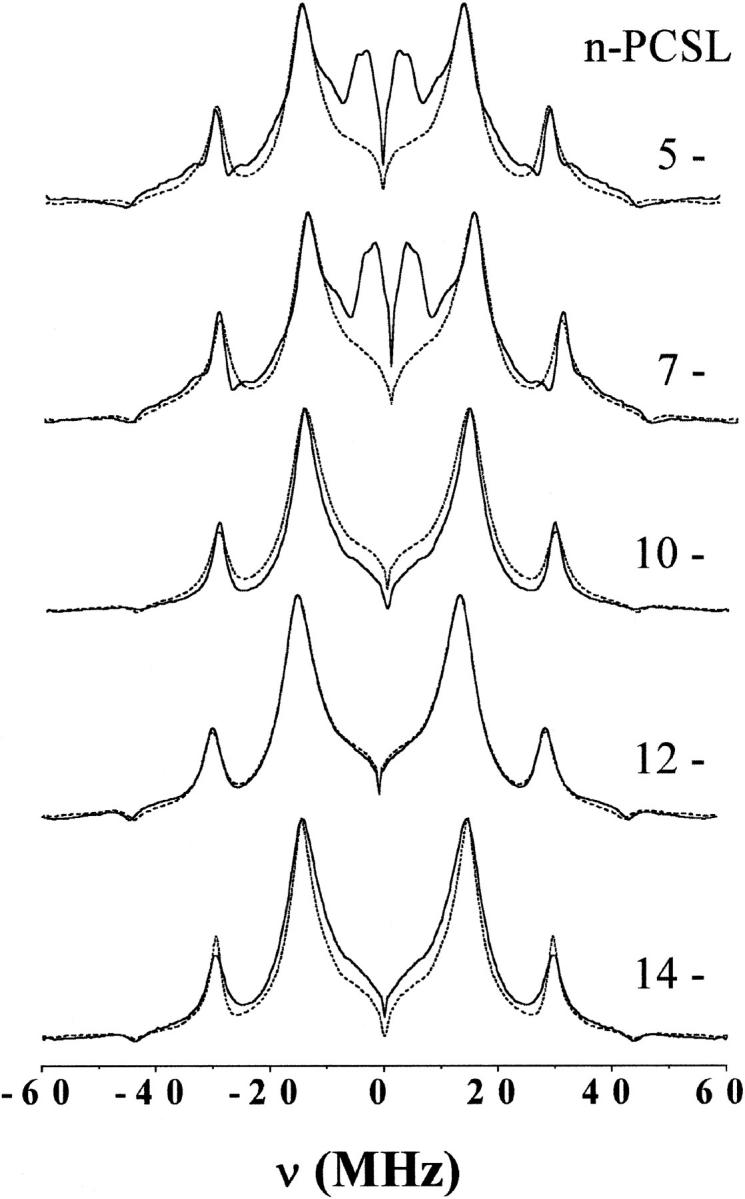
Fourier transform of the relaxation-corrected echo decays from n-PCSL spin-labeled phosphatidylcholines in bilayer membranes of dipalmitoyl phosphatidylcholine with 50 mol% cholesterol. Solid lines are for membranes dispersed in D2O and dashed lines are for membranes dispersed in H2O. The position, n, of spin labeling in the sn-2 chain is indicated on the figure. Spectra are normalized to the maximum peak height.
Similar ESEEM spectra are obtained also at temperatures of 190 K and 230 K (data not shown), although, in the latter case, broadening is present for some label positions. At temperatures higher than 230 K, the echo becomes difficult to detect because of the strongly reduced phase memory time, T2M. Three-pulse ESEEM spectra of 5-PCSL were recorded for samples in D2O. The delay between the first two pulses (τ ≈ 200 ns) was adjusted to suppress proton modulations. These spectra contained only a single sharp line, at a position very close to the free deuterium frequency of νI = 2.2 MHz.
2H-ESEEM amplitudes
As a measure of the amplitude, Δh, of the 2H-ESEEM spectrum, we have taken the height of the deuterium peak at νD = 2.87 MHz from samples dispersed in D2O, minus the corresponding height at 2.87 MHz in the ESEEM spectrum from samples dispersed in H2O, where both are normalized to the height, ho, of the proton peak at νH = 14.4 MHz (see Figs. 2 and 3). These normalized, baseline-corrected amplitudes, Δh/ho, are given in Table 1 for the n-PCSL spin labels in membranes with and without cholesterol. Note that an internal standard, which is offered here by the proton peak, is necessary for these experiments. Determining the transmembrane profile requires comparison of different spin-label positional isomers in entirely separate samples, all of which are inhomogeneous membrane dispersions.
TABLE 1.
Normalized amplitudes (Δh/ho) of the 2H-ESEEM spectra (Figs. 2 and 3),* and 14N-hyperfine splittings (2Azz) in field-swept echo-detected ESR spectra (Fig. 4), from n-PCSL phospholipid spin labels in DPPC membranes, with and without 50 mol% cholesterol, that are dispersed in D2O. T = 150 K*
| DPPC
|
+50 mol% cholesterol
|
|||
|---|---|---|---|---|
| n-PCSL | Δh/ho | 2Azz(G) | Δh/ho | 2Azz(G) |
| 5-PCSL | 0.39 | 69.5 | 0.49 | 71 |
| 7-PCSL | 0.21 | 69 | 0.58 | 71 |
| 10-PCSL | (0.18)† | (68.5)† | −0.1 | 65.6 |
| 12-PCSL | −0.09 | 66 | −0.02 | 65 |
| 14-PCSL | −0.06 | 65 | 0.05 | 65 |
ESEEM spectra from samples in D2O and H2O are normalized to the proton peak at νH = 14.4 MHz (see Figs. 2 and 3). The amplitude, Δh of the D2O peak at νD = 2.87 MHz, relative to the spectral amplitude at 2.87 MHz in the spectrum from the sample in H2O, is divided by the normalized amplitude, ho, at 14.4 MHz.
Uncertain, because of the distortion in the spectrum from the sample in H2O, due to spin-spin broadening (see text).
The value of Δh/ho for 10-PCSL in DPPC is uncertain, because of the problems with spin-spin-broadening for the sample dispersed in H2O that were mentioned already above. The value given in parentheses in Table 1 is referred to the spectrum of 12-PCSL (and of 7-PCSL) in H2O, and is much greater than the corresponding value of Δh/ho for 10-PCSL in DPPC + 50 mol% cholesterol. The presence of a definite peak in the νD-region for 10-PCSL in DPPC (see Fig. 2) shows that the local water concentration at 10-PCSL is greater than in membranes containing cholesterol (see Fig. 3), the 10-PCSL spectra of which are not spin-spin broadened. Evidently, partial phase separation of 10-PCSL in DPPC alone at low temperature results in a higher local water concentration than would be the case if 10-PCSL were distributed uniformly in the unlabeled lipid host.
The notable features of the 2H-amplitudes in Table 1 are that Δh/ho is relatively large at the C-5 and C-7 positions, both in the presence and absence of cholesterol, and is reduced to zero at the C-10 to C-14 positions (with the exception of the anomalous behavior of spin-spin broadened 10-PCSL in DPPC alone). The transition region between these two regimes is relatively sharp. Finally, the values of Δh/ho at the C-5 and C-7 positions are considerably larger in membranes containing cholesterol than in those without cholesterol.
14N-hyperfine splittings
Fig. 4 gives the conventional field-swept EPR spectra of the n-PCSL spin labels in DPPC membranes, with and without 50 mol% cholesterol, at 150 K. These spectra are obtained by taking the first derivative of the echo-detected absorption spectra. The splitting, 2Azz, between the two outer 14N-hyperfine peaks decreases on going from chain position C-5 to C-14. At the C-5 and C-7 positions, the outer hyperfine splitting is greater for membranes of DPPC + 50 mol% cholesterol than for membranes without cholesterol, but is the same in both cases at the C-12 and C-14 positions.
FIGURE 4.
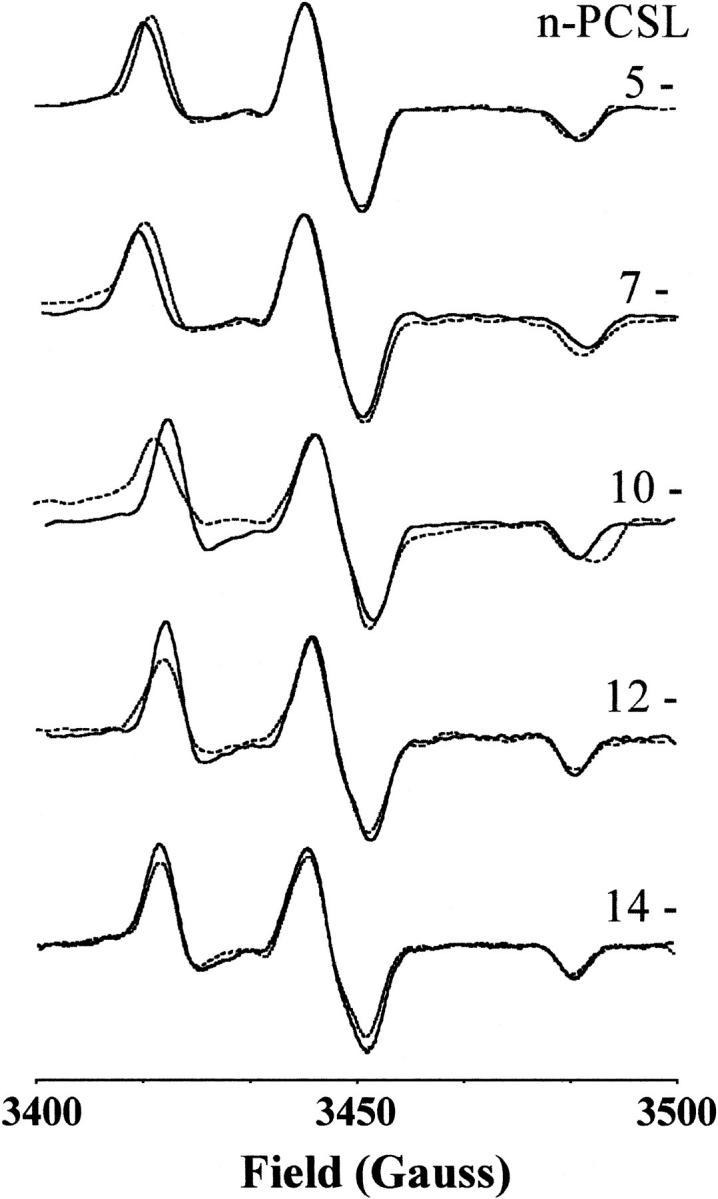
First derivatives of the echo-detected field-swept ESR spectra of n-PCSL spin labels in membranes of DPPC (dotted lines) and DPPC + 50 mol% cholesterol (solid lines) at 150 K. The spin-label position, n, in the sn-2 chain is indicated in the figure.
Table 1 gives the values of the Azz-hyperfine tensor element recorded at 150 K. At this low temperature motional contributions to the 14N-hyperfine splittings are very small and the dependence on spin-label position n is dominated by variations in the local polarity (Griffith et al., 1974; Marsh, 1981). The positional profiles of Azz, and their difference between membranes with and without cholesterol, very clearly mirror those of the 2H-ESEEM intensities, Δh/ho, from D2O associated with these membranes.
The values of 2Azz in Table 1 are for samples in H2O. At the C-5 and C-7 positions, the values of 2Azz for samples in D2O are up to 1 G lower. At the C-14 position they are identical for samples in D2O and H2O. Differences between H2O and D2O, because of the difference in relative H-bonding strengths, can be expected only where water penetration into the membrane is appreciable.
DISCUSSION
The amplitude of a two-pulse ESEEM spectrum is given by the modulation depth parameter, kM (Schweiger and Jeschke, 2001):
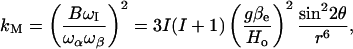 |
(1) |
where B represents the strength of the dipolar interaction, r is the distance between the electron and nuclear spins, and θ is the angle between the magnetic field and the interspin vector. All other symbols have their usual meaning as defined in Schweiger and Jeschke (2001). For interaction with several D2O molecules, it is necessary to integrate over the distribution of nuclear spins, which have quantum number I = 1. A somewhat similar situation was analyzed recently for the T1-relaxation enhancement of spin-labeled lipids by paramagnetic ions (Livshits et al., 2001). For water molecules in the bulk and adsorbed at the membrane surface, the 1/r6 term dominates over angular factors and the depth parameter kM varies as 1/Rm, where R is the distance of the spin label from the membrane surface and m = 3 or 4 for bulk or surface-adsorbed water, respectively (Páli et al., 1993). For water molecules within the membrane, on the other hand, integration is over the water distribution surrounding the spin label and is determined by the contact distance, rWL, and the local water concentrations, nW(z), at distance z along the membrane normal. The integrated value for the modulation depth parameter then becomes (Livshits et al., 2001):
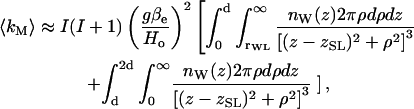 |
(2) |
where zSL is the vertical position of the spin-label group and d is the membrane half-width. The lower limit of the radial integral over ρ extends down to rWL for water molecules in the membrane half that contains the spin label. Note that the profile nW(z) has reflection symmetry about the membrane midplane (z = d).
Clearly, the results given in Table 1 correspond neither to a 1/R3 nor to a 1/R4 dependence (Páli et al., 1993). Therefore, the dominant contribution to the ESEEM spectrum with D2O must arise from deuterium-labeled water molecules within the membrane, for which the modulation depth is given by Eq. 2. Indeed, the profile with chain position that is represented by the data in Table 1 shows remarkable similarity to the trough-like ao-polarity profile established by CW-ESR with fluid lipid membranes (Marsh, 2001). As already noted, it is also mirrored exactly by the polarity-dependent contributions to the Azz-hyperfine elements of n-PCSL in the frozen membranes that are included in Table 1. The amplitude of the 2H-ESEEM spectrum is relatively large for spin labels at the C-5 and C-7 positions and then drops rapidly, over a relatively narrow transition, to zero at the C-12 and C-14 positions in the hydrophobic core of the membrane. Further, the amplitude at C-5 and C-7 is greater for DPPC membranes containing 50 mol% cholesterol than for those without cholesterol. This again is in agreement with the relative ao-polarity profiles in the presence and absence of cholesterol (Marsh, 2001). These effects of cholesterol, and the corresponding changes in Azz that are found in Table 1, are attributed to increased spacing of the lipid headgroups, resulting from interdigitation of cholesterol between the phospholipids (Marsh, 2001). Unlike the situation in fluid membranes, however, there is no penetration of water into the center of frozen membranes that do not contain cholesterol. This difference from the fluid state has been demonstrated recently also by high-field CW-ESR measurements of the polarity-dependent gxx-tensor elements in frozen samples of membranes with and without cholesterol (Kurad et al., unpublished results). It is also explicit in the polarity dependent Azz-hyperfine tensor elements of the frozen membranes that are given in Table 1.
The profiles for permeation of water into membranes, of the type established here, are related directly to the membrane permeability. Integration, across the lipid bilayer, of the inverse permeation profile gives the barrier to water transport that is presented by the hydrophobic interior of the membrane (Diamond and Katz, 1974). For fluid membranes, the predicted reduction in water permeability by cholesterol accords with that observed experimentally (Marsh, 2001). On the other hand, the nonvanishing water concentration in membrane regions corresponding to the upper parts of the lipid chains will have a marked thermodynamic influence on the transmembrane insertion of integral proteins. Tryptophan residues, rather than residues with either aliphatic or polar side chains, are known to be concentrated at the membrane interface.
The nuclear modulation frequencies, να and νβ, from the upper and lower superhyperfine manifolds are given by (Mims, 1972a,b):
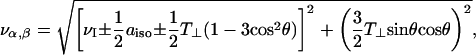 |
(3) |
where νI is the free nuclear Larmor frequency, aiso is the isotropic hyperfine coupling constant, and T⊥ (=−gegNβeβN/r3) is the perpendicular element of the dipolar hyperfine tensor. The overall lineshape of the ESEEM spectrum is given by: ∼kM(θ)sinθ(∂θ/∂να,β). For small dipolar hyperfine anisotropies (|T⊥|≪νI), as apparently is the case here, the spectral maximum in the overall powder pattern occurs for spin labels oriented at the magic angle (i.e., cos2θ=1/3). It is seen from Eq. 3 that electron-nuclear dipolar interactions then give rise only to second order shifts in the modulation frequency. This explains why, although a nonvanishing dipolar interaction is required to observe the echo modulation, the ESEEM frequencies lie close to νI, and no separation of the να and νβ frequencies is observed in the 3-pulse spectrum. The ESEEM spectrum apparently is dominated by D2O molecules that are not immediately adjacent to the spin label. These have smaller dipolar interactions, but are considerably more numerous and contribute additively to the spectral intensity at the unperturbed nuclear frequency (Eq. 2).
Measurements on the interaction of D2O molecules with spin-labeled lipids by 2H-ESEEM therefore demonstrate the penetration of water molecules into the hydrocarbon-chain region of the membrane directly. This substantiates the previous interpretation of the ao-polarity profiles obtained by conventional CW-ESR as a water permeation profile (Marsh, 2001). The conventional measurements of the isotropic ao-hyperfine splittings then give a more precise indication of the local water permeation profile because they are determined by direct hydrogen bonding of water to the spin-label nitroxide group. 2H-ESEEM intensities, on the other hand, are determined by integration of the dipolar interactions (i.e., Eq. 2) over all water molecules in the membrane (Livshits et al., 2001). Although this gives a coarser profile of local water concentration in the membrane, the pulsed-ESR measurements are essential in establishing the origin of the conventional ao-polarity profile across the membrane. Even if it is assumed that water penetrates the membrane, corrections for the local dielectric constant (based on Onsager theory) require the additional assumption that there is no ordering of water molecules in the membrane, to interpret the ao-profiles (Marsh, 2002b). The present results with pulsed ESR demonstrate that water penetrates the membrane with a sigmoidal transmembrane profile, which hitherto was not established directly.
Acknowledgments
We thank Dr. D. Erilov for recording 3-pulse echo spectra and Brigitta Angerstein for spin-label synthesis.
This work was performed in the framework of the project CIPE-MIA26-WP3.
References
- Diamond, J. M., and Y. Katz. 1974. Interpretation of nonelectrolyte partition coefficients between dimyristoyl lecithin and water. J. Membr. Biol. 17:121–154. [DOI] [PubMed] [Google Scholar]
- Fajer, P., A. Watts, and D. Marsh. 1992. Saturation transfer, continuous wave saturation, and saturation recovery electron spin resonance studies of chain-spin labeled phosphatidylcholines in the low temperature phases of dipalmitoyl phosphatidylcholine bilayers. Effects of rotational dynamics and spin-spin interactions. Biophys. J. 61:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagua, A. V., G. G. Malenkov, and V. P. Timofeev. 1978. Hydrogen-bond contribution to isotropic hyperfine splitting constant of a nitroxide free-radical. Chem. Phys. Lett. 56:470–473. [Google Scholar]
- Griffith, O. H., P. J. Dehlinger, and S. P. Van. 1974. Shape of the hydrophobic barrier of phospholipid bilayers. Evidence for water penetration in biological membranes. J. Membr. Biol. 15:159–192. [DOI] [PubMed] [Google Scholar]
- Livshits, V. A., B. G. Dzikovski, and D. Marsh. 2001. Mechanism of relaxation enhancement of spin labels in membranes by paramagnetic ion salts: dependence on 3d and 4f ions and on the anions. J. Magn. Reson. 148:221–237. [DOI] [PubMed] [Google Scholar]
- Marsh, D. 1981. Electron spin resonance: spin labels. In Membrane Spectroscopy. Molecular Biology, Biochemistry and Biophysics, Vol. 31. E. Grell, editor. Springer-Verlag, Berlin, Heidelberg, New York. 51–142. [DOI] [PubMed]
- Marsh, D. 2001. Polarity and permeation profiles in lipid membranes. Proc. Natl. Acad. Sci. USA. 98:7777–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, D. 2002a. Polarity contributions to hyperfine splittings of hydrogen-bonded nitroxides - the microenvironment of spin labels. J. Magn. Reson. 157:114–118. [DOI] [PubMed] [Google Scholar]
- Marsh, D. 2002b. Membrane water-penetration profiles from spin labels. Eur. Biophys. J. In press. [DOI] [PubMed]
- Marsh, D., and A. Watts. 1982. Spin-labeling and lipid-protein interactions in membranes. In Lipid-Protein Interactions, Vol. 2. P. C. Jost and O. H. Griffith, editors. Wiley-Interscience, New York. 53–126.
- Mims, W. B. 1972a. Envelope modulation in spin-echo experiments. Phys. Rev. B. 5:2409–2419. [Google Scholar]
- Mims, W. B. 1972b. Amplitudes of superhyperfine frequencies displayed in electron-spin echo envelope. Phys. Rev. B. 6:3543–3545. [Google Scholar]
- Páli, T., R. Bartucci, L. I. Horváth, and D. Marsh. 1993. Kinetics and dynamics of annealing during sub-gel phase formation in phospholipid bilayers. A saturation transfer electron spin resonance study. Biophys. J. 64:1781– 1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger, A., and G. Jeschke. 2001. Principles of pulse electron paramagnetic resonance. Oxford University Press, Oxford.
- Subczynski, W. K., A. Wisniewska, J. J. Yin, J. S. Hyde, and A. Kusumi. 1994. Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry. 33:7670–7681. [DOI] [PubMed] [Google Scholar]
- Szajdzinska-Pietek, E., R. Maldonado, L. Kevan, and R. R. M. Jones. 1984. Electron spin resonance and electron spin echo modulation studies of N, N, N′, N′-tetramethylbenzidine photoionization in anionic micelles: structural effects of tetramethylammonium cation counterion substitution for sodium cation in dodecyl sulfate micelles. J. Am. Chem. Soc. 106:4675–4678. [Google Scholar]