Abstract
The photocycle of the photophobic receptor from Natronobacterium pharaonis, NpSRII, is studied by static and time-resolved step-scan Fourier transform infrared spectroscopy. Both low-temperature static and time-resolved spectra resolve a K-like intermediate, and the corresponding spectra show little difference within the noise of the time-resolved data. As compared to intermediate K of bacteriorhodopsin, relatively large amide I bands indicate correspondingly larger distortions of the protein backbone. The time-resolved spectra identify an intermediate L-like state with surprisingly small additional molecular alterations. With the formation of intermediate M, the Schiff-base proton is transferred to the counterion Asp-75. This state is characterized by larger amide bands indicating larger distortions of the protein. We can identify a second M state that differs only in small-protein bands. Reisomerization of the chromophore to all-trans occurs with the formation of intermediate O. The accelerated decay of intermediate M caused by azide results in another red-shifted intermediate with a protonated Schiff base. The chromophore in this state, however, still has 13-cis geometry. Nevertheless, the reisomerization is still as slow as under the conditions without azide. The results are discussed with respect to mechanisms of the observed proton pumping and the possible roles of the intermediates in receptor activation.
INTRODUCTION
Archeabacterial phototaxis is mediated by the two photoreceptors sensory rhodopsin I and II. These two pigments enable bacteria like those of Halobacterium salinarum to seek optimal light conditions above 500 nm and to avoid harmful ultraviolet (UV) light as well as prevent photooxidative stress in case of high oxygen concentrations in bright sunlight. This latter property is conferred by the photophobic receptor sensory rhodopsin II (reviewed by Schäfer et al., 1999 and Spudich et al., 2000). Generally, the signal transduction chain is based on the two-component system that is well characterized for the chemotactic signaling cascade in e.g., Escherichia coli (Rudolph and Oesterhelt, 1996). After the receptor is activated by light, the signal is transferred to its cognate transducer, which in turn triggers the cytoplasmic components that signal the chain and thereby switches the flagellar motor.
Sensory rhodopsin II, originally identified in H. salinarum (HsSRII), has also been isolated from Natronobacterium pharaonis (NpSRII; Imamoto et al., 1991; Scharf, Pevec, et al., 1992). Because of its superior chemical stability over HsSRII (Scharf, Pevec, et al., 1992), NpSRII has been widely used in recent physiological and biophysical studies. Its crystal structure has been determined to display a fold that is similar to that of the light-activated proton pump bacteriorhodopsin (Luecke et al., 2001; Royant et al., 2001). Differences are found in the cytoplasmic and extracellular channels. The latter changes are mainly due to the replacement of the Asp-96 that is present in bacteriorhodopsin by Phe-86, which is located at a homologous position in NpSRII.
The light-activated photocycle of NpSRII has been studied in great detail (Hirayama et al., 1992; Chizhov et al., 1998). The general mechanism is quite similar to that of bacteriorhodopsin, including the archetypical spectral states KNpSRII, LNpSRII, MNpSRII, NNpSRII, and ONpSRII. Noteworthy is the observation of two MNpSRII states (M1NpSRII and M2NpSRII). M1NpSRII decays to M2NpSRII in ∼2 ms. This transition, which represents in respect to the UV-visible (UV-vis) spectral range a truly spectrally silent transition, is probably the switch between the ground state and the signaling state. Recent experiments using electron-spin resonance spectroscopy revealed that helix F in a outwardly directed flaplike motion changes the mobility of residues on the cytoplasmic side during the lifetime of MNpSRII (Wegener et al., 2000), which could be later correlated with the M1NpSRII → M2NpSRII transition (H. J. Steinhoff and M. Engelhard, unpublished observation).
Further insight into the molecular events that occur during the photocycle have been obtained by static Fourier transform infrared (FTIR) spectroscopy experiments. It can be shown that the proton from the Schiff base has already been transferred to Asp-75 in the M intermediate, which indicates that this neutralization of the Schiff base takes place during the LNpSRII → MNpSRII transition (Scharf, Englehard, and Siebert, 1992; Engelhard et al., 1996). For HsSRII, this reaction was also confirmed by an analysis using the corresponding Asp/Asn mutant (Bergo et al., 2000). The retinal chromophore adopts an all-trans configuration in the ground state. A light-dark adaptation cannot occur, because the 13-cis isomer of retinal is not bound by the protein (Hirayma et al., 1995). In the KNpSRII state, retinal has isomerized to the 13-cis configuration (Kandori et al., 2001). It is not yet known at what time the back-isomerization takes place.
For the ion pumps bacteriorhodopsin and halorhodopsin, static time-resolved FTIR spectroscopy has contributed considerably to the understanding of the pumping mechanism (Maeda, 1995; Hackmann et al., 2001). Because it is sensitive to structural changes of both the chromophore and the protein, it is especially suitable for addressing questions about the isomeric state of the chromophore and protein structural changes during the photocycle of NpSRII. By incorporating isotopic labels at specific sites of the protein backbone of bacteriorhodopsin, we provide evidence that in the FTIR difference spectra, the amide I bands reflect local distortions (Hauser et al., 2002). Previously, using time-resolved step-scan FTIR spectroscopy, we unequivocally identified small-protein structural changes during the spectrally silent transitions of bacteriorhodopsin (Rödig et al., 1999) and N. pharaonis halorhodopsin (Hackmann et al., 2001) thereby reflecting such backbone distortions, and we also determined the chromophore geometry in the O state of the latter pigment to all-trans.
In the present paper, the complete photocycle is analyzed using step-scan FTIR spectroscopy. The data reveal the molecular nature of the M1NpSRII → M2NpSRII transition and the isomeric state of retinal in the ONpSRII intermediate without and in the presence of azide. The latter information provides an explanation for a postulated two-photon process under steady-state illumination (Schmies et al., 2000).
MATERIALS AND METHODS
NpSRII was expressed in E. coli and purified as described (Shimono et al., 1997; Hohenfeld et al., 1999). The solubilized photoreceptor was reconstituted into purple membrane lipids in accordance with standard procedures (Wegener et al., 2000). These samples did not contain the transducer.
In all infrared measurements, hydrated film samples of NpSRII reconstituted into purple membrane lipids were used. A suspension containing ∼100 μg of protein and 500 nM phosphate buffer, pH 8, was dried onto a BaF2 window, and the film was rehydrated via the water-vapor phase. For the measurements with azide, the molar ratio of azide to NpSRII was 3.3, and the buffer was adjusted to pH 6.5. For measurements in 2H2O, the films were completely dried, and 5 μl of 2H2O was deposited via the vapor phase in the sealed sample cuvette. This process was repeated five times, after which the final hydration was adjusted.
The low-temperature (103 K) FTIR measurements were performed as described previously (Hackmann et al., 2001) using light of wavelengths between 450 and 500 nm for irradiation. The methods for obtaining time-resolved step-scan infrared spectra were essentially as used before (Rödig et al., 1999; Hackmann et al., 2001). For the largest part of our studies, we used our slower detection system (effective rise time, ∼600 ns). Because the photocycle is slow even at 37°C, the repetition rate for signal averaging was 0.5 Hz. To avoid overly long measuring times for a single step-scan run, only four signals were averaged at each sampling position of the interferogram. To achieve a sufficient signal-to-noise ratio, several measurements (16–20) were averaged, which corresponds to 64–80 averages per sampling position. To obtain information about earlier intermediates, we also performed measurements with our fast-detection system (effective rise time, ∼30 ns). Because the noise was very large in the early time range of the corresponding time-resolved spectra, a reliable kinetic analysis could not be performed; instead, the spectra of several time-slices were averaged. For sample excitation, the output of an optical parametric oscillator (OPO; Lambda Physik, Göttingen) pumped by the tripled output of a Nd:YAG laser (Brilliant, Quantel) was tuned to 490 nm. Pulse duration was 4 ns. Using neutral-density filters, the pulse energy was reduced to ∼1.5 mJ at the sample position. Spectral resolution for the static FTIR measurements was 4 cm−1 and for the step-scan measurements was 8 cm−1.
The time-resolved spectra obtained with the slower instrumentation were fitted to a sum of exponentials as described earlier (Rödig et al., 1999; Hackmann et al., 2001). To avoid distortions from the detection rise time, the fit was started 1 μs after the laser flash. The amplitude spectra were further evaluated using the unidirectional reaction scheme without back-reaction as described recently (Rödig et al., 1999; Hackmann et al., 2001). This scheme, which describes transitions between possible fast equilibria of pure states, was used here only as a suitable and direct means of describing the molecular events. For a consistent description, it was necessary to order the reaction sequence according to the increasing time constants derived from the global fit.
RESULTS
General description of the intermediate spectra
Because the cycling time of the NpSRII photoreaction was quite slow, measurements were performed at 37°C, which allowed for a repetition rate of 0.5 Hz for signal averaging during the step-scan measurements. In addition, the transient concentration of ONpSRII increased, which probably played an important role in determining the duration of the photocycle (Chizhov et al., 1998). Although the repetition rate could be enhanced, it was still quite low as compared to that used in our step-scan FTIR measurements of bacteriorhodopsin (Rödig et al., 1999) or halorhodopsin (Hackmann et al., 2001), which made the measurements more difficult and caused a poorer signal-to-noise ratio. This must be taken into account in the analysis of the step-scan data.
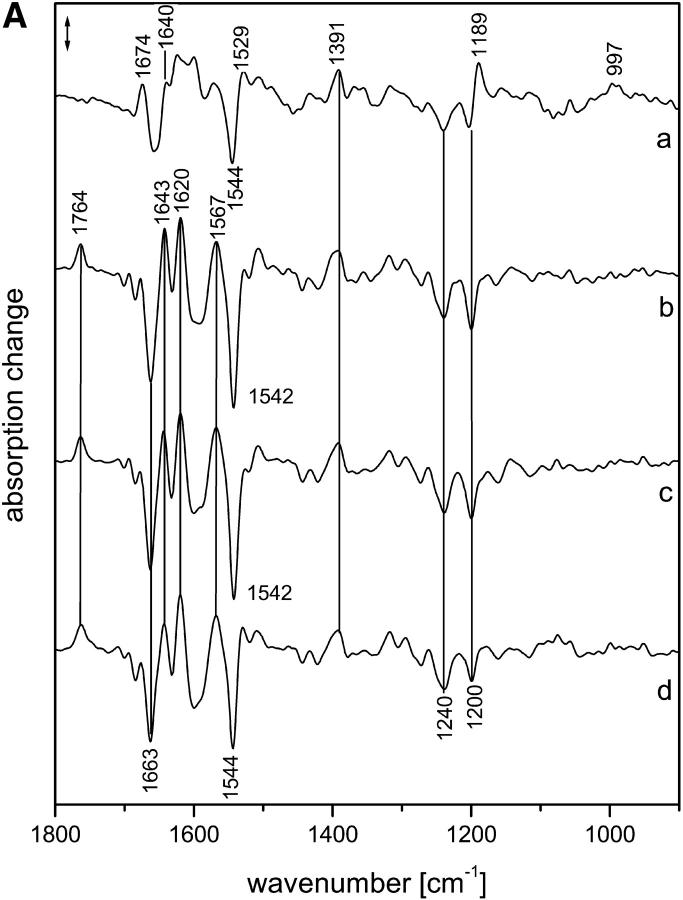
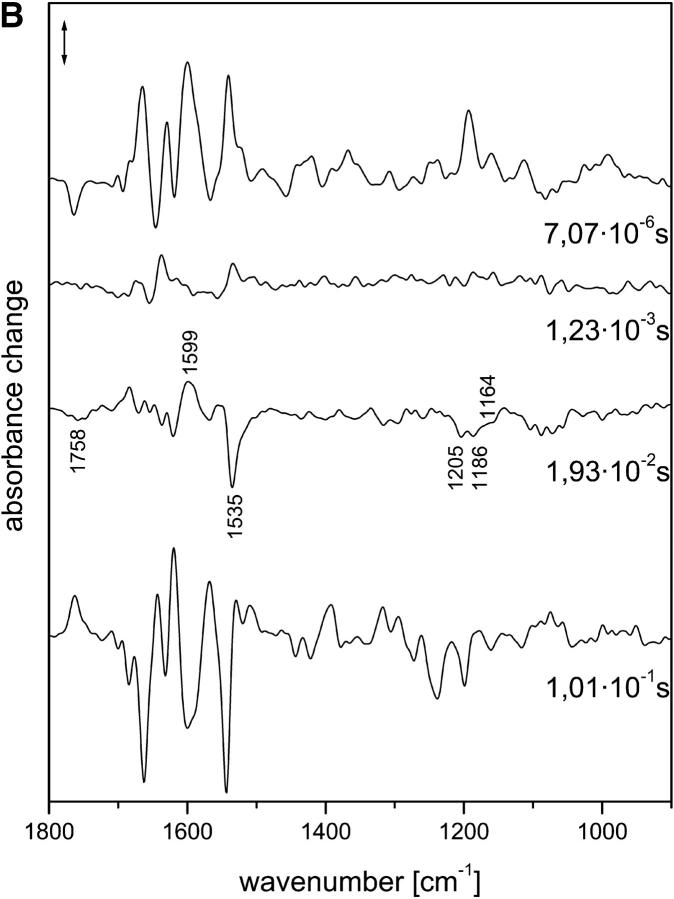
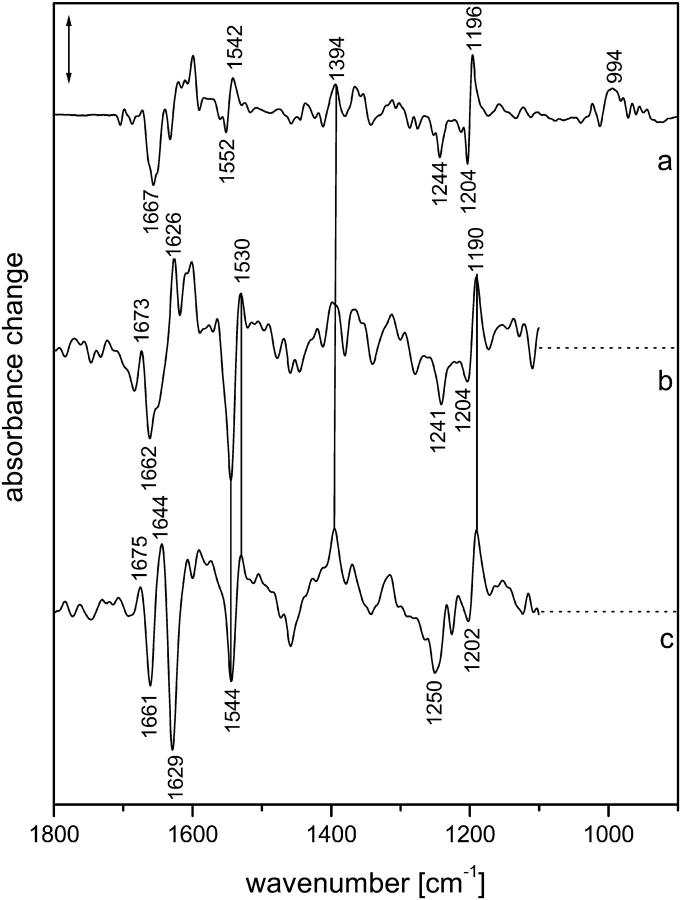
Fig. 1 A shows the intermediate spectra correlated to the four half-times derived from the global fit, i.e., 7 μs and 1.2, 19, and 101 ms (for evaluation of the data, see Materials and Methods). The corresponding amplitude spectra are shown in Fig. 1 B. According to recent time-resolved UV-vis experiments (Chizhov et al., 1998), the photocycle at 37°C is characterized by half-times of 150 ns (extrapolated), 4.5 and 13 μs, and 1, 33, 80, 150, and 350 ms, and we used these data as a reference. The smaller number of exponentials obtained from the time-resolved infrared spectra is due to the larger noise in the infrared data. Comparing the two data sets, it is likely that the 7-μs (FTIR) half-time represents an average of the transitions with half-times of 4.5 and 13 μs that were observed in the UV-vis range, with the larger noise smearing out the two time constants.
FIGURE 1.
(A) Intermediate spectra of NpSRII obtained at 37°C with H2O. Spectra are labeled with the decay half-times of the intermediates: (a) 7.07 μs; (b) 1.23 ms; (c) 19.3 ms; (d) 101 ms. Spectral resolution, 8 cm−1. (B) Amplitude spectra of NpSRII obtained at 37°C with H2O from the time-resolved infrared spectra by global fitting to a sum of exponentials. The spectra are labeled with the respective half-times of the exponentials. Spectral resolution, 8 cm−1. Method used to derive the intermediate spectra (A) from the amplitude spectra (B) is described in Materials and Methods.
The first intermediate identified with the time resolution of 700 ns has to be assigned to the LNpSRII state (Fig. 1 A(a)). The next intermediate that appeared with a half-time of 7 μs consisted, according to the UV-vis data, of a mixture of mainly MNpSRII and a small amount of LNpSRII, the latter decreasing with higher temperature. Only the next intermediate, which appeared with 13 μs, represents a pure MNpSRII state. However, at 37°C, the amount of LNpSRII was very small, and it is plausible that we were unable to detect the transition with a half-time of 13 μs. Thus, we conclude that the second spectrum (Fig. 1 A (b)) already represented a pure MNpSRII state. Its signatures are the positive band at 1764 cm−1 and the lack of a positive band at 1189 cm−1. The former band represents the protonation of the counterion Asp-75 with the deprotonation of the Schiff base (Scharf, Englehard, and Siebert, 1992; Engelhard et al., 1996), whereas the latter indicates the deprotonation of the Schiff base, which caused a drastic reduction of the intensity of the fingerprint bands of the chromophore (Siebert and Mäntele, 1980).
The next transition, with a half-time of 1.2 ms, was also observed in the UV-vis data (1 ms). Its infrared spectrum (Fig. 1 A (c)) was very similar to that of the previous one. However, subtle differences became evident in the corresponding amplitude spectrum (Fig. 1 B, labeled 1.2 ms), which essentially describes the spectral changes between the two intermediates. Only bands in the amide I/II spectral range showed up, which indicates that the protein underwent small distortions of the backbone, but that the chromophore binding pocket was not altered. This observation is in agreement with the UV-vis data, from which a spectrally silent transition was deduced. Obviously, the characterization of this transition as spectrally silent holds true only for the UV-vis spectral range.
Beyond 1 ms, the comparison of the decay times of the infrared vs. the UV-vis measurements reveals larger deviations. Nevertheless, the half-time of 19 ms probably corresponds to that of 30 ms, which characterizes the transition to a mixture of MNpSRII and ONpSRII. From the infrared spectrum of this intermediate (Fig. 1 A (d)), the contribution of ONpSRII is not directly obvious, although the high temperature should have increased its yield. We also provide evidence that the infrared data indicate a contribution of ONpSRII (see M intermediates), although it is smaller than expected based on the UV-vis measurements. In our data, the half-time of 100 ms describes the transition back to the initial state. However, the half-time of 80 ms observed in the UV-vis experiments describes a transition from the MNpSRII/ONpSRII mixture to an MNpSRII/NNpSRII/ONpSRII equilibrium. Therefore, the process with the half-time of 150 ms, which describes to a large extent the back-reaction to the initial state, better agrees with the last transition that we observed. On the other hand, the last process observed in the UV-vis measurements has no correspondence in the infrared data. Because the amplitude was rather small, this might be due to the larger noise in the infrared data. Later (see Discussion), we discuss possible causes for the deviations in the photocycles if measured using infrared or UV-vis.
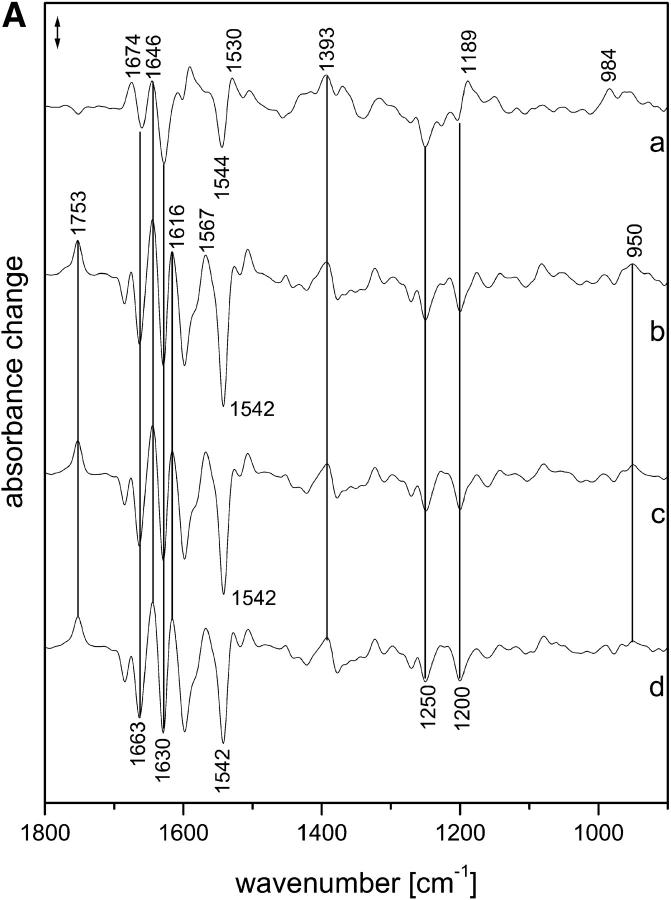
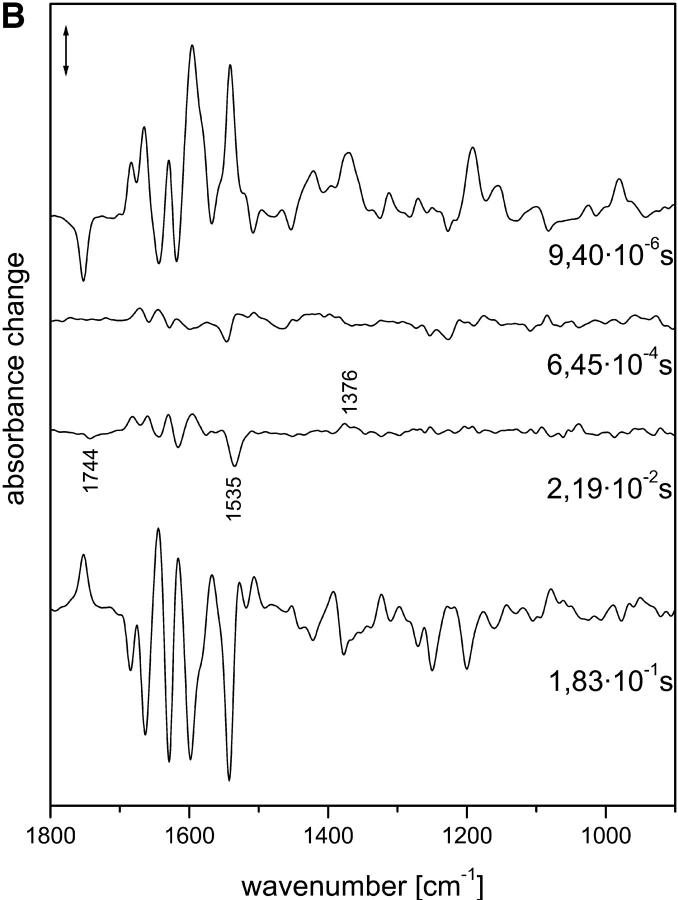
The intermediate and amplitude spectra of samples in the presence of 2H2O are shown in Fig. 2, A and B, respectively. Although the half-times partly differ from the values obtained for samples hydrated with H2O, the spectra demonstrate that they describe the same transitions (see below).
FIGURE 2.
(A) Intermediate spectra of NpSRII obtained at 37°C with 2H2O. Spectra are labeled with the decay half-times of the intermediates: (a) 9.4 μs; (b) 0.645 ms; (c) 21.9 ms; (d) 183 ms. Spectral resolution, 8 cm−1. (B) Amplitude spectra of NpSRII obtained at 37°C with 2H2O from the time-resolved infrared spectra by global fitting to a sum of exponentials. The spectra are labeled with the respective half-times of the exponentials. Spectral resolution, 8 cm−1. Method used to derive the intermediate spectra (A) from the amplitude spectra (B) is described in Materials and Methods.
Description of the intermediates
KNpSRII and LNpSRII states
We also measured the photocycle with our faster detection system, which enabled a time resolution of 30 ns (Rödig et al., 1999). However, because of the large size of noise in the data, we were unable to reliably deduce half-times <1 μs. To still be able to deduce an approximate spectrum of KNpSRII, we averaged the spectra that were obtained with high time resolutions over the range from 30 to 100 ns for measurements in H2O (Fig. 3 (b)) and 2H2O (Fig. 3 (c)). These averaged spectra are very similar to the first intermediate spectra that we identified (Figs. 1 A (a) and 2 A (a)). The main differences are the shift of the positive band at 1527 cm−1 in the LNpSRII spectra to 1530 cm−1 in the spectra of KNpSRII and the larger size of the bands at 1674 and 1640 cm−1 in LNpSRII, which both probably represent alterations of amide I bands, because they are little influenced by H/2H exchange. Because 2H2O shifts the C N stretch of the protonated Schiff base of the initial state from 1657 to 1633 cm−1, the remaining bands between 1680 and 1640 cm−1 in the 2H2O spectrum can be assigned to amide I spectral changes. These bands are considerably more pronounced than those in intermediate K of bacteriorhodopsin, which indicates larger changes of the protein backbone.
N stretch of the protonated Schiff base of the initial state from 1657 to 1633 cm−1, the remaining bands between 1680 and 1640 cm−1 in the 2H2O spectrum can be assigned to amide I spectral changes. These bands are considerably more pronounced than those in intermediate K of bacteriorhodopsin, which indicates larger changes of the protein backbone.
FIGURE 3.
Comparison of (a) the low-temperature KNpSRII spectrum obtained at 103 K with H2O, and the time-resolved KNpSRII spectra obtained at 37°C, averaged over 30–100 ns with (b) H2O or (c) 2H2O. Spectral resolution, 4 cm−1 (a) or 8 cm−1 (b) and (c). Owing to the large noise below 1100 cm−1, this spectral range was omitted in (b) and (c).
The low-temperature KNpSRII spectrum obtained at 103 K (Fig. 3 (a)) and the time-resolved KNpSRII spectrum are, within the signal-to-noise ratio of the latter, very similar. In the low-temperature spectrum, the small size of the ethylenic difference band 1552 (−)/1542 (+) as compared to the fingerprint bands between 1270 and 1150 cm−1 shows that the ethylenic bands largely overlap and thus the absorption maximum is only slightly red-shifted (Ottolenghi, 1980). This is in agreement with the time-resolved UV-vis data, from which a red-shift of only 11 nm has been deduced (Chizhov et al., 1998), resulting in a shift of the ethylenic mode of only 3 cm−1 (see Ottolenghi, 1980 for a correlation of the ethylenic frequencies and absorption maxima). Therefore, the separation of the peaks of the difference band is essentially determined by the half-width of the two ethylenic bands. In contrast to this observation, the time-resolved spectrum exhibits a large negative band at 1544 cm−1 and a small positive band at 1530 cm−1. We concluded that the large negative band cannot be assigned to the ethylenic mode of the dark state. Resonance Raman experiments locate this mode at 1548 cm−1 (Gellini et al., 2000), which is in agreement with the absorption maximum at 500 nm. Probably an amide II band superimposes at a slightly lower position. This would also reduce the size of the positive ethylenic mode and shift it to lower frequencies, as observed. A further difference between the two spectra (Fig. 3, a and b) can be seen in the amide I region: the band at 1626 cm−1 has considerably lower intensity in the low-temperature spectrum. Thus, both differences are indications for larger distortions of the protein backbone in the time-resolved KNpSRII state which were taken at 37°C. In both KNpSRII states, the amide I bands at 1674 and 1640 cm−1 are smaller than those in LNpSRII, which demonstrates that during the KNpSRII → LNpSRII transition, the protein undergoes additional structural changes. The positive band at 1527 cm−1 in LNpSRII cannot be assigned to the ethylenic stretch, because the absorption maximum is at 500 nm. Therefore, we assign it to protein changes (e.g., amide II or tyrosine). It appears plausible that in KNpSRII the band at 1530 cm−1 results from a superposition of the ethylenic stretch and this protein band. It should be noted that a low-temperature KNpSRII spectrum that agrees well with the spectrum shown here has been published before (Kandori et al., 2001). In agreement with those studies, we were unable to stabilize LNpSRII at low temperature.
Surprisingly, in the spectrum of the low-temperature K state, the main hydrogen out-of-plane (HOOP) mode at 997 cm−1 is not altered in the L state (although the larger noise in the time-resolved KNpSRII spectrum impedes a reliable identification of this mode, it can be tentatively assigned to a band at the same position; data not shown). This is in contrast to the corresponding K intermediates in bacteriorhodopsin (Weidlich and Siebert, 1993), where the modes of the low-temperature state differ considerably from those of time-resolved KL and L states, and it has been concluded that the chromophore geometries differ considerably. In the latter two states, the twist is confined to the Schiff-base region (Rödig et al., 1999). Because the band in the LNpSRII intermediate is shifted down to 984 cm−1 by Schiff-base deuteration (Fig. 2 A (a)), we assigned it to the 15-HOOP mode. Thus it can be concluded that as in the L state of bacteriorhodopsin, the chromophore in LNpSRII is twisted around the C14–C15 single bond.
It was found recently that the HOOP modes of the low-temperature K state of bacteriorhodopsin could also be induced in KNpSRII if the amino acids of the retinal binding pocket were changed to those of bacteriorhodopsin (Shimono et al., 2002). This shows that it is mainly the reduced steric hindrance that allows the chromophore to adopt a more relaxed conformation in the KNpSRII intermediate of wild-type NpSRII.
Measurements after H/2H exchange often allow an assignment of the C N stretching mode of the protonated Schiff base, which is shifted down by deuteration. In the spectrum of the LNpSRII intermediate (Fig. 1 A), a negative band at 1657 cm−1 lost intensity upon deuteration, and instead, a negative band at 1630 cm−1 gained intensity (Fig. 2 A). Thus we assigned these bands to the C
N stretching mode of the protonated Schiff base, which is shifted down by deuteration. In the spectrum of the LNpSRII intermediate (Fig. 1 A), a negative band at 1657 cm−1 lost intensity upon deuteration, and instead, a negative band at 1630 cm−1 gained intensity (Fig. 2 A). Thus we assigned these bands to the C N stretch of the protonated and deuterated Schiff base of the initial state. In the later intermediates, this shift could still be discerned, although the band at 1657 cm−1 was superimposed by another negative band that resulted in a negative band at 1663 cm−1 and a positive band at 1643 cm−1. These additional bands were assigned to amide I changes because they were also observed in the presence of 2H2O (Fig. 2 A). In low-temperature spectra of the KNpSRII intermediate, the band of the deuterated Schiff base was found at 1633 cm−1 (data not shown, and see Kandori et al., 2001) within the spectral resolution, which is in agreement with the time-resolved data and also approximately in agreement with resonance Raman spectra (Gellini et al., 2000). However, it was more difficult to assign the corresponding modes to bands of LNpSRII. H/2H exchange caused pronounced alterations of the positive bands between 1623 and 1600 cm−1, and it was tempting to assign this positive feature to the C
N stretch of the protonated and deuterated Schiff base of the initial state. In the later intermediates, this shift could still be discerned, although the band at 1657 cm−1 was superimposed by another negative band that resulted in a negative band at 1663 cm−1 and a positive band at 1643 cm−1. These additional bands were assigned to amide I changes because they were also observed in the presence of 2H2O (Fig. 2 A). In low-temperature spectra of the KNpSRII intermediate, the band of the deuterated Schiff base was found at 1633 cm−1 (data not shown, and see Kandori et al., 2001) within the spectral resolution, which is in agreement with the time-resolved data and also approximately in agreement with resonance Raman spectra (Gellini et al., 2000). However, it was more difficult to assign the corresponding modes to bands of LNpSRII. H/2H exchange caused pronounced alterations of the positive bands between 1623 and 1600 cm−1, and it was tempting to assign this positive feature to the C N stretch of LNpSRII. However, these H/2H-induced changes persisted in the MNpSRII intermediate (the first amplitude spectrum was little influenced by H/2H exchange (Fig. 1 B vs. Fig. 2 B)), and the positive bands were already present in KNpSRII (H2O). Thus the C
N stretch of LNpSRII. However, these H/2H-induced changes persisted in the MNpSRII intermediate (the first amplitude spectrum was little influenced by H/2H exchange (Fig. 1 B vs. Fig. 2 B)), and the positive bands were already present in KNpSRII (H2O). Thus the C N stretch of LNpSRII cannot be assigned, and its intensity must be very low.
N stretch of LNpSRII cannot be assigned, and its intensity must be very low.
M intermediates
In the transition from LNpSRII to MNpSRII, besides the characteristic features of the MNpSRII state discussed already, additional molecular changes took place. A band at 1643 cm−1 gained intensity overcompensating the negative band at 1657 cm−1 (C N stretch), and the LNpSRII band at 1674 cm−1 lost intensity. Probably these bands represented alterations of amide I modes and indicated distortions of the protein backbone. The LNpSRII band at 1600 cm−1 disappeared, and instead, a broad negative band appeared. This is the largest spectral feature of the LNpSRII → MNpSRII transition. In this spectral range, the antisymmetric CO2− stretch of deprotonated carboxyl groups contributes. Because Asp-75 becomes protonated in the M intermediate, the sign of the spectral changes would be in agreement with such a process. However, the observed spectral changes appear to be far too large to represent only this single protonation step. The largest part, i.e., the positive band at 1600 cm−1 in LNpSRII disappearing in MNpSRII, must be caused by additional, yet-unidentified molecular changes. On the other hand, the spectral change around 1360 cm−1 that is especially evident in the first amplitude spectrum as a positive band (Fig. 1 B) could be caused by the symmetric CO2− stretch of deprotonated Asp-75 disappearing in the transition to MNpSRII. This is corroborated by the measurements in 2H2O (Fig. 2 B). Further changes were observed at 1567 cm−1 (positive), which probably represent larger amide II spectral changes in MNpSRII. However, the ethylenic mode of the deprotonated Schiff base chromophore could also contribute. On the other hand, the band at 1527 cm−1 of LNpSRII, tentatively assigned to amide II spectral changes, had reduced intensity in MNpSRII. A further large change in the LNpSRII → MNpSRII transition involved the ethylenic mode: in the MNpSRII spectrum, the negative band at 1542 cm−1 gained intensity. This indicates that a band absorbing in this region disappeared and confirms our earlier conclusion that the ethylenic modes of the initial state and LNpSRII exhibited considerable overlap. (With the formation of the M intermediate, i.e., deprotonation of the Schiff base, there is a large upshift and intensity loss of the ethylenic mode that relieves the overlap between the ground and photoproduct states.) All the spectral features of the LNpSRII → MNpSRII transition were especially evident in the amplitude spectrum, where the positive bands represented the LNpSRII state and the negative ones the MNpSRII state. It is noteworthy that this amplitude spectrum was little influenced by H/2H exchange (Fig. 1 B vs. Fig. 2 B). This shows that the involved modes coupled only little, if at all, to exchangeable hydrogens.
N stretch), and the LNpSRII band at 1674 cm−1 lost intensity. Probably these bands represented alterations of amide I modes and indicated distortions of the protein backbone. The LNpSRII band at 1600 cm−1 disappeared, and instead, a broad negative band appeared. This is the largest spectral feature of the LNpSRII → MNpSRII transition. In this spectral range, the antisymmetric CO2− stretch of deprotonated carboxyl groups contributes. Because Asp-75 becomes protonated in the M intermediate, the sign of the spectral changes would be in agreement with such a process. However, the observed spectral changes appear to be far too large to represent only this single protonation step. The largest part, i.e., the positive band at 1600 cm−1 in LNpSRII disappearing in MNpSRII, must be caused by additional, yet-unidentified molecular changes. On the other hand, the spectral change around 1360 cm−1 that is especially evident in the first amplitude spectrum as a positive band (Fig. 1 B) could be caused by the symmetric CO2− stretch of deprotonated Asp-75 disappearing in the transition to MNpSRII. This is corroborated by the measurements in 2H2O (Fig. 2 B). Further changes were observed at 1567 cm−1 (positive), which probably represent larger amide II spectral changes in MNpSRII. However, the ethylenic mode of the deprotonated Schiff base chromophore could also contribute. On the other hand, the band at 1527 cm−1 of LNpSRII, tentatively assigned to amide II spectral changes, had reduced intensity in MNpSRII. A further large change in the LNpSRII → MNpSRII transition involved the ethylenic mode: in the MNpSRII spectrum, the negative band at 1542 cm−1 gained intensity. This indicates that a band absorbing in this region disappeared and confirms our earlier conclusion that the ethylenic modes of the initial state and LNpSRII exhibited considerable overlap. (With the formation of the M intermediate, i.e., deprotonation of the Schiff base, there is a large upshift and intensity loss of the ethylenic mode that relieves the overlap between the ground and photoproduct states.) All the spectral features of the LNpSRII → MNpSRII transition were especially evident in the amplitude spectrum, where the positive bands represented the LNpSRII state and the negative ones the MNpSRII state. It is noteworthy that this amplitude spectrum was little influenced by H/2H exchange (Fig. 1 B vs. Fig. 2 B). This shows that the involved modes coupled only little, if at all, to exchangeable hydrogens.
As already discussed in the general description of the spectra, the transition to the next intermediate involved only small-protein structural changes. Thus this state also had all the characteristics of an M state. The earlier state is called M1NpSRII; this later one is termed M2NpSRII. Unfortunately, it is presently impossible to interpret these spectral changes in precise molecular terms. Because amide bands show up, we can conclude that the protein backbone undergoes small distortions.
In the subsequent transition, larger spectral changes occurred. We now provide evidence that the ONpSRII state was formed. ONpSRII has an absorption maximum at 535 nm (Chizhov et al., 1998), and thus an ethylenic mode of the chromophore around 1534 cm−1 is expected (Ottolenghi, 1980). Indeed, in the corresponding intermediate spectrum, the increase in absorbance at 1529 cm−1 could be caused by such a band. But better evidence was obtained from the amplitude spectrum, where the negative band at 1535 cm−1 was clearly visible (formation of ONpSRII). The comparison of the last two intermediate spectra shows that the contribution of ONpSRII was small. Therefore, the involved spectral features are better discussed with regard to the amplitude spectrum. Further large changes could be seen around 1597 cm−1 and in the amide I spectral range. A peculiar band of the ONpSRII state rose around 1757 cm−1 (broad negative band). In the presence of 2H2O, this band was shifted to 1744 cm−1 (Fig. 2 B), which is typical for the C O stretch of a protonated carboxyl group. Because in both amplitude spectra no corresponding positive band was observed, we assigned the negative band to the protonation of a carboxyl group. Especially, a shift of the band caused by Asp-75 to lower frequencies can be excluded. Thus the positive band observed at 1597 cm−1 in the presence of H2O and 2H2O could represent the disappearance of the corresponding antisymmetric CO2− stretching mode of deprotonated carboxyl groups. (Evidence for the disappearance of the corresponding symmetric one is obtained from Fig. 2 B (22 ms) at 1376 cm−1. However, because this is not so clearly observed in Fig. 1 B (19 ms), the assignment is only tentative.) The lack of a clear positive band around 1567 cm−1 demonstrates that the corresponding band that rose with the formation of MNpSRII (Fig. 1 A (b)) cannot represent the ethylenic stretch of the deprotonated Schiff base, but probably must be ascribed to amide II spectral changes. The isomeric state of the chromophore in ONpSRII can be deduced from the fingerprint modes in this amplitude spectrum. The three negative bands at 1164, (shoulder) 1186, and 1205 cm−1 are similar to corresponding bands observed at 1168, 1185, and around 1210 cm−1 in the transition from N to O in bacteriorhodopsin (Hessling et al., 1993; Rödig et al., 1999). One has to recall that the amplitude spectrum described here reflects the transition from M2NpSRII to ONpSRII, i.e., from an unprotonated Schiff base to a protonated one. Therefore, the negative fingerprint bands essentially represent those of the protonated Schiff base. Because this pattern of fingerprint bands is characteristic of an all-trans chromophore, we can conclude that, like in the O intermediate of bacteriorhodopsin and halorhodopsin (Smith et al., 1983; Hackmann et al., 2001), the chromophore is also in this geometry in ONpSRII. In Fig. 2 B, these three bands cannot be seen. This is probably due to the larger noise in this spectral region caused by the strong absorbance of 2H2O.
O stretch of a protonated carboxyl group. Because in both amplitude spectra no corresponding positive band was observed, we assigned the negative band to the protonation of a carboxyl group. Especially, a shift of the band caused by Asp-75 to lower frequencies can be excluded. Thus the positive band observed at 1597 cm−1 in the presence of H2O and 2H2O could represent the disappearance of the corresponding antisymmetric CO2− stretching mode of deprotonated carboxyl groups. (Evidence for the disappearance of the corresponding symmetric one is obtained from Fig. 2 B (22 ms) at 1376 cm−1. However, because this is not so clearly observed in Fig. 1 B (19 ms), the assignment is only tentative.) The lack of a clear positive band around 1567 cm−1 demonstrates that the corresponding band that rose with the formation of MNpSRII (Fig. 1 A (b)) cannot represent the ethylenic stretch of the deprotonated Schiff base, but probably must be ascribed to amide II spectral changes. The isomeric state of the chromophore in ONpSRII can be deduced from the fingerprint modes in this amplitude spectrum. The three negative bands at 1164, (shoulder) 1186, and 1205 cm−1 are similar to corresponding bands observed at 1168, 1185, and around 1210 cm−1 in the transition from N to O in bacteriorhodopsin (Hessling et al., 1993; Rödig et al., 1999). One has to recall that the amplitude spectrum described here reflects the transition from M2NpSRII to ONpSRII, i.e., from an unprotonated Schiff base to a protonated one. Therefore, the negative fingerprint bands essentially represent those of the protonated Schiff base. Because this pattern of fingerprint bands is characteristic of an all-trans chromophore, we can conclude that, like in the O intermediate of bacteriorhodopsin and halorhodopsin (Smith et al., 1983; Hackmann et al., 2001), the chromophore is also in this geometry in ONpSRII. In Fig. 2 B, these three bands cannot be seen. This is probably due to the larger noise in this spectral region caused by the strong absorbance of 2H2O.
Measurements in the presence of azide
Because azide considerably increases the steady-state electric current, which is interpreted as a steady-state proton translocation (Schmies et al., 2000), we studied the photoreaction in the presence of azide. It has been reported that azide accelerates the reprotonation of the Schiff base (Takao et al., 1998), but without shortening of the photocycle (Schmies et al., 2000). It was concluded that the increase of the proton-pumping rate cannot be explained by the acceleration of the slow photocycle. The pH dependence of the azide effect showed that it is the protonated form of azide that is involved in proton translocation.
In performing the measurements, we faced the problem that to obtain unequivocal spectra, the molar amount of azide should be larger than the molar amount of NpSRII. Owing to the low water content of the infrared samples, this led to an azide concentration exceeding the 1 M level, and according to the time-resolved UV-vis measurements, to a very fast reprotonation of the Schiff base. In addition, the high salt concentration caused a low infrared transmission of the sample and therefore increased the noise in the spectra. It turned out that the spectra showed only small changes in time. The high azide concentration caused such a fast reprotonation of the Schiff base that the MNpSRII intermediate did not accumulate. Together with the larger noise, this made the global fitting procedure of the time-resolved spectra to a sum of exponentials unreliable. To still be able to deduce the main temporal variations, we formed time-averaged spectra whereby the corresponding time ranges were selected from the half-times obtained by the approximate global fit. The last half-time, which described the reformation of the initial state, was reliably determined to 120 ms at 37°C, thereby confirming the observation that azide does not accelerate the photocycle. The results are shown in Fig. 4.
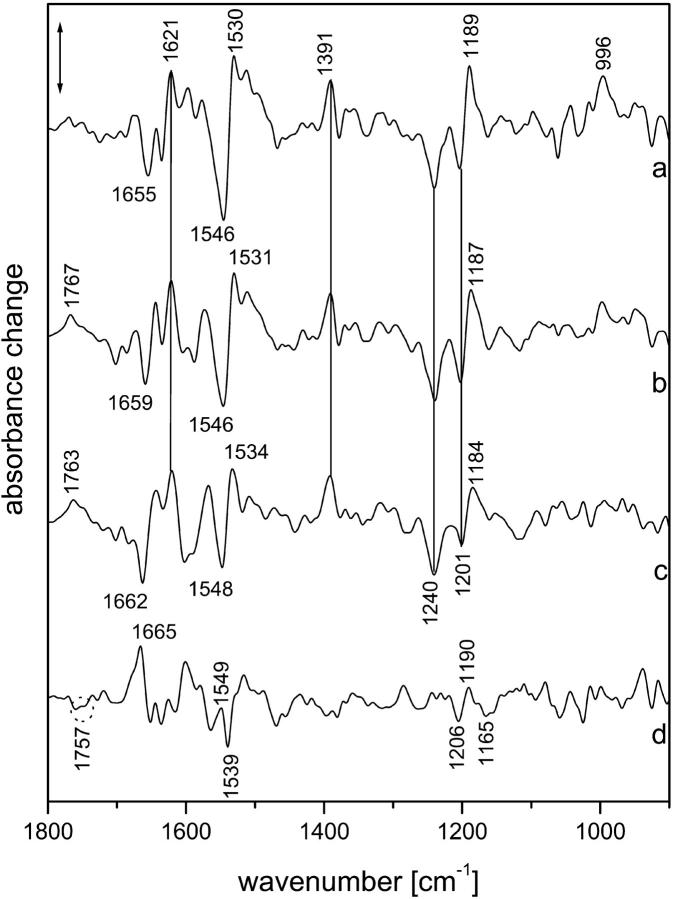
FIGURE 4.
Time-resolved difference spectra obtained in the presence of azide at 37°C. Molar ratio of azide to NpSRII was 3.3:1. (a) Spectrum averaged between 100 and 1040 ns; (b) spectrum averaged between 25 and 61 μs; (c) spectrum averaged between 40 and 100 ms; (d): weighted difference between (b) and (c), using the negative band at 1240 cm−1 for normalization. Spectral resolution, 8 cm−1.
The first spectrum (Fig. 4 (a)), which was averaged over the period from 100 to 1040 ns, was very similar to the LNpSRII spectrum shown in Fig. 1 A (a). The deviations, especially in the amide I spectral range and between 1100 and 1000 cm−1, can be explained by the larger noise. However, the larger intensities of the bands between 1530 and 1500 cm−1 represent real spectral differences. Whether they are caused by larger amide II alterations or by a downshifted and increased ethylenic stretching mode is difficult to decide. However, it should be noted that this feature even persisted at later times when Asp-75 became protonated. The fingerprint band characteristic of the 13-cis configuration at 1189 cm−1 is evident. Although the noise was quite large in the spectral range between 1100 and 1020 cm−1 and below 930 cm−1, the HOOP mode at 996 cm−1 was by far above the noise level.
The next spectrum, which was averaged between 25 and 61 μs, showed (as expected) the band due to protonation of Asp-75 at 1767 cm−1 (Fig. 4 (b)). However, in contrast to the MNpSRII spectrum of Fig. 1 A (b), the positive fingerprint band, although slightly shifted to 1187 cm−1, was still present. This clearly shows that the Schiff base is protonated. This is corroborated by the increase of the band at 1531 cm−1, which was caused by the rise of the corresponding ethylenic stretching mode. The corresponding absorption maximum must therefore be similar to that of the ONpSRII intermediate, which is in agreement with earlier studies of the influence of azide on the photoreaction (Schmies et al., 2000). The spectra between 1 and 25 μs only show the direct transition from the earlier spectrum (Fig. 4 (a)) to this one, without a transient intensity loss of the fingerprint band around 1187 cm−1. Because the time range for protonation of Asp-75 is similar to that observed without azide, we must conclude that immediately after proton transfer from the Schiff base to Asp-75, the Schiff base becomes protonated via protonated azide. Therefore, the species with an unprotonated Schiff base cannot accumulate. The amide I bands indicate that the distortions of the protein backbone are similar to those of MNpSRII (Fig. 1 A (b)). In contrast to the all-trans chromophore geometry in ONpSRII, in this species, the chromophore was still 13-cis as indicated by the positive fingerprint band at 1187 cm−1. Therefore, we will call this species MONpSRII, similar to the NO state identified for mutations of Leu-93 in bacteriorhodopsin (Subramaniam et al., 1991). It is remarkable that the HOOP mode at 996 cm−1 was still present in this intermediate, i.e., the chromophore was similarly twisted as in the precursor intermediate and as in LNpSRII in the measurements without azide.
It is tempting to also assign in the first spectrum the larger intensity of the band at 1530 cm−1 to the ethylenic mode. This would indicate that in the presence of azide, the precursor of MNpSRII (or MONpSRII) has a red-shifted absorption maximum. However, earlier studies on the influence of azide provide no evidence for this, at least up to a 1 M azide concentration. But it appears possible that in our infrared samples, azide was already bound to the cytoplasmatic channel, close to the Schiff base, which would enable its very fast reprotonation. This could cause a red-shift of the absorption maximum of LNpSRII or even bypass its formation.
The analysis of the time-resolved spectra showed that further spectral changes occurred in the 0.1–20-ms time range. Therefore, the last spectrum was averaged over a period from 50 to 100 ms (Fig. 4 (c)). The smaller size of the bands is explained by the partial decay to the initial state. The changes with respect to the earlier spectrum can be best estimated by forming the difference between the two spectra using the negative band at 1240 cm−1 for normalization. This is shown in Fig. 4 (d). It resembles the last amplitude spectrum shown in Fig. 1 B (101 ms). The negative bands in the fingerprint region (1205 and 1165 cm−1) show that the chromophore underwent reisomerization to all-trans. Here, one must consider that this difference spectrum reflects the transition from MONpSRII to ONpSRII. Therefore, the strong fingerprint band of MONpSRII at 1187 cm−1 showed up as a positive band around 1190 cm−1. The band at 1757 cm−1 indicates that an additional carboxyl group became protonated. Thus, the spectral changes reflect the transition to the ONpSRII intermediate. Interestingly, the ethylenic mode was probably upshifted to 1539 cm−1, indicating that ONpSRII was slightly blue-shifted as compared to MONpSRII. The larger ethylenic band indicates that considerably more ONpSRII was formed as compared with the measurements without azide (Fig. 1 A (d)). From our approximate fitting procedure, we obtained a half-time for the MONpSRII decay of ∼25 ms at 37°C. Thus the rate-limiting step of the photocycle was not the back-isomerization of the chromophore (although it was slower than in bacteriorhodopsin), but the relaxation of the protein must control the reformation of the initial state. The last intermediate still contained a large fraction of MONpSRII, which indicates that the lifetime of protein states that favor the 13-cis geometry of the chromophore is rather long.
DISCUSSION
In the analysis of the time-resolved infrared spectra obtained at 37°C, we were able resolve four intermediates: LNpSRII, M1NpSRII, M2NpSRII, and MONpSRII. The analysis of the spectra obtained with 30-ns time resolution also provided evidence for the existence of a KNpSRII state that is very similar to the LNpSRII state, with the exception of small alterations in the amide I/II spectral range. Up to several milliseconds, the analysis was in good agreement with earlier time-resolved UV-vis studies (Chizhov et al., 1998). The deviations observed for the later times can probably be explained by the low water content of the infrared samples. Preliminary experiments with steady-state illumination using a diamond-attenuated total-reflection cell or the sandwich-sample technique (Vogel and Siebert, 2001), which both guarantee a natural aqueous environment, showed that as compared with the film samples, considerably more ONpSRII accumulated as expected from the photocycle. Thus, hydration sensitively influenced the photocycle after formation of the MNpSRII intermediates.
The time-resolved UV-vis spectra showed only a small red-shift (11 nm) for KNpSRII (Chizhov et al., 1998). This is in contrast to earlier published low-temperature spectra, which indicated red-shifts of 25 and 35 nm for two KNpSRII species (Hirayama et al., 1992). As discussed, the small size of the ethylenic modes in the low-temperature infrared spectrum presented here is in better agreement with the small red-shift. It is not clear what caused the differences, but the different sample preparations, namely, NpSRII reconstituted into lipids vs. solubilized NpSRII, may have contributed. The time-resolved spectra of KNpSRII of samples hydrated with 2H2O where the C N mode of the Schiff base was shifted to 1630 cm−1 clearly reveal amide I bands between 1700 and 1640 cm−1 that are considerably larger than those observed for the K intermediate of bacteriorhodopsin. This indicates that in this early intermediate, the protein backbone underwent correspondingly larger distortions. Interestingly, photoacoustic measurements provided evidence for considerable volume changes for KNpSRII, but a much smaller change has been deduced for K of bacteriorhodopsin (Losi et al., 1999).
N mode of the Schiff base was shifted to 1630 cm−1 clearly reveal amide I bands between 1700 and 1640 cm−1 that are considerably larger than those observed for the K intermediate of bacteriorhodopsin. This indicates that in this early intermediate, the protein backbone underwent correspondingly larger distortions. Interestingly, photoacoustic measurements provided evidence for considerable volume changes for KNpSRII, but a much smaller change has been deduced for K of bacteriorhodopsin (Losi et al., 1999).
We have clearly identified a transition (M1NpSRII → M2NpSRII) that only involves changes of the protein that is in agreement with the UV-vis studies describing a spectrally silent transition (in the UV-vis) with the same half-time. A similar transition has been described for halorhodopsin, and the corresponding protein changes have been invoked as an indication for the accessibility change of the anion from the extracellular to the cytoplasmic side (Hackmann et al., 2001; Chizhov and Engelhard, 2001). The functional role of this transition in NpSRII is not yet clear. It could be that in view of the influence of hydration on the millisecond part of the photocycle, the differences between M1NpSRII and M2NpSRII are actually larger if excess water is present. In view of these data, it is noteworthy that recent electron paramagnetic resonance experiments indicate an outward movement of helix F during the M1NpSRII → M2NpSRII transition (M. Engelhard and J. Steinhoff, unpublished results). An unequivocal correlation of the amide-band changes and the helix-F movement may be accomplished by using site-specific isotope labeling of peptide bonds. Using bacteriorhodopsin as a model system, we recently showed by selective incorporation of a 13C label into a single peptide group (carbonyl position) that especially positions in the middle to extracellular part of helices 3 and 6 contribute to the amide I changes in the N state (Hauser et al., 2002). This has been interpreted by the assumption that at these positions, the movements of these helices cause distortions in the backbone.
The most striking observation is the identification of the 13-cis geometry of the chromophore in the MONpSRII state accumulated in the presence of azide, whereas in the subsequent ONpSRII intermediate the conformation was turned to all-trans. This observation could explain the increase in steady-state proton pumping in the presence of azide, which has been explained by a two-photo process (Schmies et al., 2000). Because the Schiff base was already reprotonated, the long-lived MONpSRII intermediate could easily absorb a second photon. This process probably caused the cis-trans back-isomerization of the retinal chromophore and thereby shortened the photocycle. The higher turnover number would result in an increased photostationary current as was actually observed.
At present, we cannot identify the second carboxyl group that became protonated with the formation of ONpSRII. In the case of bacteriorhodopsin, some evidence has been obtained for protonation of Asp-212 with the formation of O. One interpretation has been that the proton is transferred from Asp-85 to Asp-212 (Dioumaev et al., 1999). Such a proton exchange between two carboxyl groups can be excluded, because in the corresponding amplitude spectrum (Fig. 1 B (19 ms)) in the spectral range where protonated carboxyl groups show up, we observed only a negative band. A proton circulation involving an additional titratable group has been proposed for Schiff-base reprotonation (Sasaki and Spudich, 1999). However, in this case, a deprotonation would have been expected. The band position at 1758 cm−1 indicated that the protonated carboxyl group was not strongly hydrogen-bonded, thereby excluding a group exposed to the aqueous phase. The only internal carboxyl group is Asp-201, the equivalent of Asp-212 in bacteriorhodopsin. If the band were caused by this residue, it would not pick up the proton from Asp-75. The positive charge of the Schiff base and the neutralization of Asp-75 and Asp-201 would probably require a compensation of the positive charge of Arg-72. Thus it could be that there is proton transfer from this residue to Asp-201. Interestingly, the protonation state of Arg-72 has been recently suggested as taking part in controlling the pKa of the Schiff base in the dark state (Iwamoto et al., 2002). Because Asp-201 is close to the Schiff base, a proton transfer from Arg-72 to Asp-201 appears possible if the corresponding pKa values were regulated by the protein environment. The trigger for this proton transfer could be conformational changes that relocate either of these residues or both in a less-polar environment. However, it has to be emphasized that this interpretation is speculative. The small size of the band is probably due to the small amount of O formed in this transition. A more thorough analysis of the molecular events involved with the formation of O requires its considerably larger accumulation. This can be achieved, as mentioned above, at a much higher degree of hydration that is obtainable with sandwich samples. Such experiments are in progress.
It is remarkable that the reprotonation of the Schiff base in MONpSRII does not, as compared to M2NpSRII, accelerate the reisomerization, in that the barrier of the 13,14 double bond is reduced. This shows that it is the protein conformation that determines the rate of back-isomerization. Furthermore, because ONpSRII is in equilibrium with considerable amounts of either MNpSRII or MONpSRII, the back-isomerization is essentially controlled by the long lifetime of the state with M conformation, which is thought to represent the signaling state. This temporal stability may be important to convey the information of light absorption by the receptor to the interacting transducer.
Acknowledgments
The authors thank M. Schumacher for excellent technical help and T. Savopol for critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grants AZ EN 87/10-4,5 (to M. Engelhard) and AZ SI 278/18-3,4 (to F. Siebert) and by Fonds der Chemischen Industrie (to F. Siebert).
References
- Bergo, V., E. N. Spudich, K. L. Scott, J. L. Spudich, and K. J. Rothschild. 2000. FTIR analysis of the SII540 intermediate OD sensory rhodopsin II: Asp-73 is the Schiff base proton acceptor. Biochemistry 39:2823–2830. [DOI] [PubMed] [Google Scholar]
- Chizhov, I. and M. Engelhard. 2001. Temperature and halide dependence of the photocycle of halorhodopsin from Natronobacterium pharaonis. Biophys. J. 81:1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhov, I. V., G. Schmies, R. Seidel, J. R. Sydor, B. Lüttenberg, and M. Engelhard. 1998. The photophobic receptor from Natronobacterium pharaonis: temperature and pH dependence of the photocycle of sensory rhodopsin II. Biophys. J. 75:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioumaev, A. K., L. S. Brown, R. Needleman, and J. K. Lanyi. 1999. Fourier transform infrared spectra of a late intermediate of the bacteriorhodopsin photocycle suggest transient protonation of Asp-212. Biochemistry 38:10070–10078. [DOI] [PubMed] [Google Scholar]
- Engelhard, M., B. Scharf, and F. Siebert. 1996. Protonation changes during the photocycle of sensory rhodopsin II from Natronobacterium pharaonis. FEBS Lett. 395:195–198. [DOI] [PubMed] [Google Scholar]
- Gellini, C., B. Luttenberg, J. Sydor, M. Engelhard, and P. Hildebrandt. 2000. Resonance Raman spectroscopy of sensory rhodopsin II from Natronobacterium pharaonis. FEBS Lett. 472:263–266. [DOI] [PubMed] [Google Scholar]
- Hackmann, C., J. Guijarro, I. Chizhov, M. Engelhard, C. Rödig, and F. Siebert. 2001. Static and time-resolved step-scan FTIR investigations of the photoreaction of halorhodopsin from Natronobacterium pharaonis: consequences for the anion translocation mechanism. Biophys. J. 81:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, K., M. Engelhard, N. Friedman, M. Sheves, and F. Siebert. 2002. Interpretation of amide I difference bands observed during protein reactions using site-directed isotopically labeled bacteriorhodopsin as a model system. J. Phys. Chem. A 106:3553–3559. [Google Scholar]
- Hessling, B., G. Souvignier, and K. Gerwert. 1993. A model-independent approach to assigning bacteriorhodopsin's intramolecular reactions to photocycle intermediates. Biophys. J. 65:1929–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama, J., Y. Imamoto, Y. Shichida, N. Kamo, H. Tomioka, and T. Yoshizawa. 1992. Photocycle of phoborhodopsin from haloalkaliphilic bacterium (Natronobacterium pharaonis) studied by low-temperature spectrophotometry. Biochemistry 31:2093–2098. [DOI] [PubMed] [Google Scholar]
- Hirayma, J., N. Kamo, Y. Imamoto, Y. Shichida, and T. Yoshizawa. 1995. Reason for the lack of light-dark adaptation in pharaonis phoborhodopsin: reconstitution with 13-cis-retinal. FEBS Lett. 364:168–170. [DOI] [PubMed] [Google Scholar]
- Hohenfeld, I. P., A. A. Wegener, and M. Engelhard. 1999. Purification of histidine tagged bacteriorhodopsin, pharaonis halorhodopsin, and pharaonis sensory rhodopsin II functionally expressed in Escherichia coli. FEBS Lett. 442:198–202. [DOI] [PubMed] [Google Scholar]
- Imamoto, Y., Y. Shichida, T. Yoshizawa, H. Tomioka, T. Takahashi, K. Fujikawa, N. Kamo, and Y. Kobatake. 1991. Photoreaction cycle of phoborhodopsin studied by low-temperature dpectrophotometry. Biochemistry 30:7416–7424. [DOI] [PubMed] [Google Scholar]
- Iwamoto, M., Y. Furutani, Y. Sudo, K. Shimono, H. Kandori, and N. Kamo. 2002. Role of Asp193 in chromophore-protein interaction of pharaonis phoborhodopsin (sensory rhodopsin II). Biophys. J. 83:1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandori, H., K. Shimono, Y. Sudo, M. Iwamoto, Y. Shichida, and N. Kamo. 2001. Structural changes of pharaonis phoborhodopsin upon photoisomerization of the retinal chromophore: infrared spectral comparison with bacteriorhodopsin. Biochemistry 40:9238–9246. [DOI] [PubMed] [Google Scholar]
- Losi, A., A. A. Wegener, M. Engelhard, W. Gärtner, and S. E. Braslavsky. 1999. Time-resolved absorption and photothermal measurements with recombinant sensory rhodopsin II from Natronobacterium pharaonis. Biophys. J. 77:3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke, H., B. Schobert, J. K. Lanyi, E. N. Spudich, and J. L. Spudich. 2001. Crystal structure of sensory rhodopsin II at 2.4 Å resolution: insights into color tuning and transducer interaction. Science 293:1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, A. 1995. Application of FTIR spectroscopy to the structural study on the function of bacteriorhodopsin. Isr. J. Chem. 35:387–400. [Google Scholar]
- Ottolenghi, M. 1980. The photochemistry of rhodopsin. In Advances in Photochemistry, Vol. 12. J. N. Pitts, G. S. Hammond, K. Gollnik, and D. Grosjean, editors. Wiley-Interscience, New York. 97–200.
- Rödig, C., I. V. Chizhov, O. Weidlich, and F. Siebert. 1999. Time-resolved step-scan FTIR spectroscopy reveals differences between early and late M intermediates of bacteriorhodopsin. Biophys. J. 76:2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royant, A., P. Nollert, K. Edman, R. Neutze, E. M. Landau, E. Pebay-Peyroula, and J. Navarro. 2001. X-ray structure of sensory rhodopsin II at 2.1-Å resolution. Proc. Natl. Acad. Sci. USA 98:10131–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph, J. and D. Oesterhelt. 1996. Deletion analysis of the operon in the archeon Halobacterium salinarium. J. Mol. Biol. 258:548–554. [DOI] [PubMed] [Google Scholar]
- Sasaki, J. and J. L. Spudich. 1999. Proton circulation during the photocycle of sensory rhodopsin II. Biophys. J. 77:2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, G., M. Engelhard, and V. Müller. 1999. Bioenergetics of the archaea. Microbiol. Mol. Biol. Rev. 63:570–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf, B., M. Engelhard, and F. Siebert. 1992. A carboxyl group is protonated during the photocycle of the photophobic receptor psR-II from Natronobacterium pharaonis. In Structure and Function of Retinal Proteins, Vol. 221. J.-L. Rigaud, editor. Colloque INSERM/John Libbey Eurotext Ltd. 317–320.
- Scharf, B., B. Pevec, B. Hess, and M. Engelhard. 1992. Biochemical and photochemical properties of the photobic receptors from Halobacterium halobium and Natronobacterium pharaonis. Eur. J. Biochem. 206:359–366. [DOI] [PubMed] [Google Scholar]
- Schmies, G., B. Lüttenberg, I. V. Chizhov, M. Engelhard, A. Becker, and E. Bamberg. 2000. Sensory rhodopsin II from the haloalkaliphilic Natronobacterium pharaonis: light-activated proton transfer reactions. Biophys. J. 78:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono, K., Y. Furutani, H. Kandori, and N. Kamo. 2002. A pharaonis phoborhodopsin mutant with the same retinal binding site residues as in bacteriorhodopsin. Biochemistry 41:6504–6509. [DOI] [PubMed] [Google Scholar]
- Shimono, K., M. Iwamoto, M. Sumi, and N. Kamo. 1997. Functional expression of pharaonis phoborhodopsin in Escherichia coli. FEBS Lett. 420:54–56. [DOI] [PubMed] [Google Scholar]
- Siebert, F. and W. Mäntele. 1980. Investigation of the rhodopsin/meta I and rhodopsin/meta II transitions of bovine rod outer segments by means of kinetic infrared spectroscopy. Biophys. Struct. Mech. 6:147–164. [DOI] [PubMed] [Google Scholar]
- Smith, S. O., J. A. Pardoen, P. P. J. Mulder, B. Curry, J. Lugtenburg, and R. A. Mathies. 1983. Chromophore structure in bacteriorhodopsin's O640 photointermediate. Biochemistry 22:6141–6148. [Google Scholar]
- Spudich, J. L., C.-S. Yang, K.-H. Jung, and E. N. Spudich. 2000. Retinylidene proteins: structures and functions from archae to humans. Annu. Rev. Cell Dev. Biol. 16:365–392. [DOI] [PubMed] [Google Scholar]
- Subramaniam, S., D. A. Greenhalgh, P. Rath, K. J. Rothschild, and H. G. Khorana. 1991. Replacement of leucine-93 by alanine or threonine slows down the decay of the N and O intermediates in the photocycle of bacteriorhodopsin: implications for proton uptake and 13-cis-retinal—all-trans-retinal reisomerization. Proc. Natl. Acad. Sci. USA 88:6873–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao, K., T. Kikukawa, T. Araiso, and N. Kamo. 1998. Azide accelerates the decay of M-intermediate of pharaonis phoborhodopsin. Biophys. Chem. 73:145–153. [DOI] [PubMed] [Google Scholar]
- Vogel, R. and F. Siebert. 2001. Conformations of the active and inactive states of opsin. J. Biol. Chem. 276:38487–38493. [DOI] [PubMed] [Google Scholar]
- Wegener, A. A., I. Chizhov, M. Engelhard, and H. J. Steinhoff. 2000. Time-resolved detection of transient movement of helix F in spin-labelled pharaonis sensory rhodopsin II. J. Mol. Biol. 301:881–891. [DOI] [PubMed] [Google Scholar]
- Weidlich, O. and F. Siebert. 1993. Time-resolved step-scan FT-IR investigations of the transition from KL to L in the bacteriorhodopsin photocycle: identification of chromophore twists by assigning hydrogen-out-of-plane (HOOP) bending vibrations. Appl. Spectrosc. 47:1394–1400. [Google Scholar]