Abstract
G-Protein activated, inwardly rectifying potassium channels (GIRKs) are important effectors of G-protein β/γ-subunits, playing essential roles in the humoral regulation of cardiac activity and also in higher brain functions. G-protein activation of channels of the GIRK1/GIRK4 heterooligomeric composition is controlled via phosphorylation by cyclic AMP dependent protein kinase (PKA) and dephosphorylation by protein phosphatase 2A (PP2A). To study the molecular mechanism of this unprecedented example of G-protein effector regulation, single channel recordings were performed on isolated patches of plasma membranes of Xenopus laevis oocytes. Our study shows that: (i) The open probability (Po) of GIRK1/GIRK4 channels, stimulated by coexpressed m2-receptors, was significantly increased upon addition of the catalytic subunit of PKA to the cytosolic face of an isolated membrane patch. (ii) At moderate concentrations of recombinant Gβ1/γ2, used to activate the channel, Po was significantly reduced in patches treated with PP2A, when compared to patches with PKA-cs. (iii) Several single channel gating parameters, including modal gating behavior, were significantly different between phosphorylated and dephosphorylated channels, indicating different gating behavior between the two forms of the protein. Most of these changes were, however, not responsible for the marked difference in Po at moderate G-protein concentrations. (iv) An increase of the frequency of openings (fo) and a reduction of dwell time duration of the channel in the long-lasting C5 state was responsible for facilitation of GIRK1/GIRK4 channels by protein phosphorylation. Dephosphorylation by PP2A led to an increase of Gβ1/γ2 concentration required for full activation of the channel and hence to a reduction of the apparent affinity of GIRK1/GIRK4 for Gβ1/γ2. (v) Although possibly not directly the target of protein phosphorylation/dephosphorylation, the last 20 C-terminal amino acids of the GIRK1 subunit are required for the reduction of apparent affinity for the G-protein by PP2A, indicating that they constitute an essential part of the off-switch.
INTRODUCTION
Humoral regulation of the heartbeat in mammals, including humans, is mediated by means of the release of neurotransmitter, such as acetylcholine, from the parasympathetic nervous system (Löwi, 1921). In the sinus node, the atrioventricular node, and other parts of the atrium, an inwardly rectifying potassium channel is abundant, which is activated by G-proteins via neurotransmitter receptors (IK.ACh; see Dascal, 1997 and Yamada et al., 1998, for review). The effective contribution of this G-protein effector to the humoral regulation of the heartbeat in vivo was not exactly known until recently, when Wickman et al. (1998) generated a mouse strain, lacking IK.ACh currents, by knocking out the gene encoding an essential subunit of the protein, underlying IK.ACh (see also Dobrev et al., 2001, and Kovoor et al., 2001). In the brain, G-protein activated inwardly rectifying potassium channels (GIRKs) are pivotal for postsynaptic inhibition, mediated by numerous neurotransmitters, and possibly also involved in presynaptic events. In several other tissues, mRNAs encoding GIRK subunits, as well as functional GIRK channels, have been detected (see (Yamada et al., 1998), for review). Molecular cloning of the genes encoding GIRK channels revealed four structurally related genes, termed GIRK1 to GIRK4 in mammals, plus an amphibian GIRK5 subunit (see Dascal, 1997, for review). It has been demonstrated that the GIRK1 subunit, which was the first to be cloned and expressed (Dascal et al., 1993; Kubo et al., 1993), is unable to form functional homooligomeric ion channels when expressed alone. Instead, GIRK1 requires additional isoforms to form functional heterooligomeric channels (Hedin et al., 1996). In the atrium, IK.ACh has been shown to be composed of GIRK1 plus GIRK4 subunits (Krapivinsky et al., 1995).
The membrane delimited activation of IK.ACh via heterotrimeric G-proteins has been recognized to be mediated by the G-protein β/γ dimer (as reviewed by Dascal, 1997, and Yamada et al., 1998). The G-protein α-subunit, however, which was originally thought to activate the channel, also interacts with the GIRK protein and seems to be involved in the regulation and specificity of Gβ/γ action (Yatani et al., 1990; Schreibmayer et al., 1996; Yakubovich et al., 2000; Peleg et al., 2002). In addition to their activation by Gβ/γ, GIRK channels are regulated by a multitude of physiological pathways, including advisory proteins such as regulators of G-protein signaling (RGS; see Doupnik et al., 1997, and Saitoh et al., 1997), the internal Na+ concentration (see Dascal, 1997 and Yamada et al., 1998, for review), derivatives of arachidonic acid (Kim and Clapham, 1989; Kurachi et al., 1989; Scherer and Breitwieser, 1990; Nakajima et al., 1991; Ramos-Franco et al., 1993; Scherer et al., 1993; Lohberger et al., 2000) and specific phospholipids, such as phosphatidyl-inositol-bis-phosphate (PIP2; see Hilgemann et al., 2001, and Mark and Herlitze, 2000, for review). Most remarkably, GIRK channels are regulated by intracellular adenosine triphosphate (ATP; reviewed up to 1997 in: Dascal, 1997; Yamada et al.; 1998; and Pleumsamran et al., 1998). In most cases, hydrolysis of ATP is required for the activation of GIRK channels. In the living cell, ATP is acting as a universal equivalent for metabolic energy as well as a phosphate donor for the covalent modification of proteins and other biomolecules. According to this ubiquitous role, several distinct mechanisms result in alterations of GIRK channel behavior by intracellular ATP: (i) Transphosphorylation between adenosine- and guanine-nucleotides via NDPK, (ii) formation of PIP2 via hydrolysis of ATP, and (iii) direct protein phosphorylation. Activation of IK.ACh by phosphorylation via cyclic AMP-dependent protein kinase (PKA) has been shown to occur in native atrial cells as well as in heterologous expression systems (Kim, 1990; Müllner et al., 2000). Correspondingly, dephosphorylation via protein phosphatase 2A (PP2A), greatly impaired the ability of recombinant Gβ/γ to activate IK.ACh (Medina et al., 2000). Moreover, phosphorylation/dephosphorylation of IK.ACh was shown to act as a molecular switch that regulates the ability of neurotransmitter receptors to activate the channel (Medina et al., 2000; Müllner et al., 2000). The current study aims to investigate the molecular mechanism by which phosphorylation and dephosphorylation regulate the G-protein activation of this G-protein effector, an unprecedented example of cellular regulation.
MATERIALS AND METHODS
Frog oocytes
Xenopus laevis oocytes were prepared as described (Dascal and Lotan, 1992) and the following amounts of cRNA were injected (ng/oocyte): 1.5 muscarinic m2 receptor, 0.003 GIRK1, 0.0025 GIRK1ΔC20, and 0.015 GIRK4. To knock out the endogenously existing GIRK5 subunit, 20 ng/oocyte of the phosphorothioated antisense oligonucleotide KHA2 was injected one day before cRNA injection (Hedin et al., 1996). Oocytes were incubated in NDE solution (see below) at 19°C for 4–10 days before electrophysiological recordings.
Electrophysiology
Immediately before electrophysiological recording, oocytes were placed shortly into PG180Ca solution for shrinking and the vitteline layer was removed with fine forceps (Swiss No. 5). The devittelinized oocytes were placed into a recording chamber and mounted on an inverted microscope (Zeiss IM35, Germany). Gigaseals were produced with borosilicate pipettes (outer diameter: 1.6 mm, inner diameter: 1.0 mm; 5–12 MΩ), coated with Sylgard (Sylgard 184, Dow Corning), fire-polished with a custom-made microforge and filled with pipette filling solution, containing 200 μM GdCl3 to block endogenous stretch-activated channels. Seal resistance ranged between 10 and 200 GΩ. When a single GIRK1/GIRK4 channel was detected in the membrane patch, the patch was excised into the bathing solution. Frequently, short air exposures of the pipette tip immediately after patch excision were required to prevent vesicle formation at the tip of the pipette. Membrane potential was held at −80 mV. Increasing amounts of recombinant Gβ1/γ2 were added to the bathing solution to activate the channels. The number of channels in a given patch was estimated from the periods of overlap of channel openings observed at fully activating concentrations of Gβ1/γ2 (100 nM). Traces were digitized at 20 kHz by an IBM-compatible PC equipped with the Labmaster TL-1-125 hardware (Axon Instruments) using the Axotape 2.0 program (Axon Instruments). Single channel traces were digitally filtered at 2 kHz and events lists were generated using the Fetchan 6.0 software (Axon Instruments). Events lists were further processed using the MatLab5.0 environment, as described in Yakubovich et al. (2000). Briefly, histograms of open times were fitted using two exponential functions. Closed times of fully activated channels were fitted with five exponents, whereas two exponents were adequate for resting conditions. Experimental parameters obtained under different conditions were compared using the Student t-Test or the Mann-Whitney Rank Sum Test (SigmaStat 2.0), as appropriate. Modal gating was analyzed by dividing single channel traces into 400-ms segments and the frequency of openings (fo) was determined for each interval. Distributions of fo were fitted by the sum of one to three geometrical functions according to Eq. 1:
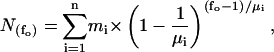 |
(1) |
where 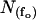 denotes the number of segments as a function of fo, mi the number of segments at fo = 0, μi the characteristic frequency of the geometrical function, and n the number of geometrical functions. Actual fitting was performed by excluding segments with
denotes the number of segments as a function of fo, mi the number of segments at fo = 0, μi the characteristic frequency of the geometrical function, and n the number of geometrical functions. Actual fitting was performed by excluding segments with 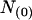 (defined as the number of silent segments without channel openings). For statistical comparison of parameters obtained by fitting the entire dataset to the sum of geometrical functions, the 95% confidence intervals were calculated. Parameters were regarded as statistically significantly different when the 95% confidence intervals did not overlap. As an additional indicator for modal gating, the mean open time (to,s) at a given fo was calculated according to Eq. 2:
(defined as the number of silent segments without channel openings). For statistical comparison of parameters obtained by fitting the entire dataset to the sum of geometrical functions, the 95% confidence intervals were calculated. Parameters were regarded as statistically significantly different when the 95% confidence intervals did not overlap. As an additional indicator for modal gating, the mean open time (to,s) at a given fo was calculated according to Eq. 2:
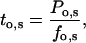 |
(2) |
where Po,s represents the open probability at a given fo (Yakubovich et al., 2000).
Molecular biology
Plasmid vectors were linearized (Sambrook and Russel, 2001) and cRNA was synthesized as described previously (Dascal and Lotan, 1992). Plasmid vectors encoding the m2-muscarinic acetylcholine receptor (m2R; Lim et al., 1995), GIRK1 and GIRK4 (Silverman et al., 1996) were used. The GIRK1ΔC20 mutation was generated by performing a PCR reaction using GIRK1wt as a template, utilizing the appropriate forward and backward primers containing the desired parts of the coding sequence preceded or followed respectively by the appropriate restriction enzyme recognition sequences. The PCR product was digested and ligated via the EcoRI and HindIII sites into pGEMHE.
Solutions
Solutions were (in mM) PG180Ca: K-Aspartate (180), KCl (37.5), CaCl2 (1), MgCl2 (1), and HEPES (10) buffered with KOH to pH 7,4. Bathing solution: K-Aspartate (120), KCl (20), MgCl2 (4), NaCl (10), EGTA (1), ATP (1), and HEPES (10) buffered with KOH to pH 7.4. Pipette filling solution: KCl (150), MgCl2 (2), CaCl2 (1), GdCl3 (0.2), and HEPES (10) buffered with KOH to pH 7.4. CHAPS-buffer: CHAPS (11.4), NaCl (50), DTT (3), and HEPES (20) buffered with KOH to pH 8,0. NDE: NaCl (96), KCl (2), MgCl2 (1), CaCl2 (1.8), pyruvate (2.5), antibiotics (0.1%; Sigma, Germany, G-1397; 1000× stock), and HEPES (5) buffered with NaOH to pH 7.4.
Reagents
Recombinant Gβ1/γ2 was prepared as described previously (Iniguez-Lluhi et al., 1992; Kozasa and Gilman, 1995), shock-frozen in liquid nitrogen and stored in small aliquots at −70°C. Immediately before use, the aliquots were thawed at 30°C, diluted in the appropriate amount of CHAPS-buffer and applied to the recording chamber. Aliquots were kept on ice and used up to 6 h after thawing. PKA-cs was obtained from Boehringer Mannheim (Cat. No.: 1529 307) and PP2A from Calbiochem (Cat. No.: 539508). Other reagents were reagent grade throughout.
RESULTS
Due to the high density of GIRK1/GIRK4 channels in mammalian atrial cells, membrane patches containing single IK.ACh channels are almost impossible to obtain. Accordingly, few data exist on single channel behavior in native tissue (Ivanova-Nikolova et al., 1998; Ivanova-Nikolova and Breitwieser, 1997; Nemec et al., 1999). Heterologous expression of cRNA-encoding GIRK channels allows the adjustment of channel density by regulation of the amount of cRNA injected (Yakubovich et al., 2000; Bard et al., 2000). Therefore, we used this approach to obtain single channel records.
Facilitation of GIRK1/GIRK4 channel activity by protein kinase A
To detect the effects of PKA-dependent phosphorylation on GIRK channels, patches were isolated from oocytes expressing GIRK1/GIRK4 channels and the m2-muscarinic receptor. The pipette solution contained 10−5 M acetylcholine and the bathing solution 50 μM–1 mM ATP and 100 μM GTP to establish and maintain channel activity. Upon patch excision, open probability (Po) reached a steady state level after 2–5 min. When, at this stage, the catalytic subunit of PKA (PKA-cs) was added to the bath solution facing the cytosolic side of the membrane patch, a significant increase in Po could be observed (Fig. 1). Similar results were obtained in oocytes expressing channels composed of the GIRK1/GIRK5 subunits (Dascal and Schreibmayer, unpublished observations).
FIGURE 1.
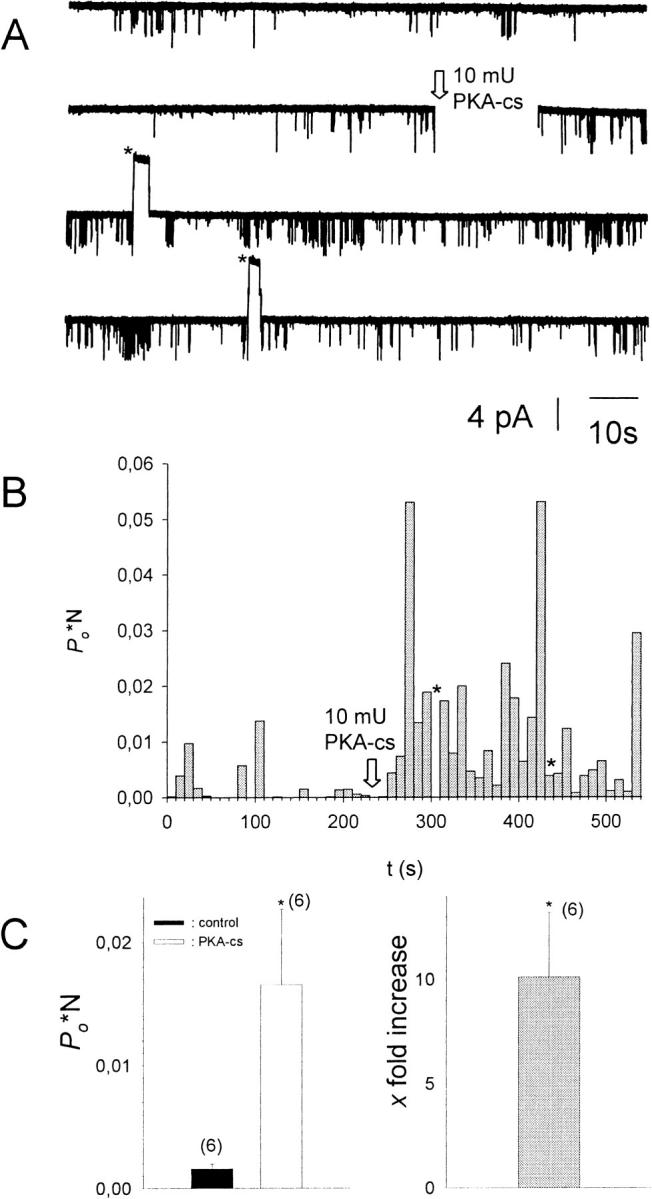
Addition of PKA-cs to the cytosolic solution results in an increase of Po*N. (A) Current recorded from a multichannel i.o. patch at −80 mV (N = 3). 10−5 M acetylcholine was present in the pipette solution, 1 mM ATP, and 0.1 mM GTP in the bath solution. Channel openings are represented by downward deflections of the current trace at −80 mV. The arrow indicates addition of 20 mU/ml PKA-cs to the (intracellular) bath solution (total bath volume was 500 μl). Asterisks signify time spans when the membrane potential was switched to +80 mV to test for inward rectification of channel openings. (B) Po*N, calculated for 10-s intervals, versus time, shown for the entire current trace visible in A. (C, left) Average Po*N before (control) and after addition of 20 mU/ml PKA-cs (PKA-cs), obtained from six different patches. (C, right) Average increase in Po*N upon addition of PKA-cs (n = 6). *, The change in Po*N compared to control conditions is statistically significant at the p < 0.05 level.
Activation of phosphorylated/dephosphorylated GIRK1/GIRK4 channels by constant concentrations of Gβ/γ
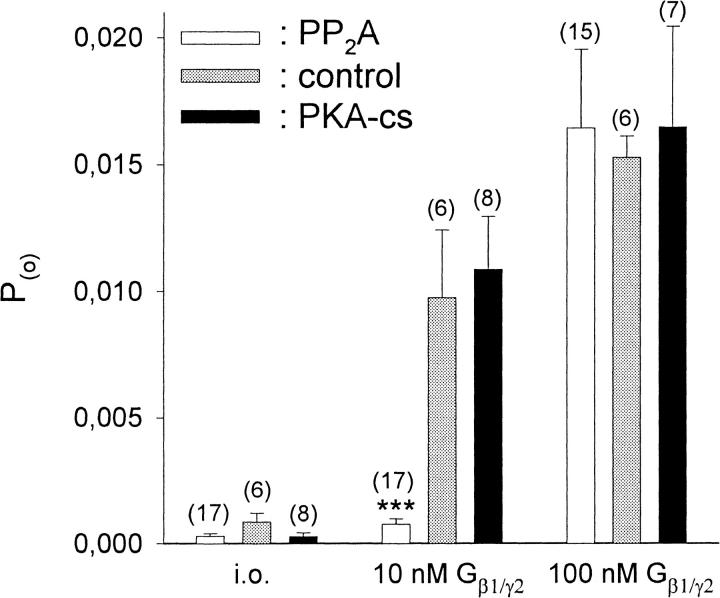
PKA-dependent facilitation as well as protein phosphatase 2A (PP2A) catalyzed inhibition of GIRK1/GIRK4 activation by G-proteins has been reported (Medina et al., 2000; Müllner et al., 2000). To study the effect of phosphorylation/dephosphorylation on the activation of GIRK channels by recombinant Gβ1/γ2, patches were excised into a bathing solution containing PKA-cs or PP2A or without enzymes. In five of the patches not treated with enzymes, 10 nM Gβ1/γ2 exerted profound effects on Po, whereas in one patch it was almost ineffective, resembling patches treated with PP2A. This is in accordance with a previous study, where in the absence of enzymes, 10 nM Gβ1/γ2 was shown to activate heterologously expressed as well as native atrial GIRK1/GIRK4 channels almost maximally (Schreibmayer et al., 1996). After patch excision into bathing solution containing PP2A, however, application of 10 nM Gβ1/γ2 resulted only in a modest activation of GIRK channel activity, whereas activation was almost full when PKA-cs was present instead of PP2A. At 100 nM Gβ1/γ2, activation was maximal in all cases (Figs. 2 and 3). This difference in activation by Gβ1/γ2 upon treatment with either PP2A or PKA-cs was statistically significant.
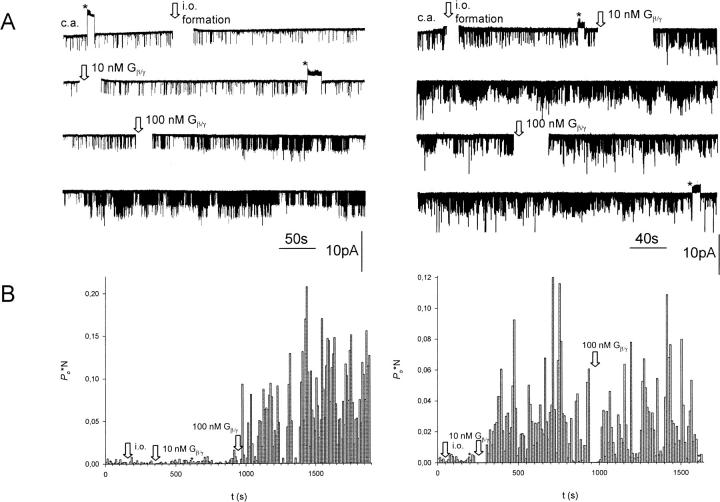
FIGURE 2.
Effect of dephosphorylation/phosphorylation by PP2A/PKA-cs treatment on the activation of GIRK1/GIRK4 channels by Gβ1/γ2. (A, left) Current recorded from a multichannel i.o. patch at −80 mV (N = 3). 1 mM ATP and 134 mU/ml PP2A were present in the bath solution. Channel openings are represented by downward deflections of the current trace at −80 mV. First, currents were recorded in the cell attached (c.a.) configuration. The arrows indicate formation of an isolated (i.o.) patch, addition of 10 nM Gβ1/γ2, and later 100 nM Gβ1/γ2 to the bathing solution. Asterisks signify time spans when the membrane potential was switched to +80 mV to test for inward rectification of channel openings. (A, right) Same experimental paradigm as for current trace shown in the left panel, but 20 mU/ml PKA-cs was present in the bath solution instead of PP2A (N = 2). (B) P(o)*N, calculated for 10-s intervals, versus time, shown for the entire current traces visible in A.
FIGURE 3.
Channel activity (Po) at different concentrations of Gβ1/γ2 for patches treated with PP2A, PKA-cs, or no enzyme added. Number of experiments (n) is given in parentheses above each bar. Number of channels in the patch (N ≥ 1). ***, The average Po for PP2A-treated patches deviates significantly at the p < 0.001 level from Po in patches treated with PKA-cs.
Single channel analysis
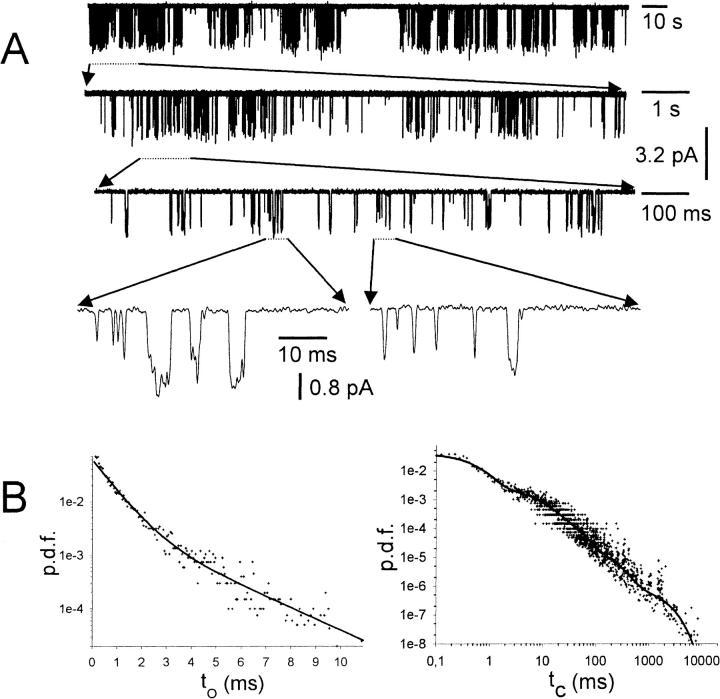
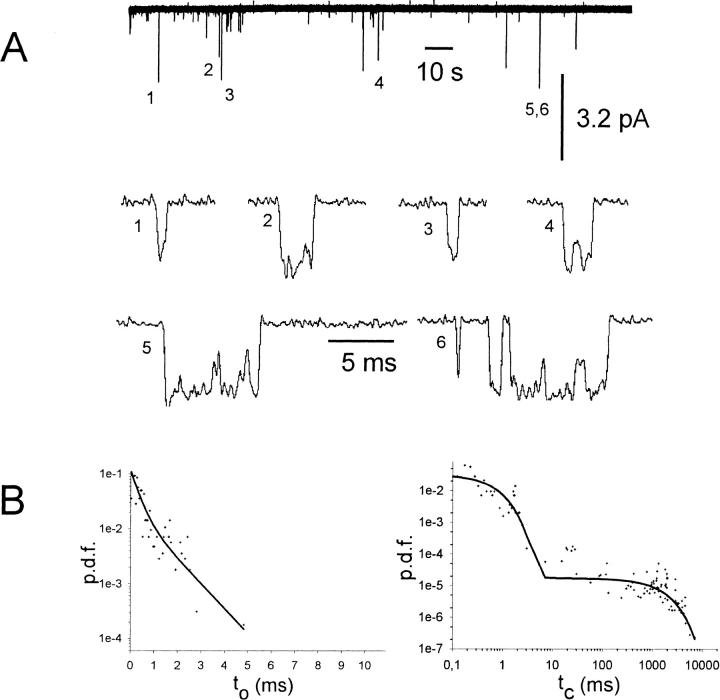
To distinguish between channel populations that are either completely phosphorylated or dephosphorylated, we restricted the single channel analysis to patches where one of the enzymes was present. In the case of PP2A treatment, seven patches, and in the case of PKA-cs treatment, four patches, contained one single GIRK1/GIRK4 channel (N = 1; see Methods for the assessment of the number of individual channels in a given patch). When activated by Gβ1/γ2, single GIRK1/GIRK4 channel proteins exerted complex gating behavior: active periods with frequent channel openings interspersed with silent periods, which surmounted up to several seconds. Even within active periods, multiple, kinetically distinct closed states were observed, as can be seen by direct inspection of a single channel trace at different time resolutions (Fig. 4; the GIRK1/GIRK4 channel had been activated by 100 nM Gβ1/γ2 in advance). Altogether, five closed states with different time constants sufficed to fit closed time distributions, as has been described previously (Yakubovich et al., 2000). Accordingly, open times also were fit by two exponential functions. When gating parameters were evaluated for single channel recordings either in the presence of PKA-cs or PP2A under basal conditions as well as under saturating concentrations of Gβ1/γ2, it was obvious that, besides almost identical Po values, other parameters such as state durations, their contributions to total closed or open times, and the frequency of openings were quite similar and statistically not different (see Tables 1 and 2). Under basal conditions, openings of single channels occurred sparsely, with silent intervals between them that lasted for many seconds (Fig. 5). When once the channel complex opened, the opening was either to a single burst (e.g., Fig. 5, openings 1–4), or included a few reopenings, interrupted by closed durations in the millisecond range (e.g., Fig. 5, openings 5 and 6). Hence, closed times could be clearly subdivided into two different groups. Also the conducting state was kinetically heterogeneous and exhibited two states with short and long lifetimes, respectively. When single channel gating parameters from traces recorded in the presence of PKA-cs were compared to those recorded from patches bathed in medium containing PP2A, also in the absence of added Gβ1/γ2, the differences were negligible. The exception here was a significantly different ratio between short- and long-lived open states, indicating that phosphorylation/dephosphorylation might result in slightly different gating behavior, without significant contributions to macroscopic ensemble currents. A striking difference was observed when single channel traces were recorded in the presence of 10 nM Gβ1/γ2: in the presence of PKA-cs the single channel parameters were practically indistinguishable from the recordings obtained at 100 nM Gβ1/γ2. However, with 10 nM Gβ1/γ2, Po was markedly reduced in the presence of PP2A when compared to recordings obtained in the presence of PKA-cs (Figs. 3 and 6). By far, the most profound effect was a significantly lower frequency of openings of GIRK1/GIRK4 channels in the presence of PP2A. Open state kinetics, on the other hand, were almost unaffected by treatment with the phosphatase.
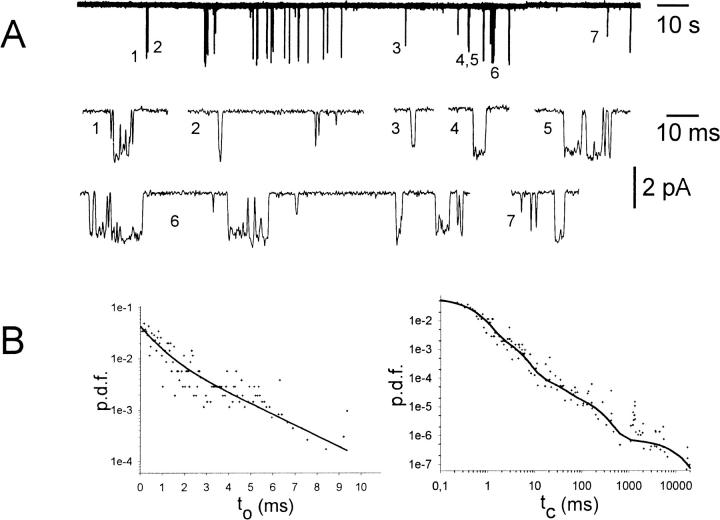
FIGURE 4.
Single channel trace recorded from an inside-out patch treated with PKA-cs and 100 nM Gβ1/γ2. (A, top) 200 s of history in the lifetime of a single GIRK1/GIRK4 channel, phosphorylated by PKA-cs and activated by Gβ1/γ2. (A, lower traces) Segments of the current trace shown on the top of A, expanded at increasing timescales, to demonstrate the multiplicity of channel closed time distributions over several orders of magnitude in durations. At the most expanded timescale, short and longer lived openings also can be seen. (B, left) Probability density function (p.d.f.) of open time distribution for the current trace shown in A. +, experimental data; (solid line), maximum likelihood regression obtained by fitting the data to the sum of two exponentials. (B, right) The p.d.f. of closed time distributions. Data were fitted by the sum of five exponentials.
TABLE 1.
Single channel open state parameters in inside-out patches
| Basal
|
10 nM Gβ1/γ2
|
100 nM Gβ1/γ2
|
||||
|---|---|---|---|---|---|---|
| PKA-cs | PP2A | PKA-cs | PP2A | PKA-cs | PP2A | |
| τo1 (ms) | 0.22 ± 0.06 | 0.34 ± 0.01 | 0.66 ± 0.15 | 0.36 ± 0.08 | 0.73 ± 0.10 | 0.41 ± 0.03 |
| τo2 (ms) | 2.03 ± 0.51 | 1.67 ± 0.29 | 1.89 ± 0.14 | 1.86 ± 0.25 | 1.92 ± 0.14 | 2.29 ± 0.58 |
| ao1 | 0.83** ± 0.12 | 0.20 ± 0.11 | 0.61 ± 0.14 | 0.67† ± 0.10 | 0.72 ± 0.10 | 0.69 ± 0.08 |
| ao2 | 0.17 ± 0.12 | 0.80 ± 0.11 | 0.39 ± 0.14 | 0.33 ± 0.10 | 0.28 ± 0.10 | 0.31 ± 0.08 |
| Po × 10−2 | 0.016 ± 0.006 | 0.022 ± 0.004 | 1.14*,†† ± 0.24 | 0.055†† ± 0.023 | 2.17 ± 0.54 | 1.61‡ ± 0.07 |
| fo (s−1) | 0.41 ± 0.16 | 0.22 ± 0.07 | 11.8*,† ± 3.34 | 0.48 ± 0.12 | 60.0 ± 41.6 | 13.4‡ ± 3.91 |
| Nevents | 327 | 316 | 7471 | 1140 | 24143 | 18416 |
| No. patches | 4 | 7 | 4 | 7 | 4 | 5 |
τo1 and τo2 represent the mean time constants from exponential functions fitted to the probability density functions of channel open times, ao1 and ao2 the magnitude of the contribution to an exponential function, Po the open probability, fo the frequency of openings, Nevents the total number of events, and No. patches the number of individual patches used for the calculation of the parameters. Mean values ± standard error of mean (SEM) are given. *, (**), the difference in the mean value is statistically significant at the p < 0.05 (0.01) level, in comparisons between PKA-cs- and PP2A-treated patches, at the same internal concentration of Gβ/γ. †, (††), the difference in the mean value is statistically significant at the p < 0.05 (0.01) level, in comparisons between recordings obtained at 0 nM and 10 nM Gβ/γ. ‡, the difference in the mean value is statistically significant at the p < 0.05 level, in comparisons between recordings obtained at 10 nM and 100 nM Gβ/γ.
TABLE 2.
Single channel closed state parameters in inside-out patches
| Basal
|
10 nM Gβ1/γ2
|
100 nM Gβ1/γ2
|
||||
|---|---|---|---|---|---|---|
| PKA-cs | PP2A | PKA-cs | PP2A | PKA-cs | PP2A | |
| τc1 (ms) | 0.85 ± 0.22 | 0.94 ± 0.28 | 0.44 ± 0.03 | 0.58 ± 0.14 | 0.51 ± 0.03 | 0.52 ± 0.02 |
| τc2 (ms) | n.d. | n.d. | 4.37 ± 1.29 | 5.14 ± 1.25 | 6.48 ± 0.62 | 4.24 ± 0.96 |
| τc3 (ms) | n.d. | n.d. | 22.1 ± 3.9 | 54.6 ± 13.9 | 31.7* ± 3.0 | 41.9 ± 2.9 |
| τc4 (ms) | n.d. | n.d. | 192 ± 27 | 979 ± 329 | 179 ± 19 | 333 ± 107 |
| τc5 (s) | 4.47 ± 1.32 | 11.0 ± 3.2 | 1.42** ± 0.37 | 11.2 ± 2.0 | 3.09 ± 1.27 | 2.85‡‡ ± 0.50 |
| ac1 | 0.17* ± 0.07 | 0.43 ± 0.07 | 0.33 ± 0.06 | 0.32 ± 0.07 | 0.40 ± 0.08 | 0.47 ± 0.09 |
| ac2 | n.d. | n.d. | 0.17 ± 0.04 | 0.17 ± 0.05 | 0.27 ± 0.03 | 0.17 ± 0.04 |
| ac3 | n.d. | n.d. | 0.27* ± 0.05 | 0.07 ± 0.03 | 0.23 ± 0.04 | 0.21 ± 0.06 |
| ac4 | n.d. | n.d. | 0.16 ± 0.06 | 0.14 ± 0.04 | 0.09 ± 0.01 | 0.12 ± 0.05 |
| ac5 | 0.83 ± 0.07 | 0.57 ± 0.07 | 0.07**;††† ± 0.02 | 0.30 ± 0.07 | 0.01 ± 0.00 | 0.03‡‡ ± 0.01 |
τc1–τc5 represent the mean time constants from exponential functions fitted to the probability density functions of channel closed times and ac1–ac5 the magnitude of the contribution to an individual exponential function, respectively. Mean values ± standard error of mean value (SEM) are given. *, (**), the difference in the mean value is statistically significant at the p < 0.05 (0.01) level, in comparisons between PKA-cs- and PP2A-treated patches, at the same internal concentration of Gβ/γ. †††, the difference in the mean value is statistically significant at the p < 0.001 level, in comparisons between recordings obtained at 0 nM and 10 nM Gβ/γ. ‡‡, the difference in the mean value is statistically significant at the p < 0.01 level, in comparisons between recordings obtained at 10 nM and 100 nM Gβ/γ. n.d., states could not be detected.
FIGURE 5.
Single channel trace recorded from an inside-out patch under basal conditions. (A, top) 200 s of history in the lifetime of a single GIRK1/GIRK4 channel excised into a bathing solution containing PKA-cs. No recombinant Gβ1/γ2 was present. (A, lower traces) Single openings of the current trace shown on top, shown at expanded timescale. Single (1,2,3,4) as well as multiple openings (5,6) could be detected within a burst, resulting in at least two different kinetically distinguishable closed states. (B) Probability density functions of open (left) and closed (right) time distributions. +, experimental data; solid line, maximum likelihood fit. Both probability density functions (p.d.f.) were fit by the sum of two exponentials.
FIGURE 6.
Single channel trace recorded from an inside-out patch treated with PP2A and 10 nM Gβ1/γ2. (A, top) 200 s of history in the lifetime of a single GIRK1/GIRK4 channel, dephosphorylated by PP2A and activated by 10 nM Gβ1/γ2. (A, lower traces) Single and multiple openings of the current trace shown on top and shown below at expanded timescale. (B, left) Probability density function (p.d.f.) of open time distribution for the current trace shown in A. +, experimental data; (solid line), least square regression obtained by fitting the data to the sum of two exponentials. (B, right) The p.d.f. of closed time distribution. Data were fitted by the sum of five exponentials.
Does phosphorylation/dephosphorylation affect modal gating?
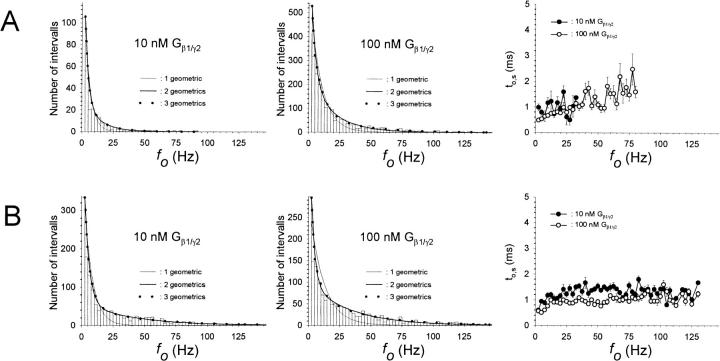
Several modes of activity have been described for active GIRK1/GIRK4 channels (Ivanova-Nikolova and Breitwieser, 1997; Ivanova-Nikolova et al., 1998, and Yakubovich et al., 2000). Hence, it was of interest to investigate whether modal gating was affected by phosphorylation/dephosphorylation of the channel. Distributions of opening frequencies (fo) were obtained from the pooled original data (Fig. 7; Table 3). Whereas one geometric function did not suffice to describe the frequency distributions, fitting of experimental data by the sum of two geometrical functions gave satisfactory results. Therefore, at least two different modes of gating of active channels were detected in the prevalent study. The characteristic frequency of the high frequency mode was significantly lower when the membrane patches were treated with PP2A, when compared to patches treated with PKA-cs. This fact indicates that changes in gating kinetics indeed occurred due to the covalent modification of the channel protein by phosphate residues. Another interesting observation was that the relative contribution of the high frequency gating mode was increased by higher concentrations of Gβ1/γ2 at the cytosolic side of the patch (this increase was significant for PKA-cs-treated patches). Significant differences in the calculated mean open time (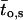 ) support the finding that modal gating was to some extent affected by both phosphorylation/dephosphorylation as well as by the G-protein concentration. By far the most prominent effect with respect to contribution to overall Po was, however, the large contribution of silent segments to gating observed at 10 nM Gβ/γ with PP2A treatment. This fact, again, indicates a reduced ability of moderate concentrations of the G-protein to activate the channel and is unrelated to different modes of the activated channel.
) support the finding that modal gating was to some extent affected by both phosphorylation/dephosphorylation as well as by the G-protein concentration. By far the most prominent effect with respect to contribution to overall Po was, however, the large contribution of silent segments to gating observed at 10 nM Gβ/γ with PP2A treatment. This fact, again, indicates a reduced ability of moderate concentrations of the G-protein to activate the channel and is unrelated to different modes of the activated channel.
FIGURE 7.
Modal gating of single phosphorylated/dephosphorylated GIRK1/GIRK4 channels. (A) Frequency distributions obtained from patches treated with PP2A at 10 nM Gβ1/γ2 (left) and at 100 nM Gβ1/γ2 (middle). Mean channel open time (to,s) as a function of fo is shown for both 10 nM and 100 nM Gβ1/γ2 (right). (B) Frequency distributions and distributions of to,s, but for patches treated with PKA-cs.
TABLE 3.
Modal gating of phosphorylated/dephosphorylated channels
| 10 nM Gβ1/γ2
|
100 nM Gβ1/γ2
|
|||
|---|---|---|---|---|
| PKA-cs | PP2A | PKA-cs | PP2A | |
| μ1 (Hz) | 4.15 ± 0.37 | 3.11 ± 0.84 | 3.38‡ ± 0.42 | 4.26 ± 0.63 |
| μ2 (Hz) | 39.1 ± 6.7 | 13.4* ± 7.5 | 33.8 ± 4.0 | 20.0* ± 2.9 |
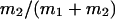 |
0.58 ± 0.03 | 0.46 ± 0.17 | 0.74‡ ± 0.02 | 0.67 ± 0.06 |
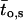 (ms) (ms) |
1.05 ± 0.02 | 0.95* ± 0.07 | 0.83 ± 0.01 | 0.79‡‡ ± 0.02 |
| %silent segments | 47.6 ± 5.3 | 94.6** ± 0.7 | 45.6 ± 3.1 | 56.1‡‡‡ ± 7.7 |
μ1 and μ2 designate the characteristic frequencies of slow and fast gating modes, 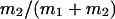 the contribution of the fast gating mode to the segments at fo = 0,
the contribution of the fast gating mode to the segments at fo = 0, 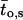 the mean open time in all segments and %silent segments the percentage of segments with no openings (see Methods section). Mean values ± standard error of mean value (SEM) are given for
the mean open time in all segments and %silent segments the percentage of segments with no openings (see Methods section). Mean values ± standard error of mean value (SEM) are given for 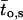 and %silent segments, whereas the borders of the 95% confidence intervals are given for μ1, μ2, and
and %silent segments, whereas the borders of the 95% confidence intervals are given for μ1, μ2, and 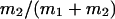 . *, (**): the difference in the mean value is statistically significant at the p < 0.05 (0.01) level, in comparisons between PKA-cs- and PP2A-treated patches, at the same internal concentration of Gβ/γ. †††, the difference in the mean value is statistically significant at the p < 0.001 level, in comparisons between recordings obtained at 0 nM and 10 nM Gβ/γ. ‡‡, (‡‡‡), the difference in the mean value is statistically significant at the p < 0.01 (0.001) level, in comparisons between recordings obtained at 10 nM and 100 nM Gβ1/γ2.
. *, (**): the difference in the mean value is statistically significant at the p < 0.05 (0.01) level, in comparisons between PKA-cs- and PP2A-treated patches, at the same internal concentration of Gβ/γ. †††, the difference in the mean value is statistically significant at the p < 0.001 level, in comparisons between recordings obtained at 0 nM and 10 nM Gβ/γ. ‡‡, (‡‡‡), the difference in the mean value is statistically significant at the p < 0.01 (0.001) level, in comparisons between recordings obtained at 10 nM and 100 nM Gβ1/γ2.
Role of GIRK1 C-terminus
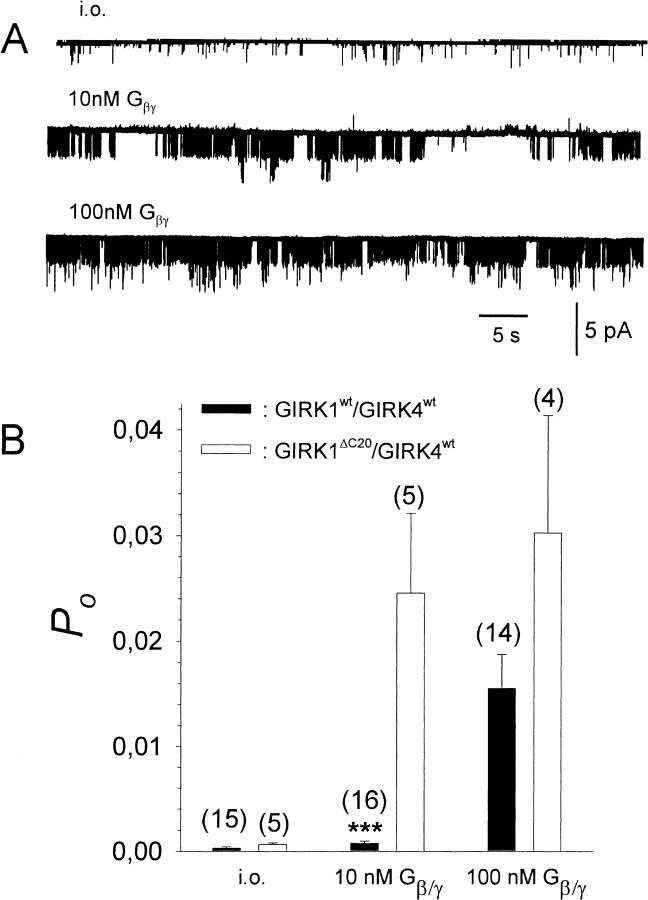
The carboxy-terminal tail of the GIRK1 subunit has been shown to be important for the gating of the GIRK1/GIRK4 channel (Luchian et al., 1997). Hence it was of interest to investigate whether this C-terminal part also plays a role in the regulation of channel activity by phosphorylation/dephosphorylation. To do so, a truncated form of the GIRK1 subunit, lacking the last 20 C-terminal amino acids (GIRK1ΔC20) was expressed together with GIRK4wt. Inside-out patches were isolated and exposed to an intracellular bathing solution containing PP2A. Gβ1/γ2 was added at concentrations of 10 and 100 nM. Interestingly, PP2A treatment of the patch was ineffective when channels had the GIRK1Δ20/GIRK4wt composition (Fig. 8).
FIGURE 8.
Effect of deletion of the last 20 C-terminal amino acids on the activation by Gβ1/γ2 in the presence of PP2A. (A) Current trace recorded at −80 mV from an i.o. patch containing multiple GIRK1ΔC20/GIRK4wt channels (N = 3) at increasing concentrations of Gβ1/γ2. (B) Comparisons of Po at different concentrations of recombinant Gβ1/γ2 for GIRK1wt/GIRK4wt and GIRK1ΔC20/GIRK4wt channels. ***, The average Po for patches containing GIRK1wt/GIRK4wt channels deviates significantly (p < 0.001) from patches containing channels of the GIRK1ΔC20/GIRK4wt composition.
DISCUSSION
The current study clearly corroborates the regulation of heterooligomeric inwardly rectifying G-protein activated potassium channels of the GIRK1/GIRK4 composition via phosphorylation by protein kinase A (PKA) and dephosphorylation by protein phosphatase 2A (PP2A). The findings support previous studies that report functional modulation by PKA (Kim, 1990; Müllner et al., 2000), as well as by PP2A (Medina et al., 2000). In addition, we present evidence for the molecular mechanism of facilitation of G-protein regulation by GIRK phosphorylation and its inhibition by dephosphorylation.
Several studies so far reported the requirement of intracellular ATP for normal function of GIRK channels (see Yamada et al., 1998, for review up to 1998; Sui et al., 1998; Kim and Bang, 1999). This requirement seems only partly to be due to direct phosphorylation of the GIRK1/GIRK4 protein. Accordingly, the functional effects observed are different: Kim (1991) found an increase in IK.ACh channels in rat atrial cells by the intracellular application of ATP. Whereas hydrolysable analogs of ATP were required to produce the functional effect, the increase in current was due to a mechanism different from the one observed in this study: application of ATP acted via a significant increase of channel open time but the frequency of openings was not affected. Application of the nonspecific alkaline phosphatase resulted in the reversal of the effect. Moreover, the effect of ATP was not blocked by specific inhibitors for PKA (see also Pleumsamran et al., 1998, and Kim and Pleumsamran, 2000). In the present study, however, where intracellular ATP was present throughout the experiment, we observed an increase in open probability of GIRK1/GIRK4 channels, activated by acetylcholine via m2 receptors, upon addition of PKA-cs to the cytosolic face of the membrane patch. Moreover, dwell time in the open state was unaffected by phosphorylation/dephosphorylation as well as by increasing the concentration of Gβ1/γ2 in the bathing solution, for both open states observed. The relative contribution of the short-lived open state under basal conditions (no Gβ1/γ2 added) was significantly larger for PKA-cs-treated patches than for PP2A-treated patches. This fact indicates that phosphorylation/dephosphorylation by the enzymes changed intrinsic parameters of channel gating, that are, however, of no importance for the marked and significant difference in Po observed at moderate concentrations of Gβ1/γ2. Similarly, at saturating concentrations of Gβ1/γ2, also the dwell time in the C3 state and, under basal conditions, the relative contribution of the C1 state, differed significantly between PP2A-treated and PKA-cs-treated membrane patches. Both for PP2A-treated and PKA-cs-treated patches the primary mode of action by which increasing amounts of Gβ1/γ2 activated GIRK1/GIRK4 channels was a marked increase in the frequency of openings fo, accompanied by a clear reduction of dwell time in the long-lasting C5 state (which accounts for the majority of the time a single GIRK1/GIRK4 channel resides in the nonconducting mode). These findings agree with previous observations on IK.ACh channels recorded from bullfrog atrial cells, where activation by increasing the concentration of the agonist resulted in an increase of Po within bursting phases, mainly by an increase in fo (Ivanova-Nikolova and Breitwieser, 1997; Ivanova-Nikolova et al., 1998).
At moderate concentrations of Gβ1/γ2 (10 nM), the predominant effect of dephosphorylation of the GIRK1/GIRK4 channel by PP2A when compared to phosphorylation by PKA-cs was a substantial reduction of fo, accompanied by a significant increase in the dwell time in the C5 state. Thus, PP2A action resembled results with low concentrations of the G-protein, i.e., dephosphorylation apparently reduced the affinity of Gβ1/γ2 for its target, rather than resulting in gross changes of gating kinetics of the channel. Our data thus confirm the view of GIRK1/GIRK4 as a G-protein effector, whose accessibility to activation by the G-protein is switched on or off as needed via phosphorylation/dephosphorylation. On the functional level, other studies (Ivanova-Nikolova and Breitwieser, 1997; Ivanova-Nikolova et al., 1998) support the idea that the GIRK1/GIRK4 channel protein possesses an intrinsic gate, which keeps the channel closed under resting conditions whereas activation by Gβ1/γ2 results merely in a relief from this intrinsic block. In fact it has been shown previously that: (i) treatment of atrial i.o. patches containing IK.ACh with trypsin from the cytosolic side resulted in IK.ACh activation (Kirsch and Brown, 1989), (ii) the entire cytosolic C-terminus of the GIRK1 subunit acts as a blocker of GIRK1/GIRK4 currents (Dascal et al., 1995; Kunkel and Peralta, 1995; Pessia et al., 1995), and (iii) the last 20 C-terminal amino acids of the GIRK1 subunit suffice to effectively block channel activity (Luchian et al., 1997). Medina et al. (2000) have shown that the GIRK1 subunit, but not the GIRK4 subunit, is a target of phosphorylation by PKA, PKC, CaMKI, and CamKII in vitro and in vivo. PP2A has been shown to be most effective in dephosphorylating the GIRK1 subunit. Site-directed mutagenesis in combination with phosphorylation in vivo and in vitro revealed that, again, the C-terminus is the target for serine/threonine protein kinases and that the serines/threonines responsible are located between amino acids 373–419 (numbering according to the human GIRK1 subunit; Dascal, 1997). When seven serines/threonines were mutated within this region, the GIRK1 subunit was not susceptible to phosphorylation. Moreover, a functionally active, truncated form of GIRK1, lacking amino acids 373–501, did not react to PP2A treatment. As shown in the previous studies, the domain of the GIRK1 structure responsible for this action lies within the last 20 C-terminal amino acids, the same domain that acts as an effective blocking particle for the entire channel complex. Because these amino acids are possibly not a target for phosphorylation (Medina et al., 2000), phosphorylation of the GIRK1 subunit may be one part of the switch, whereas the very C-terminus may be another indispensable physical part of its molecular machinery.
The major molecular mode of action of phosphorylation/dephosphorylation of GIRK1/GIRK4 channels has now been identified as a shift in the apparent affinity of the G-protein effector for the G-protein. It will be interesting in the future to localize the exact positions of the serines/threonines that act as the initial steps of this molecular switch.
Acknowledgments
We thank N. Dascal (Tel Aviv, Israel), N. McCarty (Atlanta, Georga, USA), and B. Steinecker (Graz, Austria) for critically reading the manuscript. Ms. A. Gorischeck (Graz, Austria) provided excellent technical support.
W.S. is supported by the Austrian Research Foundation (SFB007/F708; P13724-GEN) and the Austrian Ministry of Science (UGP4).
References
- Bard, J., M. T. Kunkel, and E. G. Peralta. 2000. Single channel studies of inward rectifier potassium channel regulation by muscarinic acetylcholine receptors. J. Gen. Physiol. 116:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal, N., and I. Lotan. 1992. Expression of exogenous ion channels and neurotransmitter receptors in RNA-injected Xenopus oocytes. In Methods in Molecular Neurobiology, Vol. 13. A. Longstaff and P. Revest, editors. Humana Press, Totowa, New Jersey. 205–225.
- Dascal, N. 1997. Signalling via the G protein-activated K+ channels. Cell. Signal. 9:551–573. [DOI] [PubMed] [Google Scholar]
- Dascal, N., C. A. Doupnik, T. Ivanina, S. Bausch, W. Wang, C. Lin, J. Garvey, C. Chavkin, H. A. Lester, and N. Davidson. 1995. Inhibition of function in Xenopus oocytes of the inwardly rectifying G-protein-activated atrial K channel (GIRK1) by overexpression of a membrane-attached form of the C-terminal tail. Proc. Natl. Acad. Sci. USA. 92:6758–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal, N., W. Schreibmayer, N. F. Lim, W. Wang, C. Chavkin, L. DiMagno, C. Labarca, B. L. Kieffer, C. Gaveriaux-Ruff, D. Trollinger, H. A. Lester, and N. Davidson. 1993. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc. Natl. Acad. Sci. USA. 90:10235–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev, D., E. Graf, E. Wettwer, H. M. Himmel, O. Hala, C. Doerfel, T. Christ, S. Schuler, and U. Ravens. 2001. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 104:2551–2557. [DOI] [PubMed] [Google Scholar]
- Doupnik, C. A., N. Davidson, H. A. Lester, and P. Kofuji. 1997. RGS proteins reconstitute the rapid gating kinetics of Gβ/γ activated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. USA. 94:10461–10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin, K. E., N. F. Lim, and D. E. Clapham. 1996. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IK.ACh currents in oocytes. Neuron. 16:423–429. [DOI] [PubMed] [Google Scholar]
- Hilgemann, D. W., S. Feng, and C. Nasuhoglu. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. RE19. [DOI] [PubMed]
- Iniguez-Lluhi, J. A., M. I. Simon, J. D. Robishaw, and A. G. Gilman. 1992. G protein β/γ subunits synthesized in Sf9 cells. Functional characterization and the significance of prenylation of γ. J. Biol. Chem. 267:23409–23417. [PubMed] [Google Scholar]
- Ivanova-Nikolova, T. T., E. N. Nikolov, C. Hansen, and J. D. Robishaw. 1998. Muscarinic K+ channel in the heart. Modal regulation by G protein β/γ subunits. J. Gen. Physiol. 112:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova-Nikolova, T. T., and G. E. Breitwieser. 1997. Effector contributions to Gβ/γ—mediated signaling as revealed by muscarinic potassium channel gating. J. Gen. Physiol. 109:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. 1990. β-adrenergic regulation of the muscarinic-gated K+ channel via cyclic AMP-dependent protein kinase in atrial cells. Circ. Res. 67:1292–1298. [DOI] [PubMed] [Google Scholar]
- Kim, D. 1991. Modulation of acetylcholine-activated K+ channel function in rat atrial cells by phosphorylation. J. Physiol. (Lond.). 437:133–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D., and H. Bang. 1999. Modulation of rat atrial G protein-coupled K+ channel function by phospholipids. J. Physiol. 517:59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D., and D. E. Clapham. 1989. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 244:1174–1176. [DOI] [PubMed] [Google Scholar]
- Kim, D., and A. Pleumsamran. 2000. Cytoplasmic unsaturated free fatty acids inhibit ATP-dependent gating of the G protein-gated K(+) channel. J. Gen. Physiol. 115:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, G. E., and A. M. Brown. 1989. Trypsin activation of atrial muscarinic K+ channels. Am. J. Physiol. 257:H334–H338. [DOI] [PubMed] [Google Scholar]
- Kovoor, P., K. Wickman, C. T. Maguire, W. Pu, J. Gehrmann, C. I. Berul, and D. Clapham. 2001. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J. Am. Coll. Cardiol. 37:2136–2143. [DOI] [PubMed] [Google Scholar]
- Kozasa, T., and A. G. Gilman. 1995. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of α12 and inhibition of adenylyl cyclase by αz. J. Biol. Chem. 270:1734–1741. [DOI] [PubMed] [Google Scholar]
- Krapivinsky, G., L. Krapivinsky, B. Velimirovic, K. Wickman, B. Navarro, and D. Clapham. 1995. The cardiac inward rectifier K+ channel subunit, CIR, does not comprise the ATP-sensitive K+ channel, IK.ATP. J. Biol. Chem. 270:28777–28779. [DOI] [PubMed] [Google Scholar]
- Kubo, Y., E. Reuveny, P. A. Slesinger, Y. N. Jan, and L. Y. Jan. 1993. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 364:802–806. [DOI] [PubMed] [Google Scholar]
- Kunkel, M. T., and E. G. Peralta. 1995. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 83:443–449. [DOI] [PubMed] [Google Scholar]
- Kurachi, Y., H. Ito, T. Sugimoto, T. Shimizu, I. Miki, and M. Ui. 1989. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 337:555–557. [DOI] [PubMed] [Google Scholar]
- Lim, N. F., N. Dascal, C. Labarca, N. Davidson, and H. A. Lester. 1995. A G protein-gated K channel is activated via β2-adrenergic receptors and Gβ/γ subunits in Xenopus oocytes. J. Gen. Physiol. 105:421–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohberger, B., K. Groschner, H. Tritthart, and W. Schreibmayer. 2000. IK.ACh activation by arachidonic acid occurs via a G-protein- independent pathway mediated by the GIRK1 subunit. Pflugers Arch. 441:251–256. [DOI] [PubMed] [Google Scholar]
- Löwi, O. 1921. Über die humorale Übertragbarkeit der Herznervenwirkung. Pflugers Arch. 189:239–242. [Google Scholar]
- Luchian, T., N. Dascal, C. Dessauer, D. Platzer, N. Davidson, H. A. Lester, and W. Schreibmayer. 1997. A C-terminal peptide of the GIRK1 subunit directly blocks the G protein-activated K+ channel (GIRK) expressed in Xenopus oocytes. J. Physiol. (Lond.). 505:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark, M. D., and S. Herlitze. 2000. G-protein mediated gating of inward-rectifier K+ channels. Eur. J. Biochem. 267:5830–5836. [DOI] [PubMed] [Google Scholar]
- Medina, I., G. Krapivinsky, S. Arnold, P. Kovoor, L. Krapivinsky, and D. E. Clapham. 2000. A switch mechanism for Gβ/γ activation of IK.ACh. J. Biol. Chem. 275:29709–29716. [DOI] [PubMed] [Google Scholar]
- Müllner, C., D. Vorobiov, A. K. Bera, Y. Uezono, D. Yakubovich, B. Frohnwieser-Steinecker, N. Dascal, and W. Schreibmayer. 2000. Heterologous facilitation of G protein-activated K(+) channels by β-adrenergic stimulation via cAMP-dependent protein kinase. J. Gen. Physiol. 115:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, T., T. Sugimoto, and Y. Kurachi. 1991. Platelet-activating factor activates cardiac GK via arachidonic acid metabolites. FEBS Lett. 289:239–243. [DOI] [PubMed] [Google Scholar]
- Nemec, J., K. Wickman, and D. E. Clapham. 1999. Gβ/γ binding increases the open time of IK.ACh: kinetic evidence for multiple Gβ/γ binding sites. Biophys. J. 76:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg, S., D. Varon, T. Ivanina, C. W. Dessauer, and N. Dascal. 2002. Gαi Controls the gating of the G protein-activated K(+) channel, GIRK. Neuron. 33:87–99. [DOI] [PubMed] [Google Scholar]
- Pessia, M., C. T. Bond, M. P. Kavanaugh, and J. P. Adelman. 1995. Contributions of the C-terminal domain to gating properties of inward rectifier potassium channels. Neuron. 14:1039–1045. [DOI] [PubMed] [Google Scholar]
- Pleumsamran, A., M. L. Wolak, and D. Kim. 1998. Inhibition of ATP-induced increase in muscarinic K+ current by trypsin, alkaline pH, and anions. Am. J. Physiol. 275:H751–H759. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco, J., C. F. Lo, and G. E. Breitwieser. 1993. Platelet-activating factor receptor-dependent activation of the muscarinic K+ current in bullfrog atrial myocytes. Circ. Res. 72:786–794. [DOI] [PubMed] [Google Scholar]
- Saitoh, O., Y. Kubo, Y. Miyatani, T. Asano, and H. Nakata. 1997. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 390:525–529. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., and D. W. Russel. 2001. Molecular Cloning - A Laboratory Manual. 3rd ed. CSH Press, Cold Spring Harbor, New York.
- Scherer, R. W., and G. E. Breitwieser. 1990. Arachidonic acid metabolites alter G protein-mediated signal transduction in heart. Effects on muscarinic K+ channels. J. Gen. Physiol. 96:735–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, R. W., C. F. Lo, and G. E. Breitwieser. 1993. Leukotriene C4 modulation of muscarinic K+ current activation in bullfrog atrial myocytes. J. Gen. Physiol. 102:125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer, W., C. W. Dessauer, D. Vorobiov, A. G. Gilman, H. A. Lester, N. Davidson, and N. Dascal. 1996. Inhibition of an inwardly rectifying K+ channel by G-protein alpha-subunits. Nature. 380:624–627. [DOI] [PubMed] [Google Scholar]
- Silverman, S. K., H. A. Lester, and D. A. Dougherty. 1996. Subunit stoichiometry of a heteromultimeric G protein-coupled inward-rectifier K+ channel. J. Biol. Chem. 271:30524–30528. [DOI] [PubMed] [Google Scholar]
- Sui, J. L., J. J. Petit, and D. E. Logothetis. 1998. Activation of the atrial KACh channel by the beta gamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. USA. 95:1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman, K., J. Nemec, S. J. Gendler, and D. E. Clapham. 1998. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 20:103–114. [DOI] [PubMed] [Google Scholar]
- Yakubovich, D., V. Pastushenko, A. Bitler, C. W. Dessauer, and N. Dascal. 2000. Slow modal gating of single G-protein-activated K+ channels expressed in Xenopus oocytes. J. Physiol. 524:737–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, M., A. Inanobe, and Y. Kurachi. 1998. G protein regulation of potassium ion channels. Pharmacol. Rev. 50:723–760. [PubMed] [Google Scholar]
- Yatani, A., K. Okabe, L. Birnbaumer, and A. M. Brown. 1990. Detergents, dimeric G beta gamma, and eicosanoid pathways to muscarinic atrial K+ channels. Am. J. Physiol. 258:H1507–H1514. [DOI] [PubMed] [Google Scholar]