We were very interested in the paper by Swain and Siggia (2002) on the possible role of multiple phosphorylation in amplification of signal transduction specificity. By specificity, they mean the ability of a protein kinase (e.g., Ste7, a member of the MAPKK class) preferentially activates, via phosphorylation, its proper substrate protein (i.e., Fus3, a member of the MAPK class, which is the preferred substrate) instead of other proteins due to improper cross talk (e.g., Kss1, another member of the MAPK class). By amplification, they mean in living cells the preferential activation exceeds the mere difference in equilibrium binding affinities between the proper and improper protein substrates. This is a nonequilibrium biological phenomenon which has been best understood in the kinetic proofreading mechanism for increasing the accuracy of cellular protein biosynthesis (Hopfield, 1974; Ninio, 1975). Swain and Siggia proposed a similar kinetic model based on the widely observed dual phosphorylation of MAPK (Canagarajah et al., 1997).
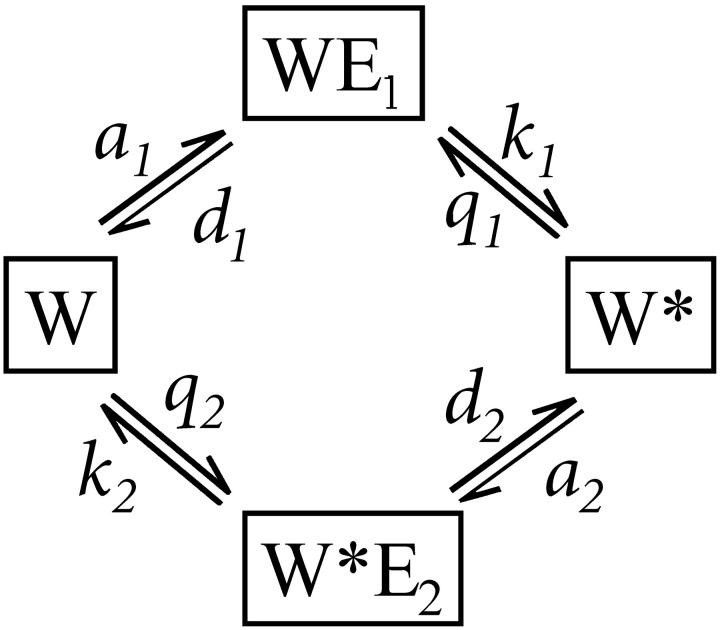
Phosphorylation-dephosphorylation cycle (PdPC, Fig. 1), with its zero-order ultrasensitivity, is well known to exhibit sensitivity amplification (Goldbeter and Koshland, 1981). Its activation has a switchlike behavior with high Hill coefficient, which is sensitive to stimulation and inhibition, expressed in terms of the respective kinase and phosphatase activities. Swain and Siggia have pointed out another important aspect of PdPC kinetics which so far has attracted less attention. They showed how PdPC can also discriminate against nonspecific cross talk in signal transduction. In quantitative terms, sensitivity is reflected in [W*], the phosphorylated protein substrate, as a function of σ, and specificity is defined as [W*] as a function of K (Fig. 1), the affinity between the kinase and its protein substrate.
FIGURE 1.
A kinetic schematics for PdPC. E1 and E2 represent kinase and phosphatase. The substrate, protein W, is phosphorylated by the kinase to become W*, which in turn is dephosphorylated by E2. A complete reaction cycle hydrolyzes one 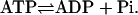 Hence,
Hence, 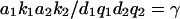 where
where 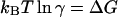 is the phosphorylation potential. Both enzymatic reactions are nearly irreversible:
is the phosphorylation potential. Both enzymatic reactions are nearly irreversible: 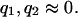 Let
Let 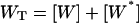 be the total substrate concentration neglecting the small amount of enzyme-substrate complexes, E1T and E2T the total enzyme concentrations for E1 and E2. Goldbeter and Koshland (1981) showed that the number of model parameters can be reduced, with nondimensionalization, to four key parameters:
be the total substrate concentration neglecting the small amount of enzyme-substrate complexes, E1T and E2T the total enzyme concentrations for E1 and E2. Goldbeter and Koshland (1981) showed that the number of model parameters can be reduced, with nondimensionalization, to four key parameters: 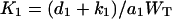 ,
, 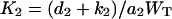 are the Michaelis-Menten constants, and
are the Michaelis-Menten constants, and 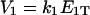 ,
, 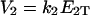 are maximal velocities, for E1 and E2 respectively.
are maximal velocities, for E1 and E2 respectively. 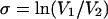 is a measure of the strength of stimuli/inhibition. K1 and K2 are directly related to the affinity of the enzymes to their respective substrates.
is a measure of the strength of stimuli/inhibition. K1 and K2 are directly related to the affinity of the enzymes to their respective substrates.
One of the key assumptions in Swain and Siggia's analysis is that the MAPK undergoes dual phosphorylation and its activation requires both of them. This is supported by laboratory experiments (Canagarajah et al., 1997; Anderson et al., 1990). However, not every protein with dual phosphorylation requires both for its activation. The best known example for the latter is glycogen phosphorylase (Fischer et al., 1971; Kreb, 1981). Part of the differences might be the tyrosine kinase family versus the serine kinase family.
The most unique feature of kinetic proofreading is its energy expenditure (Hopfield, 1974) which is present for any PdPC, either with single or dual phosphorylation (Goldbeter and Koshland, 1987). We have recently investigated the thermodynamic energetics of PdPC and shown how the quality of sensitivity amplification decreases with diminishing intracellular phosphorylation potential (Qian, 2002). Furthermore, we have also discovered that the high amplification in zero-order ultrasensitivity is mechanistically related to proofreading kinetics; both utilize multiple kinetic cycles in time to gain temporal cooperativity, in contrast to allosteric cooperativity that utilizes multiple subunits in a protein (Qian, 2002).
Inspired by the work of Swain and Siggia, we naturally ask whether a PdPC can have some specificity amplification without the multiple phosphorylation. To our surprise, we observe a significantly amplified specificity even in the system with single phosphorylation (Fig. 1). Following Goldbeter and Koshland (1981), we denote the fraction of phosphorylated protein by 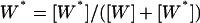 . It has been shown that (Goldbeter and Koshland, 1981, Qian, 2002) as a function of the stimuli σ expressed through activating a kinase and inhibiting a phosphatase, W* can rise from 0.1 to 0.9 within σ = 0.89 to 1.12 when both enzymes are highly saturated. This is the quantitative statement about amplified sensitivity.
. It has been shown that (Goldbeter and Koshland, 1981, Qian, 2002) as a function of the stimuli σ expressed through activating a kinase and inhibiting a phosphatase, W* can rise from 0.1 to 0.9 within σ = 0.89 to 1.12 when both enzymes are highly saturated. This is the quantitative statement about amplified sensitivity.
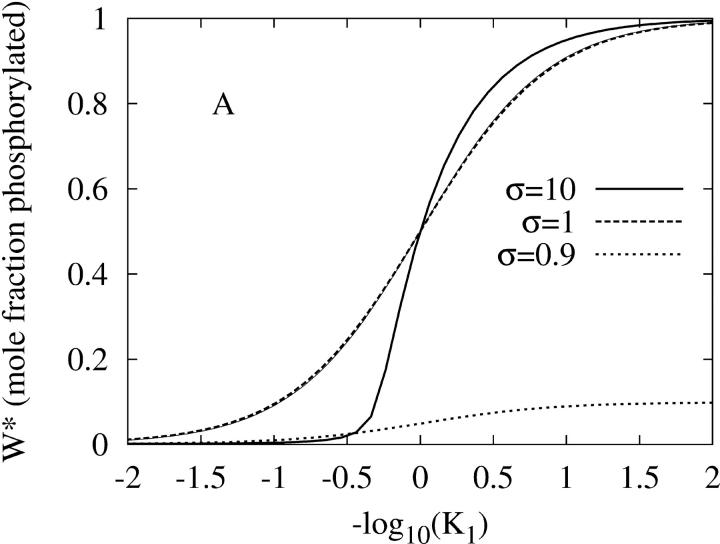
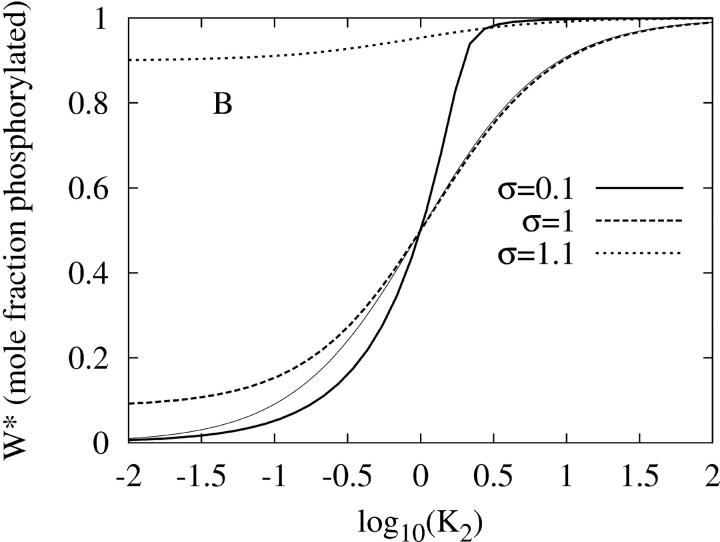
Fig. 2 shows that the PdPC given in Fig. 1 also exhibits amplified specificity. Fig. 2 A shows that the phosphorylation of W to W* by the kinase (E1) depends on 1/K1, the binding affinity between the kinase and its protein substrate. It is seen that, with sufficient stimulation σ = 10, there is an increase in discrimination against substrates with weaker affinity (larger K1). Similarly in Fig. 2 B, the dephosphorylation by the phosphatase (E2) also exhibits amplified specificity when there is sufficient inhibition σ = 0.1. As a control, Fig. 2 also shows that specificity amplification disappears when there are no significant signals for activation nor inhibition (σ = 1). On the other hand, if the kinase activity as stimulus is low (σ ≤ 0.9), then there is no significant level of phosphorylated W* no matter how large 1/K1 is. Similarly, if the phosphatase activity is low (σ ≥ 1.1), then there is always a high level of W* no matter how large 1/K2 is.
FIGURE 2.
The amplified specificity of PdPC with single phosphorylation when phosphorylation potential 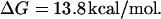 (A) The steady-state level of activation, W*, as function of K1, the dissociation constant between kinase E1 and its protein substrate W. K2 is kept at 0.01. The thick solid, dashed, and dotted lines are for σ = 10, 1, and 0.9 respectively. The first two curves have Hill coefficients of 2 and 1. Calculations for σ > 10 are indistinguishable from the solid line. For comparison, the thin solid line is for nonamplified specificity:
(A) The steady-state level of activation, W*, as function of K1, the dissociation constant between kinase E1 and its protein substrate W. K2 is kept at 0.01. The thick solid, dashed, and dotted lines are for σ = 10, 1, and 0.9 respectively. The first two curves have Hill coefficients of 2 and 1. Calculations for σ > 10 are indistinguishable from the solid line. For comparison, the thin solid line is for nonamplified specificity: 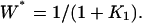 (B) Steady-state W* as function of K2, the dissociation constant between phosphatase E2 and its protein substrate W*. K1 is kept at 0.01. The thick solid, dashed, and dotted lines are for σ = 0.1, 1, and 1.1, respectively. Calculations for σ < 0.1 are indistinguishable from the solid line. Again, the thin solid line is for nonamplified specificity with Hill coefficient 1:
(B) Steady-state W* as function of K2, the dissociation constant between phosphatase E2 and its protein substrate W*. K1 is kept at 0.01. The thick solid, dashed, and dotted lines are for σ = 0.1, 1, and 1.1, respectively. Calculations for σ < 0.1 are indistinguishable from the solid line. Again, the thin solid line is for nonamplified specificity with Hill coefficient 1: 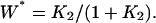
Wite energy expenditure from physiological ATP hydrolysis, the zero-order PdPC is capable of both sensitivity amplification, i.e., ultrasensitivity with respect to the stimuli in terms of the kinase activity (Goldbeter and Koshland, 1981), and specificity amplification which discriminates against nonspecific cross talk in signal transduction processes.
References
- Anderson, N. G., J. L. Maller, N. K. Tonks, and T. W. Sturgill. 1990. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 343:651–653. [DOI] [PubMed] [Google Scholar]
- Canagarajah, B. J., A. Khokhlatcher, M. H. Cobb, and E. J. Goldsmith. 1997. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 90:859–869. [DOI] [PubMed] [Google Scholar]
- Fischer, E. H., L. M. G. Heilmeyer, and R. H. Haschke. 1971. Phosphorylase and the control of glycogen degradation. Curr. Top. Cell. Reg. 4:211–251. [Google Scholar]
- Goldbeter, A., and D. E. Koshland. 1981. An amplified sensitivity arising from covalent modification in biological systems. Proc. Natl. Acad. Sci. USA. 78:6840–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter, A., and D. E. Koshland. 1987. Energy expenditure in the control of biochemical systems by covalent modification. J. Biol. Chem. 262:4460–4471. [PubMed] [Google Scholar]
- Hopfield, J. J. 1974. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl. Acad. Sci. USA. 71:4135–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreb, E. G. 1981. Phosphorylation and dephosphorylation of glycogen phosphorylase: a prototype for reversible covalent enzyme modification. Curr. Top. Cell. Reg. 18:401–419. [DOI] [PubMed] [Google Scholar]
- Ninio, J. 1975. Kinetic amplification of enzyme discrimination. Biochimie. 57:587–595. [DOI] [PubMed] [Google Scholar]
- Qian, H. (2002). Thermodynamic and kinetic analysis of sensitivity amplification in biological signal transduction. http://xxx.lanl.gov/abs/physics/0207049 [DOI] [PubMed]
- Swain, P. S., and E. D. Siggia. 2002. The role of proofreading in signal transduction specificity. Biophys. J. 82:2928–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]