Abstract
We have measured the initial propagation velocity of the sperm-induced Ca2+ wave in the egg of Xenopus laevis and have compared it with the initial propagation velocities of the inositol triphosphate (IP3)-induced and Ca2+-induced Ca2+ waves. The initial mean propagation velocity of the sperm-induced wave (13 μm/s) is very similar to that of the IP3-induced waves (12.3 μm/s) and two times faster than the mean Ca2+-induced wave velocity (6.6 μm/s). We have generated realistic simulations of the fertilization wave in the frog egg using a computational technique based on the finite difference method. Modeling refinements presented here include equations for the production, degradation, and diffusion of IP3, a description for Ca2+ dynamics in the endoplasmic reticulum, and a highly concentrated endoplasmic reticulum in the egg cortex. We conclude that models incorporating sperm-induced IP3 generation fit the data best and those involving the influx of either Ca2+ or a diffusible sperm factor fit the data poorly. This independence from Ca2+ influx is also supported by electrophysiological data indicating that Ca2+ influx is not needed to maintain open Cl− channels that generate the fertilization potential.
INTRODUCTION
Fertilization triggers one or more waves of increased cytosolic calcium concentration starting at the site of sperm-egg fusion in all organisms studied. These waves generate both the fast and slow blocks to polyspermy and play an important role in releasing the egg from cell cycle arrest (Nuccitelli, 1991; Stricker, 1999; Ciapa and Chiri, 2000; Runft et al., 2002). However, the mechanism by which these waves are themselves initiated upon fertilization is not well understood. Presently there are three popular hypotheses: 1), The “calcium bomb” hypothesis proposes that upon fertilization Ca2+ enters the egg either from stores in the sperm itself or through channels in the sperm's plasma membrane (Jaffe, 1983). This creates a bolus of elevated calcium concentration that triggers a wave of Ca2+ release from the endoplasmic reticulum (ER). This wave propagates further by the self-sustaining mechanism of calcium-induced calcium release (CICR). 2), The “sperm-factor” hypothesis proposes that a diffusible activator of calcium release enters the egg from the sperm after fusion with the egg (Swann, 1990). 3), The sperm receptor hypothesis proposes that the interaction of sperm and egg membranes activates a special class of receptors on the egg surface that results in the production of inositol trisphosphate (IP3). This second messenger opens IP3-dependent calcium channels (IP3 receptors or IP3Rs) in the egg's endoplasmic reticulum to release calcium near the fertilization site. In both mammalian and amphibian eggs, integrins have been proposed as putative sperm receptors (Almeida et al., 1995; Shilling et al., 1997; Shilling et al., 1998). In the frog egg, peptides targeted to integrins on the egg surface were able to activate the calcium wave (Iwao and Fujimura, 1996; Shilling et al., 1998).
Our goal is to determine which of the above mechanisms are most likely to be responsible for triggering the fertilization Ca2+ wave in the Xenopus egg. We assume that different initiation mechanisms will exhibit distinctive dynamics during the initial stages of the fertilization wave. Therefore we have made accurate quantitative measurements of this dynamic behavior and have compared them with predictions made by realistic computer models that incorporate different initiation mechanisms.
MATERIALS AND METHODS
Obtaining gametes
Xenopus laevis eggs were obtained by gentle massage of ovaries of females that had been induced to ovulate by injection with 300–500 IU human chorionic gonadotropin 8–10 h before massage.
Male frogs were anesthetized in tricaine methanesulfonate (1.5 g/l) and then were decapitated and pithed. The testes were removed and stored in OR2 (82.5 mM NaCl, 2.5 mM KCl, 1.0 mM CaCl2 1.0 mM MgCl2, 1.0 mM Na2HPO4, 5.0 mM Hepes; pH 7.8) supplemented with L15 culture media at 4°C for up to a week.
Microinjection of eggs
Mature, albino eggs were microinjected using an oil-filled line attached to a threaded syringe as previously described by Nuccitelli et al, (1993) with approximately 3 nl of 10 mM Ca-green-1-dextran (10,000 mol wt), in an intracellular injection buffer (109.0 mM KCl, 5.0 mM HEPES, 5.0 mM CaCl2, 10 mM EGTA; pH 7.3) to yield an approximate final concentration of 60 μM inside the egg. Microinjection was conducted in a chlorobutanol solution (10% chlorobutanol in calcium-free OR2 (82.5 mM NaCl, 2.0 mM KCl, 5.0 mM HEPES, 20 mM MgCl2, 0.1 mM EGTA; pH 7.4) to reduce the likelihood of activation of the eggs. Once injected, the eggs were held in the chlorobutanol solution for ∼1 min and were then placed in F1 solution (41.25 mM NaCl, 1.75 mM KCl, 0.5 mM NaHPO4, 1.9 mM NaOH, 2.5 mM HEPES, 0.25 mM CaCl2, 0.063 mM MgCl2; pH 7.8) and the injected dye was allowed to diffuse for 1–3 h. Some eggs were also microinjected with various amounts of 1G9 in conjunction with the Calcium Green Dextran. The antibody was allowed to diffuse throughout the egg for at least 2.5 h.
Electrophysiology and Ca2+ influx blockers
The calcium channel blockers used were gadolinium, nickel, and cobalt. The solutions containing these blockers were all made in standard F1. The solutions prepared were 100 μM solutions of GdCl3 and of GdCl3·6H2O, 1 mM solutions of NiCl2·6H2O, and CoCl2·6H2O. These were made from 10 mM stock solutions containing the appropriate channel blockers. All solutions were in the pH range of 7.30–7.8.
Glass micropipettes were made from 0.75 mm or 0.78 mm inner diameter borosilicate capillary tubing with an inner filament. Each micropipette was filled with 3M KCl and had a resistance between 4.7 and 28.2 MΩ when measured in standard F1, with the majority having resistances between 14.0 and 20.0 MΩ. A new electrode was used for each egg. Measurements were made using the GeneClamp 500 (Axon Instruments, Foster City, CA) and a chart recorder. The F1 solution was exchanged with a solution containing the appropriate calcium channel blocker immediately after fertilization had occurred through a vacuum- and gravity-driven flow system that was attached to the low volume chamber (Warner Instruments, RC-10 chamber). This low volume chamber was used to allow for quick exchange of the solutions. After the recovery period following the solution exchange, some of the eggs were removed from the chamber and placed in standard F1 where they were observed to develop through the swimming tadpole stage.
Dejellied eggs
Eggs were collected and dejellied using the 0.3% β-mercaptoethanol in 1/3 modified Ringer (MR) solution. A piece of testis was cut off and macerated in 100 μl of jelly water. It sat in the jelly water for at least 15 min before being used and was kept on ice. The jelly water-treated sperm were then centrifuged for 2 min at 2000 × g before being used to fertilize the egg to separate the sperm from the particulate matter of the testis.
Using EGTA
A stock solution of 250 mM, pH 7.83, was made in distilled water. This was diluted to 5 mM in Ca2+-free F1 at pH 7.82. Upon fertilization of the egg, the bath of normal F1 was exchanged for the Ca2+-free F1 containing the EGTA.
Fluorescence imaging
The eggs were placed on a Zeiss LSM 410 Invert Laser Scan confocal microscope for all of the experiments described here. The Zeiss LSM 410 Invert was equipped with an argon/krypton excitation laser (Zeiss, 110 V, 25 mW, 488/586 nm) and an ultraviolet laser (Coherent, 220 V, 50 mW, 351/364 nm). We used a 10× Fluar lens (Zeiss, 0.5 N.A.) for all experiments. Filters for calcium-green-1-dextran were a 510-nm dichroic for excitation and a 515–555 nm band pass for emission.
Egg activation by IP3 injection
Eggs microinjected with fluorescent dye were dejellied using a 2% β-mercaptoethanol solution (pH 8.9), agitated for ∼1 min. The eggs were then rinsed three times in MR pH 6.5 followed by three rinses in MR pH 7.8. Eggs were placed on the confocal stage and impaled with a micropipette containing 10 μM IP3. After determining that the impalement did not activate or otherwise damage the egg, the IP3 was injected while recording images.
MODEL AND SIMULATIONS
Model of calcium handling
The model of calcium handling that we use here is an extension of the model used in the earlier work on fertilization waves in Xenopus by our group (Wagner et al., 1998). It is based on the assumption that the dominant handling mechanisms for intracellular Ca2+ are IP3-sensitive channels and SERCA pumps in the ER. We chose parameter values to account for the experimentally observed bistability of calcium handling system and observed wave speed. The kinetic scheme of regulation of the IP3 receptor by calcium and IP3 is based on the De Young-Keizer model (De Young and Keizer, 1992) as simplified by Li and Rinzel (Li and Rinzel, 1994). In this scheme IP3 works as a permissive switch. Calcium opens the channel when it binds to the activating site (fast) and causes it to close again by binding to an inhibitory site (slow). Here we have extended the earlier model by including an equation that describes the dynamics of calcium concentration within the ER. We obtain the following set of equations for calcium concentration:
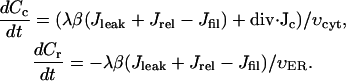 |
(1a) |
And the equation for dynamics of uninhibited channels:
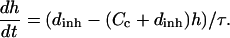 |
(1b) |
Here Cc and Cr denote cytoplasmic and ER calcium concentrations, respectively. Jrel is the rate of calcium release into the cytoplasm and Jfil is the rate at which calcium is being moved back into the ER by SERCA pumps. λ scales the density of channels and pumps in the ER membrane and β is the fraction of calcium that remains free (not bound to buffers). These rates are related to the unit of total intracellular volume. To account for the fact that only part of total egg volume is available to calcium in each compartment (the cytoplasm and the ER) we normalized the source terms in Eqs. 1a and 1b by the fractions of intracellular volume occupied by these compartments: υcyt and υER respectively. Here we assume that υcyt + υER = 1. Jc is the diffusion flux of Ca2+ in the cytoplasm. Finally, h is the fraction of channels that are not inhibited by calcium, h∞ is its equilibrium value at a given calcium concentration, and τ is time constant of inhibition. Discussion of the specific form of these terms is provided in Appendix 1. Here we only note that most of them depend on IP3 and Ca2+ concentration in a nonlinear fashion. The parameters of the calcium-handling model are given in Table 2.
TABLE 2.
Parameters of calcium handling model
| dI | Dissociation constant for IP3 binding site | 0.025 μM |
| dact | Dissociation constant for activating Ca2+ binding site | 1.2 μM |
| dinh | Dissociation constant for inhibiting Ca2+ binding site | 4.0 μM |
| vrel | Ca2+ release rate constant | 1.0 s−1 |
| vL | Leak rate constant | 0.0005 s−1 |
| Vmax | Maximum pump rate | 0.12 μM/s |
| kP | Michaelis constant for the pump | 0.4 μM |
| λ | Scaling parameter for density of pump and channels | 30.0 |
| β | Fraction of Ca2+ that is free | 0.02 |
| τ | Inhibition time constant | 100 s |
| D0 | Effective diffusion constant of Ca2+ | 40 μm2/s |
Model of IP3 metabolism
In this study we have integrated a realistic model of IP3 metabolism into our simulation framework for the first time. This model is based on a mechanism in which IP3 is generated by phospholipase C (PLC) and degraded by two parallel pathways: dephosphorylation to IP2 by 5-phosphotase and phosphorylation to IP4 by 3-kinase. This mechanism is based on a study of IP3 metabolism in Xenopus oocyte (Sims and Allbritton, 1998). This study shows that under the conditions of low [IP3] (∼100 nM) and [Ca2+] > 1 μM, phosphorylation is the major pathway of IP3 removal, with a half-life time of ∼60 s. On the other hand, when the concentration of inositol trisphosphate is high (>8 μM), the second messenger is primarily removed by dephosphorylation by 5-phosphatase. At low [IP3] and low [Ca2+] (<400 nM), both pathways remove approximately equal amounts of IP3.
The Xenopus egg can also exhibit calcium wave propagation in response to other stimuli besides sperm (e.g., triggered by the injection of a bolus of calcium). Because IP3 works as a permissive switch of calcium channels we assume that there is a background level of IP3 present in the egg before fertilization. We have measured the total average amount of IP3 in 20 unfertilized eggs and calculate that this would result in an average cytoplasmic concentration of around 0.3 μM (Snow et al., 1996). In our model we have used a basal IP3 level of 0.1 μM and assume this level results from the balance between production and degradation of the second messenger in the unfertilized egg. Our model also allows for additional local production of IP3 near the site of fertilization. Finally, IP3 is diffusing within the cytosol. The resulting equation for IP3 dynamics is the following:
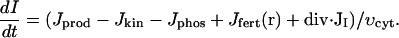 |
(2) |
Here I denotes IP3 concentration, Jprod is the stationary rate of IP3 production, Jkin and Jphos are rates of its degradation by kinase and phosphotase pathways respectively. Jfert(r) is the additional source of IP3 in the vicinity of the fertilization site. The latter term is spatially dependent and quickly declines with the distance, r, from the fertilization site. Derivation and specific form of different terms in Eq. 2 are given in Appendix 2. The parameters of the IP3 metabolism model are given in Table 3.
TABLE 3.
Parameters of IP3 metabolism model
| Vmphos | Maximum rate of 5-phosphotase | 0.02 μM/s |
| KMphos | Michaelis constant of 5-phosphotase | 30.0 μM |
| Vmkin1 | Maximum rate of Ca2+-free form of 3-kinase | 0.002 μM/s |
| KMkin1 | Michaelis constant of Ca2+-free form of 3-kinase | 2.5 μM |
| Vmkin2 | Maximum rate of Ca2+-bound form of 3-kinase | 0.007 μM/s |
| KMkin2 | Michaelis constant of Ca2+-bound form of 3-kinase | 0.4 μM |
| dkin | Dissociation constant of Ca2+ binding site on 3-kinase | 0.1 μM |
| DI | IP3 diffusion constant | 300 μm2/s |
| Iss | Basal concentration of IP3 | 0.12 μM |
| Jmax | Maximum IP3 production rate at fertilization site | 0.06 M/s |
Sperm-factor
Part of our modeling study investigates the hypothesis of a sperm-factor—a soluble, diffusible molecule that is capable of activating Ca2+ release. Little is known about the nature of this sperm-factor or about the specific mechanism by which it activates calcium release. Therefore, we assumed in our model that its mode of action is similar to that of IP3 and it binds to the receptor and works as a permissive switch for CICR. We also assume that binding to receptors does not affect its diffusion significantly. Therefore, the sperm-factor concentration is governed by a simple diffusion equation:
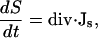 |
(3) |
where S is the concentration of the sperm-factor.
Structure of the egg
In our model we include some elements of the internal anatomy of the egg. At this stage we only consider distinct properties of the cortical region, but eventually other heterogeneities such as animal-vegetal gradients in yolk concentration will be included. Our assumptions concerning the properties of the cortical region are based upon a number of experimental observations: 1), Electron microscopy has indicated that the cortical ER becomes about twofold more concentrated in the cortex of the egg than deeper in the egg after maturation (Campanella et al., 1984; Gardiner and Grey, 1983). 2), Ca2+ imaging studies have indicated that the peak [Ca2+]i during the wave is nearly twice as high in the cortex as it is deep in the cytoplasm (Fontanilla and Nuccitelli, 1998). This concentration difference may result from a combination of factors, including the higher density of ER containing Ca2+ channels and pumps involved in calcium handling in the cortical region. Our preliminary simulations with the simplified “point system” show that although increasing channel density leads to a sharper rise in calcium concentration, this factor alone is not sufficient to account for the higher peak calcium concentration as observed in the experiments. Thus, in our model we have incorporated a spatial dependence of the volume fraction taken by the ER and density of IP3 receptors—assuming that they may be higher in the layer adjacent to the plasma membrane.
We use functions 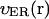 and
and 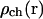 to describe this dependence. Both functions remain nearly constant within the deep parts of the egg but rise exponentially near the plasma membrane. Both are scaled by the same factor ζER:
to describe this dependence. Both functions remain nearly constant within the deep parts of the egg but rise exponentially near the plasma membrane. Both are scaled by the same factor ζER:
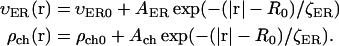 |
Here 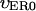 and
and 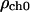 are the values deep within the egg, ζER = 10 μm, AER and Ach are the relative maximum elevations in the ER volume fraction and channel density, R0 is egg radius. We will also keep in mind that
are the values deep within the egg, ζER = 10 μm, AER and Ach are the relative maximum elevations in the ER volume fraction and channel density, R0 is egg radius. We will also keep in mind that 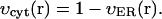
Another type of heterogeneity pertains to the diffusion of calcium. Ca2+ binds to a number of mobile and immobile buffers within the cell cytosol. It was shown that its diffusion under such conditions may be approximately described by a regular diffusion equation with an effective diffusion constant. This constant is ∼100 times smaller than that measured in water (Allbritton et al., 1992). As expected, the effective rate of calcium diffusion depends on types and concentrations of buffers (mobile or immobile). It is known that in the cortical region of the Xenopus egg there is a high density of cytoskeletal proteins and cortical granules, both of which serve as immobile buffers of cytosolic calcium. Therefore, we assume that these proteins may contribute to the lowering of the effective diffusion constant for calcium near the plasma membrane. In our model this spatial dependence is given by the expression:
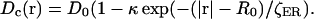 |
Here D0 is the effective diffusion constant for calcium deep within the egg and κ is the maximum value of the relative change in calcium diffusivity. For the sake of simplicity we assumed that this dependence is scaled by the same space factor, ζER, as the ER distribution. Structural parameters of the egg are given in Table 4.
TABLE 4.
Structural parameters of the egg
| υER0 | ER volume fraction at the center of the egg | 0.2 |
| AER | Maximum increase in the ER volume fraction in the cortical region | 0.26 |
| ρch0 | IP3Rs density at the center of the egg (arbitrary units) | 1.0 |
| Ach | Maximum elevation in the IP3Rs density in the cortical region (arbitrary units) | 1.3 |
| ζER | Width of the cortical region | 10 μm |
| κ | Relative change in Ca2+ effective diffusion constant in the cortical region | −0.5 |
| R0 | Egg radius | 500 μm |
Simulations
In the course of our work we used two sets of simulations. One was done with the so-called point system model in which diffusion terms were omitted. This model was further simplified in the following ways. First, due to the conservation of the total amount of calcium in the point system, we have eliminated the equation for the ER calcium concentration. Second, in these simulations we were only interested in the first few seconds of the evolution of the system. Because IP3 does not change significantly during this time period we treated it as a steady parameter. Such reduction allowed us to represent the system's behavior on a two-dimensional phase plane. The resulting two ordinary differential equations (ODE) were integrated using XPP software (Emertrout, http://www.math.pitt.edu/bard/xpp/xpp.html). We have chosen the Runge-Cutta method with a time step 0.05 s.
Three-dimensional calculations
For three-dimensional (3-D) calculations we have used our full model. Symmetry considerations were used to reduce the amount of computer time needed. There is not much known about the effect of animal-vegetal asymmetry on wave propagation. Thus we assumed that before fertilization the egg is spherically symmetrical. Fertilization destroys spherical symmetry but conserves rotational one with the axis coming through the site of fertilization and center of the egg. We have rewritten grad and div in cylindrical coordinates and eliminated angular dependence terms. Therefore, in terms of a required computational power our model is equivalent to a two-dimensional simulation in a semicircle although physically it represents the 3-D egg. We have used explicit forward Euler method for integrating this system. The equation solver was written in C++ using Overture framework (Brown, Henshaw: http://www.llnl.gov/CASC/Overture/). This software package also provides a generator for chimera grids and build-in capabilities for interpolating solutions between component grids. In our simulations we used a chimera grid that consists of a rectangular piece for the internal parts of the egg and a circular annulus for the periphery. Simulations were run under Linux on a 450-MHz Intel Pentium II processor. We used 0.07 s for a time step and 5 μm spatial resolution.
RESULTS
Experimental observation of fertilization waves
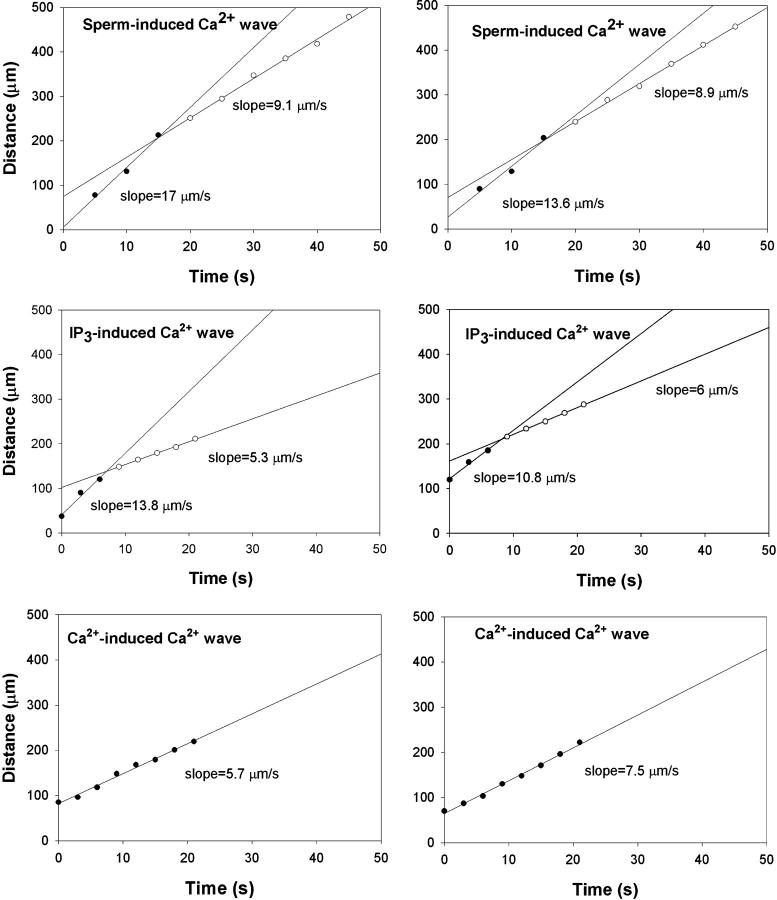
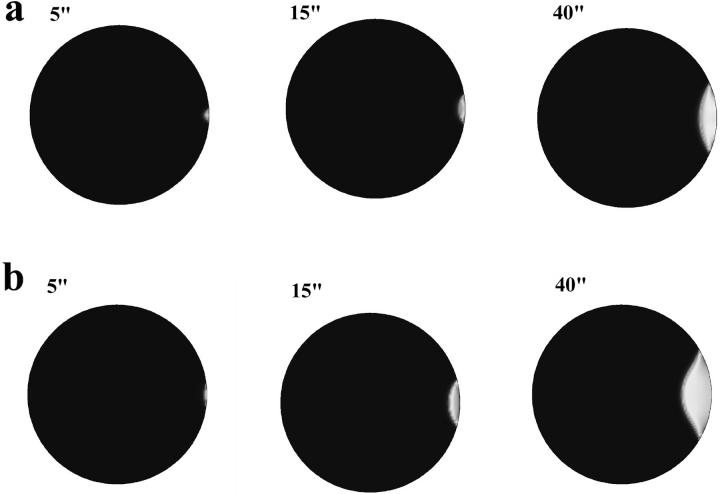
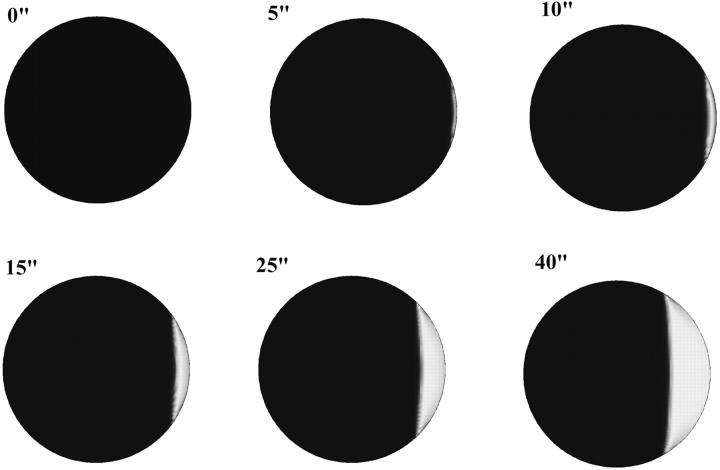
We have activated the Ca2+ wave in mature frog eggs by three different methods: fertilization, IP3 injection, and inducing a Ca2+ leak (Fig. 1). We carefully measured the initial propagation velocity of the waves triggered by each of these stimuli (Fig. 2). We found that both the sperm-induced and IP3-induced Ca2+ waves exhibited a rapid initial tangential cortical velocity (13 ± 1.2(5) and 12.3 ± 2.1(2) μm/s [mean±SE(N)], respectively) that then slowed after the first 10–15 s, whereas the Ca2+-triggered Ca2+ wave appeared to have a single, fairly slow tangential velocity (6.6 ± 0.7 μm/min [mean±SE, n = 5]). Quantification of these waves shows that after the initial fast spread, the speed of wave propagation along the cortex is only marginally higher than that of propagation through the center. We now believe that such a reduction in wave speed can be explained by a change in the mechanism from propagation driven by diffusion of IP3 to that driven by diffusion of calcium. We discuss this further in the last section of the paper.
FIGURE 1.

Time sequence of confocal images of Ca2+ wave initiation using a 40-μm thick optical section. Albino Xenopus eggs were injected with calcium green-dextran and activated by sperm (top), IP3 microinjection (middle) or Ca2+ leak (bottom). Arrows mark the leading edge of the Ca2+ wave. Both fertilization and the microinjection of IP3 produced a Ca2+ wave that traveled faster during the first 10–15 s than waves triggered by a Ca2+ leak.
FIGURE 2.
Tangential spread of [Ca2+]i wave over time. Two typical results are shown side by side for each wave type. The data for sperm-induced and IP3-induced waves are best fit by two straight lines of different slopes or velocities. Early time points (filled circles) have a steeper slope or higher velocity than later time points (open circles). In contrast, Ca2+-induced Ca2+ wave propagation is best fit with a single straight line of lower slope or velocity (bottom two examples).
To quantify propagation of fertilization Ca2+ waves we measured the position of the wave front at different times. We took these measurements in two directions: 1) along the cortex, tangent to the surface and 2) in the direction along the normal to the surface at the site of fertilization. Then we plotted the tangential measurements versus time in Fig. 2 because many of these displayed two propagation rates. Initially the rate of spread of calcium release tangentially along the cortex is much faster than the speed of the subsequent wave (13 vs. 6 μm/s). In contrast, the propagation in the normal direction is somewhat delayed and slower in the first 30–50 s (3.4 ± 0.5(7) μm/s) and never displayed two slopes. The combination of these two factors gives the fertilization wave in the Xenopus egg its distinct concave shape.
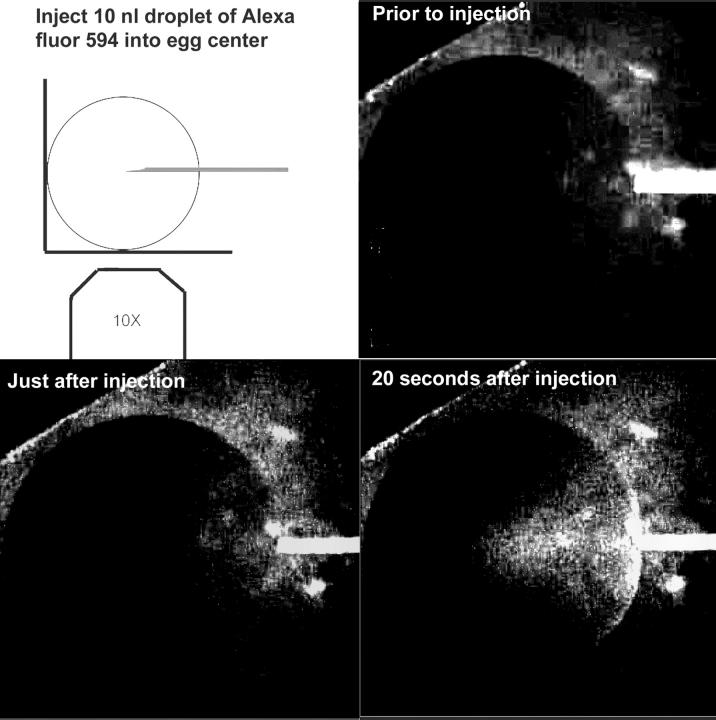
One concern about these measurements of the propagation in the normal direction is that the fluorescent signal will be attenuated as it passes through the thicker cytoplasm on route toward the center of the egg. The spherical shape of the egg visualized on an inverted microscope requires that the epifluorescent signal traverse a greater amount of cytoplasm as the wave moves from the periphery toward the egg center. To determine how deep into the cytoplasm we are able to detect fluorescence, we placed the tip of a micropipette at the center of an egg and injected a 15 nl droplet of Alexa fluor 594 hydrazide (50 mM). One would predict that this amount of dye would initially form a sphere with a 150-μm radius, but in fact the dye tends to move along the outside of the glass pipette, forming a cone shape that is visible in Fig. 3 D. We were unable to detect the pipette at the center of the egg before or just after injection (Fig. 3). However, dye became visible just after injection at ∼360 μm from the edge of the egg. This would correspond to a cytoplasmic path length of 550 μm through which the fluorescent signal passed. Within 22 ± 3 (SE, N = 5) s after injection the dye became visible at the center of the optical section of the egg. Given its molecular weight of 759, its diffusion constant in water would be ∼2 × 10−6 cm2/s and the dye would be expected to diffuse by no more than 150 μm in a 20-s interval. This is an upper limit because the egg cytoplasm is much more viscous than water. Given the 600 μm radius of the egg, this observation implies that signals as deep as 450 μm from the egg's surface can be detected in our system using a very bright fluor at 50 mM. Because the injected Ca2+ green-dextran used was 10 mM and diffused throughout the egg to a final concentration of 100 μM, we would not expect to be able to detect the signal from this dye as deep as that from 50 mM Alexa. However, we believe that the signal we observe is representative of Ca2+ changes at least 300 μm below the surface. Observing a spherical egg on an inverted microscope, fluorescence from a horizontal plane passing through the center of the egg will pass through 300 μm of cytoplasm when the wave has traveled 80 μm inward from the plasma membrane. Therefore, Ca2+ waves propagating into the cytoplasm from the plasma membrane should be reliably detected within 80 μm of the plasma membrane. This means that our conclusion regarding the initial velocity profiles tangential and normal to the plasma membrane is reliable. It also raises the question of what we are measuring when we see the wave travel across the egg. Because we are not able to detect light emitted from cytoplasm deeper than 300 μm, we must be detecting fluorescence emitted from an outer shell of cytoplasm ∼300 μm thick.
FIGURE 3.
Microinjection of a 10 nl droplet of 50 mM Alexa fluor 594 into the center of a Xenopus egg. The dye is not visible in the center of the egg but can be detected after diffusing for 20 s.
Preliminary modeling study using a point system model
We began our modeling study by investigating the behavior of a “point system.” Such a simplified model describes a well-stirred medium with no diffusion so equations are reduced to the set of ODEs. Although one obviously cannot observe any spatial effects such as a wave in such a system, it is a very useful approximation for a preliminary study. First, it allows us to illustrate how spontaneous Ca2+ release can be triggered. Second, it helps to estimate the values of parameters that are needed for it, therefore reducing the number of costly simulations using a full-fledged spatial model. Here it is also convenient to treat the IP3 concentration as a control parameter rather than as a dynamic variable. This way the model is reduced to two equations and its behavior can be easily analyzed on a phase plane.
Phase diagrams
A convenient way to represent the behavior of a two-component dynamic system is by way of a phase diagram because the state of such a system is fully defined by the values of its two dynamic variables (Fig. 4). In our case, these variables are the calcium concentration (abscissa) and the fraction of uninhibited IP3 receptors (ordinate). As values of these variables change over time in the course of Ca2+ release, consecutive states of the system form a curve on this plot called a “phase trajectory” (thick lines). These trajectories are essentially parametric representations of the system's dynamics.
FIGURE 4.
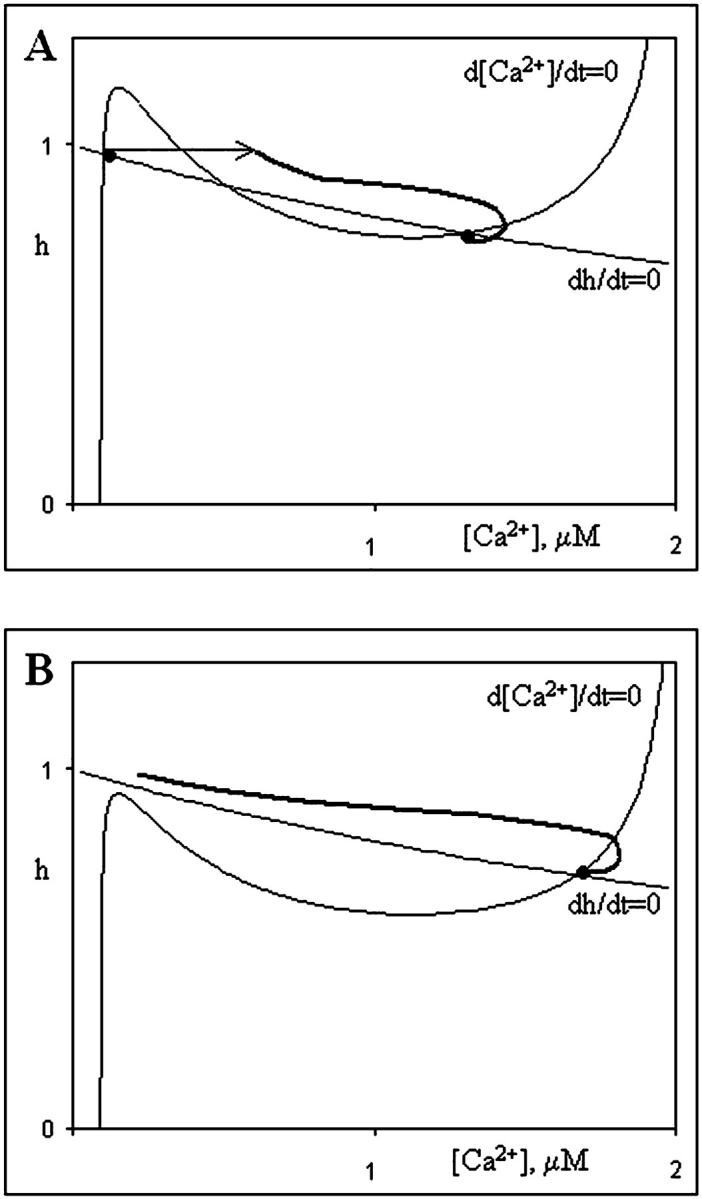
Nullclines of the simplified point system and two scenarios of wave initiation. a) Calcium release is initiated by a perturbation, such as Ca2+ injection. b) Shift in parameters (increase of IP3 level) leads to the loss of low-calcium steady state. Calcium concentration inevitably rises to attain the only remaining high-calcium steady state.
An additional feature of a phase diagram is the depiction of nullclines, representing the points on the phase diagram for which the rate of change for a given variable is equal to zero because the right side of the corresponding differential equation is zero at those points. The intersection of two nullclines corresponds to a steady state where neither variable changes. Such a steady state can be either stable or unstable with respect to a small perturbation so perturbation analysis is employed to determine stability.
Fig. 4 shows phase diagrams for possible scenarios of Ca2+ signal initiation that correspond to different hypotheses. In this simplified system, the concentration of IP3 is clamped at 100 nM and we draw nullclines and phase trajectories. The N-shaped curve corresponds to points where d[Ca2+]/dt = 0 and the monotonically decreasing curve corresponds to dh/dt = 0. In Fig. 4 A nullclines intersect at two points that correspond to steady state. Therefore the system is bistable. The first steady state corresponds to low- and the second to high-calcium concentrations. Both steady states are stable with respect to small perturbation. If a relatively large (superthreshold) perturbation is applied while the system is in the low-calcium state (Fig. 4 A, thin arrow), the calcium concentration continues to rise (thick solid line) until it reaches the high steady state value. Such a perturbation mimics an injection of external Ca2+ into the egg.
The other two initiation scenarios can be represented by the shift of parameters near the site of fertilization. Both the action of a putative activator and a rise in IP3 concentration leads to a higher fraction of channels available for calcium release from the ER. The bulk effect of these changes is an increase in Ca2+ release flux (Jrel). In our simplified point system model they are indistinguishable. Both scenarios result in a shift of the nullclines, as shown on Fig. 4 B. The low calcium steady state is lost when the N-shaped nullcline (that corresponds to cytosolic calcium dynamics) shifts downward. In its new position it has only one intersection with the other nullcline, and the system therefore has only one steady state (high calcium). Inevitably, the Ca2+ concentration rises to attain this steady state (thick solid line). Our next task is to incorporate the three proposed mechanisms into our full 3-D model of the Xenopus egg.
Fertilization waves in 3-D computer simulations
We have investigated the three proposed initiation mechanisms with our 3-D model of the Xenopus egg Ca2+ wave. Simulation results were then compared with the experimental measurements of wave dynamics to find which of them provided the best fit.
Ca2+ bomb hypothesis
When we introduce a bolus of Ca2+ in our model that is sufficient to elevate the local concentration to a 1μM level within a semispherical area with a 10 μm radius adjacent to the boundary (Fig. 5 a), the wave retains the convex shape of the initial perturbation for a relatively long time due to the relatively slow diffusion rate of Ca2+. It does not spread rapidly in the cortex as IP3 does. Only later does it develop a concave profile apparently due to heterogeneity of the egg. Comparing this model with experimental observations we find that it predicts the slower initial wave speed quite well but does not predict the overall spatial pattern, which looks quite similar to both sperm- and IP3-induced release (Fig. 1).
FIGURE 5.
Local injection of (a) calcium (1 μM) and (b) slow diffusing sperm-factor (D = 30 μm2/s) initiate calcium waves in computer simulations. They fail, however, to reproduce fast initial spread of calcium release along the egg cortex as seen in the experiments (compare to Fig. 1, top).
Calcium channel blockers
We conducted another experimental test of the Ca2+ bomb hypothesis. If Ca2+ influx through a sperm channel is occurring after sperm-egg fusion, the blockage of Ca2+ channels or removal of external Ca2+ should reduce this influx and the local increase in [Ca2+]i. Because the fertilization potential is generated by a Ca2+-gated Cl− channel, it too should be disrupted by reducing Ca2+ influx. We fertilized the eggs in normal F1 medium and as soon as we could be sure that the fertilization potential had begun its upward rise, we changed the medium in the chamber holding the eggs to one containing either a Ca2+ channel blocker or Ca2+ chelator. However, we observed no change in the fertilization potential of the Xenopus egg upon addition of these channel blockers, indicating no change in Cl− channel conductivity had occurred (Fig. 6; Table 1). This suggests that Ca2+ influx is not required for Cl− channel gating as early as we could completely change the medium (ranging from 36 to 72 s after fertilization).
FIGURE 6.
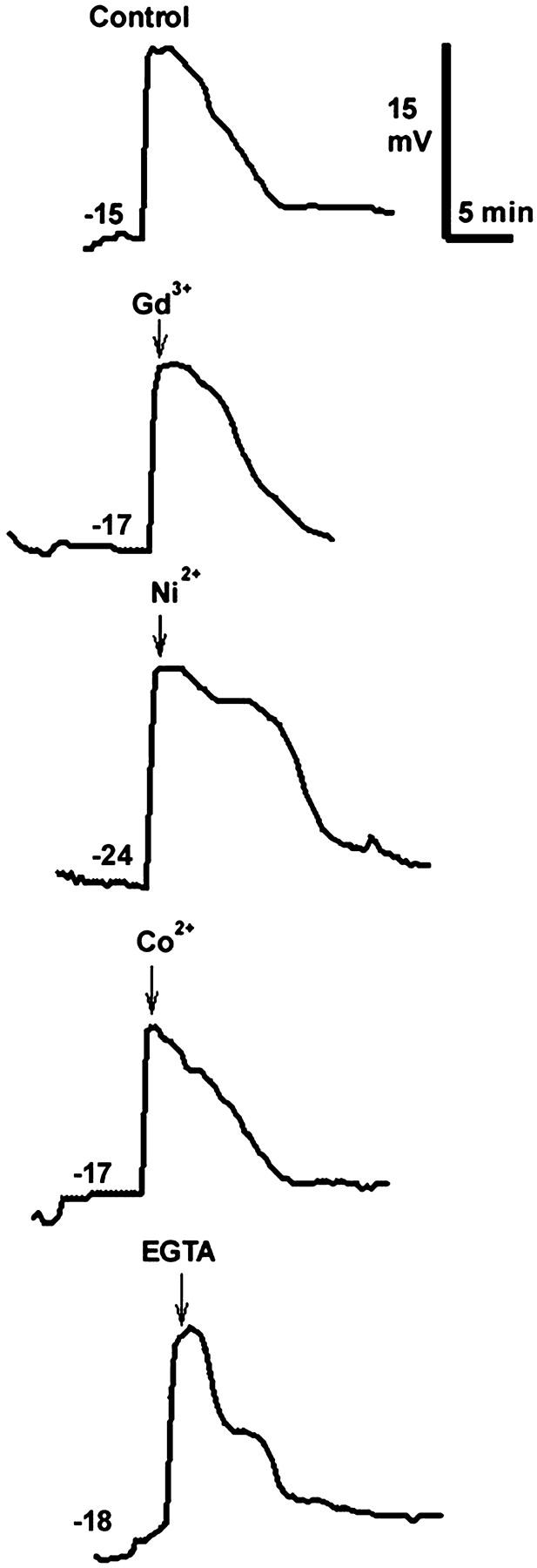
Typical fertilization potentials measured in Xenopus eggs under different conditions. The resting membrane potential (mV) before fertilization is indicated on each recording and the voltage and time scales are on the upper right. At the time indicated by each arrow, the medium containing the indicated blocker or chelator completely replaced the F1 medium in the chamber holding the egg (100 μM Gd3+, 1 mM Ni2+, and Co2+, 5 mM EGTA).
TABLE 1.
Timing of fertilization and medium changes
| Minutes between insemination and fertilization (mean ± SE (N))
|
Minutes between fertilization and medium change (mean ± SE (N))
|
|||
|---|---|---|---|---|
| Ion added | Jellied | Dejellied | Jellied | Dejellied |
| 100 μM Gd3+ | 8.2 ± 0.8(7) | 5.2 ± 0.4(3) | 1.0 ± 0.2(7) | 0.9 ± 0.2(3) |
| 1 mM Ni2+ | 5.7 ± 0.3(6) | 3.4 ± 0.8(3) | 0.8 ± 0.2(6) | 0.6 ± 0.07(3) |
| 1 mM Co2+ | 6.5 ± 1.4(6) | 6.3 ± 3.1(3) | 0.8 ± 0.2(6) | 1.2 ± 0 (2) |
| 5 mM EGTA | – | 4.2 ± 1.0(4) | – | 1.4 ± 0.2(4) |
We considered the possibility that the jelly layers might impede access of these blockers to the plasma membrane and repeated the experiments using dejellied eggs. The calcium channel blockers also had no effect on the fertilization potential of the fertilized, dejellied eggs (Table 1).
Chelating external calcium
In addition to using calcium channel blockers to test the role of external calcium on the fertilization potential of the Xenopus egg, the calcium chelator, EGTA, was used. To assure that EGTA could rapidly buffer external calcium, the jelly layer was removed from the egg's surface before impalement with the microelectrode. A procedure similar to the one above was used, with the exception of the use of 5 mM EGTA rather than calcium channel blockers during the solution change. As with the calcium channel blockers, EGTA did not change the fertilization potential of the fertilized egg (Table 1). The membrane potential trace for the EGTA trial does not deviate much from that of the control (Fig. 6).
Sperm-factor
Two popular hypotheses have been proposed for how a diffusible sperm-factor could activate eggs: 1), It could act directly as an IP3R activator; or 2), It could act indirectly to lead to the production of IP3, such as PLC would. When the introduction of a small spherical bolus of IP3R activator is modeled we see yet a different predicted Ca2+ wave shape (Fig. 5 b). Here we introduce a bolus of the same size and position as the bolus of calcium in the previous example and the initial concentration of activator within the bolus was 10,000 times higher than its assumed affinity for the IP3 receptor. The choice of the initial concentration can be justified as follows. Because the initial cortical spread of calcium signal extends to ∼150 μm, we assume that whatever causes it should still be effective at such a distance from the site of fertilization. On the other hand, the size of the initial bolus should be much less than the sperm size (∼15–20 μm long for Xenopus). If activator is to reach physiologically active levels within 150 μm bolus, then its initial concentration should be a few thousand times higher. Simulations show, however, that although sperm-factor was allowed to diffuse freely within the cytoplasm and its initial gradient was huge, it failed to spread fast enough to trigger fast cortical propagation observed in the experiment. We believe that the reason for this is a relatively low diffusion constant (we assumed it to be 40 μm2/s given a molecular weight of 30 kD).
The second popular hypothesis is that the sperm factor is indeed phospholipase C, which would generate IP3 by hydrolyzing PIP2. This mechanism could produce a wave shape similar to that resulting from IP3 production (see below) assuming that the rate of hydrolysis were as fast as the endogenous rate in the egg. However, our model does not produce the same rapid initial tangential wave because we would expect there to be a delay between sperm-egg binding and Ca2+ release due to the diffusion time for PLC to get into the egg (Fig. 5 b). Therefore we think it is unlikely that this mechanism is operating in the frog egg.
IP3 production
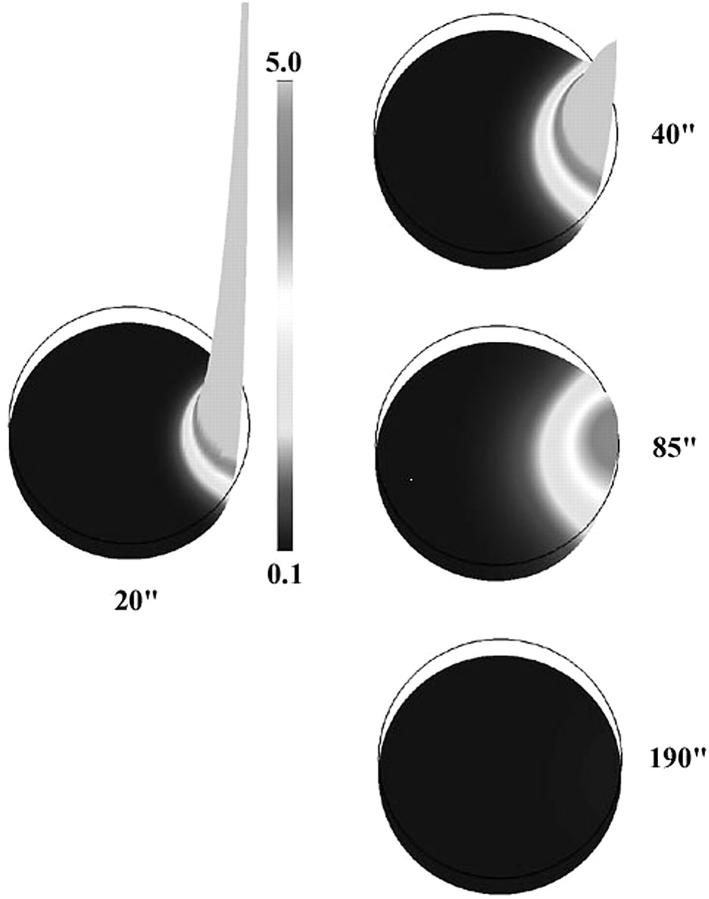
The only mechanism of the three most popular candidates that reproduces the rapid tangential cortical spread is the local production of IP3 at the fertilization site. This allows rapid production of a gradient in [IP3]. In our simulation IP3 was produced for only 3 s after fertilization. The rate of production was such that the resulting concentration at the fertilization site briefly reached 1 mM (8000 times its assumed affinity to the receptor). Due to high diffusivity (DIP3 = 300 μm2/s in our simulations) and steep initial gradient, this second messenger rapidly spreads over a larger area of the egg (Fig. 7). The combination of the elevated IP3 level and the high density of Ca2+ channels in the ER concentrated in the cortical region creates conditions for the very fast spread of calcium release in a large sector of this region (Fig. 8). The initial propagation of the calcium wave here is essentially driven by fast diffusion of IP3 rather than calcium. As we mentioned above, the latter is rather slow due to the presence of multiple calcium binding proteins in the egg cytoplasm. This release is triggered by the mechanism plotted in Fig. 4 B.
FIGURE 7.
Simulated IP3 profile for the initiation scenario shown on Fig. 8. During first few seconds there is a very high and narrow peak of IP3 concentration (in μM) near fertilization site. As it smoothes out due to diffusion and degradation larger area of elevated IP3 appears (40–90 s). Eventually, IP3 returns to its basal level (190 s).
FIGURE 8.
Computer simulations of the wave initiated by rapid production of IP3 near fertilization site. Initial rate of production is 0.06 M/s. Production decays exponentially over 3 s and is spatially limited to a 20 μm circular area on the plasma membrane. The initiation dynamics of this wave appears to be similar to that observed in the experiment. As on Fig. 1, zero time corresponds to the moment just before the first appearance of calcium release. This appears 10 s after start of IP3 production.
DISCUSSION
New findings
Careful analysis of the initial stages of [Ca2+]i wave propagation reveals a fast initial tangential wave propagation velocity in sperm-induced and IP3-induced but not Ca2+-induced waves. We have developed a refined computer model of the fertilization-induced Ca2+ wave in the frog egg that has a number of new features that significantly distinguish it from the model used in our previous work (Wagner et al., 1998). These features include a three-dimensional spatial domain, a dynamic equation for calcium concentration in the ER, a dynamic equation for [IP3] that includes degradation by two different mechanisms, and finally, a heterogeneous distribution of the ER, IP3 receptors, and calcium buffers. Using this improved model, we have generated computer simulations of these waves that are compared to quantitative experimental measurements of fertilization calcium waves. By carefully comparing the experimental data with these improved models, our simulations show that only the local production of IP3 near the site of fertilization can reproduce the experimentally observed calcium dynamics. Therefore we suggest that this is the most likely mechanism of wave initiation.
Initial phase wave propagates faster than subsequent trigger wave
In the proposed mechanism, the fast initial cortical spread that is often observed in the experiments can be attributed to a phase wave. Such a wave takes place due to the combination of two factors. First, calcium release is enhanced within a large area by rapid spread of highly diffusive IP3. Second, the cortical region has a high density of IP3 receptors and therefore, is more sensitive to the increase in IP3 level than internal parts of the egg. As a result, in a subcortical layer where these two factors overlap, the low calcium steady state disappears and calcium level inevitably rises until it reaches a high calcium steady state. The phase wave occurs because the activation of calcium release is not completely simultaneous. Due to a finite rate of IP3 diffusion it is slightly delayed in the areas further away from fertilization. Thus, calcium elevation first starts near the fertilization site whereas the phase of the adjacent points further along the cortex is slightly shifted. This creates a fast propagating wave that is observed in the experiments and simulations. Once this wave reaches the region where the IP3 concentration is not sufficient to trigger spontaneous release, the mode of propagation changes to a much slower trigger wave.
All of our experimental observations and modeling results support local IP3 production as a crucial step in the initiation of fertilization calcium waves in the Xenopus egg. It is possible however that this is not the only mechanism involved. Although it appears that the calcium bolus and sperm-factor hypotheses alone cannot explain wave dynamics, it is conceivable that they operate alongside an IP3 mechanism. One possibility, for instance, is that sperm-factor is indeed an active PLC that enhances local IP3 production. However, recent studies of sperm-egg fusion suggest that Ca2+ release occurs before dye can be seen diffusing from the egg into the sperm and are inconsistent with the diffusion of sperm factor into the egg (Nuccitelli, et al., in preparation).
No support for the Ca2+ bomb hypothesis
The Ca2+ bomb hypothesis proposes that the sperm introduces sufficient Ca2+, either from intracellular stores or through ion channels in the sperm's plasma membrane, to trigger Ca2+-induced Ca2+ release from the ER. None of our results here supports that hypothesis. Imaging eggs activated by Ca2+ influx reveals very slow tangential initiation velocities that do not resemble sperm-induced waves (Figs. 1 and 2). Modeling predicts similar slow wave velocities. In addition, our measurements of the fertilization potentials in eggs exposed to Ca2+ channel blockers or Ca2+ chelators within 50 s of sperm-egg fusion show no change in the Ca2+-gated Cl− channels. This suggests that these channels do not rely on Ca2+ influx for their activation. However, we cannot rule out the possibility that sufficient influx to trigger CICR occurred during the first 36 s before we could completely change the external medium to block Ca2+ influx.
Acknowledgments
We thank Chris Fall and John Wagner for many stimulating discussions about this work.
This work was supported by National Institutes of Health (RR10081 and HD19966).
APPENDIX 1: EQUATIONS FOR Ca2+ DYNAMICS
Our equations for calcium and channel dynamics are based on the De-Young-Keizer model for gating of the IP3R by Ca2+ and IP3 (De Young and Keizer, 1992) as simplified by Li and Rinzel (1994). According to this model calcium fluxes between the cytoplasm and the ER can be expressed in the following form:
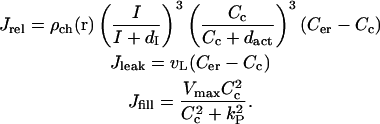 |
(A1) |
Here 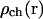 is the spatially dependent density of IP3Rs, dI and dact are dissociation constants for IP3 and activating Ca2+ binding sites on IP3 receptor, vL is calcium leak rate constant, Vmax and kP are maximum pump rate and Michaelis constant for the pump respectively.
is the spatially dependent density of IP3Rs, dI and dact are dissociation constants for IP3 and activating Ca2+ binding sites on IP3 receptor, vL is calcium leak rate constant, Vmax and kP are maximum pump rate and Michaelis constant for the pump respectively.
Eq. A1 is based on the assumption that the binding and dissociation of IP3 and Ca2+ are fast compared to changes in calcium and IP3 concentrations. Therefore, the fraction of receptors that has IP3 and activating Ca2+ bound rapidly adjusts to its equilibrium value for current concentrations of ligands. The third power in calcium release term corresponds to the assumption that it takes three subunits of the receptor to be activated for the channel to open.
Diffusion terms in our equations require special attention. This is due to the fact that we consider heterogeneity of the egg with respect to the distribution of the ER and calcium buffers. We have assumed that the effective diffusion constant for calcium is lower near the plasma membrane where more immobile buffers are present (Eq. 5). In addition to this, calcium diffusion within the cytosol is probably more obstructed where there is more volume taken by the ER. It is not clear what is the actual dependence of the diffusion constant on the volume fraction occupied by the ER, so we will simply assume that this dependence is linear. Therefore, we can write the expression for the diffusion flux of Ca2+ in the egg's cytosol:
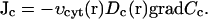 |
(A2) |
APPENDIX 2: THE MODEL OF IP3 DYNAMICS
In our model IP3 is generated throughout the egg at a constant rate and degraded by two pathways—phosphorylation to IP4 by 3-kinase and dephosphorylation by 5-phosphotase. In some simulations it can also be generated locally near the site of fertilization. First, we consider degradation pathways. According to Allbritton and co-workers, phosphorylation is a major pathway at physiological levels of IP3 (0.1–1 μM). The rate of phosphorylation also depends on calcium concentration. Here we assume that both the maximum rate and Michaelis constant of 3-kinase change upon binding Ca2+. Assuming also that calcium binding to the enzyme is fast compare to rate of changes in calcium concentration we arrive at the following expression for the rate of phosphorylation:
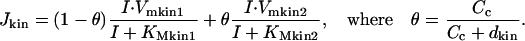 |
(A3) |
Here Vmkin1 and Vmkin2 are maximum rates of phosphorylation by calcium-free and calcium-bound forms of 3-kinase respectively, KMkin1 and KMkin2 are corresponding Michaelis constants. θ is the fraction of the kinase in calcium-bound form and dkin is the dissociation constant of calcium binding site.
Phosphorylation of IP3 by 5-phosphotase becomes essential at high (∼30 μM) concentrations of the second messenger and is calcium independent. In our model its rate is given by the expression:
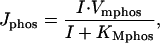 |
(A4) |
where Vmphos is the maximum rate of dephosphorylation and KMphos is Michaelis constant for 5-phosphotase.
The rate of constant IP3 production is set in such a way as to equilibrate degradation reactions if both [IP3] and [Ca2+] are at their basal levels. In some simulations we added the extra term for IP3 production to account for one of the initiation mechanisms. This term is a function of the following form:
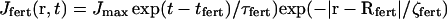 |
(A5) |
Here Jmax is maximum rate of IP3 production, tfert is the time of fertilization and Rfert is the radius vector of fertilization point. This production term quickly declines with time (τfert = 2 s) and with distance from the fertilization site (ζfert = 10 μm).
Diffusion of IP3 is treated in a similar way to that of calcium. Unlike calcium however, IP3 is not buffered in the cytosol so its effective diffusion constant depends only on the cytosolic volume fraction. The diffusion flux JI in Eq. 6 can be expressed as:
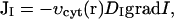 |
(A6) |
where DI is the diffusion constant of IP3 in cytosol.
Andrej Bugrim's present address is GeneGo, Inc., 101 W. Madison Ave., New Buffalo, MI 49117. E-mail: andrej@genego.com.
References
- Allbritton, N. L., T. Meyer, and L. Stryer. 1992. Range of messenger action of calcium ion and inositol 1, 4,5-trisphosphate. Science. 258:1812–1815. [DOI] [PubMed] [Google Scholar]
- Almeida, E. A. C., A.-P. J. Huovila, A. E. Sutherland, L. E. Stephens, P. G. Calarco, L. M. Shaw, A. M. Mercurio, A. Sonnenberg, P. Primakoff, D. G. Myles, and J. M. White. 1995. Mouse egg integrin α6β1 functions as a sperm receptor. Cell. 81:1095–1104. [DOI] [PubMed] [Google Scholar]
- Campanella, C., P. Andreucceti, C. Taddei, and R. Talevi. 1984. The modifications of cortical endoplasmic reticulum during in vitro maturation of Xenopus laevis oocytes and its involvement in cortical granule exocytosis. J. Exp. Zool. 229:283–293. [DOI] [PubMed] [Google Scholar]
- Ciapa, B., and S. Chiri. 2000. Egg activation: upstream of the fertilization calcium signal. Biol. Cell. 92:215–233. [DOI] [PubMed] [Google Scholar]
- De Young, G. W., and J. Keizer. 1992. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. USA. 89:9895–9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla, R. A., and R. Nuccitelli. 1998. Characterization of the sperm-induced calcium wave in Xenopus eggs using confocal microscopy. Biophys. J. 75:2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, D. M., and R. D. Grey. 1983. Membrane junctions in Xenopus eggs: their distribution suggests a role in calcium regulation. J. Cell Biol. 96:1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao, Y., and T. Fujimura. 1996. Activation of Xenopus eggs by RGD-containing peptides accompanied by intracellular Ca2+ release. Dev. Biol. 177:558–567. [DOI] [PubMed] [Google Scholar]
- Jaffe, L. F. 1983. Sources of calcium in egg activation: a review and hypothesis. Dev. Biol. 99:265–276. [DOI] [PubMed] [Google Scholar]
- Li, Y. X., and J. Rinzel. 1994. Equations for InsP3 receptor-mediated [Ca2+]i oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. J. Theor. Biol. 166:461–473. [DOI] [PubMed] [Google Scholar]
- Nuccitelli, R. 1991. How do sperm activate eggs? Curr. Top. Dev. Biol. 25:1–16. [DOI] [PubMed] [Google Scholar]
- Nuccitelli, R., D. L. Yim, and T. Smart. 1993. The sperm-induced Ca2+ wave following fertilization of the Xenopus egg requires the productin of Ins(1,4,5)P3. Dev. Biol. 158:200–212. [DOI] [PubMed] [Google Scholar]
- Runft, L. L., L. A. Jaffe, and L. M. Mehlmann. 2002. Egg activation at fertilization: where it all begins. Dev. Biol. 245:237–254. [DOI] [PubMed] [Google Scholar]
- Shilling, F. M., J. Krätzschmar, H. Cai, G. Weskamp, U. Gayko, J. Leibow, D. G. Myles, R. Nuccitelli, and C. P. Blobel. 1997. Identification of metalloprotease/disintegrins in Xenopus laevis testis with a potential role in fertilization. Dev. Biol. 186:155–164. [DOI] [PubMed] [Google Scholar]
- Shilling, F. M., C. R. Magie, and R. Nuccitelli. 1998. Voltage-dependent activation of frog eggs by a sperm surface disintegrin peptide. Dev. Biol. 202:113–124. [DOI] [PubMed] [Google Scholar]
- Sims, C. E., and N. L. Allbritton. 1998. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate by the oocytes of Xenopus laevis. J. Biol Chem. 273:4052–4058. [DOI] [PubMed] [Google Scholar]
- Snow, P., D. L. Yim, J. D. Leibow, S. Saini, and R. Nuccitelli. 1996. Fertilization stimulates an increase in inositol trisphosphate and inositol lipid levels in Xenopus eggs. Dev. Biol. 180:108–118. [DOI] [PubMed] [Google Scholar]
- Stricker, S. A. 1999. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 211:157–176. [DOI] [PubMed] [Google Scholar]
- Swann, K. 1990. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development. 110:1295–1302. [DOI] [PubMed] [Google Scholar]
- Wagner, J., Y.-X. Li, J. Pearson, and J. Keizer. 1998. Simulation of the fertilization Ca2+ wave in Xenopus laevis eggs. Biophys. J. 75:2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]