Abstract
The mechanism of pH-triggered destabilization of liposomes composed of a polyethyleneglycol-orthoester-distearoylglycerol lipid (POD) and phosphatidyl ethanolamine (PE) has been studied using an ANTS/DPX leakage and a lipid-mixing assay. We developed a kinetic model that relates POD hydrolysis to liposome collapse. This minimum-surface-shielding model describes the kinetics of the pH-triggered release of POD/PE liposomes. In the model, when acid-catalyzed hydrolysis lowers the mole percentage of POD on the liposome surface to a critical level, intervesicular lipid mixing is initiated, resulting in a burst of contents release. Two phases of content leakage are observed: a lag phase and a burst phase. During the lag phase, less than 20% of liposomal contents are released and the leakage begins to accelerate when approaching to the transition point. During the burst phase, the leakage rate is dependent on interbilayer contact. The burst phase occurs when the surface density of the PEG lipid is 2.3 ± 0.6 mol%, regardless of the pH. Vesicles containing 4 mol% of a pH-insensitive PEG-lipid conjugate and 10% POD did not leak contents or collapse at any pH. These data are consistent with the stalk theory to describe the lamellar-to-inverted hexagonal phase transition and set a lower bound of ∼16 PE lipids on the external monolayer as the contact site required for lipid mixing between two bilayers.
INTRODUCTION
Contact between lipids in apposed bilayers is the prerequisite for a lamellar-hexagonal phase transition, contact-induced bilayer leakage, and membrane fusion. Biophysical models of this process have been explicated by Siegel (1993; 1999), Siegel and Epand (1997), and others (for reviews see Epand, 2000; Burger, 2000). These models provide insight into membrane fusion in cells and a strategy for the design of lipid vesicles that can deliver their contents into the cytoplasm. One tactic to enable such contents delivery from vesicles is to devise a pH-sensitive trigger for membrane destabilization (Thomas and Tirrell, 1992; Chu and Szoka, 1994; Gerasimov et al., 1997; Liang and Hughes, 1998a,b; Song and Hollingsworth, 1999; Zhu et al., 2000; Drummond et al., 2000).
Recently, we described an acid-labile conjugate of polyethylene glycol and distearoyl glycerol via a diortho ester linkage (POD, Fig. 1, inset; Guo and Szoka, 2001). At neutral pH, POD was relatively stable and its presence provided a steric hindrance to prevent bilayer contact between DOPE-rich vesicles. The steric hindrance also interfered with the interaction of serum components with the bilayer. When the pH was decreased, the POD was rapidly hydrolyzed, leading to the aggregation and leakage of the PE-rich lipid vesicles. A minimum-surface-shielding model was proposed to account for the pH-dependent phase changes in POD/DOPE lipid vesicles (Fig. 1; Guo and Szoka, 2001). The lamellar structures of POD/DOPE vesicles are thought to remain intact until the proton-catalyzed POD hydrolysis lowered the number of PEG groups on the liposome surface to a critical level, at which the PE-rich bilayers are no longer sufficiently shielded from interbilayer contact. When the bilayers contact, vesicle aggregation, membrane mixing, and contents leakage are triggered (Bentz et al., 1983; Ellens et al., 1984) in lipid vesicles prepared from a variety of hexagonal phase-competent lipids. However, in DOPE liposomes stabilized by BVEP, an acid-labile diplasmenyl lipid conjugate of PEG5000 (Boomer and Thompson, 1999), contents release and phase transition are independent of intervesicular lipid mixing. Inasmuch as membrane mixing before fusion plays a critical role in the intracellular delivery of macromolecules encapsulated in lipid vesicles (Drummond et al., 2000), it is important to learn if the pH-triggered contents release requires interbilayer contact in the POD liposomes or if they behave like the BVEP-stabilized liposomes.
FIGURE 1.
Collapse of PE liposome as the POD lipid hydrolyzes to a minimum-surface-shielding concentration (Ac).
Our previous observation on the relationship between the lag time of liposome leakage and the incubation pH (Guo and Szoka, 2001) supported the minimum-surface-shielding model, but the experimental data were insufficient to determine either the rate of POD hydrolysis at the surface of POD/PE vesicles, or the minimum level of POD required to maintain the PE-rich bilayers. The rate of POD hydrolysis and the minimum percent of POD required to stabilize the PE bilayer are now determined. Moreover, we employ the POD lipid to investigate the lamellar-hexagonal phase transition. When the results of the experiments reported in this manuscript are interpreted in the context of the stalk hypothesis of membrane mixing (Siegel and Epand, 1997; Siegel, 1999), they provide an estimate that 16 PE lipids at the point of bilayer contact are required to initiate the lamellar-hexagonal transition. This estimate is consistent with the number predicted by Siegel and co-workers (Siegel and Epand, 1997; Siegel, 1999), providing experimental support to the stalk mechanism of lamellar-hexagonal transitions.
MATERIALS AND METHODS
General techniques
The pH-sensitive lipid-PEG conjugate (POD) was synthesized as previously described (Guo and Szoka, 2001). PEG-distearoyl glycerol conjugate (PEG-DSG; Shimada et al., 1995) was a generous gift from Dr. A. Suginaka (NOF Corp., Tokyo, Japan). Dioleoylphosphatidylethanolamine (DOPE) and 1-Palmitoyl-2-oleoylphosphatidylethanolamine (POPE), n-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (NBD-PE) and Dipalmitoyl-phosphatidylethanolamine-lissamine rhodamine B (Rh-PE) were purchased from Avanti Polar Lipids (Birmingham, AL). 8-Aminonaphthalene-1,2,3-trisulfonic acid (ANTS) and p-Xylenebis(pyridinium) bromide (DPX) were purchased from Molecular Probes (Junction City, OR). MilliQ water (Millipore, Bedford, MA) was used to prepare all the aqueous buffers. All other chemical reagents and solvents were purchased from Sigma (St. Louis, MO) or Fisher (Tustin, CA). Ratios of lipid components in liposomes are in mole units.
Liposome preparation
Liposomes were prepared by the freeze-thawing method based on the procedure of Monnard and co-workers (1997). A chloroform solution of POD, PEG-DSG, POPE, and/or DOPE in desired molar ratio (10 μmol total lipid) was added to a Pyrex brand glass tube. For lipid-mixing assays, the chloroform solution was mixed with NBD-PE and Rh-PE (1 mol% each in sample vesicles; 0.167 mol% each in control vesicles) in chloroform. Chloroform was evaporated under reduced pressure (27 mm Hg) at room temperature to form a lipid film at the bottom of the Pyrex tube. The lipid film was placed under high vacuum for 1 h to remove residual chloroform. The film was then hydrated with an appropriate aqueous buffer by 20 min of intermittent agitation with a vortex at 4°C. The tube containing the lipid suspension was then filled with argon and sealed. The lipid suspension was rapidly frozen by submergence into liquid nitrogen, followed by melting by incubation in water at room temperature for 15 min. The freeze-thawing cycle was repeated 10 times and the resultant liposomes were extruded five times through a 0.2-μm polycarbonate membrane (Nucleopore, Pleasanton, CA) with a hand-held extrusion device (Avestin, Ottawa, Ontario, Canada).
Liposomes for leakage assays were prepared using an alkaline buffer with the ANTS fluorophore (50 mM ANTS, 50 mM DPX, and 5 mM HEPES at pH 8.5), and the extruded vesicles were separated from the unencapsulated material using a Sephadex G-75 column with an elution buffer composed of 5 mM HEPES and 145 mM NaCl, pH 8.5. Liposomes for lipid-mixing assays were prepared in 5 mM HEPES and 145 mM NaCl, pH 8.5. Freeze-thawed liposomes for lipid-mixing assays were used after extrusion without further purification.
All freshly prepared liposomes had mean diameters ranging from 170 to 200 nm (cumulant results) and a polydispersity index of less than 0.2 as measured by a Malvern (Southborough, MA) Zeta1000 Dynamic Light Scattering Instrument using the PCS 1.32a software. The automatic algorithm was employed for data analysis. Lipid concentrations were determined based on lipid phosphorus by a modification of the Bartlett method (Bartlett, 1959).
Determination of encapsulated volume of liposomes
A small aliquot (20–150 nmol total lipids) of a liposome preparation for leakage assay was lysed in 3 mL of an alkaline buffer (5 mM HEPES and 145 mM NaCl, pH 8.5) supplemented with 200 μL C12E8 solution in water (1%, w/v). The fluorescence at 550 nm was then measured (excitation wavelength = 467 nm) with a Spex Fluorolog photon counting instrument (Model FL1/2, 150-W xenon light source, Jobin Yvon, Edison, NJ) to determine the ANTS concentration of the stock liposome preparation. The ANTS/DPX buffer (50 mM ANTS, 50 mM DPX, and 5 mM HEPES at pH 8.5) was used as the standard. With the anticipation that most of the ANTS in a liposome preparation is encapsulated within the vesicles and that the concentration of ANTS inside the vesicles (50 mM) does not change significantly during the encapsulation, the encapsulated volume of a liposome preparation can be determined using the equation
 |
(1) |
where V is the encapsulated volume in μL/(μmol lipids), CF is the concentration (mM) of ANTS in the liposome solution, and CL is concentration (mM) of total lipids.
Liposome leakage assay
The ANTS/DPX fluorescent assay (Ellens et al., 1984) was used to measure the contents release of the liposomes. One data point of fluorescent intensity was collected each second except for pH 7 and 7.4, where measurements were taken every 30 min and the samples were incubated in the dark between the measurements to minimize the exposure of the sample to the excitation light source. The leakage assays for Fig. 2 were carried out in 5 μM lipid concentration; the leakage assays for Fig. 4 were carried out at 25 μM lipid concentration; the leakage assays for Fig. 5 were carried out in a series of lipid concentrations (Ellens et al., 1984) as specified in the respective traces. There was no significant difference in the encapsulation volume among vesicles prepared with POD, PEG-DSG, or a mixture of the two.
FIGURE 2.

pH-dependent leakage. (A), Percentage of leakage over time at pH 5.0 and 5 μM lipid: a and b, POD/POPE/DOPE (10/50/40); c, PEG-DSG/POD/POPE/DOPE (4/10/50/36); d, PEG-DSG/POPE/DOPE (10/50/40); a, c, and d in acetate buffer; b in citrate buffer. (B), Percentage of leakage over time at 5 μM lipid in acetate buffer (pH 4.5, far left trace) and phosphate buffer (pH 4.5, 5.0, 5.5, 6.0, 7.0, and 7.4). (C), Logarithm of lag time (tl in seconds) of POD/POPE/DOPE (10/50/40) vesicles at various pH in acetate (⋄), citrate (♦), glucuronate (□), and phosphate (▪) buffers. Model: Log10(tl) = pH + Log10(ln(Ao/Ac)/k). Observed: Log10(tl) = (0.819 ± 0.067) × pH – (1.881 ± 0.395), r = 0.949, and P < 0.0001.
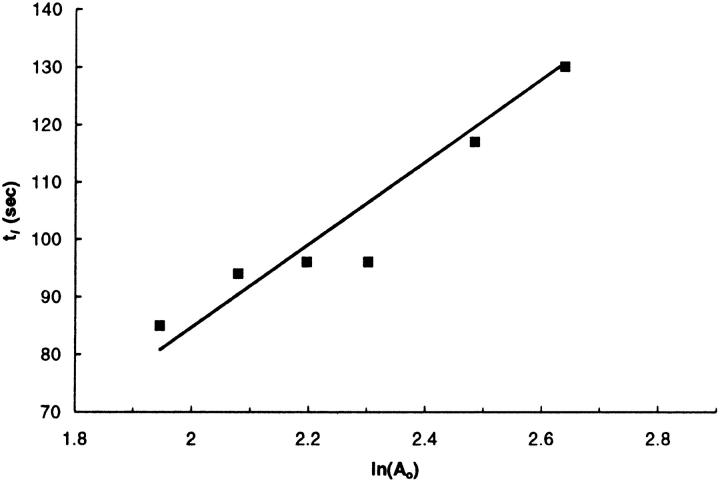
FIGURE 4.
Dependence of lag time (tl) of POD/POPE/DOPE (Ao/50/(50−Ao)) liposomes on the natural logarithm of initial mol% of POD at pH 5.0. Model: tl = ln(Ao) 10pH/k − ln(Ac) 10pH/k. Observed: k = 1390 ± 232 s−1 M−1, Ac = 2.3 ± 0.6%, r = 0.949, and P = 0.001.
FIGURE 5.

Leakage of POD/POPE/DOPE (10/50/40) vesicles at burst phase is dependent on interbilayer contact. (A), Percentage of leakage at different lipid concentrations (μM) over incubation time (seconds) at pH 5.0 in acetate buffer. (B), leakage percentage at burst phase over the product of lipid concentration and elapsed time post-transition point. The lipid concentrations are labeled adjacent to the corresponding fluorescent trace. (C), duration of lag phase (tl) of vesicles of different lipid concentrations in pH 5.0 acetate (□) and phosphate (▪) buffer.
Several buffers were utilized at different pH:acetate (50 mM sodium acetate, 100 mM NaCl), phosphate (50 mM sodium phosphate, 100 mM NaCl), glucuronate (50 mM glucuronic acid, 100 mM NaCl), and citrate (50 mM citric acid, 100 mM NaCl). Buffers were titrated by the addition of NaOH or HCl; pH and buffering agent are indicated on each figure.
Lipid-mixing assay
Membrane mixing between liposomes was monitored by a modified lipid-mixing assay based on the method of Struck et al. (1981), employing fluorescence resonance energy transfer. Labeled vesicles were prepared to contain NBD-PE and Rh-PE (1 mol% each) in 5 mM HEPES and 145 mM NaCl, pH 8.5. Labeled and unlabeled vesicles were premixed in a 1:5 molar ratio, and a small aliquot (∼20 μL) was injected with a Hamilton syringe into a magnetically stirred quartz cuvette containing 2 mL of an appropriate aqueous buffer (50 mM NaOAc/HOAc and 100 mM NaCl, pH < 6; 50 mM NaH2PO4/Na2HPO4 and 100 mM NaCl, pH 6 and above) at 37°C. The final concentration of the total lipids in the cuvette was 150 μM in all the lipid-mixing assays. The starting time of liposome incubation (to) at a given pH was set 1–5 s after the addition of the vesicles when the fluorescence signal first became stable. An increase of NBD-PE fluorescence indicates a decrease in the quenching of NBD-PE fluorescence by Rh-PE due to the dilution of the two membrane-bound probes during the lipid-mixing between labeled and unlabeled vesicles. Fluorescence measurements were made with a Spex Fluorolog photon counting instrument (Model FL1/2, Jobin Yvon). Excitation was at 467 nm (4.5-nm bandpass). The 90° emission signal at 550 nm (18-nm bandpass) was observed through a Corning 3-68 nm cutoff filter (>530 nm). One data point of fluorescent intensity was collected per s except for pH 7.0 or 7.4, where measurements were taken every 30 min and the samples were incubated in the dark between the measurements to minimize the exposure of the sample to the excitation light source. To compensate for the interference from liposome aggregation and precipitation, we prepared the control liposomes of the same composition as that of their corresponding sample liposomes except for 0.167 mol% of NBD-PE and Rh-PE. Such mole percentages of the probes are expected when the lipid mixing of the sample liposomes reaches its theoretical maximum. The fluorescence of each control over time at different pHs was measured in the same manner as the corresponding sample and used to normalize the fluorescence of the sample to percentage of infinite probe dilution.
The raw fluorescent data were converted into ASCII data files and mathematically processed by Microsoft Excel. To determine the lipid mixing as a percentage of infinite probe dilution, F% is defined as the percentage of the sample fluorescence over the fluorescence of its control. F% is calculated using the following formula,
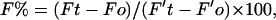 |
(2) |
where Ft and F′t are the sample fluorescence and the control fluorescence at a given incubation time, respectively; Fo is the fluorescence of the blank buffer before adding the sample; and Fo′ is the fluorescence of the blank buffer before adding the control liposome. The lipid mixing as a percentage of infinite probe dilution (M%) is then determined using the following formula,
 |
(3) |
where F%to is F% at the starting time of incubation (to).
Determination of the duration of lag phase
In the previous studies on POD/DOPE vesicles (Guo and Szoka, 2001), the duration of the lag phase (tl) for each leakage assay was determined by a visual estimation of the intersection point of two lines: a line tangent to the trace of the lag phase and a line tangent to the steepest slope of the burst phase. In this dataset, we used a mathematically stricter derivation method to reduce bias. Inasmuch as slow leakage during the lag phase and fast leakage during the burst phase are reflected, respectively, by a shallow slope and a steep slope of the fluorescent trace, the transition point between the two phases can be considered as the point when the change of the leakage rate, or the change of the slope of the fluorescent trace, reaches the maximum. Mathematically, this maximum point is equivalent to the maximum point of the secondary derivative of the fluorescent trace.
To obtain the first and second derivatives from raw fluorescence data, a window of width (tw) was centered on each time point (t). Assuming the data within the window is linear, the derivative was determined for this point by linear regression. At time points later than 0.5 × tw, the derivative was obtained for each time point to obtain the first derivative curve. To account for different leakage kinetics, the linear window time (tw) was defined as 20% of the time required for 50% leakage. This method scales tw between experiments, and computes a smooth derivative trace with sharp peaks for all data sets. The analogous method was used to obtain second derivative curves from the first derivative curves, using the same tw for obtaining the corresponding first derivative curves. The time point where the second derivative reached a maximum was taken as the transition point between the lag phase and the burst phase; the elapsed time between the transition point and to is taken as the lag time (tl).
Statistical analyses using the minimum-surface-shielding model
Estimates for the parameters of the minimum-surface-shielding model, i.e., POD hydrolysis rate constant (k) and critical percentage of POD (Ac), were obtained by nonlinear regression using Origin Windows version 6.1 (Northampton, MA). As shown in Fig. 4, the lag time versus ln(Ao) (following Eq. 8) gives a linear plot where the slope contains the kinetic rate constant, k, and the intercept contains an approximation of Ac. Buffered at pH 5.0, tl data from initial POD percentages of 7, 8, 9, 10, 12, and 14% were fit to lag following equation:
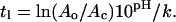 |
(4) |
The parameters obtained, Ac and k, were successfully used to predict the length of the lag phase as shown in Table 2.
TABLE 2.
Comparison of predicted and observed lag times (seconds)
| Initial Percentage of POD, Ao (mol%)*†‡
| ||||||
|---|---|---|---|---|---|---|
| pH | 7 | 8 | 9 | 10 | 12 | 14 |
| 4.5 | 26 (N.D.§) | 29 (N.D.§) | 31 (N.D.§) | 34 (63) | 38 (N.D.§) | 41 (74) |
| 4.7 | 41 (N.D.§) | 45 (N.D.§) | 50 (N.D.§) | 53 (63) | 60 (N.D.§) | 66 (84) |
| 5.0 | 81 (85) | 90 (94) | 99 (96) | 106 (97) | 120 (117) | 131 (138) |
| 5.5 | 256 (176) | 286 (211) | 313 (223) | 337 (220) | 378 (287) | 413 (332) |
| 6.0 | 808 (N.D.§) | 904 (N.D.§) | 989 (N.D.§) | 1065 (1603) | 1196 (N.D.§) | 1307 (1819) |
| 6.3 | 1613 (N.D.§) | 1804 (N.D.§) | 1974 (N.D.§) | 2125 (1645) | 2387 (N.D.§) | 2608 (2222) |
| 7.0 | 8083 (N.D.§) | 9044 (N.D.§) | 9891 (N.D.§) | 10650 (11700) | 11962 (N.D.§) | 13071 (16560) |
| 7.4 | 20303 (N.D.§) | 22717 (N.D.§) | 24846 (N.D.§) | 26750 (21600) | 30046 (N.D.§) | 32833 (37800) |
POD/POPE/DOPE (Ao/50/(50−Ao)) liposomes were prepared by freeze-thawing method. The observed lag times and the predicted lag times are listed in pairs. The observed values are in parentheses and at the right of the corresponding predicted values.
Experimental data are from the ANTS leakage assay followed by derivation of the fluorescent trace.
Predictions are by the minimum-surface-shielding model; k = 1390 ± 232 s−1 M−1, Ac = 2.3 ± 0.6%, R2 for entire table is 0.96.
Not determined experimentally.
RESULTS
Preparation of POD/PE liposomes
As an initial model of pH-sensitive liposomes composed of POD, we used the reverse-phase evaporation method (Guo and Szoka, 2001; Szoka and Papahadjopoulos, 1978) to prepare vesicles composed of 10 mol% of POD and 90 mol% of the fusogenic lipid DOPE. However, given the tendency of POD to undergo hydrolysis in aqueous media, it is suspected that the reverse-phase evaporation method involving a sonication step is not optimal for preserving the composition of the liposomes during the preparation. The freeze-thawing method (Monnard et al., 1997; Pick, 1981) is an attractive alternative due to the relative mildness of the procedure as well as the large encapsulation volume of the resultant vesicles.
Another aspect for the improvement of the POD-stabilized fusogenic vesicles would be the lipid composition. Although DOPE has been chosen in numerous pH-sensitive liposomes to trigger lamellar-to-hexagonal phase change, both of its side chains are unsaturated with a kink from the cis-double bond, and hence may have difficulty in mixing with the saturated stearoyl side chains of POD in bilayer structures (Gennis, 1989). Indeed, when we attempted to prepare the POD/DOPE liposomes by freeze-thawing, the hydration of the lipid film required lengthy agitation and was often incomplete. The encapsulated volumes of POD/DOPE vesicles (0.2–0.35 μL/μmol) are also lower than those of the reported reverse-phase evaporation vesicles (REV (Ellens et al., 1984; Szoka and Papahadjopoulos, 1978). To circumvent such difficulties, another PE lipid, POPE, was introduced into the formulation. Inasmuch as POPE has one saturated palmitoyl side chain and one unsaturated oleoyl side chain, it is miscible with both the saturated PEG-lipid conjugates and DOPE, and hence would facilitate the formation of POD/PE vesicles with uniform distribution of the lipid components in the bilayer structures. The lipid films composed of POD/POPE/DOPE or PEG-DSG/POPE/DOPE in the molar ratios of (4∼14)/50/(43∼36) for our studies were easily hydrated and the vesicles readily prepared after freeze-thawing and extrusion. As shown in Table 1, the freeze-thawed POD/POPE/DOPE vesicles are 160–200 nm in diameter, as expected after extrusion through 200-nm membranes. The freeze-thawed POD/POPE/DOPE liposomes possess large encapsulated volumes in the range of 2.5 ∼ 3.5 μl/μmol lipid, although there are probably some fraction of oligolamellar vesicles in the preparations.
TABLE 1.
Physical properties of POD/POPE/DOPE liposomes
| POD/POPE/DOPE*Ao/50/(50− Ao)
|
|||
|---|---|---|---|
| Ao† (mol%) | D‡ (nm) | P.I.§ | V¶ (μL/μmol) |
| 7 | 194 | 0.124 | 3.46 |
| 8 | 188 | 0.116 | 3.39 |
| 9 | 195 | 0.125 | 3.52 |
| 10 | 188 | 0.163 | 3.28 |
| 12 | 179 | 0.150 | 2.54 |
| 14 | 176 | 0.187 | 2.63 |
POD/POPE/DOPE liposomes were prepared by freeze-thawing method. All the data in the table are the mean of three measurements.
The initial mole percentage of POD in the lipid mixture.
The diameter of the liposomes based on the cumulant results of Photon Correlation Spectrometry measurements. The standard error is within 2% of the mean.
Polydispersity index reflecting the homogeneity of the liposomes. The standard error is within 15% of the mean.
Encapsulated volume of the liposomes reported as microliter of encapsulated aqueous liposome interior per micromole of lipids. The standard error is within 7% of the mean.
As a control for the influence of the hydrolysis products generated from the POD on vesicle structure, we prepared vesicles with various mole fractions of a pH-insensitive lipidic PEG derivative, PEG-DSG. In this control lipid, the length of the PEG is 2000 and the hydrophobic anchor is distearoyl glycerol. Thus PEG-DSG has physical-chemical properties that are analogous to POD. Vesicles containing the PEG-DSG encapsulated similar aqueous volumes as did the POD vesicles (data not shown).
Effect of pH on contents leakage
We have shown that contents release from POD/DOPE liposomes consist of two phases—a lag phase and a burst phase (Guo and Szoka, 2001). The lag phase is inversely correlated with the proton concentration; the logarithm of the duration of the lag phase shows a linear relationship with the buffer pH. During the burst phase the vesicles coalesce into large aggregates. We proposed a minimum-surface-shielding model to account for the linearity of the pH-collapse relationship and the value of the slope (Fig. 1).
To obtain a more precise estimate of the critical mole percent of POD required to stabilize the vesicles and to eliminate the possibility that hydrolysis products from the POD (the PEG, linker, or distearoyl glyceride; Fig. 1) were responsible for the leakage or collapse, we examined the pH release profile in vesicles composed of POPE/DOPE containing either the pH-sensitive POD or a pH-insensitive PEG-DSG derivative. The PEG-DSG had a similar MW PEG (2000) as POD, the same hydrophobic anchor distearoyl glyceride, and, like POD, had no charge. Vesicles composed of 10% PEG-DSG/POPE/DOPE did not leak contents (Fig. 2 A, traced) nor collapse at any pH tested, in any buffer used for these experiments (acetate, phosphate, citrate, or glucuronate; data not shown). PE vesicles stabilized either by 4% PEG-DSG, or 4% PEG-DSG and 10% POD, also did not leak contents (Fig. 2 A, tracec) nor collapse at any pH tested. Furthermore, vesicles stabilized with 3% PEG-DSG and containing 7% distearoyl glyceride also did not leak contents or collapse when the pH was lowered (data not shown). This later control was included because one of the breakdown products from POD is distearoyl glyceride. This confirms that the PE vesicles are stabilized by the PEG coat at all pH values and that hydrolysis products from POD do not cause content release.
Vesicles composed of 10% POD/POPE/DOPE underwent a pH-dependent leakage which was slightly faster in acetate buffer (Fig. 2 A, tracea) than in citrate buffer, a relatively membrane-impermeant buffer (Fig. 2 A, traceb). The POD vesicles underwent collapse into large aggregates in the same time frame as they leaked their contents (Guo and Szoka, 2001; data not shown). The pH-dependent contents-release profiles of POD/POPE/DOPE liposomes are shown in Fig. 2 B and conform to the model. The pH release profile was generated in a variety of buffers (acetate, citrate, glucuronate, and phosphate). The data shown in Fig. 2 B at pH 4.5 was generated in both acetate buffer and phosphate buffer. The phosphate buffer was used to trigger POD hydrolysis and contents release at the other pH values. We examined the effect of a variety of buffers on the contents leakage (Fig. 2 C). The lag time for contents release measured in acetate buffer was consistently shorter, albeit only slightly, than the lag time measured in the less membrane-permeant buffers (Fig. 2, A−C and Fig. 5 A). This suggests that either acetate buffer is more effective at reducing the pH in the immediate vicinity of the ortho ester linkage causing collapse from intervesicular bilayer contact on a faster time scale, or that acetate as acetic acid crosses the bilayer, and leads to hydrolysis of POD on the inside monolayer. Removal of PEG by hydrolysis of POD on the inside of the vesicles might lead to more rapid contents release. Alternatively hydrolysis of POD on the inside of oligolamellar vesicles could result in more rapid contents leakage due to membrane contact between the exterior bilayer and interior bilayers, i.e., collapse from within. At present, we are unable to differentiate among these possibilities.
The contents release profile as a function of pH, in an acetate buffer of POD/POPE/DOPE liposomes composed of 10% and 14% POD, respectively, was linear with respect to the buffer pH, with a correlation coefficient value higher than 0.99 (data not shown). The slope of the regressed lines for both formulations was 0.95 or greater and close to the theoretical value of 1. The slope of the regressed line for the combined data generated in all buffers, with all preparations examined, was 0.82 with a correlation coefficient of 0.94 (Fig. 2 C).
Effect of pH on bilayer mixing
The aggregation of the POD/DOPE liposomes occurs in a pH-dependent manner. This suggests that the leakage of POD/PE liposomes involves bilayer contact. A fluorescence resonance energy transfer assay (Struck et al., 1981; Hafez and Cullis, 2000) was thus carried out using the membrane probes NBD-PE and Rh-PE to examine if lipid mixing between POD/PE vesicles takes place during the process of pH-triggered liposome leakage.
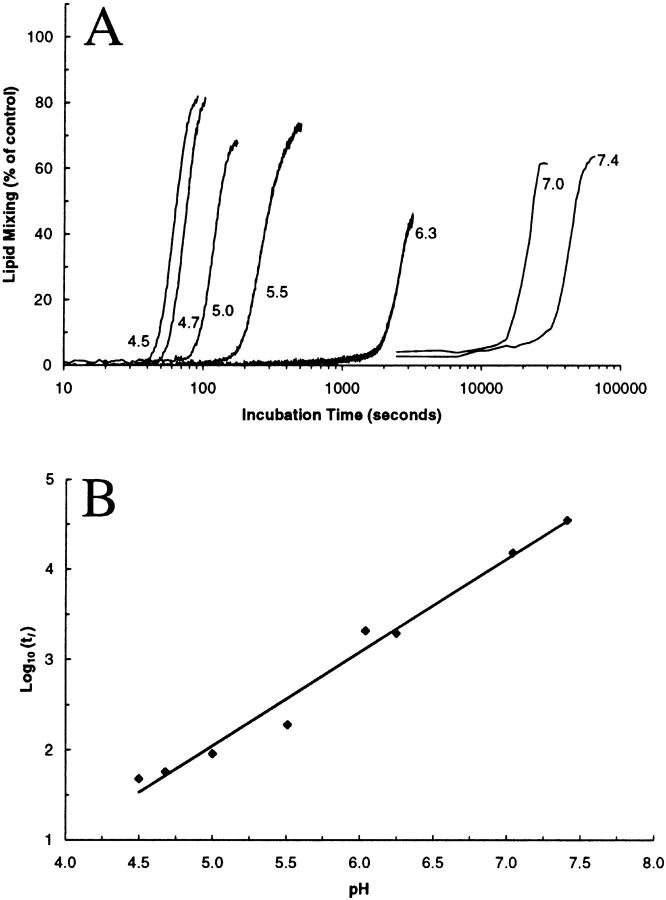
Similar to the leakage profile, the membrane mixing between POD/POPE/DOPE (10/50/40) liposomes (Fig. 3 A) exhibited kinetics comprised of two phases: a lag phase, when there was virtually no lipid mixing, and a burst phase, when up to 80% of lipid mixing was observed. The lag phase is inversely correlated with pH and the logarithm of the duration of lag phase (tl) has a linear relationship with the incubation pH (Fig. 3 B). The slope of the regressed line is 1.031 ± 0.058, which is the same within experimental error as the value predicted by the minimum-surface-shielding model. These findings demonstrate that lipid mixing is involved in the pH-dependent destabilization of POD/PE vesicles.
FIGURE 3.
pH-dependent lipid mixing of POD/POPE/DOPE (10/50/40) liposomes. (A), Percentage of leakage over time at 150 μM total lipids in acetate buffer (pH 4.5, 4.7, 5.0, 5.5) and phosphate buffer (pH 6.3, 7.0, and 7.4); (B), Logarithm of lag time (tl) in seconds at different buffer pH. Model: Log10(tl) = pH + Log10(ln(Ao/Ac)/k). Observed: Log10(tl) = (1.031 ± 0.058) × pH − (3.111 ± 0.339), r = 0.991, and P < 0.0001.
During the lag phase, the POD/POPE/DOPE liposomes released up to 20% of the encapsulated contents when acetate was the buffer used to adjust the pH, whereas there was virtually no lipid mixing during the lag phase (compare Fig. 2 B with Fig. 3 A). The leakage during the lag phase was much less pronounced when a membrane impermeant buffer (glucuronate, citrate, or phosphate) was used to adjust the pH. This observation indicates that the slow leakage during the lag phase when acetate is the buffer does not come from the collapse of a small population of less stable liposomes.
Hydrolysis rate and minimum stabilization percentage of POD
The hydrolysis of POD on liposome surfaces at a constant pH can be described by the following equation,
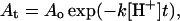 |
(6) |
where At is the percentage of POD on liposome surface at the incubation time t, Ao is the percentage of POD at the starting time of incubation, k is the POD hydrolysis rate constant, and [H+] is proton concentration. At the transition point between the lag phase and the burst phase, the equation can be written as
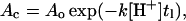 |
(7) |
where Ac is the minimum percentage of POD required on liposome surface to stabilize the bilayer structures and tl is the duration of lag phase. Taking the natural logarithm on both sides of the equation followed by rearrangement yields
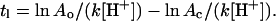 |
(8) |
Equation 8 shows that the length of lag time should have a linear relationship with the natural logarithm of the initial percentage of POD on liposome surface. Furthermore, the equation predicts that, by measuring the tl of POD/POPE/DOPE liposomes comprised of different Ao at known pH values followed by an appropriate regression of the data, one can deduce the rate constant of POD hydrolysis (k) as well as the minimum percentage of POD (Ac) that is required to stabilize the lamellar structures. POD/POPE/DOPE liposomes containing different mole percentages of POD (Table 1) were thus prepared by freeze-thawing and their leakage measured at multiple pH (Table 2).
Equation 8 also predicts that the change of tl in response to Ao is relatively small. Therefore, a precise determination of tl values is needed to obtain reasonable estimations of k and Ac. Two approaches were taken to improve the data quality for tl values. First, all the leakage data in this report were processed by an automated derivation method to remove bias during the extraction of tl values from the fluorescent traces. Second, inasmuch as tl is independent of lipid concentrations in both acetate and phosphate buffers (Fig. 5 C), more concentrated liposome samples were utilized for leakage assays (25 μM here versus 5 μM in Fig. 2) to obtain smoother traces with sharper increase of the fluorescent signal at the burst phase. The lag times of POD/POPE/DOPE liposomes with different Ao are listed in Table 2 and the data for pH 5.0 is plotted against the natural logarithm of Ao in Fig. 4.
As shown in Fig. 4, the duration of the lag time shows a linear relationship with the natural logarithm of the starting POD mole percentage at pH 5.0 (r = 0.972, P = 0.001), demonstrating that the minimum-surface-shielding model adequately describes pH-triggered contents release of POD/PE liposomes. To estimate the kinetic parameters (k and Ac), nonlinear regression was performed to fit the tl data in Fig. 4 to Eq. 4, which is obtained by minor rearrangement of Eq. 8. The parameters derived were k = 1390 ± 232 s−1 M−1 and Ac = 2.3 ± 0.6%.
Contact-dependent leakage at burst phase
Bentz and co-workers (Bentz et al., 1983) have shown by a mass action kinetic model that if the plots of leakage versus Xot (where Xo is the initial concentration of the liposomes, or in equivalent, the initial lipid concentration for a homogeneous liposome sample, and t is the incubation time) lie on the same curve regardless of the lipid concentration, then the liposomes are stable until they contact with one another. Ellens and co-workers (Ellens et al., 1984) have successfully utilized this theory to demonstrate that the acid-triggered leakage of CHEMS/DOPE liposomes requires interbilayer contact. Inasmuch as the contents release and lipid mixing of the POD/POPE/DOPE (10/50/40) vesicles exhibited very similar two-phase kinetic profiles, it was hypothesized that the destabilization of such liposomes at the burst phase is also dependent on bilayer contact. The leakage assays of POD/POPE/DOPE (10/50/40) liposomes at different concentrations were thus carried out at pH 5.0 in either acetate or phosphate buffers in an effort to test the hypothesis.
The contents release from a given liposome sample (POD/POPE/DOPE = 10/50/40) at different concentrations possesses approximately the same duration of lag phase (tl) of ∼95 s when acetate was the buffer (Fig. 5, A and C). This conforms with the minimum-surface-shielding model, which predicts that the lag time before the collapse of POD/PE bilayers should be a function of pH and the initial number of PEG groups on the liposome surface, but not a function of the liposome concentration (Guo and Szoka, 2001). A small increase of the tl for 1-μM and 2-μM lipid concentrations may be attributed in part to the low signal-to-noise ratio of these measurements. Thus, for all of the fluorescence traces in Fig. 5 A, we assign a common transition point (tl = 95 s) between the lag phase and the burst phase and define the incubation time at this point as to′. The fluorescent intensity at a given time point (t) after to′ is normalized to percentage of leakage in the burst phase (L%′) using the equation
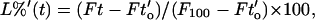 |
(5) |
where Ft is the fluorescent intensity of the trace at t, Fto′ is the fluorescent intensity at to′, and F100 is the fluorescent intensity after the liposomes were lysed with the detergent C12E8. L%′ was then plotted against Xo(t−to′) in Fig. 5 B. The replotted leakage graphs lie on the same line at 5 μM or lower concentrations, demonstrating that the bilayer contact is required for liposome leakage during the burst phase. At lipid concentrations higher than 5 μM, the curves lie lower in all values of Xo(t−to′), indicating that contents release, rather than inter-liposomal contact, becomes the limiting step of the dequenching of ANTS fluorescence. This is because the frequency of interbilayer contact increases proportionally with the square of lipid concentration whereas the leakage of ANTS is limited by the rate of bilayer mixing and lamellar-to-hexagonal phase changes after the contact (Ellens et al., 1984). Similar concentration-dependent contents-release profiles were obtained when a phosphate buffer was used to hydrolyze the POD, although the lag time increased (Fig. 5 C).
DISCUSSION
A principal objective of this study was to test the minimum-surface-shielding model (see Fig. 1 for a detailed illustration), which was proposed to account for the kinetic behavior of contents release observed in the POD/PE vesicles as a function of pH (Guo and Szoka, 2001). The model is based upon the observation in a number of model systems that PE bilayers must come into contact with another PE bilayer to transform into the hexagonal phase (Ellens et al., 1984; Cullis et al., 1991). PEG lipids stabilize PE-rich lipid compositions in the lamellar phase (Holland et al., 1996a). This effect is attributed to two factors: shape complementarity between the PEG lipid and the PE and steric hindrance to bilayer contact (Holland et al., 1996b). The model postulates that the PE bilayers are kept from coming into contact by the PEG polymers attached to the liposome surface. When a sufficient amount of PEG is removed from the vesicle surface, the surface becomes accessible to contact with another PE surface; when contact is made between two bilayers, they rapidly convert into a hexagonal phase and the encapsulated contents are rapidly released. Thus, we can use the data derived from the model to estimate the number of PE lipids involved in the initial step of membrane mixing.
In the case where the PEG is removed by a hydrolytic reaction, the stability of POD/PE liposomes is a function of the initial mole fraction of the POD in the liposome bilayer and the hydrolysis rate of the POD. For all liposome preparations used in this report to test the model, we assume that the fraction of POD on the outer monolayer is the same as the fraction of POD in the initial lipid mixture; that the POD mixes ideally with the other lipids; and that the collapse of the bilayer occurs with similar properties regardless of the lipid composition of the bilayer. When the duration of the lag phase at various pHs (Fig. 2) and the duration of the lag phase at a constant pH from liposomes containing various POD mole percentages (Fig. 4) were measured, the hydrolysis rate of POD and the critical POD mole percentage needed to stabilize PE in the lamellar phase can be computed.
We tested the assumption that bilayer collapse is responsible for contents release by measuring lipid mixing with a fluorescent resonance energy transfer lipid-mixing assay (Struck et al., 1981; and see Fig. 3). The slope of the logarithm of the lag time for membrane mixing as a function of pH was 1 and the correlation coefficient was greater than 0.99. The lag time for lipid mixing was highly correlated with the lag time for rapid release; thus hydrolysis of the ortho ester results in bilayer collapse, not increased permeability through the bilayer.
In Fig. 5 B, the leakage of POD/PE liposomes during the burst phase was extracted from that during the lag phase by “resetting” the zero percent leakage as well as the zero incubation time at the transition point. Redrawn against the product of lipid concentration and the modified incubation time, the leakage at different vesicle concentrations up to 5 μM overlapped with each other, demonstrating that most, if not all, of the liposome leakage at the studied pH values is membrane contact-dependent during the burst phase (Ellens et al., 1984).
Indeed, the kinetic data fits the model quite satisfactorily. The linear correlation coefficient is 0.98 for contents leakage of the POD/DOPE vesicles versus pH (Guo and Szoka, 2001), 0.95 for contents leakage of the POD/POPE/DOPE vesicles versus pH (Fig. 2), 0.99 for fusion of the POD/POPE/DOPE vesicles versus pH (Fig. 3), and 0.95 for contents leakage of the POD/POPE/DOPE vesicles versus the natural logarithm of the initial mol% of POD (Fig. 4).
We developed the indirect contents leakage method to determine the parameters of k and Ac for two reasons: 1), it is difficult to prepare liposomes with the critical density of PEG on their surface; and 2), direct determination of the hydrolysis rate of POD requires the separation of surfactants and could introduce complications from the degradation of POD during its isolation and quantification. Although we had directly estimated the pH-dependent hydrolysis rate of POD using thin layer chromatography in our previous studies (Guo and Szoka, 2001), the precision of this assay was insufficient to obtain more than an order-of-magnitude estimate of the rate constant. Using the indirect method that models the relationship between tl and the initial mol% of POD (data in Fig. 4), the estimated hydrolysis rate of POD in POD/POPE/DOPE (Ao/50/(50 − Ao)) vesicles is 1390 ± 232 s−1 M−1 and the estimated critical POD percentage is 2.3 ± 0.6 mol%.
The latter value is slightly greater than that found to prevent calcium-induced fusion of phosphatidylserine-PE bilayers (Holland et al., 1996b), where at 2 mol% the vesicles were stable but as the PEG-content was reduced to ∼1 mol% there was a slight increase in calcium-induced fusion that was significantly accelerated as the PEG content was reduced further to ∼0.5 mol%. The difference between the mole percent for fusion onset observed by Holland and co-workers (1996b) and the findings here may be due to the fact that the POD is an uncharged lipid whereas the PEG-PE carries a negative charge at neutral pH; hence, electrostatic effects inhibiting close apposition of bilayers may be more pronounced in the calcium-induced fusion model. To overcome this barrier a greater fraction of PEG-PE might have to be removed from the bilayer to enable vesicle fusion.
The kinetic parameters derived from fitting tl data to the model permit us to predict the lag time of a POD/POPE/DOPE liposome preparation if we know the pH. Moreover, at any pH, the lag time can be adjusted by altering the initial percentage of POD in the lipid composition. In Table 2, all the experimentally observed tl values of POD/POPE/DOPE vesicles under different conditions are compared with their corresponding predicted values based on the minimum-surface-shielding model using the above-estimated k and Ac values. Given the large ranges of pH and tl values, the model provides satisfactory predictions of the experimental data with an R2 of 0.96. This correlation coefficient is similar to that of the linear regressions in Fig. 4, from which data k and Ac are derived. The relatively poor predictions of tl values at pHs lower than 5 may be attributed to the following two factors. First, the POD hydrolysis at these low pHs is so rapid that it is no longer the rate-limiting step of liposomal leakage; the membrane mixing and collapse of the PE bilayers may, instead, be the major factors to control the contents release of the vesicles. Second, the low tl values at these pHs may induce additional errors to the derivation method, which uses the time for 50% leakage (t50) to determine the window of data smoothing. Twenty percent of t50 may be too big a time window at these low pH levels.
An interesting consequence arising from these experiments relates to the minimal surface area on two PE bilayers that must come in contact in order for the PE to undergo a lamellar-hexagonal transition (Ellens et al., 1984; Holland et al., 1996a,b). Siegel and Epand (Siegel and Epand, 1997; Siegel 1999) have proposed that such transition occurs via a stalk that progresses to a transmonolayer contact site (TMC). In the bilayer, the stalk is proposed to be a nonequilibrium structure that proceeds onto a TMC which is the initial nexus of the transition from a lamellar to hexagonal phase (Siegel and Epand, 1997; Siegel, 1999). There is surprisingly little experimental data on the structure of the stalk or TMC intermediates. In fact, Siegel and Epand (1997) state that “we cannot directly demonstrate the structure of the first processes with cryotransmission electron microscopy,” and go on to conclude that the intermediates are less than 10 nm. Since the submission of this manuscript, structures resembling the putative stalk intermediate have been captured using x-ray diffraction in diphytanylphosphatidylcholine multilayers at low hydration (Yang and Huang, 2002). The dimension of the unit cell perpendicular to the membrane is 3.95 nm. We can compute a lower bound of the area of the early intermediate from the data measured in this work by assuming a uniform surface coverage of the PE bilayer by POD. If the critical surface coverage is 2.3 mol%, approximately one in every 43 lipid molecules contains a PEG chain. Inasmuch as two liposomes must come in contact, the average surface density of PEG2000 is one polymer for every 22 lipids. Thus 22 lipids would constitute the minimum number of PE on one surface required to initiate a lamellar-to-hexagonal transition if the PEG chain did not obstruct the surface. However, the PEG attached to POD has a mol wt ≈ 2000 and, assuming it is in the mushroom regime and packs as a sphere on the vesicle surface, the projected area of this sphere is 438 Å2 (Kenworthy et al., 1995; Evans et al., 1996; Needham et al., 1999). Inasmuch as each diacyl PE occupies a projected area of ∼70 Å2, 22 lipids would occupy 1540 Å2, and at the contacting area, two liposomes would have 438 ÷ 1540 × 100% = 28.4% of the available bilayer surface occluded by a PEG polymer chain. The minimal surface area on two PE bilayers that must come in contact for the PE to undergo a lamellar-hexagonal transition would then be ∼1540 Å2 − 438 Å2 = 1102 Å2, the area of ∼16 lipids.
The calculated values are consistent with the findings of other groups (Needham et al., 1999; Siegel and Epand, 1997) on the mechanisms of the membrane mixing between PE bilayers and the shielding effect of PEG polymers (Holland et al., 1996a). The computed radius of 1.9 nm for the initial intermediate covering 1102 Å2 as estimated by the present data is also consistent with the dimensions for the stalk intermediate measured by x-ray diffraction (Yang and Huang, 2002), albeit in a different lipid. The precise exposed surface area depends upon the exact conformation of the PEG chains associated with the surface. Rex and co-workers (Rex et al., 1998) have concluded that the grafted 2000-mol wt PEG chains exist as a mushroom over this regime and the average cross-sectional areas of the PEG2000 chains are 257 Å2. They proposed the PEG moiety occurs in a relatively elongated conformation; if this is the case, a slightly different number of PE lipids could be involved in stalk formation.
Bilayers are separated between 5 and 10 nm at various PEG2000-PE mole percents (Kenworthy et al., 1995), whereas separations between 4 and 6 nm have been measured when an uncharged PEG2000 lipid is incorporated into the bilayer (Efremova et al., 2000). Therefore, the PEG polymer extends beyond the putative height of the TMC intermediate (3–5 nm; Siegel and Epand, 1997). Thus, for membrane mixing between bilayers to go to completion, it would seem that the following conditions would have to be met: the polymer coat would have to be sufficiently sparse to allow the initial bilayer contact; the stalk would have to form; and the appropriate number of PE would have to be available to form the TMC. It is possible that by judiciously mixing the POD with a pH-insensitive PEG-DSG and then removing the POD lipid at low pH, it might be possible to trap the system in a metastable state where the early intermediates have formed but are unable to coalesce so the system is unable to progress into the hexagonal phase. Thus one might observe only outer monolayer mixing with little change in vesicle diameter and less than 100% contents release.
A number of pH-sensitive liposome systems have been introduced over the years and have been employed for studies involving lamellar-hexagonal transitions or to provide a triggered release system for drug delivery. The feature of rapid, membrane contact-dependent release of contents from POD/PE liposomes at the burst phase is similar to what has been observed with the CHEMS/DOPE vesicles (Ellens et al., 1984), oleic acid/PE liposomes (Duzgunes et al., 1985), and homocysteine/PE liposomes (Connor et al., 1984). The pH-induced neutralization of the CHEMS/DOPE liposomes immediately increases the exposure of the PE headgroups, leading to membrane contact-dependent leakage kinetics. The leakage of CHEMS/DOPE liposomes has no lag phase, because the protonation of CHEMS headgroups is almost instantaneous compared with the rate of lipid fusion. The destabilization kinetics of POD/PE vesicles differs from that found with the BVEP/DOPE vesicles whose leakage occurs at a much faster rate compared with the lipid mixing upon acid-catalyzed hydrolysis of BVEP (Boomer and Thompson, 1999). This difference between the POD/PE and BVEP/DOPE liposomes may be attributed to the different hydrolysis patterns of POD and BVEP. The hydrolysis at either of the two ortho ester groups of POD leads to the immediate cleavage of the PEG2000 headgroup from the liposome surface and the exposure of PE headgroups. In the case of BVEP, the hydrolysis of one of the two vinyl ether linkages generates an alkyl aldehyde and a conjugate of PEG5000 and a single alkyl side chain. Thus, the PEG5000 headgroup would be retained on the liposome surface until the hydrolysis of the second vinyl ether group takes place. Therefore, during the early stage of BVEP hydrolysis, the resultant single-chain detergents may be sufficient to induce structural defects in the bilayers, leading to contents release and even the collapse of the vesicles. However, the retained PEG5000 groups on their surfaces still protect the colloids from the membrane contact necessary for collapse via an hexagonal transition.
Recently, Zhu and co-workers (Zhu et al., 2000) reported two acid-labile cationic lipids containing an ortho ester linker based on the structure of 3,5,8-trioxabicyclo[2.2.2]octane. Due to the particular configuration of the linker, the initial two fast hydrolysis steps of the ortho ester functionality do not fragment the cationic lipids, but rather add two hydroxy groups near the cationic headgroup region. It is only after the final slower step of the hydrolysis, which is the cleavage of an ester group, that each lipid molecule converts to two single-chain, membrane-destabilizing detergents. Such a hydrolysis pattern may complicate the kinetics of bilayer destabilization by these pH-sensitive lipids. Moreover, the cationic headgroup remains in one of the single-chain detergents, which would both partition into the bilayer as well as diffuse into the aqueous phase. Thus a clean separation of the component from the liposome will not occur.
Nonconstrained ortho ester PEG-containing surfactants have also been synthesized and have rates of hydrolysis that are slower than the POD lipid (Hellberg et al., 2000). This linkage could also be attached between PEG and hydrophobic lipid anchors to provide a pH-sensitive lipid conjugate that would have a different pH hydrolysis profile than the POD. Thus a variety of pH-sensitive derivatives are now available for the control of the phase preference of vesicles composed of PE. These pH-triggerable PEG derivatives will prove useful both in biophysical studies and in drug delivery.
CONCLUSION
The kinetics of pH-triggered destabilization of POD/PE liposomes has been studied with the ANTS/DPX leakage assay and the lipid-mixing assay. The data generated from these studies were analyzed based on the minimum-surface-shielding model. The model adequately describes the mechanism of the pH-triggered release of POD/PE liposomes and indicates that a critical surface coverage of Ac = 2.3 mol% PEG 2000 is required to stabilize the PE bilayer. Based upon this model the rate of hydrolysis of the ortho ester conjugate POD is ∼1390 ± 232 s−1 M−1. These values can be used to predict the stability of PE liposomes as a function of the pH of the environment and the initial mol% of POD in the lipid composition. These values also suggest that 16 PE lipids are sufficient to initiate a lamellar-hexagonal transition between apposed PE surfaces.
Acknowledgments
We thank Dr. Jorge Heller for the diketene acetal used to prepare the POD, and Dr. A. Suginaka for the PEG-DSG.
J.A.M. is a recipient of a Howard Hughes Medical Institute predoctoral fellowship. This work was partially supported by National Institutes of Health grants DK 46052 and GM61851.
Abbreviations used: Ac, critical POD mole percentage or minimum POD percentage necessary to stabilize a bilayer structure; Ao, initial mole percentage of POD in the bilayer; ANTS, 8-aminonaphthalene-1,2,3-trisulfonic acid; CHEMS, cholesteryl hemisuccinate; DOPE, dioleoyl-phosphatidylethanolamine; DPX, p-xylenebis(pyridinium) bromide; HEPES, (hydroxyethyl)piperazine-n-2-ethanesulfonic acid; k, hydrolysis rate constant of POD in M−1sec−1; NBD-PE, n-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine; PCS, Photon Correlation Spectrometry; PEG, polyethylene glycol; POD, polyethylene glycol 2000-diortho ester-distearoyl glycerol conjugate; PEG-DSG, polyethylene glycol 2000-distearoyl glycerol conjugate; POPE, 1-palmitoyl-2-oleoylphosphatidylethanolamine; Rh-PE, dipalmitoylphosphatidyl-ethanolamine-lissamine rhodamine B; tl, lag time or length of lag phase.
References
- Bartlett, G. R. 1959. Phosphorous assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- Bentz, J., S. Nir, and J. Wilschut. 1983. Mass action kinetics of vesicle aggregation and fusion. Colloids Surf. 6:333–363. [Google Scholar]
- Boomer, J. A., and D. H. Thompson. 1999. Synthesis of acid-labile diplasmenyl lipids for drug and gene delivery applications. Chem. Phys. Lipids. 99:145–153. [DOI] [PubMed] [Google Scholar]
- Burger, K. N. J. 2000. Greasing membrane fusion and fission machineries. Traffic. 1:605–613. [DOI] [PubMed] [Google Scholar]
- Chu, C.-J., and F. C. Szoka. 1994. pH-Sensitive Liposomes. J. Liposome Res. 4:361–395. [Google Scholar]
- Connor, J., M. B. Yatvin, and L. Huang. 1984. pH-sensitive liposomes: acid-induced liposome fusion. Proc. Natl. Acad. Sci. USA. 81:1715–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis, P. R., C. P. Tilcock, and M. J. Hope. 1991. Lipid polymorphism. In Membrane Fusion. J. Wilschut, and D. Hoekstra, editors. Marcel Dekker, Inc., New York, Basel, Hong Kong. 35–64.
- Drummond, D. C., M. Zignani, and J.-C. Leroux. 2000. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 39:409–460. [DOI] [PubMed] [Google Scholar]
- Duzgunes, N., R. M. Straubinger, P. A. Baldwin, D. S. Friend, and D. Papahadjopoulos. 1985. Proton-induced fusion of oleic acid-phosphatidylethanolamine liposomes. Biochemistry. 24:3091–3098. [DOI] [PubMed] [Google Scholar]
- Efremova, N. V., B. Bondurant, D. F. O'Brien, and D. E. Leckband. 2000. Measurements of interbilayer forces and protein adsorption on uncharged lipid bilayers displaying poly(ethylene glycol) chains. Biochemistry. 39:3441–3451. [DOI] [PubMed] [Google Scholar]
- Ellens, H., J. Bentz, and F. C. Szoka. 1984. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 23:1532–1538. [DOI] [PubMed] [Google Scholar]
- Epand, R. M. 2000. Membrane fusion. Biosci. Rep. 20:435–441. [DOI] [PubMed] [Google Scholar]
- Evans, E., D. J. Klingenberg, W. Rawicz, and F. Szoka. 1996. Interactions between polymer-grafted membranes in concentrated solutions of free polymer. Langmuir. 12:3031–3037. [Google Scholar]
- Gennis, R. B. 1989. Acyl chain configuration and packing in the bilayer. In Biomembranes: Molecular Structure and Function. Springer-Verlag New York Inc., New York, Berlin, Heidelberg, London, Paris, Tokyo. 48–50.
- Gerasimov, O. V., A. Schwan, and D. H. Thompson. 1997. Acid-catalyzed plasmenylcholine hydrolysis and its effect on bilayer permeability: A quantitative study. Biochim. Biophys. Acta. 1324:200–214. [DOI] [PubMed] [Google Scholar]
- Guo, X., and F. C. Szoka. 2001. Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG-diorthoester-lipid conjugate. Bioconjug. Chem. 12:291–300. [DOI] [PubMed] [Google Scholar]
- Hafez, I. M., and P. R. Cullis. 2000. Cholesteryl hemisuccinate exhibits pH sensitive polymorphic phase behavior. Biochim. Biophys. Acta. 1463:107–114. [DOI] [PubMed] [Google Scholar]
- Hellberg, P.-E., K. Bergstrom, and M. Juberg. 2000. Nonionic cleavable ortho ester surfactants. J. Surf. Deterg. 3:369–379. [Google Scholar]
- Holland, J. W., P. R. Cullis, and T. D. Madden. 1996a. Poly(ethylene glycol)-lipid conjugates promote bilayer formation in mixtures of non-bilayer-forming lipids. Biochemistry. 35:2610–2617. [DOI] [PubMed] [Google Scholar]
- Holland, J. W., C. Hui, P. R. Cullis, and T. D. Madden. 1996b. Poly(ethylene glycol)-lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 35:2618–2624. [DOI] [PubMed] [Google Scholar]
- Kenworthy, A. K., K. Hristova, D. Needham, and T. J. McIntosh. 1995. Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol). Biophys. J. 68:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, E., and J. Hughes. 1998a. Characterization of a pH-sensitive surfactant, dodecyl-2-(1′- imidazolyl) propionate (DIP), and preliminary studies in liposome mediated gene transfer. Biochim. Biophys. Acta. 1369:39–50. [DOI] [PubMed] [Google Scholar]
- Liang, E., and J. A. Hughes. 1998b. Membrane fusion and rupture in liposomes: effect of biodegradable pH- sensitive surfactants. J. Membr. Biol. 166:37–49. [DOI] [PubMed] [Google Scholar]
- Monnard, P. A., T. Oberholzer, and P. Luisi. 1997. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta. 1329:39–50. [DOI] [PubMed] [Google Scholar]
- Needham, D., D. V. Zhelev, and T. J. McIntosh. 1999. Surface chemistry of the sterically stabilized PEG-liposome, general principles. In Liposomes—Rational Design. A. S. Janoff, editor. Marcel Dekker, Inc., New York, Basel. 13–62.
- Pick, U. 1981. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch. Biochem. Biophys. 212:186–194. [DOI] [PubMed] [Google Scholar]
- Rex, S., M. J. Zuckermann, M. Lafleur, and J. R. Silvius. 1998. Experimental and Monte Carlo simulation studies of the thermodynamics of polyethyleneglycol chains grafted to lipid bilayers. Biophys. J. 75:2900–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, K., A. Miyagishima, Y. Sadzuka, Y. Nozawa, Y. Mochizuki, H. Ohshima, and S. Hirota. 1995. Determination of the thickness of the fixed aqueous layer around polyethyleneglycol-coated liposomes. J. Drug Target. 3:283–289. [DOI] [PubMed] [Google Scholar]
- Siegel, D. P. 1993. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate structures. Biophys. J. 65:2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, D. P. 1999. The modified stalk mechanism of lamellar/inverted phase transitions and its implications for membrane fusion. Biophys. J. 76:291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, D. P., and R. M. Epand. 1997. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys. J. 73:3089–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J., and R. I. Hollingsworth. 1999. Synthesis, conformational analysis, and phase characterization of a versatile self-assembling monoglucosyl diacylglycerol analog. J. Am. Chem. Soc. 121:1851–1861. [Google Scholar]
- Struck, D. K., D. Hoekstra, and R. E. Pagano. 1981. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 20:4093–4099. [DOI] [PubMed] [Google Scholar]
- Szoka, F., Jr., and D. Papahadjopoulos. 1978. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA. 75:4194–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. L., and D. A. Tirrell. 1992. Polyelectrolyte-sensitized phospholipid vesicles. Account. Chem. Res. 25:336–342. [Google Scholar]
- Yang, L., and H. W. Huang. 2002. Observation of a membrane fusion intermediate structure. Science. 297:1877–1879. [DOI] [PubMed] [Google Scholar]
- Zhu, J., R. J. Munn, and M. H. Nantz. 2000. Self-cleaving ortho ester lipids: a new class of pH-vulnerable amphiphiles. J. Am. Chem. Soc. 122:2645–2646. [Google Scholar]