Abstract
The filamentous bacteriophage Pf3 consists of a covalently closed DNA single strand of 5833 nucleotides sheathed by ∼2500 copies of a 44-residue capsid subunit. The capsid subunit contains a single tryptophan residue (Trp-38), which is located within the basic C-terminal sequence (–RWIKAQFF) and is essential for virion assembly in vivo. Polarized Raman microspectroscopy has been employed to determine the orientation of the Trp-38 side chain in the native virus structure. The polarized Raman measurements show that the plane of the indolyl ring is tilted by 17° from the virion axis and that the indolyl pseudo-twofold axis is inclined at 46° to the virion axis. Using the presently determined orientation of the indolyl ring and side-chain torsion angles, χ1 (N–Cα–Cβ–Cγ) and χ2,1 (Cα–Cβ–Cγ–Cδ1), we propose a detailed molecular model for the local structure of Trp-38 in the Pf3 virion. The present Pf3 model is consistent with previously reported Raman, ultraviolet-resonance Raman and fluorescence results suggesting an unusual environment for Trp-38 in the virion assembly, probably involving an intrasubunit cation-π interaction between the guanidinium moiety of Arg-37 and the indolyl moiety of Trp-38. Such a C-terminal Trp-38/Arg-37 interaction may be important for the stabilization of a subunit conformation that is required for binding to the single-stranded DNA genome during virion assembly.
INTRODUCTION
The filamentous bacteriophage Pf3 infects strains of Pseudomonas aeruginosa harboring the RP1 plasmid. About 2500 copies of a 44-residue α-helical subunit plus a few copies of several minor proteins coat the single-stranded Pf3 genome of 5833 deoxynucleotides to form a filament of ∼700-nm length and ∼6-nm diameter. The capsid subunits are assembled around the covalently closed DNA strand as a single-start helix of ∼5.4 subunits per turn (Welsh et al., 1998). The amino acid sequence of the Pf3 capsid subunit (MQSVITDVTG10 QLTAVQADIT20 TIGGAIIVLA30 AVVLGIRWIK40 AQFF), like the capsid subunit sequences of other filamentous bacteriophages, contains an acidic N-terminal region forming the outer surface of the particle, a hydrophobic central domain representing the subunit packing interface, and a basic C-terminal region lining the DNA-filled capsid core (Day et al., 1988; Pederson et al., 2001). The single tryptophan residue (Trp-38) of the Pf3 subunit is located within a turn of the α-helix that also contains two basic side chains (Arg-37, Lys-40) and is proximal to the packaged DNA molecule. This region of the subunit is thought to play a role in electrostatic stabilization of the packaged genome. Further details of filamentous virus architecture have been given elsewhere (Day et al., 1988; Marvin, 1998).
Structural models of several filamentous viruses have been proposed on the basis of fiber x-ray diffraction analyses of oriented specimens. Filaments exhibiting so-called class I symmetry (C5S2), which includes the Ff strains (fd, f1, M13), as well as If1 and IKe, have been studied extensively and yield highly informative diffraction patterns (Marvin et al., 1994; Symmons et al., 1995). The Pf3 and Pf1 virions, which are of class II symmetry (C1S5.4), have also been investigated (Welsh et al., 1998; Welsh et al., 2000). Although the fiber x-ray diffraction approach does not resolve local environments and orientations of subunit side chains and provides no direct information about interactions of side chains with one another or with the packaged DNA molecule, such information has been obtained from Raman, polarized Raman, ultraviolet-resonance Raman (UVRR), and Raman optical activity studies (Overman et al., 1996; Tsuboi et al., 1996a; Takeuchi et al., 1996; Wen et al., 1997; Matsuno et al., 1998; Tsuboi et al., 2000; Tsuboi et al., 2001; Wen et al., 1999; Wen and Thomas, 2000; Wen et al., 2001; Arp et al., 2001; Blanch et al., 1999; Blanch et al., 2001). These spectroscopic results have led to significant refinements of initially proposed x-ray based structural models for Ff and Pf1 virions (Marvin, 1998). They have also helped to provide a basis for structural modeling of the highly thermostable filamentous phage PH75, which infects Thermus thermophilus (Pederson et al., 2001).
Recent UVRR and Raman studies of Pf3 (Wen and Thomas, 2000; Wen et al., 2001) have demonstrated highly unusual indolyl marker bands for the Trp-38 residue in subunits of the assembled virion. The unusual indolyl markers of Trp-38—particularly the Raman bands at 763, 1228, 1370 and 1773 cm−1—are clearly distinct from the corresponding indolyl markers observed for detergent-disassembled Pf3 subunits, class I filamentous viruses, most globular proteins, and aqueous l-tryptophan. A strongly quenched fluorescence spectrum was also observed for Trp-38 in the assembled Pf3 particle (Wen and Thomas, 2000). Collectively, these spectroscopic findings suggest an environment for the Trp-38 indolyl moiety that is specific to the Pf3 assembly and not encountered in solvent exposed tryptophans. Additional evidence was presented to suggest that similarly anomalous fluorescence, UVRR, and Raman signatures may be diagnostic of the so-called cation-π interaction recently proposed for tryptophans in the hydrophobic cores of globular proteins (Gallivan and Dougherty, 1999; Ma and Dougherty, 1997). In the case of the Pf3 subunit, an intrasubunit cation-π interaction could involve either Arg-37 or Lys-40 as the cation donor and the indolyl ring of Trp-38 as the π acceptor.
Here, we report and interpret polarized Raman spectra of oriented fibers of the Pf3 filamentous virus to determine the specific orientation of the Trp-38 side chain in the subunit of the native virion assembly. A detailed structural model is developed for the Pf3 capsid subunit in the vicinity of the Trp-38 residue. The model permits assessment of the feasibility of a cation-π interaction involving the Trp-38 indolyl ring with either the guanidinium group of Arg-37 or the quaternary ammonium group of Lys-40. Tryptophan side-chain (Trp-38) geometry in the present Pf3 structural model is also compared with that of Trp-26 in the previously reported Ff structural model (Tsuboi et al., 1996a).
MATERIALS AND METHODS
Sample preparations
Pf3 was grown on Pseudomonas aeruginosa (strain PAO1 bearing the RP1 plasmid) in Luria-Bertani medium using stocks obtained originally from Dr. Loren A. Day, Public Health Research Institute, New York, NY. Growth media, reagents and l-tryptophan were purchased from Sigma (St. Louis, MO). The deuterated amino acid, l-tryptophan-2′, 4′, 5′, 6′, 7′-d5 (l-Trp-d5), was obtained from Cambridge Isotope Laboratories (Woburn, MA).
Mature viral particles extruded through the bacterial membrane and into the growth medium were collected by polyethylene glycol precipitation followed by centrifugation at 10,000 g. The virus particles were purified by repeated cycles of centrifugation at 330,000 g in 10 mM Tris (pH 7.8) to yield a pellet, from which virus solutions and fibers were prepared for spectroscopic analyses. To incorporate deuterated tryptophan into the coat protein of Pf3, the host and phage were grown in M9 minimal medium containing 10 μg/mL thiamine-HCl, 1% glucose and l-tryptophan-2′, 4′, 5′, 6′, 7′-d5 plus all other l-amino acids at 0.1 mM each. Mature viral particles were isolated and purified as for unlabeled Pf3. Further details of isolation and purification procedures have been described (Thomas et al., 1983; Overman and Thomas, 1995).
Pf3 solutions in the range 50–100 mg/mL were prepared for Raman spectroscopy by resuspending the pelleted virus in 10 mM Tris buffer at pH 7.8. Pf3 fibers of ∼0.5-mm thickness were prepared for polarized Raman measurements by slowly drawing droplets of dilute Pf3 solutions in a fiber pulling device maintained at 92% relative humidity (Overman et al., 1996).
Raman spectroscopy
Aliquots (∼6 μL) of Pf3 solutions were sealed in glass capillary cells (KIMAX #34507) and mounted in the sample compartment of a single grating spectrophotometer (Spex 500M, Instruments S. A., Edison, NJ) equipped with a liquid-nitrogen-cooled charge-coupled device detector. Raman spectra of 3.5 cm−1 spectral band resolution and ±1 cm−1 band precision were excited using the 514.5-nm emission of an argon laser (Coherent Innova 70, Santa Clara, CA) operating with ∼200 mW of radiant power at the sample. Further details of instrumentation and data collection procedures have been given (Wen et al., 2001).
Oriented fibers were sealed in glass capillaries maintained at constant temperature (11 ± 1°C) and relative humidity (92%) on the sample holder of a Raman microscope with the fiber axis (c) in the horizontal plane. Polarized Raman spectra were excited at 514.5 nm and collected on a ISA/Jobin Yvon model S3000 triple spectrograph (Edison, NJ) equipped with an Olympus model BHSM microscope (Lake Success, NY) and ISA Spectraview-2D charge-coupled device detector (Benevides et al., 1993; Thomas et al., 1995). The exciting radiation with electric vector polarized along c was directed onto the fiber through an 80× objective of 15-mm focal length. The Raman scattered radiation was collected with the same objective and directed through a polarizer to the monochromator. The polarizer transmitted only the Raman scattering polarized along the same direction as that of the exciting radiation. By initially orienting c parallel to the electric vector of the exciting radiation (also parallel to the electric vector of the scattered radiation), the cc-polarized Raman spectrum (Icc) was obtained. Subsequent 90° rotation of the fiber axis yielded the bb-polarized spectrum (Ibb). Three accumulations of the polarized spectra with an integration time of 180 s each were averaged. This procedure was repeated five times and the results were again averaged to produce the Icc and Ibb spectra shown in the figures. The Raman intensities were normalized using Icc = Ibb for the phenylalanine band at 1002 cm−1, which is known to exhibit an isotropic Raman tensor with 514.5-nm excitation. The orientation of a typical Raman tensor coordinate system (xyz) for the Trp-38 indolyl ring with respect to the Pf3 fiber coordinate system (abc) is depicted in the bottom of Fig. 1. The angle of inclination of the subunit α-helix axis to the fiber axis is given by θ, shown in the top of Fig. 1.
FIGURE 1.
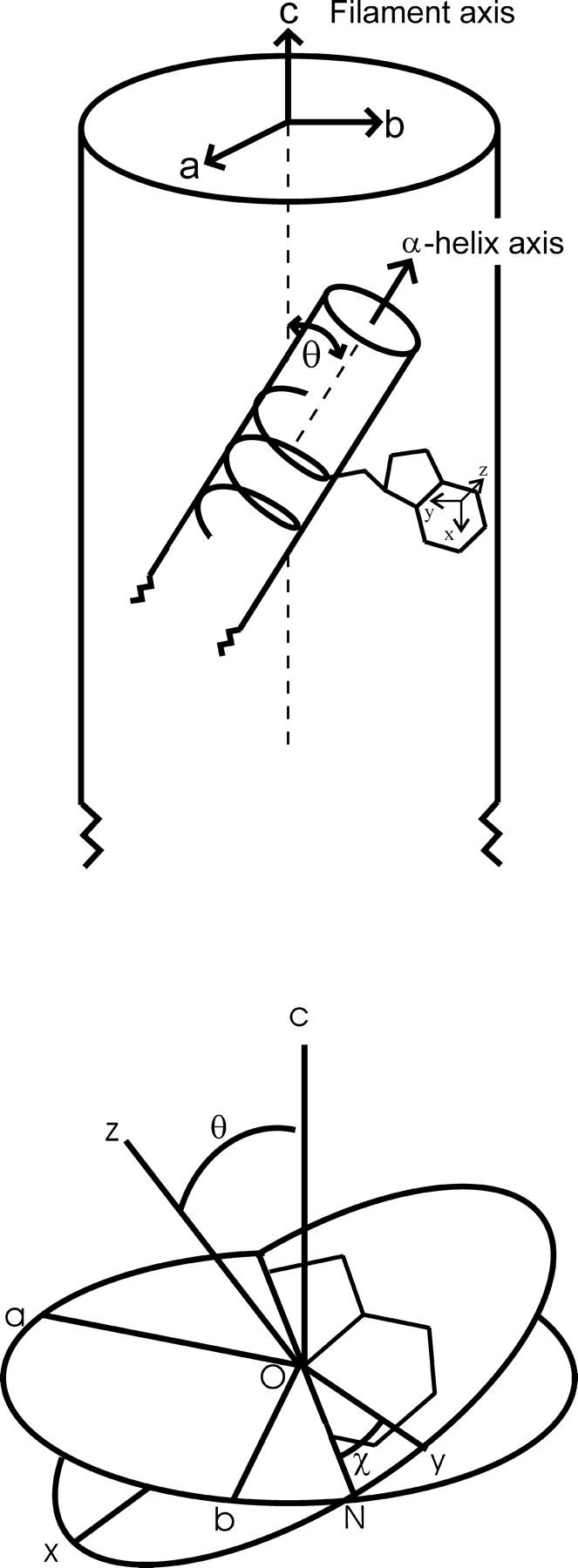
(Top) Coordinate system for the uniaxially oriented Pf3 fiber (abc) in relation to the α-helical subunit and a tryptophan side chain. The drawing (not to scale) represents a small segment of the ∼700-nm virion and a few α-helical turns of one subunit. (Bottom) The Eulerian angles θ and χ define the orientation of the coordinate system (xyz) for a tryptophan Raman tensor with respect to the (abc) system. θ is the tilt angle (c–O–z) between the Raman tensor principal axis z and the fiber axis c. χ is the angle (y–O–N) formed by the Raman tensor principal axis y and the line of intersection between the plane (ab) normal to the fiber axis and the plane (xy) of the indolyl ring.
Raman tensors of indolyl markers near 1368 cm−1 (normal mode W7″) and 1548 cm−1 (W3), which are required to calculate the Trp-38 indolyl ring orientation from polarized Raman spectra, were transferred from the previously reported analysis of N-acetyl-l-tryptophan (Tsuboi et al., 1996b). These tensor data are summarized in Table 1. The suitability of tensor transfers and previous applications to the Trp-26 indolyl ring of the Ff filamentous virus subunit have been discussed (Tsuboi et al., 1996a).
TABLE 1.
Raman tensors of selected bands of the tryptophan side chain
| Band (cm−1) | Mode | r1(≡αxx/αzz) | r2(≡αyy/αzz) | Principal axes |
|---|---|---|---|---|
| 1368 | W7″ | 1.56 | 6.13 | y: intersects Cɛ3 and Cδ1 |
| x: ⊥ to y and intersects Cζ2 | ||||
| z: ⊥ to y and x | ||||
| 1548 | W3 | 0.59 | 2.71 | y: intersects Cζ3 and Cδ2 |
| x: ⊥ to x and intersects Cζ2 | ||||
| z: ⊥ to y and x |
Determined from polarized Raman analysis of a single crystal of N-acetyl-l-tryptophan (Tsuboi et al., 1996b).
Data analysis
In the fiber-fixed coordinate system (abc) of Fig. 1, c is parallel to the virion axis and a and b are equivalent and perpendicular to c. Although a unique Raman tensor coordinate system (xyz) applies to each normal mode of vibration of the indolyl ring, such coordinate systems for all subunits are arranged symmetrically with respect to c in accordance with virion symmetry. The orientation of a given xyz system in terms of the Eulerian angles θ and χ is illustrated in the bottom of Fig. 1 (Tsuboi et al., 1996a; Overman et al., 1996; Tsuboi et al., 2001).
To each tryptophan normal mode (Raman band), we assign a Raman tensor α, which is defined as the first derivative of the molecular polarizability with respect to the vibrational normal coordinate. The tensor component αij represents the change of polarizability for radiation with incident and scattered electric vectors polarized, respectively, in the i and j directions. As previously (Tsuboi and Thomas, 1997), we need consider only the relative magnitudes of the diagonal tensor components, namely αxx/αzz ≡ r1 and αyy/αzz ≡ r2. Highly anisotropic Raman tensors are characterized by values of r1 and/or r2 differing greatly from unity, whereas isotropic tensors have r1 = r2 = 1. For Raman tensors that are axially symmetric with respect to z, r1 = r2 ≡ r (Overman et al., 1996). Tensor components associated with any vibration can be calculated from knowledge of the appropriate polarized Raman band intensities Ikl, where k and l (=a, b, c) are the directions of the incident and scattered electric vectors, respectively (Benevides et al., 1993).
The polarized Raman intensity ratio Icc/Ibb is given in terms of θ, χ, r1 and r2 by Eq 1 (Tsuboi et al., 1996a; Tsuboi et al., 1991).
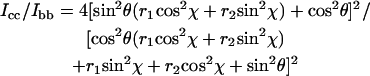 |
(1) |
The indolyl ring orientation (θ, χ) may be established from measurements of Icc/Ibb for the 1368 and 1548 cm−1 markers, designated as [Icc/Ibb]1368 and [Icc/Ibb]1548. Provided that the Raman tensors of the two bands share a common z axis, a graphical solution for θ and χ is feasible. (See the previous determination of the orientation of Trp-26 in the Ff subunit (Tsuboi et al., 1996a).)
RESULTS AND INTERPRETATION
Trp-38 Raman markers of Pf3
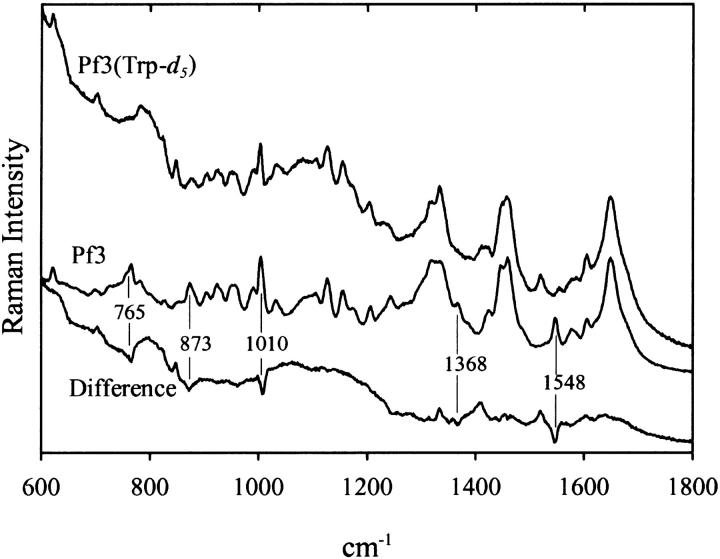
Unequivocal assignment of the Pf3 Raman bands at 1368 and 1548 cm−1 to Trp-38 was accomplished by comparing the Raman spectrum of unlabeled Pf3 with the spectrum of a labeled variant incorporating deuterated tryptophan (Trp-d5) at the Trp-38 position. Fig. 2 shows the Raman signatures of aqueous solutions of the unlabeled and labeled Pf3 virions and identifies the Raman band shifts that result from CH → CD substitution at the five indolyl ring sites. All of the observed shifts (765 → 700, 873 → 825, 1010 → 850, 1368 → 1410/1330, and 1548 → 1530 cm−1) are as expected for the indolyl CH → CD isotopic modification (Aubrey and Thomas, 1991; Overman and Thomas, 1995) and confirm assignment of the parent Raman bands in unlabeled Pf3 to the Trp-38 indolyl ring. A more comprehensive tabulation of Raman band assignments for the Pf3 virion has been given elsewhere (Wen et al., 2001).
FIGURE 2.
Raman spectra (600–1800 cm−1, 514.5-nm excitation) of solutions of unlabeled Pf3 virus (middle trace) and labeled Pf3 virus containing deuteriotryptophan (Trp-d5) at subunit residue 38 (top trace). The normalized difference spectrum is also shown (bottom trace). Samples were prepared at ∼100 mg/mL in 10 mM Tris at pH 7.8 and data were collected at 20°C.
Polarized Raman spectra of Pf3 and molecular orientations
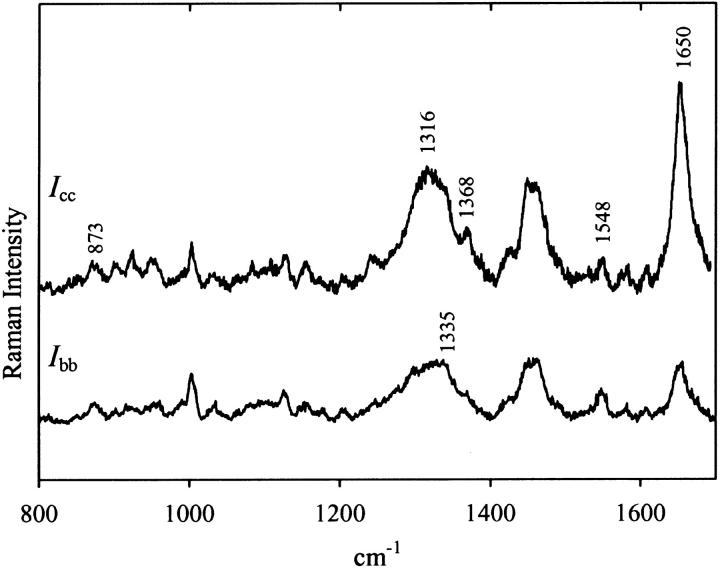
Fig. 3 shows the polarized Raman spectra (Icc and Ibb) obtained from a highly oriented Pf3 fiber. As expected, the bands in the polarized Raman spectrum correspond closely in wavenumber values to those in the solution spectrum (cf. Fig. 2, middle trace), although the relative band intensities differ in the two types of spectra. In Fig. 3 large polarization effects are evident for numerous Raman bands, including tryptophan markers near 1335 and 1368 cm−1 and the backbone amide I marker near 1650 cm−1.
FIGURE 3.
Polarized Raman spectra (800–1700 cm−1, 514.5-nm excitation) of an oriented Pf3 fiber in cc (top trace, Icc) and bb (bottom trace, Ibb) polarizations.
Orientation of the capsid subunit α-helix axis
The Raman band at 1650 cm−1 (Fig. 2) is assigned to the amide I vibration of the α-helical Pf3 subunit. As shown previously for the Ff filamentous virus (Overman et al., 1996), the polarized Raman intensity ratio for the amide I marker ([Icc/Ibb]1650) is related to the average angle of inclination of the subunit α-helix axis with respect to the virion axis (θhelix, Fig. 1 top panel). From the known amide I Raman tensor (Tsuboi et al., 1991) and the observed intensity ratio [Icc/Ibb]1650 = 3.2 ± 0.2 (Fig. 3), we obtain by use of Eq. 1 with r1 = r2, a helix tilt angle of θhelix = 16 ± 4°. This θhelix value for the Pf3 subunit is within experimental error of the values determined previously for Ff and Pf1 subunits (Overman et al., 1996; Tsuboi et al., 2000).
Orientation of the Trp-38 side chain
The Icc/Ibb values for the Trp-38 Raman markers of Pf3 at 873 (W17), 1335 (W7′), 1368 (W7″) and 1548 (W3) cm−1 (Fig. 3) are listed in Table 2. Included for comparison are the corresponding markers previously reported for the single tryptophan (Trp-26) of the Ff capsid subunit (Tsuboi et al., 1996a). The significantly different intensity polarizations observed for tryptophan Raman markers of Pf3 and Ff indicate different indolyl ring orientations in the two virion assemblies.
TABLE 2.
Tryptophan Raman bands and intensity polarizations in Pf3 and Ff viruses
| Pf3* band (cm−1) | Polarization (Icc/Ibb) | Ff† band (cm−1) | Polarization (Icc/Ibb) |
|---|---|---|---|
| 873 | ∼1.2 | 879 | ∼3 |
| 1335 | ∼0.5 | 1340 | 0.4 ± 0.2 |
| 1368 | 7 ± 2 | 1364 | 20 ± 5 |
| 1548 | 1.3 ± 0.3 | 1560 | 2.8 ± 0.2 |
Band positions are from the high resolution spectra reported previously (Wen and Thomas, 2000; Wen et al., 2001) and polarizations are from the spectra of Fig. 3.
Band positions and polarizations are as reported previously (Tsuboi et al., 1996a).
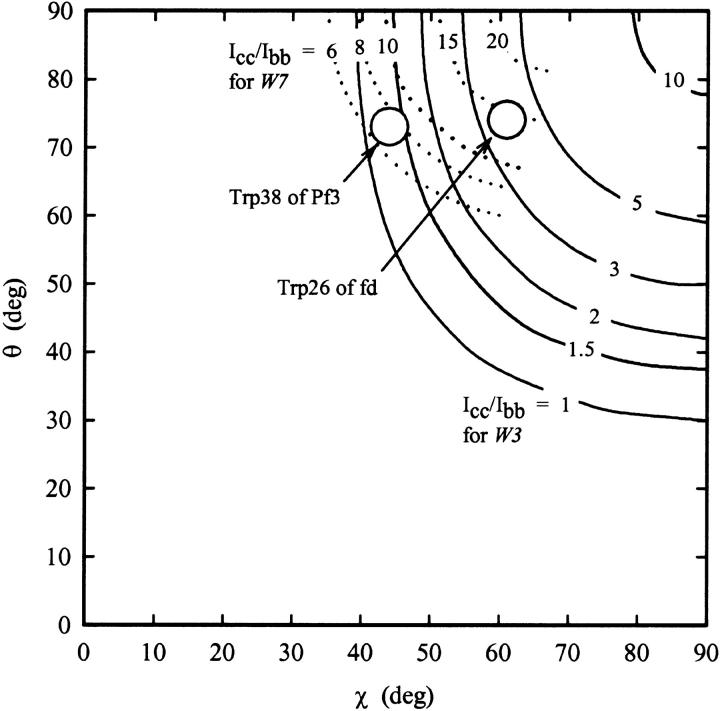
The specific geometric parameters (θ and χ, Fig. 1) that uniquely describe the Trp-38 indolyl orientation with respect to the virion axis can be determined from the data of Fig. 3 and Table 1, using the procedure outlined previously for Ff (Tsuboi et al., 1996a). In brief, we substitute the experimental value of [Icc/Ibb]1548 and the Raman tensors of the 1548 cm−1 band into Eq. 1 to find the set of (θ, χ)1548 values consistent with the data. This set defines a contour line of constant intensity ratio in (θ, χ)1548-space. The same procedure is followed for [Icc/Ibb]1368 and the Raman tensors of the 1368 cm−1 band, to yield a contour line in (θ, χ)1368-space. The two contours are then plotted in a common (θ, χ) axis system—achieved by a 22° rotation of the (θ, χ)1368 coordinate system about the χ axis (Tsuboi et al., 1996a)—to find their point of intersection, which represents the Eulerian coordinates of the indolyl moiety. The results are shown in Fig. 4.
FIGURE 4.
Isointensity contours of Icc/Ibb in (θ, χ)-space for the Trp-38 Raman markers of Pf3 at 1368 cm−1 (W7″) and 1548 cm−1 (W3). Contours were obtained by substituting the appropriate Raman tensors from Table 1 into Eq. 1. The observed Raman intensity ratios, [Icc/Ibb]1368 = 7 ± 2 and [Icc/Ibb]1548 = 1.3 ± 0.3, restrict the acceptable Eulerian angles to the circled area of (θ, χ)-space, i.e., (73 ± 4°, 44 ± 3°).
The graphical solution of Fig. 4 indicates the following Eulerian coordinates for the Trp-38 indolyl moiety: (θ, χ) = (73 ± 4°, 44 ± 3°). The uncertainties reflect the limits of error in the Raman polarization measurements.
Combining the result of Fig. 4 with the definitions of Fig. 1, we find that the plane of the indolyl ring of Trp-38 is tilted by 17° from the Pf3 virion axis (c) and that the pseudo-twofold axis of the indolyl ring (i.e., the line intersecting the Cδ1 atom and bisecting the Cζ3–Cη2 bond) is inclined at 46° from the c axis. These results, together with the previously determined magnitude of the side-chain torsion angle of Trp-38 (|χ2,1| = 90 ± 5°) (Wen and Thomas, 2000; Wen et al., 2001), provide a basis for elucidating the complete side-chain orientation of Trp-38.
Refinement of the Pf3 capsid model proposed from fiber x-ray diffraction analysis
The orientation of Trp-38 determined by polarized Raman spectroscopy differs significantly from the orientation that has been incorporated into a recently proposed Pf3 capsid model developed primarily from fiber x-ray diffraction data (Protein Data Bank entry 1IFP) (Welsh et al., 1998). The indolyl ring of the 1IFP model is characterized by χ2,1 = 142° and (θ, χ) = (34°, 29°), whereas the present experimental determination indicates |χ2,1| = 90 ± 5° and (θ, χ) = (73 ± 4°, 44 ± 3°). Interestingly, the discrepancy between the 1IFP model and the present determination can be reconciled by a single internal rotation within the Trp-38 side chain. Thus, changing the χ2,1 torsion angle of 1IFP from +142° to +93° but maintaining all other bond angles and bond lengths unchanged, yields a structure in which the Eulerian coordinates of the indolyl moiety become (θ, χ) = (77°, 45°), which is consistent with the polarized Raman results. The atomic coordinates for Trp-38 in this refined model are given in Table 3. The structures of two neighboring subunits in a refined Pf3 capsid model are shown in the top panel of Fig. 5. The refined structure is consistent with all of the available Raman, polarized Raman and UVRR data.
TABLE 3.
Revised atomic coordinates for Tryptophan 38 and Arginine 37
| Trp-38* atom (no.) | x | y | z | Arg-37† atom (no.) | x | y | z |
|---|---|---|---|---|---|---|---|
| Cγ (264) | −8.066 | −14.581 | −11.342 | Cβ (252) | −10.939 | −9.568 | −8.408 |
| Cδ1 (265) | −7.878 | −14.952 | −10.043 | Cγ (253) | −9.901 | −8.630 | −9.043 |
| Cδ2 (266) | −6.814 | −14.891 | −12.017 | Cδ (254) | −8.580 | −9.302 | −9.300 |
| Nɛ1 (267) | −6.589 | −15.434 | −9.855 | Nɛ (255) | −8.548 | −10.071 | −10.536 |
| Cɛ2 (268) | −5.911 | −15.405 | −11.076 | Cζ (256) | −7.525 | −10.829 | −10.899 |
| Cɛ3 (269) | −6.360 | −14.690 | −13.339 | Nη1 (257) | −6.463 | −10.938 | −10.126 |
| Cζ2 (270) | −4.587 | −15.768 | −11.385 | Nη2 (258) | −7.572 | −11.479 | −12.046 |
| Cζ3 (271) | −5.069 | −15.067 | −13.660 | ||||
| Cη2 (272) | −4.198 | −15.619 | −12.702 |
Obtained from the atomic coordinates of atoms 264–272 of PDB entry 1IFP by a rotation of 49° about the Cβ–Cγ bond.
Obtained from the atomic coordinates of atoms 252–258 of PDB entry 1IFP by a rotation of 125° about the Cα–Cβ bond and 108° about the Cβ–Cγ bond.
FIGURE 5.
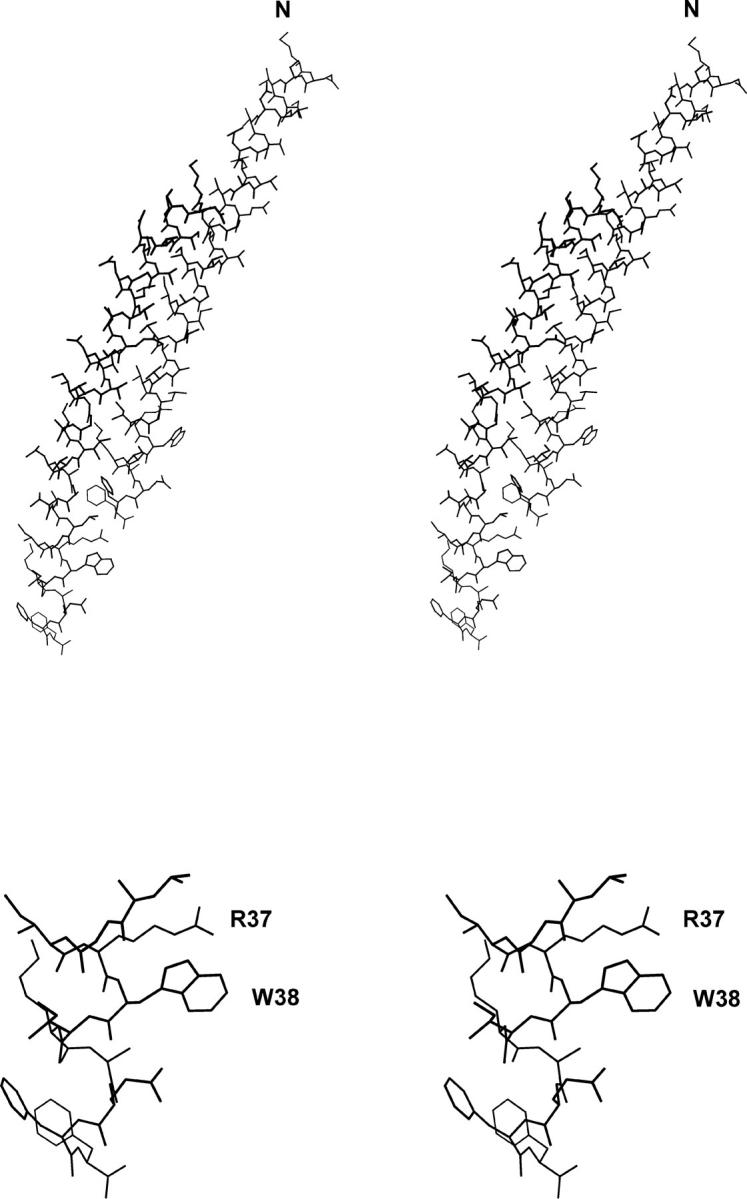
Stereo model of the refined structure of the Pf3 capsid subunit (Molscript software (Kraulis, 1991)). The top panel shows two neighboring subunits (indices 11 and 17 of the assembled capsid). The bottom panel shows residues 34–44 of the subunit C-terminus with labels indicating Arg-37 and Trp-38. In this energy-minimized model (CNS software (Brunger et al., 1998)), the guanidinium group of Arg-37 is close to the indolyl ring of Trp-38 (Nη2 to Cζ2 distance of ∼3.5 Å) implying a cation-π interaction similar to that suggested (Gallivan and Dougherty, 1999) for the tyrosine kinase domain of the insulin receptor (Hubbard et al., 1994).
Comparison of Trp-38 (Pf3) and Trp-26 (Ff) orientations
Table 2 shows that for the Ff virion the polarized Raman signature and therefore also the indolyl ring (Trp-26) orientation differ significantly from the present results on Trp-38 of Pf3. Specifically, the coordinates are (θ, χ) = (90°, 54°) for Trp-26 (Tsuboi et al., 1996a; Takeuchi et al., 1996) in contrast to (73°, 44°) for Trp-38. Thus, the plane of the Trp-26 indolyl ring in Ff is close to parallel to the virion axis (c), whereas a tilt of 17° is observed for the plane of the Trp-38 indolyl ring in Pf3; also the pseudo-twofold axis of the Trp-26 ring is inclined by 36° from c, compared to a 46° inclination for the Trp-38 ring. In addition, the magnitude of χ2,1 is quite different in the two virions, e.g., 120° for Trp-26 versus 90° for Trp-38.
Previous Raman and UVRR studies of Ff and Pf3 (Thomas et al., 1983; Overman and Thomas, 1995; Wen and Thomas, 2000; Wen et al., 2001) also demonstrate that Trp-26 and Trp-38 differ significantly with respect to the hydrophobicity of the indolyl ring environment.
Proposed cation-π interaction involving Arg-37 and Trp-38
On the basis of UVRR and fluorescence spectroscopic signatures of the Pf3 assembly, Wen and Thomas (2000) considered the prospect of an intrasubunit cation-π interaction involving the guanidinium group of Arg-37 as donor and the π-electron system of the Trp-38 indolyl ring as acceptor. Such an interaction was considered to be compatible not only with the experimental data but also with the steric expectations for cation-π stabilization in a helical protein (Ma and Dougherty, 1997; Gallivan and Dougherty, 1999). With the atomic coordinates for the Trp-38 side chain provided by the polarized Raman measurements, we examined possible orientations of the Arg-37 side chain that would be compatible with cation-π interaction and the α-helix dimensions of the 1IFP model for the Pf3 subunit (Welsh et al., 1998). We have found an acceptable conformation for the Arg-37 side chain that is sterically compatible with cation-π interaction between its guanidinium group and the Trp-38 indolyl moiety. In this conformation, the two torsions proposed in the 1IFP model, χ1 (N–Cα–Cβ–Cγ) = −55° and χ2 (Cα–Cβ–Cγ–Cδ) = +168°, are changed to +179° and −84°, respectively. All other bond angles and lengths remain unchanged. The refined coordinates of the Arg-37 atoms are listed in the right columns of Table 3. We note also that steric constraints exclude the likelihood of a cation-π interaction involving the Lys-40 and Trp-38 side chains.
Relative orientations of the Arg-37 and Trp-38 side chains in the refined model of the Pf3 capsid subunit are shown in the lower panel of Fig. 5. Here, the minimum guanidinium-indole distance is ∼3.5 Å, which is compatible with the definition of a cation-π interaction (Ma and Dougherty, 1997; Gallivan and Dougherty, 1999).
DISCUSSION AND CONCLUSIONS
The present study provides details on the orientation of the Trp-38 side chain in the capsid subunit of filamentous bacteriophage Pf3. The results also confirm the previously reported anomalous tryptophan Raman markers of the Pf3 assembly (Wen and Thomas, 2000; Wen et al., 2001), which suggest an unusual local environment for the Trp-38 indolyl ring.
In most filamentous viruses, including Pf3, Xf, PH75, Ff, If1, and IKe, the capsid subunit contains a single tryptophan residue (Thomas et al., 1983; Day et al., 1988; Pederson et al., 2001). For class I virions the subunit tryptophan is typically located in the central hydrophobic region of the α-helix, which serves as the intersubunit interface in the assembled capsid. This hydrophobic sequence is also likely to be sequestered within the cytoplasmic membrane bilayer before assembly (Webster, 1996; Feng et al., 1999; Yuen et al., 2000). Scanning and site-directed mutagenesis studies show that the tryptophan residue of the Ff subunit is essential to both capsid assembly and phage viability (Williams et al., 1995; Ridder et al., 2000). Similar results have been obtained for IKe (Williams and Deber, 1996; Williams et al., 1996).
For the class II virions Pf3 and Xf, the subunit tryptophan is not located in the central hydrophobic segment, but is within or close to the C-terminus. Although the subunit of the class II Pf1 virion contains no tryptophan, one of its two tyrosines (Tyr-40) is located within the C-terminal segment, where it may serve a structural requirement similar to that of the Trp residues of Pf3 and Xf. At present, there is no report of a successful mutation of tryptophan in any class II virion, nor of Tyr-40 in Pf1. Mutation of Trp-38 is conspicuously absent from the many mutants of Pf3 that have been developed for analysis of membrane insertion properties (Kuhn, 1995; Kiefer et al., 1997; Kiefer and Kuhn, 1999). We conclude that tryptophans present in the subunits of filamentous viruses are essential for assembly and viability. This requirement may extend also to Tyr-40 in the case of Pf1.
The structural role of the essential tryptophan residue is not fully understood for any filamentous virus assembly. However, both the location and the orientation of the tryptophan residue in class I particles suggest that the side chain is critical to proper positioning and packing of the subunits for capsid assembly and stability. In the best studied example, Ff, the indolyl ring of Trp-26 is oriented close to parallel to the virion axis (and α-helix axis) (Tsuboi et al., 1996a; Overman et al., 1996; Takeuchi et al., 1996), which is conducive to tight packing and is consistent with the hydrophobic environment indicated by Raman marker bands. Clearly, this is not the case for Pf3. The key Raman markers of Trp-38 are distinct from those of Trp-26 (Wen and Thomas, 2000; Wen et al., 2001), signaling a very different local environment. In addition, the present study shows that the side-chain orientation of Trp-38 differs significantly from that of Trp-26. The indolyl ring of Trp-38 protrudes away from the surface of the α-helix axis (Fig. 5) and appears to be unshielded by hydrophobic side chains of either the same or neighboring subunits.
The presently determined orientation of the Trp-38 side chain and a plausible orientation for the Arg-37 side chain (Table 3) demonstrate that the two side chains could form a cation-π interaction, as illustrated in the lower panel of Fig. 5. Such a cation-π interaction might stabilize the position of the guanidinium group of Arg-37 to mediate electrostatic interaction with a DNA phosphate group.
Although we have not yet investigated the orientation of the C-terminal tryptophan (Trp-39) of Xf by polarized Raman spectroscopy, the key tryptophan Raman markers of Xf, like those of Pf3, depart from the “canonical” wavenumber values observed for Ff, If1, and IKe (Thomas et al., 1983). This suggests that the C-terminal segment of the Xf subunit may also be stabilized by a cation-π interaction involving the Trp-39 indolyl moiety as a π acceptor. Several likely candidates for the role of cation donor occur in the C-terminal sequence of the Xf subunit, including Lys-35, Lys-38, Arg-41, and Arg-42. A similar situation may also occur for PH75 (Trp-37 could pair with either Lys-38, Lys-41, Arg-42, or Lys-45). In the case of Pf1, a cation-π interaction might involve the phenoxyl ring of Tyr-40 as acceptor with either Arg-44 or Lys-45 as donor. It will be interesting to evaluate these possibilities in structural models developed from future polarized Raman studies.
Finally, we note that in all filamentous viruses (Ff, Pf1, Pf3) investigated by polarized Raman methods the average orientation of the subunit α-helix axis with respect to the virion axis is close to 16°. Thus, despite differences in overall architecture between the class I (Ff) and class II (Pf1, Pf3) assemblies, the subunit axial orientations are very similar.
Acknowledgments
This research (Part LXXVIII in the series Structural Studies of Viruses by Raman Spectroscopy) was supported by National Institutes of Health (GM50776).
M. Tsuboi's permanent address is College of Science and Engineering, Iwaki-Meisei University, Iwaki, Fukushima 970-8551, Japan.
K. Nakamura's permanent address is Gifu College of Medical Technology, 795-1 Ichihiraga, Saki, Gifu 501-3892, Japan.
References
- Arp, Z., D. Autrey, J. Laane, S. A. Overman, and G. J. Thomas, Jr. 2001. Tyrosine Raman signatures of the filamentous virus Ff are diagnostic of non-hydrogen-bonded phenoxyls: demonstration by Raman and infrared spectroscopy of p-Cresol vapor. Biochemistry. 40:2522–2529. [DOI] [PubMed] [Google Scholar]
- Aubrey, K. L., and G. J. Thomas, Jr. 1991. Raman spectroscopy of filamentous bacteriophage Ff (fd, M13, f1) incorporating specifically-deuterated alanine and tryptophan side chains: assignments and structural interpretation. Biophys. J. 61:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides, J. M., M. Tsuboi, A. H. J. Wang, and G. J. Thomas, Jr. 1993. Local Raman tensors of double-helical DNA in the crystal: a basis for determining DNA residue orientations. J. Am. Chem. Soc. 115:5351–5359. [Google Scholar]
- Blanch, E. W., A. F. Bell, L. Hecht, L. A. Day, and L. D. Barron. 1999. Raman optical activity of filamentous bacteriophages: hydration of alpha-helices. J. Mol. Biol. 290:1–7. [DOI] [PubMed] [Google Scholar]
- Blanch, E. W., L. Hecht, L. A. Day, D. M. Pederson, and L. D. Barron. 2001. Tryptophan absolute stereochemistry in viral coat proteins from Raman optical activity. J. Am. Chem. Soc. 123:4863–4864. [DOI] [PubMed] [Google Scholar]
- Brunger, A. T., P. D. Adams, G. M. Clore, W. L. Delano, P. Gross, R. W. Grosse-Kunstleve, J.-S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simmonson, and G. L. Warren. 1998. Crystallography and NMR system. Acta Crystallogr. D Biol. Crystallogr. 54:905–921. [DOI] [PubMed] [Google Scholar]
- Day, L. A., C. J. Marzec, S. A. Reisberg, and A. Casadevall. 1988. DNA packing in filamentous bacteriophages. Annu. Rev. Biophys. Biophys. Chem. 17:509–539. [DOI] [PubMed] [Google Scholar]
- Feng, J. N., P. Model, and M. Russel. 1999. A trans-envelope protein complex needed for filamentous phage assembly and export. Mol. Microbiol. 34:745–755. [DOI] [PubMed] [Google Scholar]
- Gallivan, J. P., and D. A. Dougherty. 1999. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. USA. 96:9459–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, S. R., L. Wei, L. Ellis, and W. A. Hendrickson. 1994. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 372:746–754. [DOI] [PubMed] [Google Scholar]
- Kiefer, D., X. Hu, R. Dalbey, and A. Kuhn. 1997. Negatively charged amino acid residues play an active role in orienting the Sec-independent Pf3 coat protein in the Escherichia coli inner membrane. EMBO J. 16:2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer, D., and A. Kuhn. 1999. Hydrophobic forces drive spontaneous membrane insertion of the bacteriophage Pf3 coat protein without topological control. EMBO J. 18:6299–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis, P. J. 1991. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24:946–950. [Google Scholar]
- Kuhn, A. 1995. Major coat proteins of bacteriophage Pf3 and M13 as model systems for Sec-independent protein transport. FEMS Microbiol. Rev. 17:185–190. [DOI] [PubMed] [Google Scholar]
- Ma, J. C., and D. A. Dougherty. 1997. The cation-π interaction. Chem. Rev. 97:1303–1324. [DOI] [PubMed] [Google Scholar]
- Marvin, D. A. 1998. Filamentous phage structure, infection and assembly. Curr. Opin. Struct. Biol. 8:150–158. [DOI] [PubMed] [Google Scholar]
- Marvin, D. A., R. D. Hale, C. Nave, and M. H. Citterich. 1994. Molecular models and structural comparisons of native and mutant Class I filamentous bacteriophages: Ff (fd, f1, M13), If1 and IKe. J. Mol. Biol. 235:260–286. [DOI] [PubMed] [Google Scholar]
- Matsuno, M., H. Takeuchi, S. A. Overman, and G. J. Thomas, Jr. 1998. Orientations of Tyrosines 21 and 24 in coat subunits of Ff filamentous virus: determination by Raman linear intensity difference spectroscopy and implications for subunit packing. Biophys. J. 74:3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman, S. A., and G. J. Thomas, Jr. 1995. Raman spectroscopy of the filamentous virus Ff (fd, fl, M13): structural interpretation for coat protein aromatics. Biochemistry. 34:5440–5451. [DOI] [PubMed] [Google Scholar]
- Overman, S. A., M. Tsuboi, and G. J. Thomas, Jr. 1996. Subunit orientation in the filamentous virus Ff (fd, f1, M13). J. Mol. Biol. 259:331–336. [DOI] [PubMed] [Google Scholar]
- Pederson, D. M., L. C. Welsh, D. A. Marvin, M. Sampson, R. N. Perham, M. Yu, and M. R. Slater. 2001. The protein capsid of filamentous bacteriophage PH75 from Thermus thermophilus. J. Mol. Biol. 309:401–421. [DOI] [PubMed] [Google Scholar]
- Ridder, A. N., S. Morein, J. G. Stam, A. Kuhn, B. de Kruijff, and J. A. Killian. 2000. Analysis of the role of interfacial tryptophan residues in controlling the topology of membrane proteins. Biochemistry. 39:6521–6528. [DOI] [PubMed] [Google Scholar]
- Symmons, M. F., L. C. Welsh, C. Nave, D. A. Marvin, and R. N. Perham. 1995. Matching electrostatic charge between DNA and coat protein in filamentous bacteriophage. Fibre difraction of charge-deletion mutants. J. Mol. Biol. 245:86–91. [DOI] [PubMed] [Google Scholar]
- Takeuchi, H., M. Matsuno, S. A. Overman, and G. J. Thomas, Jr. 1996. Raman linear intensity difference of flow-oriented macromolecules: orientation of the indole ring of Tryptophan-26 in filamentous virus fd. J. Am. Chem. Soc. 118:3498–3507. [Google Scholar]
- Thomas, G. J., Jr., J. M. Benevides, S. A. Overman, T. Ueda, K. Ushizawa, M. Saitoh, and M. Tsuboi. 1995. Polarized Raman spectra of oriented fibers of A DNA and B DNA: anisotropic and isotropic local Raman tensors of base and backbone vibrations. Biophys. J. 68:1073–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. J., Jr., B. Prescott, and L. A. Day. 1983. Structure similarity, difference and variability in the filamentous viruses fd, If1, IKe, Pf1, Xf and Pf3. J. Mol. Biol. 165:321–356. [DOI] [PubMed] [Google Scholar]
- Tsuboi, M., T. Ikeda, and T. Ueda. 1991. Raman microscopy of a small unixial crystal: tetragonal aspartame. J. Raman Spectrosc. 22:619–626. [Google Scholar]
- Tsuboi, M., S. A. Overman, and G. J. Thomas, Jr. 1996a. Orientation of Tryptophan-26 in coat protein subunits of the filamentous virus Ff by polarized Raman microspectroscopy. Biochemistry. 35:10403–10410. [DOI] [PubMed] [Google Scholar]
- Tsuboi, M., M. Suzuki, S. A. Overman, and G. J. Thomas, Jr. 2000. Intensity of the polarized Raman band at 1340–1345 cm−1 as an indicator of protein α-helix orientation: application to Pf1 filamentous virus. Biochemistry. 39:2677–2684. [DOI] [PubMed] [Google Scholar]
- Tsuboi, M., and G. J. Thomas, Jr. 1997. Raman scattering tensors in biological molecules and their assemblies. Appl. Spectrosc. Revs. 32:263–299. [Google Scholar]
- Tsuboi, M., T. Ueda, K. Ushizawa, Y. Ezaki, S. A. Overman, and G. J. Thomas, Jr. 1996b. Raman tensors for the tryptophan side chain in proteins determined by polarized Raman microspectroscopy of oriented N-Acetyl-l-Tryptophan crystals. J. Mol. Struct. 379:43–50. [Google Scholar]
- Tsuboi, M., K. Ushizawa, K. Nakamura, J. M. Benevides, S. A. Overman, and G. J. Thomas, Jr. 2001. Orientations of Tyr 21 and Tyr 24 in the capsid of filamentous virus Ff determined by polarized Raman spectroscopy. Biochemistry. 40:1238–1247. [DOI] [PubMed] [Google Scholar]
- Webster, R. E. 1996. Biology of the filamentous bacteriophage. In Phage Display of Peptides and Proteins. B. K. Kay, J. Winter, and J. McCaffery, editors. Academic Press, London. 1–20.
- Welsh, L. C., M. F. Symmons, and D. A. Marvin. 2000. The molecular structure and structure transition of the α-helical capsid in filamentous bacteriophage Pf1. Acta Crystallogr. D Biol. Crystallogr. 56:137–150. [DOI] [PubMed] [Google Scholar]
- Welsh, L. C., M. F. Symmons, J. M. Sturtevant, D. A. Marvin, and R. N. Perham. 1998. Structure of the capsid of Pf3 filamentous phage determined from x-ray fibre diffraction data at 3.1 Å resolution. J. Mol. Biol. 283:155–177. [DOI] [PubMed] [Google Scholar]
- Wen, Z. Q., A. Armstrong, and G. J. Thomas, Jr. 1999. Demonstration by ultraviolet resonance Raman spectroscopy of differences in DNA organization and interactions in filamentous viruses Pf1 and fd. Biochemistry. 38:3148–3156. [DOI] [PubMed] [Google Scholar]
- Wen, Z. Q., S. A. Overman, P. Bondre, and G. J. Thomas, Jr. 2001. Structure and organization of bacteriophage Pf3 probed by Raman and ultraviolet resonance Raman spectroscopy. Biochemistry. 40:449–458. [DOI] [PubMed] [Google Scholar]
- Wen, Z. Q., S. A. Overman, and G. J. Thomas, Jr. 1997. Structure and interactions of the single-stranded DNA genome of filamentous virus fd: investigation by ultraviolet resonance Raman spectroscopy. Biochemistry. 36:7810–7820. [DOI] [PubMed] [Google Scholar]
- Wen, Z. Q., and G. J. Thomas, Jr. 2000. Ultraviolet-resonance Raman spectroscopy of the filamentous virus Pf3: interactions of Trp 38 specific to the assembled virion subunit. Biochemistry. 39:146–152. [DOI] [PubMed] [Google Scholar]
- Williams, K. A., and C. M. Deber. 1996. Biophysical characterization of wild-type and mutant bacteriophage IKe major coat protein in the virion and in detergent micelles. Biochemistry. 35:10472–10483. [DOI] [PubMed] [Google Scholar]
- Williams, K. A., N. A. Farrow, C. M. Deber, and L. E. Kay. 1996. Structure and dynamics of bacteriophage IKe major coat protein in MPG micelles by solution NMR. Biochemistry. 35:5145–5157. [DOI] [PubMed] [Google Scholar]
- Williams, K. A., M. Glibowicka, Z. Li, H. Li, A. R. Khan, Y. M. Chen, J. Wang, D. A. Marvin, and C. M. Deber. 1995. Packing of coat protein amphipathic and transmembrane helices in filamentous bacteriophage M13: role of small residues in protein oligomerization. J. Mol. Biol. 252:6–14. [DOI] [PubMed] [Google Scholar]
- Yuen, C. T., A. R. Davidson, and C. M. Deber. 2000. Role of aromatic residues at the lipid-water interface in micelle-bound bacteriophage M13 major coat protein. Biochemistry. 39:16155–16162. [DOI] [PubMed] [Google Scholar]