Abstract
The mechanism(s) underlying the sorting of integral membrane proteins between the Golgi complex and the plasma membrane remain uncertain because no specific Golgi retention signal has been found. Moreover one can alter a protein's eventual localization simply by altering the length of its transmembrane domain (TMD). M. S. Bretscher and S. Munro (Science. 261:1280–1281, 1993) therefore proposed a physical sorting mechanism based on the hydrophobic match between the proteins' TMD and the bilayer thickness, in which cholesterol would regulate protein sorting by increasing the lipid bilayer thickness. In this model, Golgi proteins with short TMDs would be excluded from cholesterol-enriched domains (lipid rafts) that are incorporated into transport vesicles destined for the plasma membrane. Although attractive, this model remains unproven. We therefore evaluated the energetic feasibility of a cholesterol-dependent sorting process using the theory of elastic liquid crystal deformations. We show that the distribution of proteins between cholesterol-enriched and cholesterol-poor bilayer domains can be regulated by cholesterol-induced changes in the bilayer physical properties. Changes in bilayer thickness per se, however, have only a modest effect on sorting; the major effect arises because cholesterol changes also the bilayer material properties, which augments the energetic penalty for incorporating short TMDs into cholesterol-enriched domains. We conclude that cholesterol-induced changes in the bilayer physical properties allow for effective and accurate sorting which will be important generally for protein partitioning between different membrane domains.
INTRODUCTION
Several lines of evidence show that membrane protein sorting between the Golgi complex and the plasma membrane is determined, at least in part, by the length of the proteins' transmembrane domain (TMD). First, Golgi membrane proteins tend to have shorter TMDs (∼15 AA) than plasma membrane proteins (∼20 AA) (Bretscher and Munro, 1993; Masibay et al., 1993). Second, a protein, that is normally retained in the Golgi complex, becomes targeted to the plasma membrane if the TMD is increased in length (Cole et al., 1998; Masibay et al., 1993; Munro, 1991)—but is minimally affected if the TMD is replaced by a Leu sequence of the same length as the native segment (Munro, 1991). Third, proteins that normally traffic to the plasma membrane are retained in the Golgi complex if the hydrophobic length of the TMD is shortened (Sivasubramanian and Nayak, 1987). Fourth, no specific Golgi retention signal has been identified, and the mechanism underlying the retention of Golgi proteins cannot be saturated by overexpression (Gleeson, 1998; Nilsson and Warren, 1994; Opat et al., 2001). Taken together, these results suggest that the sorting mechanism(s) underlying protein retention in the Golgi complex depend on some general physical characteristic of the bilayer-protein interactions.
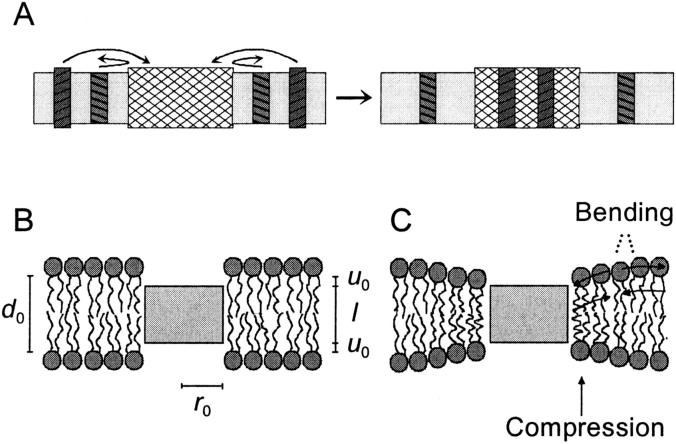
Bretscher and Munro (1993) proposed such a physical mechanism, which was based on the following observations: first, cholesterol increases the thickness of lipid bilayers (Nezil and Bloom, 1992); and second, the cholesterol content of the cellular membranes increases along the secretory pathway such that cholesterol in the plasma membrane constitutes ∼50% of the membrane lipids (van Meer, 1989). Cholesterol therefore was proposed to regulate protein sorting by a bilayer-mediated mechanism, in which proteins are targeted to bilayers whose hydrophobic thickness matches the hydrophobic length of their TMD. Sorting in the generally cholesterol-poor Golgi bilayers would involve the lateral partitioning of plasma membrane proteins with longer TMDs into cholesterol-enriched membrane domains (now called lipid rafts; Brown and London, 1998; Simons and Ikonen, 1997), whereas Golgi-resident proteins, with shorter TMDs, would be excluded from the cholesterol-enriched membrane domains (Fig. 1 A). The increase in membrane cholesterol content along the secretory pathway further was proposed to reflect a preferential incorporation of cholesterol-enriched domains into forward moving transport vesicles, which therefore would account for protein sorting.
FIGURE 1.
(A) Lateral sorting of membrane proteins (dark-hatched) between thin, cholesterol-poor bilayer domains (light gray) and thicker, cholesterol-enriched bilayer domains (cross-hatched). The proteins will tend toward the domain in which there is hydrophobic match between the protein length and the bilayer thickness. (B) In a nondeformable lipid bilayer, a mismatch between the hydrophobic thickness of the bilayer and the protein hydrophobic length leads to exposure of hydrophobic surface to the aqueous surroundings. (C) In a deformable bilayer, the hydrophobic coupling between the protein and the bilayer induces a bilayer deformation.
Because of the role of the hydrophobic length of the TMD in sorting, and further because cholesterol depletion leads to mistargeting of plasma membrane proteins, the association of membrane proteins with cholesterol-enriched domains is currently viewed as a potential sorting mechanism (Bagnat et al., 2001; Dumas et al., 1999; Keller and Simons, 1998). Although the mechanism(s) underlying the genesis and maintenance of the Golgi complex remain unresolved (cf. Check, 2002), a general feature of all sorting mechanisms is a lateral segregation of proteins between different compartments, which eventually become part of vesicles involved in forward or retrograde transport (cf. Mellman and Warren, 2000). That is, even if proteins destined for the plasma membrane move forward by cisternal maturation (Check, 2002; Munro, 1998), the latter process would involve retrograde transport of Golgi-resident proteins. Also in this case the ability of a membrane protein to associate with cholesterol-enriched domains would serve as a sorting mechanism—provided the retrograde transport vesicles are formed from phospholipid-rich domains of the Golgi bilayers (Munro, 1998).
Bilayer-based sorting, being a physical mechanism, will be operative generally; but it may not be sufficient for effective protein sorting. Targeting to different membrane compartments, for example, can involve sequence-dependent recognition signals (e.g., Bonifacino and Dell'Angelica, 1999), which will exert their action in conjunction with the bilayer-mediated sorting mechanism. The important question thus becomes: how large an impact might a bilayer-based sorting mechanism have on overall protein sorting?
General support for a bilayer-mediated sorting mechanism was obtained in studies on the insertion of hydrophobic α-helices into synthetic lipid bilayers, which correlates with the bilayer thickness and cholesterol content (Ren et al., 1997; Webb et al., 1998). Nevertheless, it is not clear if cholesterol-induced changes in bilayer thickness are sufficient to regulate protein sorting or whether changes in other bilayer properties, such as the material properties, also need to be involved. The adsorption of amphipathic peptides to a lipid bilayer thus varies as a function of the area-compression modulus (cf. Vidal et al., 2002). To address this uncertainty, we have examined the energetic feasibility of a sorting mechanism based on cholesterol-induced changes in the physical properties of lipid bilayers.
We evaluated the feasibility of a simple bilayer-based sorting mechanism by considering the energetic consequences of a mismatch between the hydrophobic thickness (d0) of a lipid bilayer and the hydrophobic length (l) of the TMD of a membrane protein. If the bilayer were rigid, and its thickness were invariant, a mismatch between d0 and l would incur an energetic cost that would arise because of the energetic penalty of exposing hydrophobic residues to water (Fig. 1 B; also see Tanford, 1980). If the bilayer were just a thin sheet of liquid hydrocarbon, stabilized by the polar headgroups, the hydrophobic coupling between the TMD and the bilayer core would cause the bilayer to adjust locally to the hydrophobic length of the TMD. But lipid bilayers are neither rigid nor thin sheets of liquid hydrocarbon; they are elastic liquid crystals with well-defined material properties (Bloom et al., 1991; Helfrich, 1973; Mouritsen and Andersen, 1998). Consequently, when d0 ≠ l, the hydrophobic mismatch will induce an elastic bilayer deformation, in which the acyl chains in the vicinity of the TMD are extended or compressed and also splayed relative to each other, which will incur an energetic cost (Fig. 1 C; also see Mouritsen and Bloom, 1984). Because the bilayer deformation energy contributes to the cost of inserting a membrane protein into a lipid bilayer domain, protein sorting will be determined by both the bilayer thickness and material properties (the resistance to compression/extension and bending/splay). As cholesterol alters these bilayer properties, it should, in principle, effect protein distribution—the question becomes whether the cholesterol-induced changes are large enough to be of consequence.
Cholesterol-enriched membrane domains also are enriched in sphingolipids (Simons and Ikonen, 1997.) In the present analysis, however, we consider only the effects of cholesterol because there are insufficient data to quantitatively evaluate the combined effects of sphingolipids and cholesterol. Based on their effects on bilayer thickness (Holthuis et al., 2001) and material moduli (McIntosh et al., 1992), however, the presence of sphingolipids will only further potentiate the effects of cholesterol.
We use the theory of elastic liquid crystal deformations (Huang, 1986) to evaluate the ability of cholesterol to regulate protein sorting by changing the physical properties of lipid bilayers. The results show that cholesterol-induced changes in bilayer thickness and material properties indeed can effect protein sorting. If cholesterol altered only the bilayer thickness, however, the energetic consequences of a hydrophobic mismatch would be rather modest—and the sorting would be less efficient. But the combined effects of the changes in bilayer thickness and material properties are substantial, and the energetic cost of a bilayer deformation is of sufficient magnitude to regulate sorting, be it alone or in combination with other sorting mechanisms (Gleeson, 1998; Opat et al., 2001). The fact that cholesterol-enriched lipid domains are also enriched in sphingolipids will further increase the effects of cholesterol on protein sorting.
THEORY
Elastic bilayer deformations and the bilayer spring constant
When the hydrophobic interactions between a symmetric bilayer and an embedded inclusion are strong enough to ensure that there is no exposure of hydrophobic residues, the depth of the deformation in each monolayer (u0), will be (d0 − l) / 2 (Fig. 1 C). The associated bilayer deformation energy (ΔGdef) will be the sum of contributions from bilayer compression, which varies with u0 and the area-compression modulus (Ka), and monolayer bending, which varies with the monolayer curvature (c) and the bending modulus (Kc) (Fig. 1 C). In addition to these continuum contributions, there will be a contribution from the local lipid packing around the protein, which will tend to increase the deformation energy above the continuum contribution (May, 2000; Nielsen and Andersen, 2000; Nielsen et al., 1998). In the following analysis we define ΔGdef as the bilayer deformation energy given by the continuum contributions when the cost of local lipid packing is neglected (see Nielsen and Andersen, 2000, for a detailed discussion of this issue).
The formal expression for the bilayer deformation energy (Dan et al., 1994; Helfrich and Jakobsson, 1990; Huang, 1986; Nielsen and Andersen, 2000; Nielsen et al., 1998) is
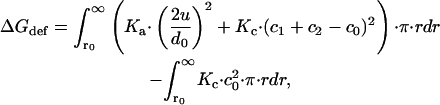 |
(1) |
where r0 denotes the radius of the inclusion, r is the distance from the center of the inclusion, c1 and c2 are the principal curvatures of the monolayer, and c0 is the equilibrium curvature of an isolated monolayer.
Eq. 1 appears forbidding; but its exact solution is a second order polynomial, which reduces to a particularly simple expression when c0 = 0,
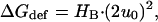 |
(2) |
where the spring constant (HB) is determined by Ka, Kc, d0, and r0. HB can be determined for any choice of Ka, Kc, r0 and d0 using the scaling relations derived by Nielsen and Andersen (2000) (see Appendix). In addition to the bilayer material constants, the value of HB is determined also by local lipid packing around the protein; and estimates for HB differ threefold depending on whether this contribution is included or not (Nielsen and Andersen, 2000).
Eq. 2 not only is the analytical solution to Eq. 1, it also describes well the effects of changes in bilayer thickness on the function of gramicidin channels (Lundbæk and Andersen, 1999). Moreover, the spring constant, determined using gramicidin channels, is in good agreement with predictions based on the elastic bilayer model using independently obtained material moduli and including the constraints on lipid packing. In an attempt to ensure that we are not overestimating the consequences of a hydrophobic mismatch, we will in the following assume that there are no constraints on local lipid packing around the protein, however. The present calculations thus should represent lower estimates of the bilayer contributions to protein sorting.
The importance of hydrophobic mismatch is a general feature of analyses of protein-bilayer interactions, and ΔGdef calculated using Eq. 2 is in general agreement with results obtained using other methods (Mouritsen and Bloom, 1984; Fattal and Ben-Shaul, 1993; Ben-Shaul et al., 1996; Bransburg-Zabary et al., 2002); but Eq. 2 provides for a particularly convenient method to evaluate the effects of cholesterol on ΔGdef, as the value of HB in the presence of cholesterol can be calculated from experimentally determined values of Ka, Kc, and d0 (Nielsen et al., 1998; Nielsen and Andersen, 2000).
How to determine HB (and the effects of cholesterol)
Membrane phospholipids tend to have a saturated acyl chain at the sn-1 position and an unsaturated acyl chain at sn-2, and 1-stearoyl-2-oleoyl-phoshatidylcholine (SOPC) has been proposed as a prototypical membrane phospholipid (Marsh, 1990; Needham, 1995). Moreover, Ka for an SOPC:Cholesterol (SOPC:Chol) bilayer at an SOPC:Chol molar ratio 1:1, 781 ± 45 pN/nm (mean ± SD) (Needham and Nunn, 1990), is comparable to Ka in red blood cell membranes, 450 pN/nm (Evans and Skalak, 1979) and in plasma membrane blebs from rabbit skeletal muscle, 490 ± 88 pN/nm (mean ± SD) (Nichol and Hutter, 1996). We therefore evaluate the ability of cholesterol to regulate membrane protein sorting, by calculating the effects of cholesterol on the ΔGdef associated with accommodating an integral membrane protein in SOPC and SOPC:Chol bilayers.
We first consider the effects of cholesterol on the sorting of an integral membrane protein with a single α-helical TMD of radius, r0 = 0.65 nm (Voegler Smith and Hall, 2001). Such a TMD will remain in an α-helical conformation irrespective of the hydrophobic mismatch with the surrounding bilayer (Zhang et al., 1992). The hydrophobic thickness, d0, of an SOPC bilayer is ∼3.0 nm (Rawicz et al., 2000), and the addition of 50% cholesterol to a phospholipid bilayer increases d0 ∼10% (Nezil and Bloom, 1992). We therefore set d0 to be 3.0 nm for the SOPC bilayer and 3.3 nm for the SOPC:Chol (1:1) bilayer. We further assume that the bilayer thickness varies as a linear function of the cholesterol mole fraction.
For the present calculations, we use the values for Ka and Kc in SOPC and SOPC:Chol (1:1) bilayers measured by Evans and Rawicz (1990) and Needham and Nunn (1990) (Table 1). These values may be underestimated by up to 20% (cf. Rawicz et al., 2000); but they were obtained using similar criteria (none of our conclusions would be affected if we used the larger values for the moduli). Using the scaling relations in Nielsen and Andersen (2000), we thus find HB to be 4.1 kcal/(mol nm2) and 13.1 kcal/(mol nm2) in SOPC and SOPC:Chol (1:1) bilayers, respectively.
TABLE 1.
Bilayer material moduli
Mean ± SD.
Material moduli measured by *Needham and Nunn (1990); †Evans and Rawicz (1990).
RESULTS
Energetics of a hydrophobic mismatch between a single α-helix and its host bilayer
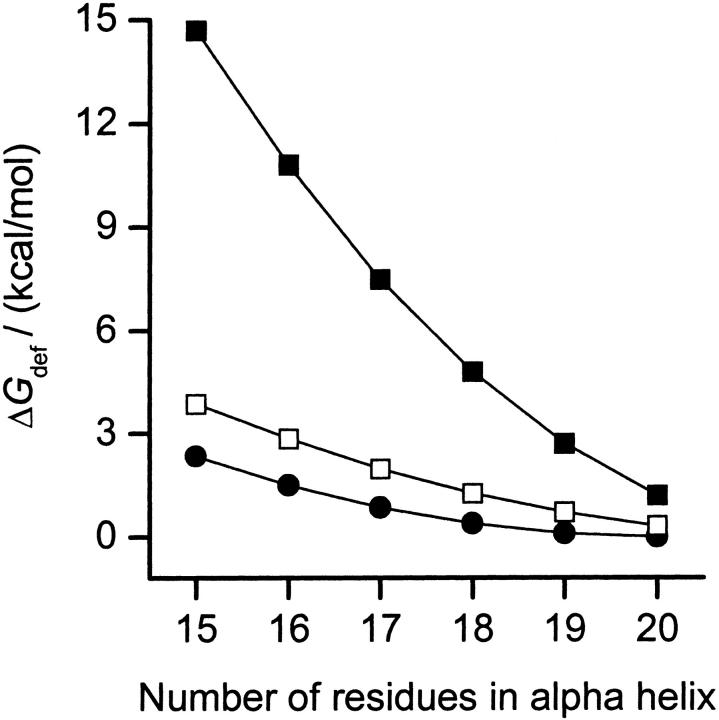
Using Eq. 2 and the above values for HB we calculate the ΔGdef contribution to the insertion energy for an α-helix in an SOPC or an SOPC:Chol (1:1) bilayer. Fig. 2 shows the results for helices of 15–20 AA, corresponding to l between 2.25 and 3.00 nm. Because the hydrophobic length of a 20 AA α-helix matches the hydrophobic thickness of SOPC, ΔGdef is zero in this bilayer; the addition of cholesterol has only a modest effect on ΔGdef, which increases to 1 kcal/mol. For the 15 and 17 AA helices, however, cholesterol causes a large increase in ΔGdef, which increases from 2 and 1 kcal/mol in the SOPC bilayer to 14 and 7 kcal/mol in the SOPC:Chol (1:1) bilayer. These energies are large enough to provide a mechanistic basis for membrane protein sorting. They also provide an estimate of the energies needed for sequence-specific sorting mechanisms to override the simple bilayer-based sorting. For comparison, the strength of a hydrogen bond is usually assumed to be ∼3 kcal/mol, and the energy released by the hydrolysis of one molecule of ATP to ADP is ∼9 kcal/mol (Veech et al., 1979).
FIGURE 2.
ΔGdef of inserting α-helices having 15–20 AA into SOPC (•); SOPC:Chol (1:1) bilayers (▪); and a bilayer with a thickness corresponding to SOPC:Chol (1:1) but with material properties as SOPC (□).
Given the above results it becomes useful to evaluate the relative importance of the cholesterol-induced changes in bilayer thickness versus the changes in bilayer material moduli. To do so we calculated ΔGdef assuming that cholesterol increased only d0, but had no effect on the material properties. In this situation ΔGdef increases only moderately relative to the values in SOPC (Fig. 2). For helices of 15, 17, and 20 AA the increase is eight-, six-, and fourfold less than the full effects caused by cholesterol.
To evaluate the concentration-dependence of the effects of cholesterol on ΔGdef we need to know how changes in the bilayer cholesterol content alter Ka, Kc, and d0. Fig. 3 A shows Ka as a function of the cholesterol mole fraction (fChol) in an SOPC bilayer (Needham and Nunn, 1990).
FIGURE 3.
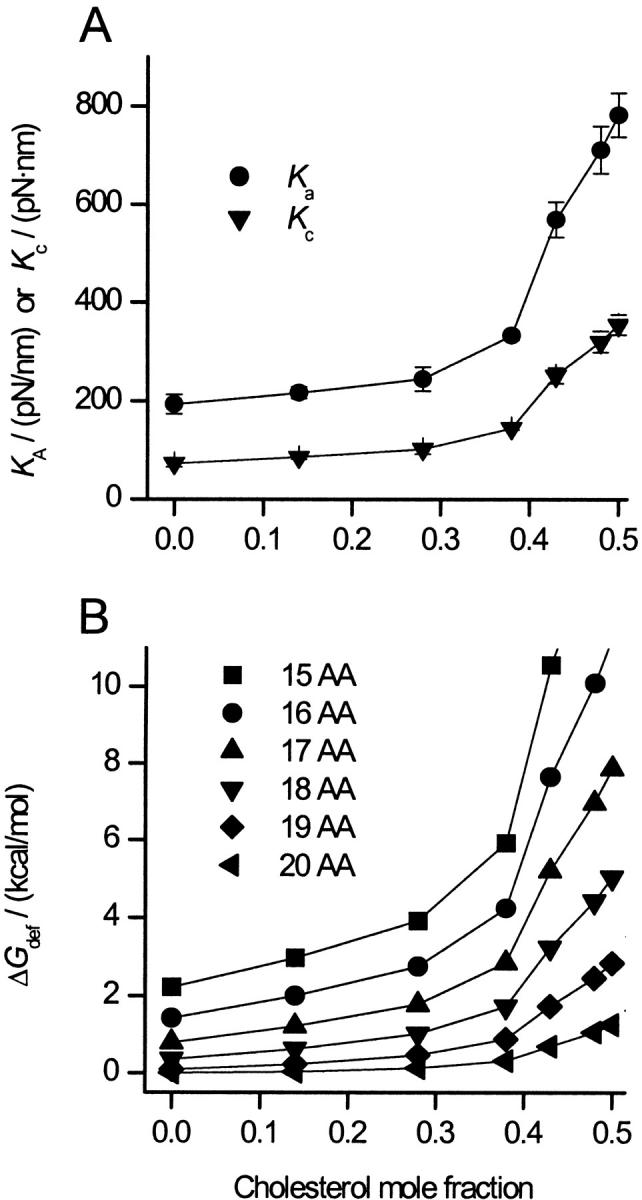
(A) Effects of cholesterol on the material moduli of SOPC bilayers having various fChol. (•) Ka measured by Needham and Nunn (1990); (▾) Kc calculated using Eq. 3. (B) The effect of cholesterol on ΔGdef for α-helices having 15–20 AA.
For fChol < 0.3, cholesterol has only modest effects on Ka; above this value, Ka rises sharply. Mechanical analysis (Evans and Skalak, 1979) show that Kc, Ka, and d0 are related by:
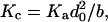 |
(3) |
where the coefficient b (24) is independent of the acyl chain length in both saturated and monounsaturated phosphatidylcholine bilayers (Rawicz et al., 2000). Needham (1995) similarly found b to be invariant among bilayers of varying composition, including cholesterol-containing bilayers. It thus is possible to estimate the cholesterol-dependent changes in Kc from the measured Ka values (see Fig. 3 A). From the changes in the material moduli, the cholesterol-dependent changes in HB and ΔGdef (Fig. 3 B) can be calculated. Because Kc for SOPC:Chol (1:1), calculated using Eq. 3, differs slightly from the measured value in Table 1, ΔGdef will also differ. This difference never exceeds 10%, however. As for the material moduli, the effects of cholesterol on ΔGdef are modest below a fChol of 0.3; above this threshold ΔGdef rises sharply.
Cholesterol-induced sorting of single α-helices
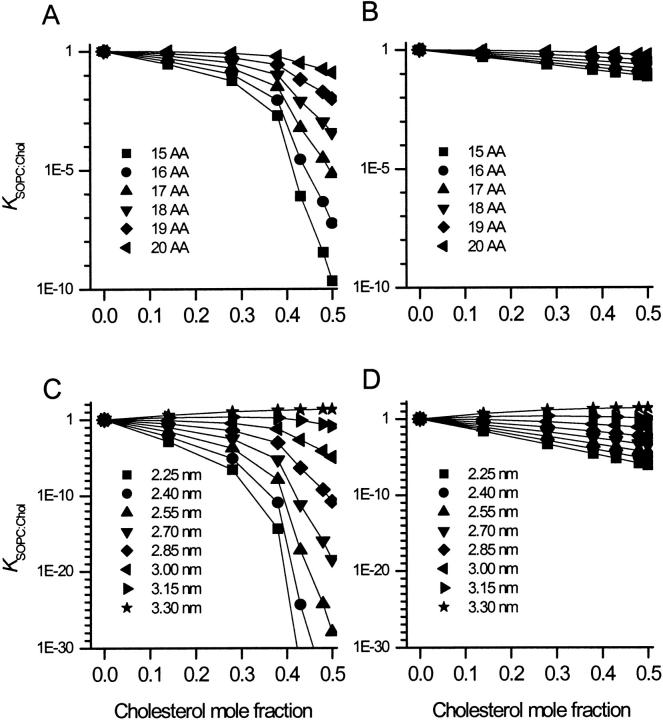
The cholesterol-induced changes in ΔGdef will affect the sorting of α-helices. Fig. 4 A shows the lateral partition coefficient between SOPC:Chol and SOPC bilayer domains (KSOPC:Chol) for α-helices of varying length, where
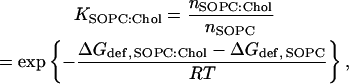 |
(4) |
and nSOPC and nSOPC:Chol denote the helix densities, and ΔGdef,SOPC and ΔGdef,SOPC:Chol denote the bilayer deformation energies in the indicated bilayer component. All values of ΔGSOPC:Chol were calculated using the Kc obtained from Eq. 3.
FIGURE 4.
(A, C) Effects of cholesterol on the lateral partition coefficient, KSOPC:Chol, of α-helices (A), and multihelical membrane proteins (C), of different length, between SOPC and SOPC:Chol bilayer domains. (B, D) Effects on the partition coefficient of α-helices (B), and multihelical membrane proteins (D), of different length, between SOPC and a bilayer domain with a thickness corresponding to SOPC:Chol, but with material properties as SOPC.
For fChol < 0.3, cholesterol has only a modest effect on the lateral distribution of single α-helices between cholesterol-free and cholesterol-enriched membrane domains, which means that sorting will be relatively inefficient (Fig. 4 A). For fChol > 0.4, cholesterol has a very strong effect. If allowed to distribute freely between SOPC and SOPC:Chol (1:1) bilayer domains, KSOPC:Chol of 20 AA, 19 AA, 18 AA, and 17 AA helices will be 10−1, 10−2, 10−4, and 10−5, respectively. Based on its effects on ΔGdef alone, cholesterol thus allows the exclusion of α-helices from a cholesterol-enriched domain. It further allows an accurate discrimination between α-helices that have only modest differences in hydrophobic length.
To evaluate the relative importance of the cholesterol-induced changes in bilayer material properties, we calculated the effects on the sorting of α-helices assuming that cholesterol effected only the bilayer thickness (Fig. 4 B). In this situation the effects of cholesterol would be much weaker, and KSOPC:Chol of the 20 AA, 19 AA, 18 AA, and 17 AA helices would be 0.6, 0.35, 0.20, and 0.15, respectively. Thus, if cholesterol altered only the bilayer thickness, KSOPC:Chol for a 20-AA helix would be fourfold that of a 17-AA helix. This is in contrast to the full effects of cholesterol, where KSOPC:Chol of the 20-AA helix is four orders-of-magnitude larger than that for the 17-AA helix (as calculated above). The cholesterol-induced changes in the bilayer material properties thus dramatically potentiate the effects of the changes in bilayer thickness.
Cholesterol-induced sorting of membrane proteins
Because HB scales with the radius of a bilayer inclusion, ΔGdef for multihelical membrane proteins will be larger than for a single α-helix. We show this for a protein with the dimensions of a nicotinic acetylcholine receptor (nAChR). The structure of the nAChR has been determined, and r0 and l are both ∼3 nm (e.g., Unwin, 2000). Using the scaling relations in Nielsen and Andersen (2000), HB is 21.2 kcal/(mol nm2) and 68.1 kcal/(mol nm2) in SOPC and SOPC:Chol (1:1) bilayers, respectively.
ΔGdef associated with accommodating a protein with the dimensions of the nAChR in different bilayers was calculated as above. As there is no hydrophobic mismatch in SOPC, ΔGdef is zero in this bilayer. In SOPC:Chol (1:1) ΔGdef is 6 kcal/mol. If l had been 2.85 nm or 2.7 nm ΔGdef would be 1 kcal/mol and 2 kcal/mol in the SOPC bilayer, and 14 kcal/mol and 25 kcal/mol in the SOPC:Chol (1:1) bilayer. In conclusion, for a protein with a radius as the nAChR, ΔGdef in a cholesterol-containing bilayer is substantially larger than for an α-helix. Further, a difference in protein hydrophobic length corresponding to only two amino acids in an α-helix (0.3 nm) leads to a difference in ΔGdef that is comparable to the energy released by hydrolysis of several ATP molecules.
Fig. 4 C shows the effects of cholesterol on the sorting of membrane proteins with radius as the nAChR and with hydrophobic lengths varying between 2.25 to 3.3 nm (KSOPC:Chol was calculated using Eq. 4). Proteins with a hydrophobic length of 3.3 nm are attracted to SOPC:Chol (1:1) because there is no hydrophobic mismatch in this bilayer domain and KSOPC:Chol is 20. In contrast, for shorter proteins with hydrophobic lengths of 3.0, 2.85, and 2.7 nm, the hydrophobic mismatch incurs an energetic penalty and KSOPC:Chol becomes 10−5, 10−11, and 10−19, respectively. In the case of a protein with a hydrophobic length that is in between the thickness of the SOPC and SOPC:Chol (1:1) bilayers, the relation between KSOPC:Chol and fChol may become biphasic (Fig. 4 C, curve for l = 3.15 nm). This result arises because, as d0 increases from the value in SOPC, the protein will tend to reside in the thicker, cholesterol-containing domains as long as 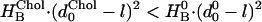 , where the HB and d0 superscripts denote the values in the absence and presence of cholesterol, respectively. Eventually, however, the increase in
, where the HB and d0 superscripts denote the values in the absence and presence of cholesterol, respectively. Eventually, however, the increase in 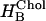 and in
and in 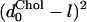 will cause the inequality to reverse and the protein will tend to reside in the thinner, cholesterol-free domains.
will cause the inequality to reverse and the protein will tend to reside in the thinner, cholesterol-free domains.
Fig. 4 D depicts the effects of an isolated change in bilayer thickness (and constant moduli) on protein sorting. If cholesterol altered only the bilayer thickness KSOPC:Chol, for proteins with a hydrophobic length of 3.3 nm and 3 nm, would be 25 and 0.06, respectively. The KSOPC:Chol for the 3.3-nm protein would thus be 400-fold larger than for the 3-nm protein—as opposed to 106-fold larger with the full effect of cholesterol. For the lateral distribution between SOPC and SOPC:Chol (1:1) bilayer domains of equal area, this means that if cholesterol affected only the bilayer thickness, the probability of finding the 3.3-nm protein in the SOPC:Chol (1:1) domain would be ∼20-fold larger than that of finding the 3-nm protein in this domain. But with the full effects of cholesterol, the probability of finding the 3.3-nm protein in the SOPC:Chol (1:1) domain is ∼5 orders-of-magnitude over that of finding the 3-nm protein in this domain.
Is hydrophobic exposure important?
Our results show that a bilayer-mediated sorting mechanism based on bilayer deformation energy is feasible. This raises the question, whether hydrophobic exposure per se (cf. Fig. 1 B) ever is important for sorting? For a sufficiently large mismatch between the hydrophobic bilayer thickness and protein length, the incremental change in ΔGdef will become so large that it becomes advantageous to expose hydrophobic surface—in the protein or the bilayer—to the aqueous phase, a situation we denote hydrophobic slippage. But the mismatch has to be extreme. When there is hydrophobic slippage, 2u0 will differ from d0 − l (compare with Fig. 1, B and C), and Eq. 2 will overestimate the energy available for protein sorting.
Following Andersen et al. (1998) and Lundbæk and Andersen (1999), the incremental change in ΔGdef is obtained by differentiating Eq. 2 with respect to u0, and hydrophobic slippage will not occur unless
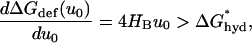 |
where 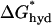 denotes the hydrophobic energy associated with exposing a unit length of the hydrophobic bilayer interior. The energetic cost of hydrophobic exposure is ∼4.7 kcal/(mol nm2) (Sharp et al., 1991), such that
denotes the hydrophobic energy associated with exposing a unit length of the hydrophobic bilayer interior. The energetic cost of hydrophobic exposure is ∼4.7 kcal/(mol nm2) (Sharp et al., 1991), such that 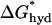 = (2π × r0) × 4.7 kcal/(mol nm2). For an α-helix with r0 = 0.65 nm,
= (2π × r0) × 4.7 kcal/(mol nm2). For an α-helix with r0 = 0.65 nm, 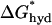 = 19 kcal/(mol nm) In SOPC and SOPC:Chol (1:1) bilayers the magnitude of
= 19 kcal/(mol nm) In SOPC and SOPC:Chol (1:1) bilayers the magnitude of 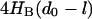 will be less than this value as long as |d0 − l| < 2.4 nm or 0.7 nm, respectively. For α-helices of 17 AA and longer, hydrophobic slippage will not occur in either bilayer; for 15- and 16-AA helices, slippage will not occur for fChol < 0.45. Similarly for a membrane protein with r0 = 3.0 nm,
will be less than this value as long as |d0 − l| < 2.4 nm or 0.7 nm, respectively. For α-helices of 17 AA and longer, hydrophobic slippage will not occur in either bilayer; for 15- and 16-AA helices, slippage will not occur for fChol < 0.45. Similarly for a membrane protein with r0 = 3.0 nm, 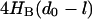 will be less than
will be less than 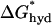 = 89 kcal/(mol nm) as long as |d0 − l| < 1.8 nm or 0.6 nm in SOPC and SOPC:Chol bilayers (1:1), respectively. This means that for membrane proteins 2.7 nm, or longer, hydrophobic slippage will not occur in either bilayer. We therefore conclude that hydrophobic exposure is unlikely to be important for protein sorting.
= 89 kcal/(mol nm) as long as |d0 − l| < 1.8 nm or 0.6 nm in SOPC and SOPC:Chol bilayers (1:1), respectively. This means that for membrane proteins 2.7 nm, or longer, hydrophobic slippage will not occur in either bilayer. We therefore conclude that hydrophobic exposure is unlikely to be important for protein sorting.
DISCUSSION
We have shown that cholesterol-induced changes in lipid bilayer physical properties are more than sufficient to support a bilayer-mediated protein sorting mechanism based on the lateral distribution of proteins between different bilayer domains. This bilayer-based protein sorting results from changes in the bilayer elastic deformation energy due to a mismatch between the protein length and the bilayer thickness, without invoking hydrophobic exposure. When compared to an isolated (cholesterol-induced) increase in bilayer thickness, the energetic consequences of the associated changes in bilayer material moduli causes a dramatic increase in the sorting efficiency. The bilayer contribution to membrane protein sorting will be operative, and of sufficient magnitude to be important, whether or not the sorting of a given protein also is under the control of other targeting signals.
Cholesterol gradients and implications for bilayer properties and protein sorting
Both cholesterol and proteins are synthesized in the endoplasmic reticulum (ER), and there is an increasing fChol in the membranes along the secretory pathway (Orci et al., 1981; Wattenberg and Silbert, 1983). Cholesterol thus constitutes ∼20% and 50% of the lipids in the Golgi complex and the plasma membranes, respectively (Evans and Hardison, 1985; van Meer, 1989). Further, relatively cholesterol-enriched bilayer domains have been demonstrated in both Golgi (Gkantiragas et al., 2001) and plasma membranes (Pike et al., 2002). The gradual increase in fChol has been proposed to reflect a selective forward transport of cholesterol (and sphingomyelin)-enriched membrane domains toward the plasma membrane (Bretscher and Munro, 1993); but it could also result from the selective retrograde transport of cholesterol/sphingomyelin-depleted vesicles (cf. Brown and London, 1998; Munro, 1998). In support of such models, the formation of COPI-coated vesicles operating in the early secretory pathway is associated with a segregation of sphingomyelin and cholesterol away from these vesicles; see Brugger et al. (2000). The precise role of these vesicles remain obscure, however; see Mellman and Warren (2000). In either case, a selective enrichment, or depletion, of a membrane protein in the cholesterol-enriched, or cholesterol-depleted, domains would enable protein sorting—as long as the transport vesicles are enriched in only one type of membrane domain. Furthermore, cholesterol depletion will lead to altered protein sorting (cf. Bagnat et al., 2001; Keller and Simons, 1998; Mayor et al., 1998), not only because the domain organization will be disrupted but also because the protein distribution among different domains will become less selective.
The effects of cholesterol on the bilayer material properties are considerable; but cholesterol-enriched lipid domains are enriched also in sphingolipids (Simons and Ikonen, 1997), which will increase both the bilayer thickness (e.g., Holthuis et al., 2001) and material moduli (McIntosh et al., 1992) above the changes induced by cholesterol alone. A bilayer-mediated sorting mechanism based on membrane deformation energy therefore would be even more efficient than indicated by our calculations as previously suggested by Gandhavadi et al. (2002). Specifically, Ka for sphingomyelin:cholesterol (1:1) bilayers is 1799 ± 234 pN/nm (McIntosh et al., 1992)—more than twofold larger than for SOPC:Chol bilayers (Table 1). Assuming that the relation between Ka and Kc in phospholipid:sphingomyelin:cholesterol mixtures is similar to that in phospholipids and phospholipid:cholesterol mixtures, HB could be twofold larger than the value we use for SOPC:Chol (1:1).
Limitations of the analysis
The present analysis is based on a symmetric bilayer but the phospholipid composition of cellular membranes is asymmetric (e.g., Masserini and Ravasi, 2001; Sprong and van Meer, 2001). It is not known to what extent cholesterol is present in the intracellular leaflet of a cholesterol-enriched lipid raft. In synthetic bilayers, however, domain formation in the two monolayers is coupled (Korlach et al., 1999), which may suggest that the cholesterol content of the two leaflets is similar also in cellular membranes. It is in this context comforting that the deduced energies are large, meaning that even two- to fourfold reductions in the deformation energies would have little impact on our general conclusion that cholesterol-dependent protein sorting, based on hydrophobic matching, is energetically feasible.
Another limitation is that a hydrophobic mismatch between a bilayer and a membrane-spanning protein may alter the lateral distribution of the bilayer lipids around the protein (Andersen et al., 1992; Sperotto and Mouritsen, 1993). In a cholesterol-containing bilayer, where d0 > l, the ensuing bilayer deformation could cause a redistribution of the lipids around the protein such that the local mole fraction of cholesterol would be less than in the bulk, unperturbed bilayer. This would occur because the reduction in bilayer material moduli (and thickness) will reduce the magnitude of ΔGdef, as compared to the situation where no redistribution has occurred, which in turn would provide the energetic basis for the redistribution. The quantitative importance of such a lipid redistribution, for the value of ΔGdef, is difficult to evaluate; but the presence of cholesterol (2:1) in a dioleoylphosphatidylcholine lipid bilayer causes a twofold increase in HB, as measured using gramicidin channels (Lundbæk et al., 1996). If the cholesterol-induced increase in the HB of SOPC bilayers (from SOPC to SOPC:Chol (1:1)) similarly were only a factor 2 (rather than the predicted factor 3), KSOPC:Chol for a 15 AA helix would be 10−5, rather than 10−10, which still would be sufficient for effective sorting.
We conclude that the present analysis constitutes a first-order approximation to the energetics of bilayer-mediated protein sorting, but that the general conclusions are unlikely to be affected by the above limitations.
Cholesterol-dependent protein sorting
Assuming that the mechanical moduli of both leaflets of the bilayer component of a cellular membrane are comparable, the lateral distribution of membrane-spanning proteins between different (cholesterol-poor and cholesterol-enriched) bilayer domains will follow the pattern in Fig. 4. That is, whereas the bilayer-based sorting mechanism is relatively inefficient at fChol < 0.3, the sorting efficiency increases as fChol is increased above 0.3. Given the change in the slope of the Ka (or Kc) versus fChol relation, (Fig. 3), there is a threshold in the sorting efficiency, meaning that bilayer-based sorting can occur between bilayer domains that have rather modest differences in their cholesterol concentration—as long as fChol in at least one of the domains is above 0.3, or so. Moreover, the threshold in the cholesterol-induced sorting would tend to enhance the tendency for the lipid composition of the cholesterol-enriched domains to change as the raft-preferring proteins partition into such domains, or when such domains coalesce into larger structures (compare with Bretscher and Munro, 1993; Dumas et al., 1997; Maer et al., 1999; Sperotto and Mouritsen, 1993). This threshold similarly will serve to strengthen retention mechanisms that rely on vesicle recycling among different compartments (cf. Ghosh et al., 1998).
There is an asymmetry to the cholesterol-induced sorting: the penalty for minor length-thickness mismatches will be significant in the cholesterol-enriched domains, but more modest in the cholesterol-poor domains. This asymmetry is important because it means that bilayer-based protein sorting fundamentally is a proofreading mechanism based on selective exclusion, meaning that proteins with short TMDs will be excluded from cholesterol-enriched bilayer domains—irrespective of the detailed amino acid sequence or structure of the TMD.
Our results provide insight into why the short TMD of Golgi-resident proteins is a conserved feature among eukaryotic cells from mammals to yeast (Holthuis et al., 2001; Levine et al., 2000). The retention of ER resident membrane proteins is likely to be determined, in part, by a similar bilayer-based sorting mechanism: elongating their TMD leads to relocation to the Golgi complex (Pedrazzini et al., 1996; Yang et al., 1997); further elongation causes the proteins to be expressed at the plasma membrane (Yang et al., 1997), and this length-dependent control of protein targeting is observed also with artificial TMDs (Honsho et al., 1998). These observations suggest that a bilayer-based sorting mechanism may be operative generally, between ER and Golgi and between Golgi and the plasma membrane (Yang et al., 1997), and even within the Golgi complex. But in the case of protein sorting between ER and Golgi, bilayer-based sorting is not the sole ER retention mechanism, as there are sequence-specific ER retention/retrieval signals (cf. Yang et al., 1997). Similarly, whereas targeting of the plasma membrane protein, Na+,K+-ATPase is controlled, at least in part, by its membrane-spanning domain (Dunbar et al., 2000) in a manner suggesting that a bilayer-based mechanism could be involved, targeting of plasma membrane proteins to apical or baso-lateral membranes generally depends also on sequence-specific signals, e.g., Rodriguez-Boulan and Nelson (1989)—indicating, again, the existence of multiple sorting mechanisms (cf. Mellman and Warren, 2000).
We finally note that bilayer-based sorting arises because biological membranes are not just fluid mosaic structure (Singer and Nicolson, 1972), but elastic bodies with material properties that allow for bilayer deformation, but at a price (cf. Mouritsen and Andersen, 1998). The bilayer elastic properties are such that a hydrophobic mismatch incurs an energetic cost that is sufficient to support bilayer-based protein sorting, without exposure of hydrophobic residues to water. Moreover, given the magnitude of the ΔGdef associated with even a modest hydrophobic mismatch, bilayer-based sorting is likely to be a general mechanism, which would be important for the lateral distribution of membrane proteins in any cellular membrane containing cholesterol/sphingolipid-enriched lipid domains. Further, bilayer-based sorting may be important for determining the lateral distribution of proteins whose TMDs vary in length, as seems to be the case for plasma membrane proteins (compare with Bretscher and Munro, 1993, their Fig. 1).
CONCLUSION
Cholesterol-induced changes in bilayer physical properties are sufficient to allow for effective sorting of membrane proteins. The effects of cholesterol are due to the combined impact of changes in bilayer thickness and material properties. The energetic consequences of the changes in the thickness per se, however, are modest; but the associated changes in material properties strongly potentiate the effects of the thickness change. The threshold in the sorting efficiency, induced by the effects on the bilayer material properties, implies that cholesterol-induced protein sorting in effect becomes a proofreading mechanism based on the exclusion of proteins with too short a TMD from the cholesterol-enriched bilayer domains.
Acknowledgments
We thank F. R. Maxfield for helpful discussions and comments on previous versions of the manuscript.
Supported by a grant from the Danish Medical Research Council (to J.A.L.); the Carlsberg Foundation (to C.N.); and grant GM21342 from the National Institutes of Health (to O.S.A.).
APPENDIX
To calculate HB we make use of the fact that the general solution to Eq. 1 is biquadratic in u0 and s, the contact slope at the protein-bilayer boundary (Nielsen et al.,1998; Nielsen and Andersen, 2000). For c0 = 0,
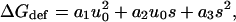 |
where a1, a2, and a3 are functions of Ka, Kc, d0, and r0, and
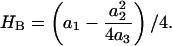 |
If the lipid packing constraints were included, s would be 0 and HB would be given by a1. To evaluate the coefficients a1, a2, and a3, we follow Nielsen and Andersen (2000), who calculated reference values, a1*, a2*, and a3* for a set of reference bilayer-inclusion parameters Ka*, Kc*, d0*, and r0*, and then derived scaling relations that could be used to calculate a1, a2, and a3 (and thus HB). For any bilayer-inclusion system, the scaling relations have the form
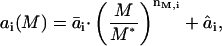 |
where ai is the resulting value of the coefficient in question (i = 1, 2, 3), M denotes the material property that is varying, nM,i is the relevant scaling exponent, and 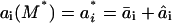 . The values for
. The values for 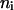 ,
, 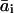 ,
, 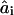 are tabulated in Nielsen and Andersen (2000, their Table 5).
are tabulated in Nielsen and Andersen (2000, their Table 5).
For any given combination of Ka, Kc, r0, and d0, we then have that
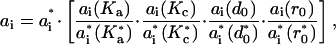 |
which allows for the determination of the ai coefficients and HB. (When Hb is calculated directly from Eq. 1—Nielsen and Andersen, 2000—we obtain values that are within 10% of the values derived using the scaling relations.)
Abbreviations used: AA, amino acids; d0, bilayer hydrophobic thickness; fChol, cholesterol mole fraction; Hb, bilayer spring constant; Ka, area-compression modulus; Kc, bending modulus; l, protein hydrophobic length; r0, protein radius; TMD, transmembrane domain.
References
- Andersen, O. S., C. Nielsen, A. M. Maer, J. A. Lundbæk, M. Goulian, and R. E. Koeppe, II. 1998. Gramicidin channels: molecular force transducers in lipid bilayers. Biol. Skr. Dan. Vid. Selsk. 49:75–82. [Google Scholar]
- Andersen, O. S., D. B. Sawyer, and R. E. Koeppe, II. 1992. Modulation of channel function by the host bilayer. In Biomembrane Structure and Function. B. Gaber and K.R.K. Easwaran, editors. Adenine Press, Schenectady. pp 227–244.
- Bagnat, M., A. Chang, and K. Simons. 2001. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell. 12:4129–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaul, A., N. Ben-Tal, and B. Honig. 1996. Statistical thermodynamic analysis of peptide and protein insertion into lipid membranes. Biophys. J. 71:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, M., E. Evans, and O. G. Mouritsen. 1991. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q. Rev. Biophys. 24:293–397. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bransburg-Zabary, S., A. Kessel, M. Gutman, and N. Ben-Tal. 2002. Stability of an ion channel in lipid bilayers: implicit solvent model calculations with gramicidin. Biochemistry. 41:6946–6954. [DOI] [PubMed] [Google Scholar]
- Bretscher, M. S., and S. Munro. 1993. Cholesterol and the Golgi aparatus. Science. 261:1280–1281. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- Brugger, B., R. Sandhoff, S. Wegehingel, K. Gorgas, J. Malsam, J. B. Helms, W. D. Lehmann, W. Nickel, and F. T. Wieland. 2000. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J. Cell Biol. 151:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check, E. 2002. Cell biology: will the real Golgi please stand up? Nature. 416:780–781. [DOI] [PubMed] [Google Scholar]
- Cole, N. B., J. Ellenberg, J. Song, D. DiEuliis, and J. Lippincott-Schwartz. 1998. Retrograde transport of Golgi-localized proteins to the ER. J. Cell Biol. 140:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan, N., A. Berman, P. Pincus, and S. A. Safran. 1994. Membrane-induced interactions between inclusions. J. Phys. II. 4:1713–1725. [Google Scholar]
- Dumas, F., M. C. Lebrun, and J. F. Tocanne. 1999. Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett. 458:271–277. [DOI] [PubMed] [Google Scholar]
- Dumas, F., M. M. Sperotto, M. C. Lebrun, J. F. Tocanne, and O. G. Mouritsen. 1997. Molecular sorting of lipids by bacteriorhodopsin in dilauroylphosphatidylcholine/distearoylphosphatidylcholine lipid bilayers. Biophys. J. 73:1940–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, L. A., P. Aronson, and M. J. Caplan. 2000. A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J. Cell Biol. 148:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E., and W. Rawicz. 1990. Entropy-driven tension and bending elasticity in condensed fluid membranes. Phys. Rev. Lett. 64:2094–2097. [DOI] [PubMed] [Google Scholar]
- Evans, E. A., and R. Skalak. 1979. Mechanics and thermodynamics of biomembranes: part 1. CRC Crit. Rev. Bioeng. 3:181–330. [PubMed] [Google Scholar]
- Evans, W. H., and W. G. Hardison. 1985. Phospholipid, cholesterol, polypeptide and glycoprotein composition of hepatic endosome subfractions. Biochem. J. 232:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattal, D. R., and A. Ben-Shaul. 1993. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys. J. 65:1795–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhavadi, M., D. Allende, A. Vidal, S. A. Simon, and T. J. McIntosh. 2002. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 82:1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, R. N., W. G. Mallet, T. T. Soe, T. E. McGraw, and F. R. Maxfield. 1998. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142:923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkantiragas, I., B. Brugger, E. Stuven, D. Kaloyanova, X. Y. Li, K. Lohr, F. Lottspeich, F. T. Wieland, and J. B. Helms. 2001. Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell. 12:1819–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson, P. A. 1998. Targeting of proteins to the Golgi apparatus. Histochem. Cell Biol. 109:517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich, P., and E. Jakobsson. 1990. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys. J. 57:1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 28C:693–703. [DOI] [PubMed] [Google Scholar]
- Holthuis, J. C. M., T. Pomorski, R. J. Raggers, H. Sprong, and G. Van Meer. 2001. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 81:1689–1723. [DOI] [PubMed] [Google Scholar]
- Honsho, M., J. Y. Mitoma, and A. Ito. 1998. Retention of cytochrome b5 in the endoplasmic reticulum is transmembrane and luminal domain-dependent. J. Biol. Chem. 273:20860–20866. [DOI] [PubMed] [Google Scholar]
- Huang, H. W. 1986. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 50:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140:1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlach, J., P. Schwille, W. W. Webb, and G. W. Feigenson. 1999. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA. 96:8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, T. P., C. A. Wiggins, and S. Munro. 2000. Inositol phosphorylceramide synthase is located in the Golgi apparatus of Saccharomyces cerevisiae. Mol. Biol. Cell. 11:2267–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek, J. A., P. Birn, J. Girshman, A. J. Hansen, and O. S. Andersen. 1996. Membrane stiffness and channel function. Biochemistry. 35:3825–3830. [DOI] [PubMed] [Google Scholar]
- Lundbaek, J. A., and O. S. Andersen. 1999. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys. J. 76:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maer, A. M., C. Nielsen, and O. S. Andersen. 1999. Gramicidin channels in bilayers formed from phosphatidylcholine mixtures with a constant average number of methylene groups per lipid molecule. Biophys. J. 76:A213. [Google Scholar]
- Marsh, D. 1990. CRC Handbook of Lipid Bilayers. CRC Press, Boca Raton, FL.
- Masibay, A. S., P. V. Balaji, E. E. Boeggeman, and P. K. Qasba. 1993. Mutational analysis of the Golgi retention signal of bovine beta-1,4- galactosyltransferase. J. Biol. Chem. 268:9908–9916. [PubMed] [Google Scholar]
- Masserini, M., and D. Ravasi. 2001. Role of sphingolipids in the biogenesis of membrane domains. Biochim. Biophys. Acta. 1532:149–161. [DOI] [PubMed] [Google Scholar]
- May, S. 2000. Protein-induced bilayer deformations: the lipid tilt degree of freedom. Eur. Biophys. J. 29:17–28. [DOI] [PubMed] [Google Scholar]
- Mayor, S., S. Sabharanjak, and F. R. Maxfield. 1998. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 17:4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, T. J., S. A. Simon, D. Needham, and C. H. Huang. 1992. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 31:2012–2020. [DOI] [PubMed] [Google Scholar]
- Mellman, I., and G. Warren. 2000. The road taken: past and future foundations of membrane traffic. Cell. 100:99–112. [DOI] [PubMed] [Google Scholar]
- Mouritsen, O. G., and O. S. Andersen., Eds. 1998. In search of a new biomembrane model. Biol. Skr. Dan. Vid. Selsk. 49:1–214. [Google Scholar]
- Mouritsen, O. G., and M. Bloom. 1984. Mattress model of lipid-protein interactions in membranes. Biophys. J. 46:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S. 1991. Sequences within and adjacent to the transmembrane segment of α-2,6-sialyltransferase specify Golgi retention. EMBO J. 10:3577–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S. 1998. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 8:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham, D. 1995. Cohesion and permeability of lipid bilayer vesicles. In Permeability and Stability of Lipid Bilayers. E.A. Disalvo. and S. A. Simon, editors. CRC Press, Boca Raton, FL. pp 49–76.
- Needham, D., and R. S. Nunn. 1990. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 58:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezil, F. A., and M. Bloom. 1992. Combined influence of cholesterol and synthetic amphiphilic peptides upon bilayer thickness in model membranes. Biophys. J. 61:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol, J. A., and O. F. Hutter. 1996. Tensile strength and dilatational elasticity of giant sarcolemmal vesicles shed from rabbit muscle. J. Physiol. 493:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., and O. S. Andersen. 2000. Inclusion-induced bilayer deformations: effects of monolayer equilibrium curvature. Biophys. J. 79:2583–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., M. Goulian, and O. S. Andersen. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, T., and G. Warren. 1994. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr. Opin. Cell Biol. 6:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opat, A. S., C. van Vliet, and P. A. Gleeson. 2001. Trafficking and localisation of resident Golgi glycosylation enzymes. Biochimie. 83:763–773. [DOI] [PubMed] [Google Scholar]
- Orci, L., R. Montesano, P. Meda, F. Malaisse-Lagae, D. Brown, A. Perrelet, and P. Vassalli. 1981. Heterogeneous distribution of filipin–cholesterol complexes across the cisternae of the Golgi apparatus. Proc. Natl. Acad. Sci. USA. 78:293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., A. Villa, and N. Borgese. 1996. A mutant cytochrome b5 with a lengthened membrane anchor escapes from the endoplasmic reticulum and reaches the plasma membrane. Proc. Natl. Acad. Sci. USA. 93:4207–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, L. J., X. Han, K. N. Chung, and R. W. Gross. 2002. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 41:2075–2088. [DOI] [PubMed] [Google Scholar]
- Rawicz, W., K. C. Olbrich, T. McIntosh, D. Needham, and E. Evans. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, J., S. Lew, Z. Wang, and E. London. 1997. Transmembrane orientation of hydrophobic α-helices is regulated both by the relationship of helix length to bilayer thickness and by the cholesterol concentration. Biochemistry. 36:10213–10220. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan, E., and W. J. Nelson. 1989. Morphogenesis of the polarized epithelial cell phenotype. Science. 245:718–725. [DOI] [PubMed] [Google Scholar]
- Sharp, K. A., A. Nicholls, R. F. Fine, and B. Honig. 1991. Reconciling the magnitude of the microscopic and macroscopic hydrophobic effects. Science. 252:106–109. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Singer, S. J., and G. L. Nicolson. 1972. The fluid mosaic model of the structure of cell membranes. Science. 175:720–731. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian, N., and D. P. Nayak. 1987. Mutational analysis of the signal-anchor domain of influenza virus neuraminidase. Proc. Natl. Acad. Sci. USA. 84:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto, M. M., and O. G. Mouritsen. 1993. Lipid enrichment and selectivity of integral membrane proteins in two component lipid bilayers. Eur. Biophys. J. 22:323–328. [DOI] [PubMed] [Google Scholar]
- Sprong, H., and G. van Meer. 2001. How proteins move lipids and lipids move proteins. Nature Rev. 2:504–513. [DOI] [PubMed] [Google Scholar]
- Tanford, C. 1980. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd Ed. Wiley-Interscience New York.
- Unwin, N. 2000. The Croonian Lecture 2000. Nicotinic acetylcholine receptor and the structural basis of fast synaptic transmission. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1813–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer, G. 1989. Lipid traffic in animal cells. Annu. Rev. Cell Biol. 5:247–275. [DOI] [PubMed] [Google Scholar]
- Veech, R. L., J. W. R. Lawson, N. W. Cornell, and H. A. Krebs. 1979. Cytosolic phosphorylation potential. J. Biol. Chem. 254:6538–6547. [PubMed] [Google Scholar]
- Vidal, A., S. A. Simon, and T. J. McIntosh. 2002. Bilayer interfacial properties modulate the binding of amphipathic peptides. Chem. Phys. Lipids. In press . [DOI] [PubMed]
- Voegler-Smith, A., and C. K. Hall. 2001. α-helix formation: discontinuous molecular dynamics on an intermediate-resolution protein model. Proteins. 44:344–360. [DOI] [PubMed] [Google Scholar]
- Wattenberg, B. W., and D. F. Silbert. 1983. Sterol partitioning among intracellular membranes. Testing a model for cellular sterol distribution. J. Biol. Chem. 258:2284–2289. [PubMed] [Google Scholar]
- Webb, R. J., J. M. East, R. P. Sharma, and A. G. Lee. 1998. Hydrophobic mismatch and the incorporation of peptides into lipid bilayers: a possible mechanism for retention in the Golgi. Biochemistry. 37:673–679. [DOI] [PubMed] [Google Scholar]
- Yang, M., J. Ellenberg, J. S. Bonifacino, and A. M. Weissman. 1997. The transmembrane domain of a carboxyl-terminal anchored protein determines localization to the endoplasmic reticulum. J. Biol. Chem. 272:1970–1975. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. P., R. N. Lewis, R. S. Hodges, and R. N. McElhaney. 1992. Interaction of a peptide model of a hydrophobic transmembrane α-helical segment of a membrane protein with phosphatidylcholine bilayers: differential scanning calorimetric and FTIR spectroscopic studies. Biochemistry. 31:11579–11588. [DOI] [PubMed] [Google Scholar]