Abstract
A clone expressing a novel immunoreactive leptospiral immunoglobulin-like protein A of 130 kDa (LigA) from Leptospira interrogans serovar pomona type kennewicki was isolated by screening a genomic DNA library with serum from a mare that had recently aborted due to leptospiral infection. LigA is encoded by an open reading frame of 3,675 bp, and the deduced amino acid sequence consists of a series of 90-amino-acid tandem repeats. A search of the NCBI database found that homology of the LigA repeat region was limited to an immunoglobulin-like domain of the bacterial intimin binding protein of Escherichia coli, the cell adhesion domain of Clostridium acetobutylicum, and the invasin of Yersinia pestis. Secondary structure prediction analysis indicates that LigA consists mostly of beta sheets with a few alpha-helical regions. No LigA was detectable by immunoblot analysis of lysates of the leptospires grown in vitro at 30°C or when cultures were shifted to 37°C. Strikingly, immunohistochemistry on kidney from leptospira-infected hamsters demonstrated LigA expression. These findings suggest that LigA is specifically induced only in vivo. Sera from horses, which aborted as a result of natural Leptospira infection, strongly recognize LigA. LigA is the first leptospiral protein described to have 12 tandem repeats and is also the first to be expressed only during infection. Thus, LigA may have value in serodiagnosis or as a protective immunogen in novel vaccines.
Leptospira interrogans causes leptospirosis (Weil's disease), a zoonotic disease that is prevalent in people, horses, cattle, and wild animals. The disease occurs widely in developing countries, such as Brazil and India, and is reemerging in developed countries. In addition to hepatic and renal failure, uveitis is sometimes a sequela to leptospiral infection (30). Although the incidence of human leptospirosis in the United States is relatively low, disease incidence in domestic animals has increased in recent years.
Leptospiral infection in people can range in severity from an inapparent infection to death from renal or hepatic failure (11). Infection is acquired either directly or indirectly from infected animals. In horses, the most common manifestations of infection are abortion and uveitis (29). L. interrogans serovar pomona type kennewicki is the predominant serovar isolated from aborted equine fetuses, whereas L. interrogans serovar grippotyphosa is found less frequently (7-9). The association of leptospires with equine recurrent uveitis (16) has been well documented, and the organism has been detected in ocular fluids by culture and PCR (31). In addition, Parma et al. demonstrated by Western blotting the reactivity of several bands in extracts of equine cornea and lens with antileptospiral sera (27, 28). Although there is a strong association between leptospiral infection and uveitis, the immunopathogenesis of leptospira-associated uveitis remains obscure.
Currently available leptospiral vaccines have low efficacy, are serovar specific, and generally produce only short-term immunity in domestic livestock. Efforts to identify immunogenic components of value in vaccines have resulted in characterization of 31-, 32-, 36-, and 41-kDa outer membrane proteins (12-15, 32, 33). Two of these proteins (31 and 41 kDa) function synergistically in the immunoprotection of hamsters, suggesting that an effective protein-based vaccine would contain several components (13). The search for protective immunogens is complicated by the possibility that important components may be produced only during infection. Supporting this possibility are recent studies that indicate that some immunogenic proteins of L. interrogans serovar pomona are upregulated at 37°C (24).
The present study was initiated to identify and characterize immunogenic Leptospira proteins that are expressed during infection. The gene for one such immunoreactive immunoglobulin-like 130-kDa protein (LigA) of L. interrogans serovar pomona type kennewicki has been characterized and shown to be expressed in vivo.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
L. interrogans serovar pomona type kennewicki was provided by M. Donahue (Diagnostic Laboratory, Department of Veterinary Science, University of Kentucky), who isolated this strain from a case of equine recurrent uveitis. Other serovars were obtained from the American Type Culture Collection and maintained in the diagnostic laboratory at Cornell University. Leptospires were grown on PLM-5 medium (Intergen) at 30°C. Growth was monitored by dark-field microscopy. Temperature regulation was carried out as previously described (24).
Sera.
Sera were obtained from mares that had recently aborted due to leptospiral infection. These sera had high titers for L. interrogans serovar pomona, as determined by the microscopic agglutination test. In most cases, the diagnosis was confirmed by microscopic detection of leptospires in fetal tissues and the placenta. Rabbit antileptospiral antibody was obtained from NVSL, Iowa (1098-LEP-FAC). Antisera to LipL32 and LipL36 were kindly provided by D. A. Haake (University of California at Los Angeles).
Genomic DNA library.
Genomic DNA was prepared from L. interrogan serovar pomona kennewicki as previously described (6). Partial restriction digestion was performed with TSP5091, and the digested fragments were ligated into predigested Lambda ZapII(Stratagene). Ligated DNA was packaged into Gigapack II Gold packaging extracts and stored in 0.3% chloroform. After transfection into Escherichia coli MRF′ XL1-Blue (Stratagene), the library was plated, amplified, and screened with convalescent mare's serum according to the manufacturer's instructions.
DNA sequencing and analysis.
DNA sequencing was done with an ABI model 377 automated nucleic acid sequencer at the Bioresource Center, Cornell University. Homology searches were performed with the NCBI database and BLAST (1). Secondary structure, hydrophobicity, and antigenic index predictions were obtained by using PCgene and DNAstar.
Expression of LigA in E. coli.
ligA without the signal sequence (deletion of the N-terminal 31 amino acids) was amplified with primers (sense [5′-GGGTTTCATATGGCTGGCAAAAGAGGC-3′] and antisense [5′-CCCTCGAGTGGCTCCGTTTTAAT-3′]) and subcloned into NdeI-XhoI sites of pET22b (Novagen, Madision, Wis.). The recombinant plasmid was transformed to E. coli BL21(DE3) and expression was induced by IPTG (isopropyl-β-d-thiogalactopyranoside) as previously described (4).
A 90-kDa truncated LigA protein was subcloned into the XhoI-BamHI sites of pET15b (Novagen) by PCR with primers (sense [5′-TCGAGGTCTCTCCAGTTTTACC-3′] and antisense [5′-GCGGATCCTGTTTTCATGTTATGGCTCC-3′]). The resulting plasmid was transformed into E. coli BL21(DE3), and the truncated recombinant LigA fusion protein was isolated from a lysate of BL21 by affinity chromatography on His-Bind resin (Novagen).
Preparation of LigA-specific rabbit antiserum.
Antiserum to a 90-kDa truncated LigA protein was prepared in adult New Zealand White rabbits. Recombinant truncate was purified from periplasmic proteins of E. coli Nova-Blue that contained pKS (Stratagene) encoding a 5-kb BamHI-SalI fragment or by affinity chromatography on Avidgel F (UniSyn Technology, Inc., Tustin, Calif.), to which immunoglobulin G (IgG) from convalescent mare's serum had been coupled. The rabbits were immunized subcutaneously with 50 μg of the 90-kDa truncated LigA mixed with complete Freund adjuvant on day 1, followed by a booster inoculum of 50 μg of protein and incomplete Freund adjuvant on days 10 and 19. On day 35, the rabbits were boosted intravenously with 50 μg of protein and then bled on day 45.
SDS-PAGE and immunoblot analysis.
Purified truncated LigA protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis as previously described (3, 6). Rabbit antiserum to truncated LigA or convalescent mares' sera were used as primary antibodies. Blots were developed with peroxidase-conjugated protein G or goat anti-horse IgG conjugated to alkaline phosphatase (KPL). Reactive bands were visualized by using 4-1-chloronaphthol (0.5 mg/ml) or nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) as appropriate.
Immunohistochemistry.
Immunohistochemistry was performed on normal and leptospiral infected hamster kidneys with biotin-streptavidin-horseradish peroxidase according to the manufacturer's instructions (Zymed Laboratories, South San Francisco, Calif.). The chromogen was Nova Red (Dako, Carpinteria, Calif.). The primary antibody was rabbit antiserum specific for truncated LigA and was titrated by using a twofold serial dilution from 1:10 to 1:320. Negative controls consisted of nonimmune rabbit serum diluted 1:10, 1:20, and 1:40. Anti-LipL32 was used as a positive control.
Kidneys were removed from leptospiral infected and normal hamsters euthanized as part of an unrelated research project. These tissues were immediately embedded in O.C.T. Compound (Miles, Elkhart, Ind.) and snap-frozen in 2-methyl butane (Sigma, St. Louis, Mo.) prechilled to the point of freezing in liquid nitrogen. Tissues were sectioned at 6 μm, mounted on Microscope Plus slides (Fisher Scientific), fixed in acetone for 2 min, and air dried. Endogenous peroxidase was quenched for 10 min in 0.3% hydrogen peroxide in 0.1% (wt/vol) sodium azide and rinsed for 3 min in 0.01 M phosphate-buffered saline (pH 7.6; PBS). Sections were then blocked with 10% heat inactivated goat serum for 10 min. The blocking serum was tipped off, and the primary antibody was applied for 60 min at room temperature. After three rinses in PBS, a 1:400 dilution of biotinylated goat anti-rabbit IgG was added for 20 min. Sections were rinsed three times and then incubated with a 1:400 dilution of the streptavidin-peroxidase reagent for 10 min. After the rinsing step, the chromogen-substrate mixture was added to the sections, and the reaction was monitored under the microscope until well developed or until background developed. The slides were again rinsed in PBS, counterstained lightly with Gill's #1 hematoxylin (ca. 30 s), and then rinsed in tap water. After dehydration in two changes of graded ethanol to 100% for 2 min each, the sections were cleared in four changes of 100% xylene for 2 min each and mounted with Fisher Permount.
PCR amplification of ligA in pathogenic serovars.
Using a primer pair specific for ligA, PCR was performed on pathogenic serovars, including L. interrogans serovar pomona type kennewicki, L. kirschneri serovar grippotyphosa, L. interrogans serovar hardjo type hardjobovis, L. interrogans serovar icterohaemorrhagiae, and L. interrogans serovar canicola. The sequence of the forward primer was 5′-GGAATTCATGTTAAAGTCACTGCT-3′, and that of the reverse primer was 5′-CCGCTCGAGGTTTTAATAGAGGC-3′. Amplification conditions were as previously described (5). PCR products were purified by using a gel purification kit (Qiagen) and digested with BamHI and HindIII to detect restriction polymorphisms.
Enzyme-linked immunosorbent assay (ELISA).
Wells of 96-well polystyrene plates (Falcon 3912 Microtest III; Becton Dickinson, Oxnard, Calif.) were coated overnight at 4°C with 0.15 μg of truncated recombinant LigA in 100 μl of PBS, washed, blocked with 2% skim milk in PBS (pH 7.2) with 0.05% Tween 20, and then incubated with a 1:100 dilution of horse serum in triplicate wells for 2 h at 37°C. After a washing step, peroxidase-conjugated protein G (1:8,000) was added (100 μl) to each well, followed by incubation for 2 h at 37°C. Finally, the plates were washed and developed with fresh substrate consisting of 0.07% ortho-phenylenediamine and 0.05% hydrogen peroxide in citric acid phosphate buffer (pH 5.0). After the reaction was stopped by the addition of 50 μl of 3 M sulfuric acid, the absorbance was read at 490 nm in an automated plate reader (Biotex, Winooski, Vt.).
Statistical analysis.
Analysis of variance was used to determine whether there was a significant difference in the mean optical density (OD) reading for each of the sera used in the present study. Multiple comparisons by using the least-significant-difference method were performed to identify which OD mean was significantly different from the other. The analysis was performed by using Statistix software (Analytical Software, Tallahassee, Fla.).
Nucleotide sequence accession numbers.
The GenBank accession number for the nucleotide sequences of ligA is AF368236.
RESULTS
Identification, sequencing, and expression of LigA.
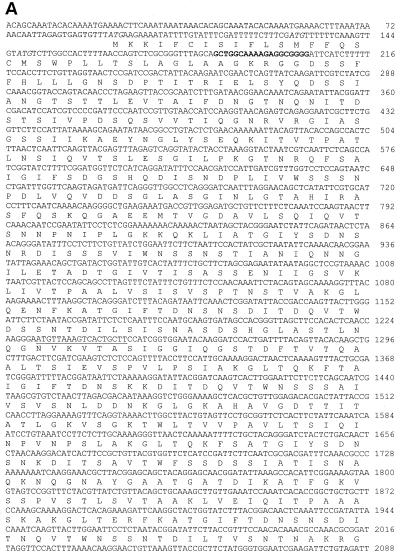
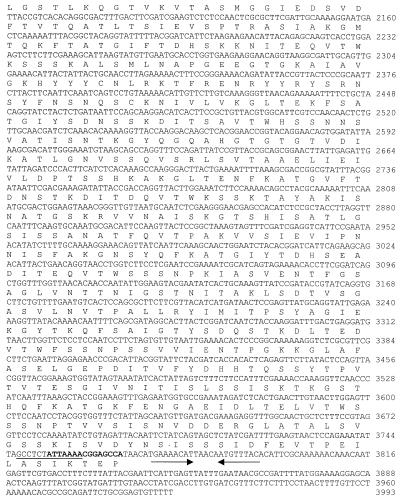
Screening of the L. interrogans genomic library with convalescent mare's serum yielded numerous positive clones, one of which contained an insert of 3,993 bp and expressed a protein that was encoded by an open reading frame of 3,675 bp (Fig. 1). The deduced sequence consisted of 1,225 amino acids with an estimated molecular mass of 129,041 Da and a pI of 6.35. An N-terminal signal sequence of 31 amino acids was predicted by using the SignalP program (25). Twelve or more tandem repeats of 90 amino acids were detected in LigA (Fig. 1B). Analysis of the sequence by using the NCBI database and BLAST revealed homology with the immunoglobulin-like domain of E. coli intimin (GenBank accession number AF252560), the putative invasin of Yersinia pestis (AJ41459), and the cell adhesion domain of Clostridium acetobutylicum (AE007823) (data not shown). LigA tandem repeats that showed homology with bacterial immunoglobulin-like domains (Ig11, CD:pfam02368; Ig12, CD:smart00635) are represented in Fig. 1B.
FIG. 1.
(A) Nucleotide sequence of ligA and its deduced amino acid sequence. Italics regions are the three possible translation start codons. Bold and underlined nucleotides indicate primer annealing sites for Fig. 2 and 6, respectively. Arrows show the potential transcription termination sequence. (B) Alignment of the predicted amino acid sequences for the 12 tandem repeats and the immunoglobulin-like domain of E. coli intimin-binding (receptor) protein (Ig11, CD:pfam02368; Ig12, CD:smart00635). Twelve repeat sequences of a 90-amino-acid sequence include residues from 136 to 218, 224 to 310, 311 to 400, 401 to 489, 490 to 580, 581 to 670, 671 to 760, 761 to 851, 852 to 942, 943 to 1033, 1034 to 1125, and 1126 to 1216, respectively.
Expression of LigA in E. coli but not in leptospiral lysates.
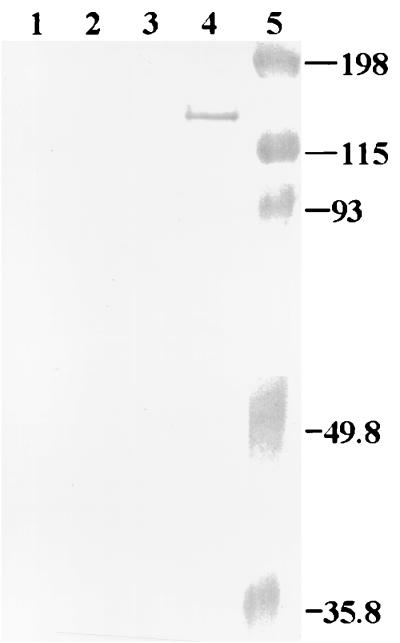
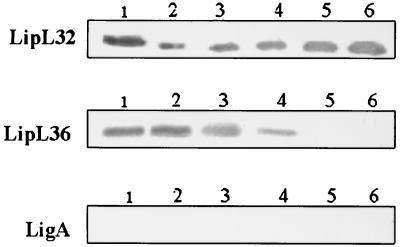
E. coli containing intact ligA without its signal sequence expressed LigA only after IPTG induction (Fig. 2), but LigA expression was toxic to E. coli, resulting in a 50-fold decrease in viability of cells (data not shown), which is similar to that found for OmpL1 of Leptospira (15). However, the expression of a 90-kDa truncated LigA was not toxic to E. coli cells (data not shown). Immunoblotting of whole-cell lysates of L. interrogans serovar pomona type kennewicki grown at 30 and 37°C with LigA-specific polyclonal rabbit serum did not show any detectable level of LigA (Fig. 3). In contrast, LipL32 was expressed by cultures grown at both 30 and 37°C, whereas LipL36 was downregulated at 37°C.
FIG. 2.
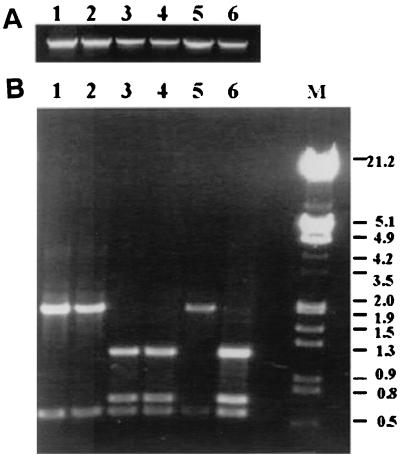
Expression of LigA in E. coli. Whole-cell lysates of E. coli were subjected to SDS-PAGE, transferred to nitrocellulose, and blotted with a 1:100 dilution of rabbit antiserum to the 90-kDa truncated LigA. Lanes 1 and 2, E. coli with vector, pET22b only; lanes 3 and 4, E. coli harboring pET22b plus ligA construct; lanes 2 and 4, E. coli was induced with 0.4 mM IPTG; lane 5, prestained molecular size markers (Bio-Rad).
FIG. 3.
LipL32 and LipL36 but not LigA expression are temperature regulated. Lane 1, whole-cell lysate of leptospires grown at 30°C; lanes 2, 3, 4, 5, and 6, cultures 2, 3, 4, 5, and 6 days old, respectively, of leptospires grown at 37°C. Each lane was loaded with ∼5.0 μg of protein.
LigA expression in vivo in Leptospira-infected hamsters.
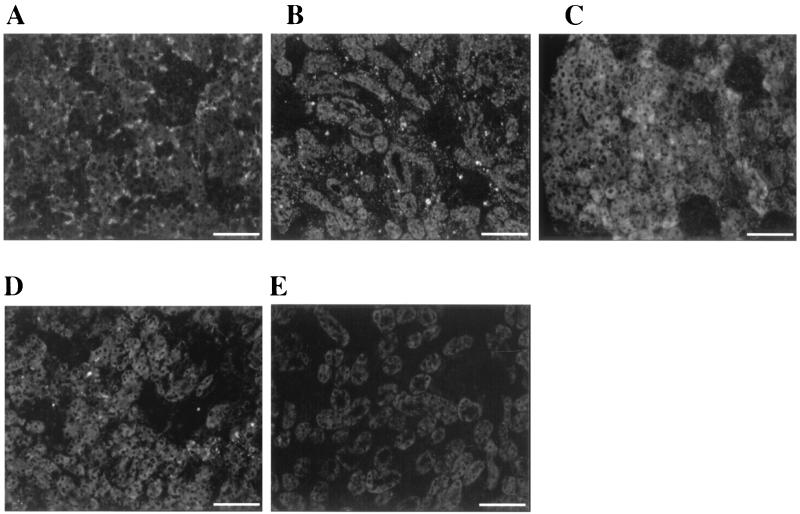
In order to examine LigA expression during leptospiral infection, immunohistochemistry was performed on kidneys from normal and leptospira-infected hamsters. LigA was expressed only in leptospira-infected hamster kidney (Fig. 4A). High-titer rabbit antileptospiral serum, as well as antiserum to LipL32, reacted with leptospires in experimentally infected kidneys (Fig. 4B and C). LipL36, which is not expressed by L. krischneri serovar grippotyphosa in infected hamster kidneys (2), was detected around the proximal convoluted tubules in L. interrogans serovar pomona-infected hamster kidney at a 1:50 dilution of antiserum to LipL36 (Fig. 4D). Preimmune rabbit serum did not react (Fig. 4E), and no immune serum reacted with normal hamster kidney (data not shown).
FIG. 4.
LigA expression in hamsters infected with L. interrogans serovar pomona. Sections of kidney were treated with rabbit antiserum specific for a 90-kDa truncated LigA (A), L. interrogans serovar pomona (B), LipL32 (C), LipL36 (D), and with preimmune serum (E). Kidney sections from noninfected hamsters were unreactive. Bar, 67 μm.
LigA-specific antibody in the sera of convalescent mares and aborted fetuses.
All convalescent-phase sera showed strong reactivity with recombinant LigA by Western blot analysis. Negative control horse sera derived from Borrelia burgdorferi (5), human granuloctyic ehrlichiosis agent (HGE) infection (4), and naive horse sera were unreactive (Fig. 5). The utilization of LigA in ELISA also showed strong reactivity to the convalescent-phase sera (Table 1). The mean OD for the leptospiral positive sera (M1 to M8) was significantly different from the negative control sera (L1 to L5) and from sera obtained from HGE (E1 and E2)- and B. burgdorferi (N1 to N4)-infected animals (P < 0.05).
FIG. 5.
Recombinant LigA protein purified by using metal affinity chromatography and subjected to SDS-PAGE separation was probed with normal horse sera (lanes 1 to 4), equine lyme disease-positive sera (lanes 5 to 9), HGE-positive sera (lanes 10 to 11), aborted mare sera (lanes 12 to 19), and rabbit serum specific for a 90-kDa truncated LigA (lane 20). Each lane was loaded with ∼0.5 μg of protein.
TABLE 1.
Reactivity in ELISA of rabbit antiserum to recombinant LigA, sera from horses infected with B. burgdorferi (L1 to L5) or E. equi (E1 and E2), normal horse sera (N1 to N4), and aborted mare's sera (M1 to M8) in an ELISA with a 90-kDa truncated LigA (200 ng/well)a
| Serum | ELISA OD at serum dilution:
|
||
|---|---|---|---|
| 1/200 | 1/400 | 1/800 | |
| Rabbit antiserum to a 90-kDa truncated LigA | 1.13 | 1.02 | 0.58 |
| L1 | 0.05 | 0.03 | 0.01 |
| L2 | 0.1 | 0.04 | 0.02 |
| L3 | 0.03 | 0.02 | 0.02 |
| L4 | 0.05 | 0.02 | 0.03 |
| L5 | 0.02 | 0.01 | 0.01 |
| E1 | 0.05 | 0.03 | 0.05 |
| E2 | 0.08 | 0.05 | 0.04 |
| N1 | 0.01 | 0.01 | 0.0 |
| N2 | 0.01 | 0.0 | 0.0 |
| N3 | 0.02 | 0.01 | 0.01 |
| N4 | 0.03 | 0.03 | 0.01 |
| M1 | 0.39 | 0.34 | 0.19 |
| M2 | 0.38 | 0.35 | 0.18 |
| M3 | 0.45 | 0.31 | 0.2 |
| M4 | 0.6 | 0.56 | 0.27 |
| M5 | 0.28 | 0.2 | 0.13 |
| M6 | 0.47 | 0.56 | 0.4 |
| M7 | 0.73 | 0.55 | 0.4 |
| M8 | 0.56 | 0.5 | 0.42 |
The ELISA OD values of sera from aborted mares were significantly higher (P < 0.05) than the values for sera from normal, B. burgdorferi- and E. equi-infected horses.
Detection of ligA in other serovars by PCR.
PCR amplification revealed the presence of ligA in genomic DNA of the pathogenic serovars hardjo, grippotyphosa, icterohaemorrhagiae, and canicola (Fig. 6A). Restriction analysis with BamHI revealed no differences in fragment patterns. However, HindIII digests revealed that ligA was more highly conserved in L. interrogans serovar pomona and L. kirchneri serovar grippotyphosa than in other serovars (Fig. 6B).
FIG. 6.
Agarose gel showing PCR products and restriction analysis of ligA from different pathogenic serovars of Leptospira. (A) PCR products of ligA. (B) HindIII-digested PCR product of ligA. Lanes: 1, L. interrogans serovar pomona type kennewicki; 2, L. interrogans serovar pomona; 3, L. interrogans serovar hardjo; 4, L. interrogans serovar icterohemorrhagiae; 5, L. kirchneri serovar grippotyphosa; 6, L. interrogans serovar wolfii.
DISCUSSION
Characterization of bacterial antigens expressed only during infection is essential in gaining a deeper understanding of infectious diseases such as leptospirosis. Immunoscreening of gene libraries with convalescent-phase serum is a powerful tool in the discovery of these in vivo-expressed immunogens, which would otherwise be difficult or impossible to identify. We have previously shown that sera from horses which aborted as a result of naturally acquired L. interrogans serovar Pomona type kennewicki infection recognize numerous periplasmic and outer membrane proteins, some of which are regulated by temperature (24). In the present study, an immunoscreening of a genomic library of L. interrogans serovar pomona type kennewicki resulted in the identification of LigA, a novel highly immunogenic protein expressed during equine infection.
LigA is mostly hydrophilic, with some hydrophobic regions located at residues 4 to 24, 306 to 326, 402 to 422, 490 to 510, and 1034 to 1054 (Fig. 1) and consists of beta sheets with a few alpha-helical regions. An Ala-Lys-Glu-Leu-Thr peptide repeat occurs at positions 416, 505, 594, and 867 corresponding to alpha-helices. LigA contains 12 or more tandem repeats of a 90-amino-acid sequence (Fig. 1B). Analysis of the nucleotide sequences by using the NCBI database and BLAST revealed no homology other than that occurring between the repeat region of LigA and the immunoglobulin-like domain of intimin-binding protein (int) of E. coli (17, 21, 23), the invasin of Y. pestis (18, 19), and a cell-binding domain of C. acetobutylicum (26). Based on these similarities, it is possible that LigA may also serve as an adhesin molecule. Further work is needed to clarify its role in adhesion. In the Streptococcus M6 protein, alteration in the number of tandem repeats changes the antigenic determinants (20). In the case of parasites such as Plasmodium, Leishmania, and Trypanosoma, a strong antibody response is induced against immunogenic proteins containing tandem repeats, suggesting that these repetitive epitopes may camouflage vulnerable parasite antigens from a “protective” immune response (10). Overall, variation in the number of tandem repeats may cause antigenic variation, immune escape, and alteration in substrate binding properties. Thus, it is plausible that Leptospira evades immune response by variation in tandem repeats of LigA.
Although sera from recently aborted mares reacted strongly with the 90-kDa truncated LigA, the protein was not detectable by immunoblot in leptospira lysates cultured at 30 and 37°C. In contrast, LipL32 is expressed at both 30 and 37°C, whereas LipL36 expression is growth phase dependent (13, 24). This indicates that LigA is not expressed or thermoregulated under in vitro culture conditions.
However, immunohistochemistry with rabbit antiserum specific for a 90-kDa truncated LigA revealed expression of LigA in kidneys of infected but not uninfected hamsters. A commercially available high-titer leptopsiral antiserum showed strong reactivity to the leptospiral organisms in infected hamster kidney. Expression of LipL32 was detected both in vitro (culture) and in vivo (leptospira-infected hamster kidney), whereas LipL36 expression has been reported only in vitro (2). Our results have also confirmed the in vivo expression of LipL32. However, we noted the reactivity of LipL36 rabbit polyclonal antibody with infected hamster kidney at a 1:50 dilution. In contrast, Barnett et al. have failed to detect expression of LipL36 in L. kirschneri serovar grippotyphosa-infected hamster kidney. It is possible that the level of LipL36 expression differs among serovars during in vivo conditions. The low level of LipL36 expression in infected hamster kidney compared to abundant expression in vitro may be a means of evading the host immune response (24). Regardless of this, these positive controls confirm that LigA is expressed only in vivo. In addition, these results suggest that LigA may be a useful antigen for differential immunodiagnosis to distinguish animals with natural infection from those that are vaccinated.
A 90-kDa protein of Leptospira has been previously shown to cross-react with polyclonal antiserum to an equine corneal protein (22). Immunohistochemistry, immunoprecipitation, and Western blot analysis revealed no reactivity of LigA specific antiserum with equine cornea, iris, vitreous, or lens (data not shown). Thus, LigA does not appear to share antigenic epitopes with equine ocular components and so it is clearly not the reactive protein (22).
PCR amplification of ligA from genomic DNA of pathogenic serovars such as hardjo, icterohaemorrhagiae, grippotyphosa, and canicola has shown that a similar sequence is widely distributed among the serovars of L. interrogans. However, restriction analysis with HindIII showed that the ligA sequence had greater similarity to that of serovars pomona and grippotyphosa than to serovars canicola and icterohaemorrhagiae. Interestingly, L. interrogans serovar pomona and L. kirchneri serovar grippotyphosa are the serovars most frequently responsible for disease in the horse.
The expression of Leptospira outer membrane proteins such as LipL32, LipL41, OmpL1, and LipL36 has been demonstrated in cultured organisms (12-15). Except for LipL36, these outer membrane proteins are expressed in infected hamsters. Interestingly, this is the first leptospiral protein that is not detectable in vitro (30 or 37°C) but is expressed in kidneys of infected hamsters. Importantly, identification of the in vivo-expressed LigA may lead to a better understanding of the molecular pathogenesis of leptospirosis and provide insights into the survival strategy of the organism in the host. Presumably, coordinate expression of a subset of genes during residence in vivo is necessary for survival and replication within the host. Therefore, identification of LigA that is expressed only in vivo may provide new insights for developing strategies to improve vaccination, diagnosis, and treatment protocols.
Acknowledgments
This work was supported in part by the Keeneland Association, the Haggard-Davidson-McGee Equine Medicine Research Fund, the Jack Lowe Equine Research Fund, and the Harry M. Zweig Memorial Fund for Equine Research.
We thank Hsiao-Wei, Fahad Hassan, and Patricia Fisher for excellent technical assistance; David Haake for his generous gift of rabbit polyclonal antisera to LipL32 and LipL36; and Mike Donahue for Leptospira strains.
Editor: D. L. Burns
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, J. K., D. Barnett, C. A. Bolin, T. A. Summers, E. A. Wagar, N. F. Cheville, R. A. Hartskeerl, and D. A. Haake. 1999. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect. Immun. 67:853-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, Y. F., M. J. Appel, R. H. Jacobson, S. J. Shin, P. Harpending, R. Straubinger, L. A. Patrican, H. Mohammed, and B. A. Summers. 1995. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect. Immun. 63:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y.-F., V. Novosol, E. Dubovi, S. J. Wong, F. K. Chu, C.-F. Chang, F. D. Piero, S. Shin, and D. H. Lein. 1998. Experimental infection of the human granulocytic ehrlichiosis agent in horses. Vet. Parasitol. 78:137-145. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y.-F., V. Novosol, S. P. McDonough, C.-F. Chang, R. H. Jacobson, T. Divers, F. W. Quimby, S. Shin, and D. H. Lein. 2000. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet. Pathol. 37:68-76. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y. F., J. Shi, D. P. Ma, S. J. Shin, and D. H. Lein. 1993. Molecular analysis of the Actinobacillus pleuropneumoniae RTX toxin-III gene cluster. DNA Cell Biol. 12:351-362. [DOI] [PubMed] [Google Scholar]
- 7.Donahue, J. M., B. J. Smith, J. K. Donahoe, C. L. Rigsby, R. R. Tramontin, K. B. Poonacha, and M. A. Wilson. 1992. Prevalence and serovars of leptospira involved in equine abortions in central Kentucky during the 1990 foaling season. J. Vet. Diagn. Investig. 4:279-284. [DOI] [PubMed] [Google Scholar]
- 8.Donahue, J. M., B. J. Smith, K. B. Poonacha, J. K. Donahoe, and C. L. Rigsby. 1995. Prevalence and serovars of leptospira involved in equine abortions in central Kentucky during the 1991-1993 foaling seasons. J. Vet. Diagn. Investig. 7:87-91. [DOI] [PubMed] [Google Scholar]
- 9.Donahue, J. M., B. J. Smith, K. J. Redmon, and J. K. Donahue. 1991. Diagnosis and prevalence of leptospira infection in aborted and stillborn horses. J. Vet. Diagn. Investig. 3:148-151. [DOI] [PubMed] [Google Scholar]
- 10.Duncan, L. R., L. S. Gay, and J. E. Donelson. 1991. African trypanosomes express an immunogenic protein with a repeating epitope of 24 amino acids. Mol. Biochem. Parasitol. 48:11-16. [DOI] [PubMed] [Google Scholar]
- 11.Feigin, R. D., and D. C. Anderson. 1975. Human leptospirosis. CRC Crit. Rev. Clin. Lab. Sci. 5:413-467. [DOI] [PubMed] [Google Scholar]
- 12.Haake, D. A., C. I. Champion, C. Martinich, E. S. Shang, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1993. Molecular cloning and sequence analysis of the gene encoding OmpL1, a transmembrane outer membrane protein of pathogenic Leptospira spp. J. Bacteriol. 175:4225-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haake, D. A., C. Martinich, T. A. Summers, E. S. Shang, J. D. Pruetz, A. M. McCoy, M. K. Mazel, and C. A. Bolin. 1998. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect. Immun. 66:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haake, D. A., M. K. Mazel, A. M. McCoy, F. Milward, G. Chao, J. Matsunaga, and E. A. Wagar. 1999. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 67:6572-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwell, R. E., T. A. Brim, M. T. Hines, D. Wolf, and F. H. White. 1985. Studies on equine recurrent uveitis. II. The role of infection with Leptospira interrogans serovar pomona. Curr. Eye Res. 4:1033-1040. [DOI] [PubMed] [Google Scholar]
- 17.Hamburger, Z. A., M. S. Brown, R. R. Isberg, and P. J. Bjorkman. 1999. Crystal structure of invasin: a bacterial integrin-binding protein. Science 286:291-295. [DOI] [PubMed] [Google Scholar]
- 18.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 19.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, K. F., S. K. Hollingshead, J. R. Scott, and V. A. Fishetti. 1988. Spontaneous M6 protein size mutants of group A streptococci display variation in antigenic and opsonogenic epitopes. Proc. Natl. Acad. Sci. USA 85:8271-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly, G., S. Prasannan, S. Daniell, K. Fleming, G. Frankel, G. Dougan, I. Connerton, and S. Matthews. 1999. Structure of the cell-adhesion fragment of intimin from enteropathogenic Escherichia coli. Nat. Struct. Biol. 6:313-318. [DOI] [PubMed] [Google Scholar]
- 22.Lucchesi, P. M., and A. E. Parma. 1999. A DNA fragment of Leptospira interrogans encodes a protein which shares epitopes with equine cornea. Vet. Immunol. Immunopathol. 71:173-179. [DOI] [PubMed] [Google Scholar]
- 23.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 24.Nally, J. E., J. F. Timoney, and B. Stevenson. 2001. Temperature-regulated protein synthesis by Leptospira interrogans. Infect. Immun. 69:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 26.Nolling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parma, A. E., A. S. Fernandez, C. G. Santisteban, R. A. Bowden, and S. I. Cerone. 1987. Tears and aqueous humor from horses inoculated with Leptospira contain antibodies which bind to cornea. Vet. Immunol. Immunopathol. 14:181-185. [DOI] [PubMed] [Google Scholar]
- 28.Parma, A. E., C. G. Santisteban, J. S. Villalba, and R. A. Bowden. 1985. Experimental demonstration of an antigenic relationship between Leptospira and equine cornea. Vet. Immunol. Immunopathol. 10:215-224. [DOI] [PubMed] [Google Scholar]
- 29.Poonacha, K. B., J. M. Donahue, R. C. Giles, C. B. Hong, M. B. Petrites-Murphy, B. J. Smith, T. W. Swerczek, R. R. Tramontin, and P. A. Tuttle. 1993. Leptospirosis in equine fetuses, stillborn foals, and placentas. Vet. Pathol. 30:362-369. [DOI] [PubMed] [Google Scholar]
- 30.Rathinam, S. R., S. Rathnam, S. Selvaraj, D. Dean, R. A. Nozik, and P. Namperumalsamy. 1997. Uveitis associated with an epidemic outbreak of leptospirosis. Am. J. Ophthalmol. 124:71-79. [DOI] [PubMed] [Google Scholar]
- 31.Roberts, S. J. 1958. Sequelae of leptospirosis in horses on a small farm. J. Am. Vet. Med. Assoc. 175:803-808. [PubMed] [Google Scholar]
- 32.Shang, E. S., M. M. Exner, T. A. Summers, C. Martinich, C. I. Champion, R. E. Hancock, and D. A. Haake. 1995. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infect. Immun. 63:3174-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang, E. S., T. A. Summers, and D. A. Haake. 1996. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect. Immun. 64:2322-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]