Abstract
The existence of unpaired electrons in the four heme groups of deoxy and methemoglobin (metHb) gives these species paramagnetic properties as contrasted to the diamagnetic character of oxyhemoglobin. Based on the measured magnetic moments of hemoglobin and its compounds, and on the relatively high hemoglobin concentration of human erythrocytes, we hypothesized that differential migration of these cells was possible if exposed to a high magnetic field. With the development of a new technology, cell tracking velocimetry, we were able to measure the migration velocity of deoxygenated and metHb-containing erythrocytes, exposed to a mean magnetic field of 1.40 T and a mean gradient of 0.131 T/mm, in a process we call cell magnetophoresis. Our results show a similar magnetophoretic mobility of 3.86 × 10−6 mm3 s/kg for erythrocytes with 100% deoxygenated hemoglobin and 3.66 × 10−6 mm3 s/kg for erythrocytes containing 100% metHb. Oxygenated erythrocytes had a magnetophoretic mobility of from −0.2 × 10−6 mm3 s/kg to +0.30 × 10−6 mm3 s/kg, indicating a significant diamagnetic component relative to the suspension medium, in agreement with previous studies on the hemoglobin magnetic susceptibility. Magnetophoresis may open up an approach to characterize and separate cells for biochemical analysis based on intrinsic and extrinsic magnetic properties of biological macromolecules.
INTRODUCTION
Early studies on the magnetic susceptibility of hemoglobin have helped to elucidate the molecular and electronic structure of the chemical bonds in the heme group depending on the oxidative state and oxygen binding of the iron atom. Early work by Pauling, Coryell, and others indicated that there is a profound change in the character of chemical bonds between the iron atom and the rest of the heme group and the globin chain as a result of oxygen binding: in deoxygenated ferrous hemoglobin, the bonds are purely ionic whereas in oxygenated ferrous hemoglobin they are more covalent in nature (Pauling and Coryell, 1936a,b). Similarly, there are ionic bonds between the Fe3+ present in methemoglobin (metHb, the oxidized form of hemoglobin), the protoporphyrin group, and the globin chain (Coryell et al., 1937). Each time the iron is attached to the surrounding protoporphyrin and histidine side chain by ionic bonds, the presence of unpaired electrons is noted. As a consequence, deoxygenated hemoglobin and metHb contain four and five unpaired electrons, respectively, per heme group, making these species paramagnetic. Due to its covalent bonds, oxyhemoglobin has no unpaired electrons and is diamagnetic. Hemoglobin's protein moiety, the globin chains, on the other hand, are diamagnetic and make no contribution to the paramagnetic moment measured in deoxyhemoglobin and metHb (Savicki et al., 1984; Spees et at., 2001).
Melville and co-workers showed in the mid 1970s the equilibrium capture of erythrocytes, reduced after treatment with a sodium dithionite solution, using a ferromagnetic wire mesh that produces a high gradient magnetic field (HGMF) induced by an external field (Melville et al., 1975). Later, Owen showed that equilibrium cell capture was also possible using metHb-containing erythrocytes (Owen, 1978). In both cases, researchers used rudimentary columns for their cell-capture experiments, made of a ferromagnetic wire mesh that generated a HGMF when placed in the vicinity of a strong magnet. These columns were the precursors of the immunomagnetic cell separation columns commercially available today in which cell selection is done with the use of monoclonal antibodies linked to ferromagnetic nanoparticles (Roath et al., 1992, 1994; Radbruch et al., 1994; Svoboda, 2000).
A different concept, however, is that of magnetophoretic mobility (MM) in which there is particle transport, imposed by a magnetic field in a viscous medium (Zborowski et al., 2002). Its physical significance is analogous to that of electrophoretic mobility in the description of particle motion in an electric field (Melcher, 1981).
Early measurements of red blood cell (RBC) MM relied on the HGMF geometry (Friedlaender et al., 1979; Plyavin and Blum, 1983). This method required precise information about the cell position relative to the magnetic field, which was a source of experimental error. Such field geometry also significantly limits the area of observation, and therefore limits the number of cells whose motion can be measured in a reasonable time. Early open-gradient experiments allowed calculating the mobile cell fraction, giving no information about the mobility of individual cells (Winoto-Morbach et al., 1994; Tchikov et al., 1999). In our laboratories, we have developed an open-gradient instrument that maintains a constant, two-dimensional magnetic field gradient over an observation area that extends 1.72 × 1.27 mm. We also have developed the image acquisition and processing hardware and software that allows simultaneous tracking and velocity calculation of tens to hundreds of cells in under a minute, which significantly increases the accuracy of our experimental results (Moore et al., 2000; Nakamura et al., 2001).
The development of cell tracking velocimetry (CTV) allowed us to make very precise measurements of the MM of various cell systems. Particularly interesting were the experiments in which we measured MM relative to cell surface antigen expression in specific cell types, and its effect on immunomagnetic cell labeling efficiency (McCloskey et al., 2000, 2001). Next, we wanted to determine whether CTV was capable of detecting differences in the MM of cells with no immunomagnetic label. We used deoxygenated as well as metHb-containing erythrocytes as our paramagnetic cell species and compared their MM with the one obtained from oxygenated erythrocytes, known to be diamagnetic (Pauling and Coryell, 1936b; Savicki et al., 1984). In RBCs, we hypothesized, different amounts of intracellular metHb would result in different degrees of cell paramagnetism, relative to that of water, and would affect the observed MM. We also wanted to know if the different MM obtained between paramagnetic and nonparamagnetic cell species were compatible with those predicted on the basis of bulk hemoglobin contents and RBC magnetic susceptibility measurements by Gouy's method (Pauling and Coryell, 1936a,b; Bozorth, 1968), superconducting quantum interference device (SQUID) (Savicki et al., 1984), and NMR methods (Weisskoff and Kiihne, 1992; Spees et al., 2001).
We report here the first successful measurement of the MM of deoxygenated and metHb-containing erythrocytes compared to oxygenated controls. We also report the close correlation of our experimental data with calculated MM values based on the physical parameters of the system.
MATERIALS AND METHODS
Red blood cell magnetophoresis
In general, cell mobility is defined as directly proportional to the particle-field interaction parameter, and inversely proportional to the cell friction coefficient (Zborowski et al., 2002). The particle-field interaction parameter represents the generalized “charge” of the particle, that is, the particle property that makes it responsive to the applied field. For instance, in the case of an electric field, the particle-field interaction parameter is equal to the effective electric charge of the molecule, the particle, or a cell in solution (Melcher, 1981). In gravitation, sedimentation, and centrifugal separations, the difference between the cell density and that of the solution, multiplied by the cell volume, is the cell-field interaction parameter, ΔρV. Analogously, when describing the motion of magnetically polarizable cells in the magnetic field, the difference between the cell volumetric susceptibility and that of the solution, multiplied by the cell volume, is the cell-field interaction parameter, ΔχV, (Table 1). The advantage of using MM to describe cell motion in a magnetic field is that MM is independent of the experimental apparatus because it is an intrinsic property of the cell.
TABLE 1.
Predicted RBC magnetophoretic mobilities based on the RBC magnetic susceptibilities
| Parameter | Symbol | Value | Units | Ref. |
|---|---|---|---|---|
| Net volumetric RBC susceptibility* | Δχ = χRBC − χH2O | −0.0147 × 10−6 (oxyHb) | 1 | Eqs. 1, 3 |
| 0.265 × 10−6 (deoxyHb) | Eqs. 1, 3 | |||
| 0.301 × 10−6 (metHb) | Eqs. 2, 3 | |||
| RBC-field interaction parameter† | ΔχV | −1.64 × 10−14 (oxyHb) | mm3 | |
| 29.5 × 10−14 (deoxyHb) | ||||
| 33.5 × 10−14 (metHb) | ||||
| RBC friction coefficient‡ | 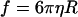 |
7.26 × 10−8 | kg/s | |
| Magnetophoretic mobility of the RBC§ | 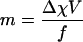 |
−0.226 × 10−6 (oxyHb) | mm3 s/kg | |
| 4.06 × 10−6 (deoxyHB) | ||||
| 4.61 × 10−6 (metHb) | ||||
| Magnetophoretic driving force¶ | 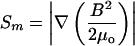 |
1.50 × 102 | kg/mm2/s2 | |
| RBC magnetic migration velocity | um = m × Sm | −0.034 (oxyHb) | μm/s | |
| +0.609 (deoxyHb) | ||||
| +0.691 (metHb) |
In cgs em units system.
In SI units system.
R = 3.85 μm, red blood cell hydrodynamic radius; η = 0.96 × 10−3 kg/m-s, aqueous solution viscosity at 20 C.
V = 88.4 μm3, red blood cell volume.
B magnetic field intensity (in tesla (T)); μ0 = 4π × 10−7 T-m/A (Spees et al., 2001; Graham, 1984).
The MM of human erythrocytes was measured using a CTV instrument developed in our laboratories for the determination of cell velocities when exposed to the magnetic and gravitational fields (Moore et al., 2000; Nakamura et al., 2001; McCloskey et al., 2000, 2001). The central part of the apparatus is the permanent magnet assembly, with specially designed pole pieces that produce a nearly constant value for the force field strength, Sm (a gradient of magnetic energy density, Table 1) over the area of observation of 1.72 × 1.27 mm, and the depth of view of ∼20 μm. Spaced 2.5-mm apart, the pole pieces conduct the magnetic flux into an air gap, into which a 0.6 × 1.7 mm ID, 0.4-mm wall square channel is placed. The orientation of the magnet and flow channel is such that the magnetic force is orthogonal to the force of gravity, to avoid sedimentation contribution to the MM. The geometry of the pole pieces, and the placement of the flow channel, ensure that the field is, essentially, two-dimensional. (However, in computing mobilities, only the horizontal component of the gradient is used). In the viewing area, the average force field strength, Sm = 150 TA/mm2 ± 0.7%; the mean field value is 1.40 T, and the mean field gradient is 0.131 T/mm.
The cell motion in the viewing area is observed with a 5× microscope objective and 2.5× photo eyepiece (Olympus, Tokyo, Japan). Light is supplied internally (epiillumination). A Cohu (San Diego, CA) CCD 4915 camera operating at a frame speed of 30 Hz, and a μ-Tech Vision 1000 PCI Bus Frame Grabber (MuTech, Billerica, MA) is used to convert the image into a 640 × 480 pixel array, where each pixel contains eight bits of gray-level information ranging from 0 (black) to 255 (white). The CTV algorithm is a modification of a three-dimensional version called particle tracking velocimetry (Guezenec and Kiritis, 1990). The current code uses five successive images to establish the most probable path for a specific particle. From this most probable path, the algorithm determines and reports the 2-D location. A linear fit of the location-time data gives velocity of each particle. The algorithm then computes statistics for the entire set of particle velocities (and mobilities), including mean, standard deviation, and confidence limits. As the CCD camera has a frame rate of 30 Hz, and the acquisition rate was set at every 60th frame for both studies on deoxygenated and metHb-containing erythrocytes, the time between acquired frames is 2.0 s. Because the number of tracked frames was always 20, therefore, the time difference between the first and last tracked frames was (20−1) × 2 = 38 s. In this time interval the red cells moved a sufficient distance to be assigned a velocity easily distinguishable from zero. We determined that the mobility discrimination power of CTV is below the noise introduced by the cell Brownian motion.
Red blood cell magnetic susceptibility
To calculate the RBC magnetic susceptibility relative to that of the solution, Δχ, (Table 1), we adapted the model described by Spees and co-workers (Spees et al., 2001). That model is based on an early work by Cerdonio et al. (1978, 1985) that was also adopted by others groups (Plyavin and Blum, 1983; Weisskoff and Kiihne, 1992; Fabry and San George, 1983; Graham, 1984). The most important difference between the models is in the number of unpaired electrons ascribed to the oxyhemoglobin heme group. Here we assume, after (Pauling and Coryell, 1936b; Savicki et al., 1984; Spees et al., 2001), that there are no unpaired electrons in the oxyhemoglobin heme group. In general, the RBC volumetric magnetic susceptibility is the weighted sum of the susceptibilities of its components:
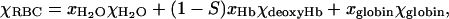 |
(1) |
where xH2O and χH2O, (1 − S)xHb and χdeoxyHb, xglobin, and χglobin are the volume fractions and volumetric magnetic susceptibilities of water, deoxyhemoglobin and the protein (globin) part of the hemoglobin molecule, respectively; and S is the oxygen saturation of oxyhemoglobin. Here xH2O = 1 − nHbνHb, where nHb = 5.5 mM is the total intracellular hemoglobin concentration, νHb = 48,277 cm3/mol is the molar volume of hemoglobin, xHbχdeoxyHb = nHbχ′deoxyHb, where χ′deoxyHb = 50,893 × 10−6 cm3/mol is the molar susceptibility of deoxyhemoglobin, and xglobinχglobin = nHbMHbχ″globin, where MHb = 64,450 is the molecular weight of hemoglobin, and χ″globin = −0.580 × 10−6 cm3/g is the specific susceptibility of globin in the hemoglobin molecule (Spees et al., 2001). The susceptibility values are given in the cgs unit system to facilitate comparison with the literature data; the conversion to values in the SI unit system requires multiplication by 4π.
The metHb contribution to the erythrocyte magnetic susceptibility has a similar form as the one above:
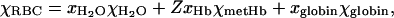 |
(2) |
where xmetHb and χmetHb are the volume fraction and the magnetic susceptibility of metHb, and Z is the fraction of hemoglobin converted to metHb. In these set of experiments, we assume that hemoglobin exists in two forms only, as metHb (ferrihemoglobin) or oxyhemoglobin. Also, xHbχmetHb = nHbχ′metHb, where χ′metHb = 57,428 × 10−6 cm3/mol (Coryell et al., 1937; Graham, 1984).
Thus, the net magnetic susceptibility of the RBC in aqueous suspension is
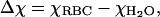 |
(3) |
where χH2O = −0.719 × 10−6 is the magnetic susceptibility of water (Spees et al., 2001).
It is interesting to note that Δχ < 0 for the fully oxygenated RBC, and Δχ > 0 for the deoxygenated and methemoglobinated RBC (Table 1); consequently, we would expect the sign for oxygenated red blood cell mobility to be opposite (negative) to the ones for deoxygenated and methemoglobinated RBCs (Table 1).
Preparation of metHb-containing erythrocytes
Blood was collected at the National Institutes of Health under an Institutional Review Board-approved protocol for blood collection from normal volunteers for research purposes. The samples were drawn in EDTA Vacutainer tubes (Becton, Dickinson, Rutherford, NJ). A 5-mM oxidant solution was prepared by dissolving sodium nitrite (Aldrich Chemical, Milwaukee, WI) in phosphate-buffered saline (PBS) pH: 7.4 without calcium chloride and without magnesium chloride (Life Technologies, Gaithersburg, MD) at room temperature. The oxidative treatment was performed by mixing 50 μl of whole blood with 4 ml of the 5 mM sodium nitrite solution. Cell mixtures were incubated for different time periods to achieve different levels of intracellular metHb. Controls were prepared by mixing 50 μl of whole blood with 4 ml of PBS. After incubation, oxidant-treated erythrocytes and controls were washed three times with PBS and resuspended in 4 ml PBS. A 300-μl aliquot of this erythrocyte suspension was mixed with 1,000 μl of distilled water to hemolyze the cells. After waiting ∼1 min for hemolysis to proceed, the samples were vortexed and centrifuged at 13,000 rpm (15,700 g) for 5 min in an Eppendorf 5415 D centrifuge (Brinkman Instruments, Westbury, NY) to pellet cell membranes. The supernatants were then transferred to plastic cuvettes and spectrophotometric readings were taken at 700, 630, 576, and 560 nm (PerkinElmer Lambda 20 UV/Vis Spectrometer, Norwalk, CT). These absorption values were used to calculate the percentage of metHb formed by the oxidative treatment in each sample (Winterbourn, 1990).
Preparation of deoxygenated erythrocytes
Red blood cells were pelleted by centrifugation at room temperature at 2000 rpm. (805 g) for 10 min in an Eppendorf 5810 R centrifuge. The cell pellet was washed twice with saline solution (0.9% sodium chloride solution for injection, Abbott Labs, North Chicago, Il) after removing plasma and buffy coat. After the second wash, enough saline solution was added to restore the sample's original volume. A diluted erythrocyte suspension was prepared by mixing150 μl of the washed red blood cells with 12 ml of saline solution-2% fetal bovine serum (FBS) (Gemini Bio-Products, Woodland, CA). This cell mixture was allowed to fully oxygenate by gentle rocking at room temperature for at least 1 h. A 4-ml aliquot of this erythrocyte cell mixture was used as the oxygenated RBC control. Another 4-ml aliquot was placed in a 10-ml glass test tube fitted to an airtight rubber top, sealed with Parafilm (American National Can, Chicago, Il) and deoxygenated by exposure to a steady flow of nitrogen coming through a 19-gauge needle inserted through the glass test tube's rubber top (and vented with a similar gauge needle), while the test tube was gently rocked in horizontal position for 45 min. Next, 200 μl of the deoxygenated erythrocyte suspension was added to 10 ml of deoxygenated saline solution-2% FBS, previously equilibrated in nitrogen gas.
CTV measurements
The CTV channel was flushed with ∼60 ml of carrier buffer before each experiment. The metHb-containing erythrocytes and oxygenated control were processed identically. Each sample was washed twice in Plasma-Lyte (Baxter Healthcare, Deerfield, IL) with 5% FBS, counted with a Coulter Z-1, and diluted in to a final concentration of 0.5 × 106 cells/ml. Cell suspensions were injected manually, valves bracketing the channel were closed, and the suspension was relaxed for 2 min. Twenty video images were then recorded with a skip frame of 59 (allowing 2.0 s between frames). Between injections, the sample syringe was mixed to assure homogeneity of the cell suspension. A minimum of eight measurement sets was processed for a total time of 30–60 min per sample.
The deoxygenated erythrocyte sample and its oxygenated control were processed as above with a few exceptions. Before the experiment, the CTV apparatus was fitted with gas-impermeable tubing. The control cells were counted and diluted in PBS for a final concentration of 0.5 × 106 cells/ml. The deoxygenated cells were neither counted nor diluted but transferred directly to a 50-cc Hamilton gas-tight syringe in a glove bag filled with nitrogen. The glass syringe was attached to the CTV injection port as quickly as possible to avoid any contact with air. Measuring time was ∼60 min per sample.
RESULTS
Magnetophoresis of metHb-containing erythrocytes
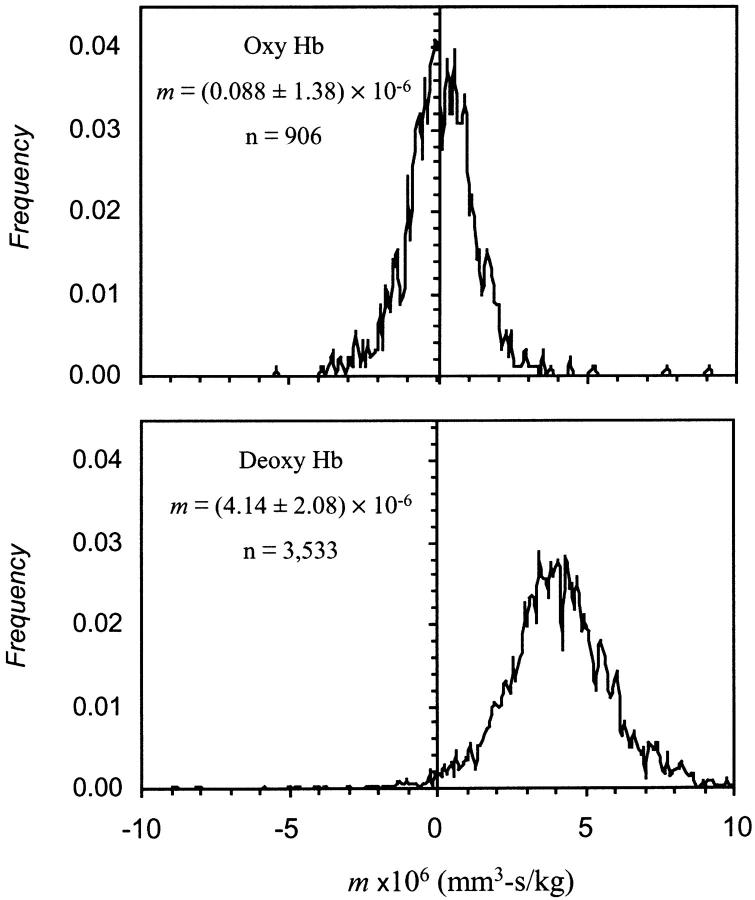
For these experiments, we used blood from five different adult individuals. Fig. 1 shows a series of RBC MM histograms obtained when four different metHb levels were tested in a single blood sample. The histograms show a monotonic increase in the mobility with increasing metHb level. There is a dispersion of the MM around the most probable value. Although the mobility distribution of control RBCs (at 0% metHb) appears narrower at half peak height than the distribution of metHb RBCs, the corresponding standard deviations of the mean are not significantly different. We ascribe this to the departure of the mobility distribution from normal. The likely source of the mobility dispersion, and the difference in its extent between control and metHb RBCs, is the heterogeneous nature of the normal erythrocyte population and the background noise introduced by the measuring apparatus. Red blood cell heterogeneity is the consequence of cell aging, which affects cell intracellular levels of different enzymatic components. The cellular contents of enzymes involved in antioxidant protection, including metHb reductase, is higher in young erythrocytes (Bartosz, 1990). We estimate that the differences in intracellular oxidative susceptibility could affect the cell ability to partially reverse hemoglobin oxidation, resulting in dispersion of the metHb content of individual cells and, consequently, the dispersion of cell MM. Control cells, on the other hand, have a narrower mobility distribution because they had not been the subject of intracellular oxidation. The contribution of the apparatus' random noise to the MM dispersion was observed using monodisperse, synthetic particles (data not shown).
FIGURE 1.
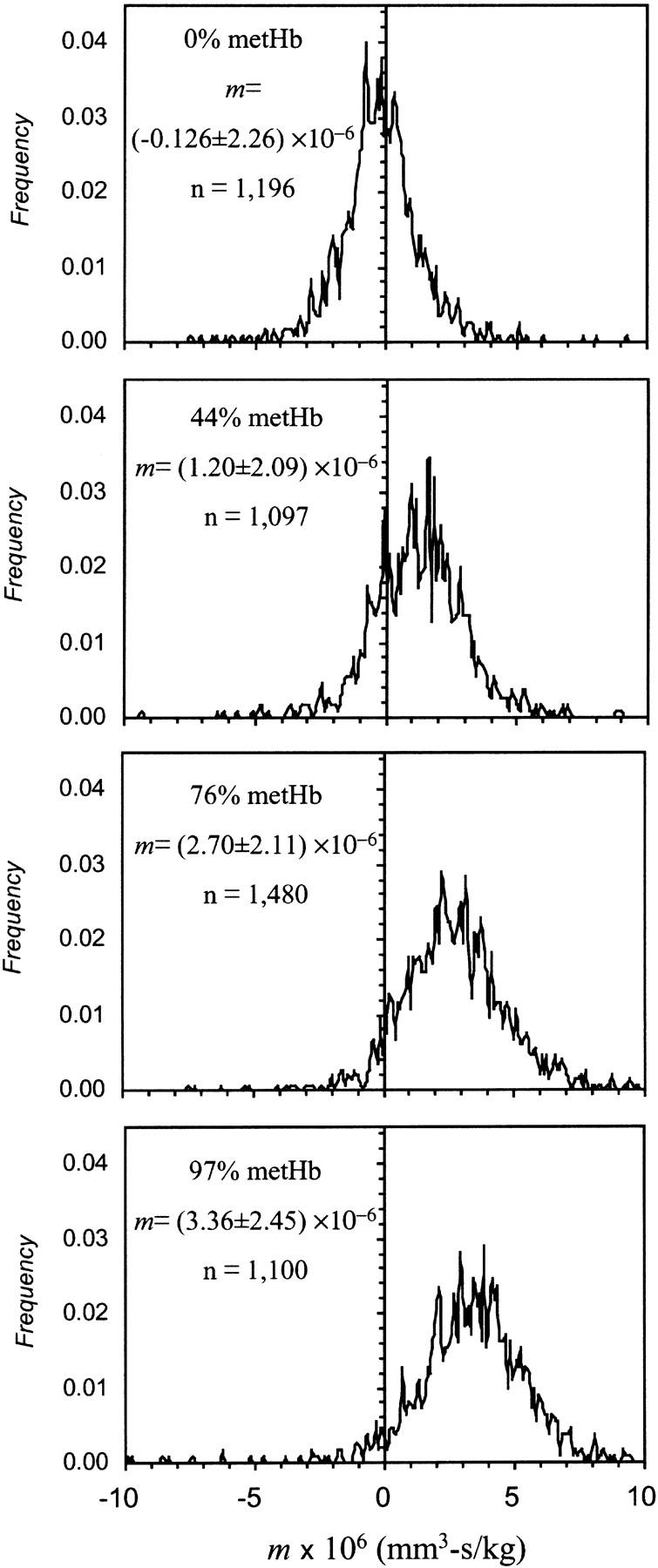
Magnetophoretic mobility histograms of erythrocytes containing different levels of methemoglobin. The data shown is a representative example from an individual.
The mobility difference between the control, fully oxygenated RBC not subjected to oxidative treatment, (Z = 0% metHb, S = 100%) and a sample with nearly full hemoglobin oxidation (at Z = 97% metHb, S ≈ 0%) was equal to approximately one-and-a-half standard deviations of the single measurement, in the representative example shown in Fig. 1. The statistical data for all samples is included in Table 2. The number of cell trajectories acquired and analyzed for a single mean mobility measurement was between 600 and 1480 individual cell measurements, with each cell trajectory consisting of 20 line segments. This produced high confidence in the mean mobility values, as illustrated by narrow 95% confidence intervals obtained and the small standard error of the mean, as can be seen in Table 2.
TABLE 2.
Observed magnetophoretic mobility of erythrocytes
| Methemoglobin-containing erythrocytes | Value |
|---|---|
| Parameter | |
| Number of observations | 16 |
| Correlation coefficient squared, R2 | 0.9796 |
| Standard error of estimate | 0.22 × 10−6 mm3 s/kg |
| Intercept (estimated mobility at Z = 0) | −0.20 × 10−6 mm3 s/kg |
| 95% C.I. of the intercept | [−0.387, −0.013] × 10−6 mm3 s/kg |
| 99% C.I. of the intercept | [−0.460, 0.060] × 10−6 mm3s/kg |
| p-value of the intercept | 0.038 |
| Slope | 3.86 × 10−6 mm3 s/kg |
| 99% C.I. of the slope | [3.42, 4.31] × 10−6 mm3s/kg |
| p-value of the slope | <10−6 |
| Estimated mobility at Z = 1 | 3.66 × 10−6 mm3 s/kg |
| Observed vs. predicted mobility (×10−6 mm3s/kg): at methemoglobin fraction: | |
| Z = 0 | −0.20 vs. −0.226 |
| Z = 1 | 3.66 vs. 4.61 |
| Deoxygenated erythrocytes | Oxygenated erythrocytes | |
|---|---|---|
| Parameter | Value | Value |
| Oxygen saturation | S = 0 | S = 1 |
| Mean/10−6 mm3 s/kg | 3.86 | 0.300 |
| SE/10−6 mm3 s/kg | 0.020 | 0.026 |
| SD/10−6 mm3 s/kg | 2.10 | 1.82 |
| Total N | 10,616 | 4,775 |
| 99% C.I./ 10−6 mm3 s/kg | [3.84, 3.89] | [0.274, 0.326] |
| t-test: two-sample assuming unequal variances | ||
| Hypothesized mean difference | 0 | |
| p (two-tail) | <10−6 | |
| Observed vs. predicted mobility (×10−6 mm3 s/kg): at oxygen saturation: | ||
| S = 0 | 3.86 vs. 4.06 | |
| S = 1 | +0.300 vs. −0.226 |
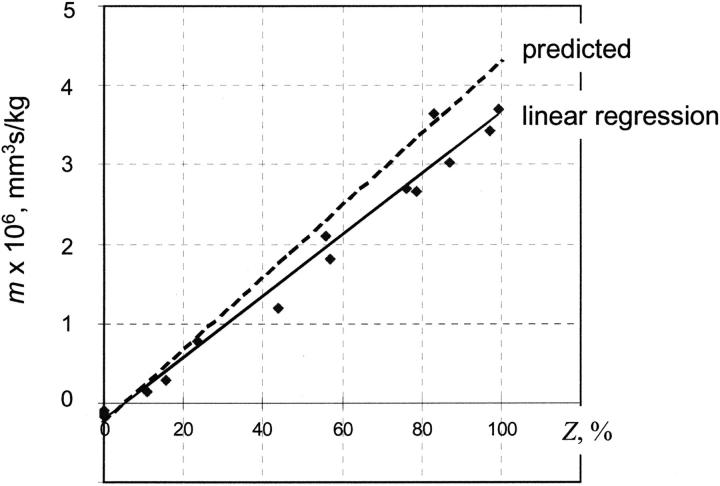
Based on the RBC magnetic susceptibility model presented in the Materials and Methods section, we expected a linear dependence of cell mobility on the RBC metHb contents (Eq. 2 and Table 1). We tested this hypothesis by regression analysis of the cell mobility on the RBC metHb level (Fig. 2 and Table 2). Both the experimental regression line and the calculated mobility dependence on metHb level (Z) are shown in Fig. 2. The calculated mobility follows closely the linear regression line obtained from the experimental data. Thus, the predicted cell mobility in the magnetic field corresponds very closely to the mobility observed experimentally. Interestingly, when interpolated to zero metHb level, the equivalent to fully oxygenated erythrocytes not treated with oxidant solution, the experimental cell mobility is slightly negative, in agreement with the RBC magnetic susceptibility model and the reported oxyhemoglobin susceptibility measurements by Gouy balance, SQUID, and the NMR methods (Table 1) (Pauling and Coryell, 1936a,b; Coryell et al., 1937; Savicki et al., 1984; Spees et al., 2001).
FIGURE 2.
Dependence of magnetophoretic mobility on the erythrocyte methemoglobin contents: a comparison of CTV experimental data and modeled calculated values.
The regression statistics and the ANOVA results indicated that the predicted dependence of the mean mobility on metHb level is highly significant: R2 = 0.9796, with a standard error of regression of 0.312·10−6 mobility units (mm3s/kg), at a significance level of p < 10−6 at N = 16 (Table 2). The regression equation of mobility, m, on the metHb fraction, Z, is:
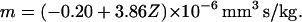 |
(4) |
The experimental mean mobility values for fully oxygenated and fully methemoglobinated RBCs are compared in Table 2. We found a remarkable agreement between the oxygenated RBC (Z = 0) mobility and the value calculated from the RBC magnetic susceptibility data (Table 1). On the other hand, in fully oxidized RBC (Z = 1), the mean mobility is lower than that predicted from the RBC susceptibility data (Table 1).
Magnetophoresis of deoxygenated erythrocytes
In this separate group of experiments, we measured the MM of deoxygenated erythrocytes obtained from five adult individuals. In this case also, a fully oxygenated, untreated RBC suspension was used as a control. We used a simplified RBC preparation protocol to maintain strict anaerobic conditions for the deoxygenated RBC mobility measurements. A representative example of the MM histograms is shown in Fig. 3. There is a noticeable shift toward higher mobility values for the deoxygenated RBCs, as predicted by Eq. 1 and Table 1. The dispersion of cell mobility around the mean value is comparable to that observed for the metHb-containing RBC mobilities. In this case, however, the likely source is slight difference in oxygen saturation between individual cells due to physiological variations in oxygen affinity, normally found during cell aging (Samaja et al., 1990; Schmidt et al., 1987). The observed shift in the MM with the decrease in oxygen saturation corresponds to approximately twice the standard deviation of the dispersion. A summary of these results is presented in Table 2. The mean MMs, each comprising from 600 to 3533 individual cell mobility measurements, were averaged over all five experiments, and the resultant average mobilities for the deoxygenated and oxygenated erythrocytes were compared (Table 2). A t-test (two-tailed) was used to test the null hypothesis stating that the deoxygenated erythrocyte mobility was not different from that of oxygenated erythrocytes. Such null hypothesis was shown to be highly improbable (p < 10−6). The observed difference between the deoxygenated and oxygenated erythrocytes is:
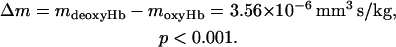 |
FIGURE 3.
Magnetophoretic mobility histograms of oxygenated and deoxygenated erythrocytes. The data shown are a representative example from an individual.
In this case, we can also see that the agreement with the calculated mobilities based on the RBC magnetic susceptibilities is very good (Table 2).
DISCUSSION
Early in the 20th century, magnetic susceptibility measurements of hemoglobin and its derivatives played an important role in the study of the molecular structure of the heme group and provided an early experimental model in quantum chemistry (Pauling and Coryell, 1936a,b; Coryell et al., 1937). Erythrocytes are an ideal cell model for physical experimentation because their structure is relatively simple, and physical parameters such as cell diameter, cell volume, and hemoglobin contents are homogeneous in the erythrocyte cell population of a particular individual (Savicki et al., 1984; Graham, 1984; Cerdonio et al., 1985; Spees et al., 2001).
We have studied the motion of RBCs in aqueous solutions induced by an external magnetic field using CTV (Moore et al., 2000; Nakamura et al., 2001). One of the advantages of this technique is that it allowed us to follow the mobility of hundreds of single cells in a relatively short period of time. The measurement of single cell mobility provides us with information that is not available by other methods based on bulk cell susceptibility measurements.
The mean RBC MM measurements we obtained agreed with values estimated from the literature's hemoglobin magnetic susceptibility data, normally provided by magnetometry, NMR, and SQUID methodologies (Pauling and Coryell, 1936a,b; Coryell et al., 1937; Savicki et al., 1984; Spees et al., 2001). This supports the notion that MM is a useful parameter in studies related to cell motion in the magnetic field and magnetic cell separation. The agreement seen between experimental and model data validated a number of assumptions underlying the definition of MM, in particular, the linear relationship between hemoglobin magnetization and magnetic field strength, at least within the range used in this study (Zborowski et al., 2002). The use of a constant field energy gradient, the isodynamic field, greatly reduced a potential source of experimental error inasmuch as it is the quantity that drives cell migration (Moore et al., 2000).
The MM measurements on erythrocytes containing from 0 to 99% of intracellular metHb led to a gradient of cell mobilities as was predicted from their magnetic susceptibilities. Moreover, the interpolation, from the experimentally obtained curve, of the RBC mobility to zero metHb content agreed with the expected mobility for fully oxygenated RBCs (Spees et al., 2001). We concluded that our mobility measurements on metHb-containing erythrocytes were well in agreement with the modeled expected mobility values.
We also compared the MM of oxygenated and deoxygenated erythrocytes. We observed significant cell mobility in the fully deoxygenated erythrocytes, as predicted by the magnetic susceptibility data. The absolute value of mean oxygenated erythrocyte mobility was weakly positive in this set of experiments. This observation contrasts with the slightly negative mean oxygenated RBC mobility obtained by extrapolation in the metHb experiments. Although the differences are minimal and do not affect our mobility measurements on deoxygenated and metHb-containing erythrocytes, our methods should allow us to address in the near future the question posed by Cerdonio (Pauling and Coryell, 1936b; Savicki et al., 1984; Cerdonio et al., 1978, 1985) about oxygenated hemoglobin's possible paramagnetic contribution.
We consider that this work contributes to our current understanding of the influence that the exposure to extremely high magnetic fields could have in physiologic systems. Although deoxygenated RBC migration velocity is zero when exposed to a homogeneous magnetic field, this velocity is significant when exposed to high magnetic gradients, as the ones generated in the proximity of highly sophisticated MRI devices. Based on our results, the expected erythrocytic migration velocity would be over 27 μm/s when exposed to a field of 10 T and a gradient of 1000 T/m. This calculated migration velocity for deoxygenated erythrocytes might induce subtle shifts in the RBC distribution in specific tissues, which may result in pathophysiological changes, particularly in those individuals carrying unusual hemoglobin variants. The effects of metHb, however, would be smaller because its production by oxidative processes, especially the interaction with nitric oxide (Gladwin et al., 1999), normally leads to very low levels in the circulation.
Magnetophoresis may also open new possibilities in the separation of specific cell types, such as erythrocytes infected with the malaria parasite. During its intraerythrocytic stage, the malaria parasite ingests intracellular hemoglobin generating hemozoin, a waste product, which is an oligomeric, high spin, ferriporphyrin complex (Egan, 2002). Erythrocytes containing hemozoin are paramagnetic, which would allow their enrichment from infected patients' blood by magnetic means (Paul et al., 1981). The cell magnetophoresis might also prove useful in the separation of nucleated erythroid cells from cell mixtures that cannot be efficiently resolved due to shared cell surface antigens. This is the case of fetal and maternal nucleated RBCs, in which different levels of intracellular metHb could be achieved due to the characteristic difference in oxidative susceptibility of their intracellular milieu (Ostera, 2001).
Acknowledgments
The authors thank Dr. Robert Ledley for early discussions on the subject, and Beth Link, R.N., for providing the blood samples for this study.
This work has been supported by grants from the National Science Foundation (BES-9731059 and BES-0124897 to J.J.C. and M.Z.) and the National Cancer Institute (R33 CA81662 to J.J.C. and R01 CA62349 to M.Z.).
References
- Bartosz, G. 1990. Erythrocyte membrane changes during aging in vivo. In Blood Cell Biochemistry, Vol. 1. J. R. Harris, editor. Plenum Press, New York. 45–79.
- Bozorth, R. M. 1968. Ferromagnetism. D. Van Nostrand, Princeton, NJ.
- Cerdonio, M., A. Congiu-Castellano, L. Calabrese, S. Morante, B. Pispisa, and S. Vitale. 1978. Room-temperature magnetic properties of oxy- and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA. 75:4916–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdonio, M., S. Morante, D. Torresani, S. Vitale, A. DeYoung, and R. W. Noble. 1985. Reexamination of the evidence for paramagnetism in oxy- and carbonmonoxyhemoglobins. Proc. Natl. Acad. Sci. USA. 82:102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell, C., F. Stitt, and L. Pauling. 1937. The magnetic properties and structure of ferrihemoglobin (methemoglobin) and some of its compounds. J. Am. Chem. Soc. 59:633–642. [Google Scholar]
- Egan, T. J. 2002. Physico-chemical aspects of hemozoin (malaria pigment) structure and formation. Review. J. Inorg. Biochem. 91:19–26. [DOI] [PubMed] [Google Scholar]
- Fabry, M. E., and R. C. San George. 1983. Effect of magnetic susceptibility on nuclear magnetic resonance signals arising from red cells: a warning. Biochemistry. 22:4119–4125. [DOI] [PubMed] [Google Scholar]
- Friedlaender, F. J., M. Takayasu, T. Nakano, and W. H. McNeese. 1979. Diamagnetic capture in single wire HGMS. IEEE Trans. Mag. MAG-15:1526–1528. [Google Scholar]
- Gladwin, M., A. Schechter, J. Shelhamer, L. Pannell, D. Conway, B. Hrinczenko, J. Nichols, M. Pease-Fye, C. Noguchi, G. Rodgers, and F. Ognibene. 1999. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. J. Clin. Invest. 104:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, M. D. 1984. Comparison of volume and surface mechanisms for magnetic filtration of blood cells. Journal de Physique, Colloque C1. 45(Suppl): C1/779–C1/784. [Google Scholar]
- Guezenec, Y. G., and N. Kiritis. 1990. Statistical investigations of errors in particle image velocimetry. Experiments in Fluids. 10:138–146. [Google Scholar]
- McCloskey, K. E., J. J. Chalmers, and M. Zborowski. 2000. Magnetophoretic mobilities correlate to antibody binding capacities. Cytometry. 40:307–315. (Erratum in Cytometry. 2000. 41:150.) [DOI] [PubMed] [Google Scholar]
- McCloskey, K. E., M. Zborowski, and J. J. Chalmers. 2001. Measurement of CD2 expression levels of IFN-alpha-treated fibrosarcomas using cell tracking velocimetry. Cytometry. 44:137–147. [PubMed] [Google Scholar]
- Melcher, J. R. 1981. Continuum Electromechanics. The MIT Press, Cambridge, MA.
- Melville, D., F. Paul, and S. Roath. 1975. Direct magnetic separation of red cells from whole blood. Nature (Lond.). 255:706. [DOI] [PubMed] [Google Scholar]
- Moore, L. R., M. Zborowski, M. Nakamura, K. E. McCloskey, S. Gura, M. Zuberi, S. Margel, and J. J. Chalmers. 2000. Magnetophoretic mobility determination of monodispersed, magnetic microspheres by cell tracking velocimetry. J. Biochem. Biophys. Methods. 44:115–130. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., M. Zborowski, L. Lasky, and J. J. Chalmers. 2001. Theoretical and experimental analysis of the accuracy and reproducibility of cell tracking velocimetry. Experiments in Fluids. 30:371–380. [Google Scholar]
- Ostera, G. 2001. Differential oxidative susceptibility of fetal and adult erythroid cells as a marker for cell separation. Ph.D. thesis. Georgetown University, Washington.
- Owen, C. S. 1978. High gradient magnetic separation of erythrocytes. Biophys. J. 22:171–178. [DOI] [PMC free article] [PubMed]
- Paul, F., S. Roath, D. Melville, D. Warhurst, and J. Osisanya. 1981. Separation of malaria infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet (N. Am. Ed.) 11:70–71. [DOI] [PubMed] [Google Scholar]
- Pauling, L., and C. Coryell. 1936a. The magnetic properties and structure of the hemochromogens and related substances. Proc. Natl. Acad. Sci. USA. 22:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling, L., and C. Coryell. 1936b. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA. 22:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyavin, Y. A., and E. Y. Blum. 1983. Magnetic parameters of blood cells and high gradient paramagnetic and diamagnetic phoresis. Magnetohydrodynamics. 19:349–359. [Google Scholar]
- Roath, S., R. J. M. Smith, A. J. Richards, and J. H. P. Watson. 1992. Positive selection of cell subpopulations using a high gradient magnetic field system. Prog. Clin. Biol. Res. 377:239–244. [PubMed] [Google Scholar]
- Roath, S., T. Thomas, J. Watson, P. Lansdorp, R. Smith, and A. Richards. 1994. Specific capture of targeted hematopoietic cells by high gradient magnetic separation by the use of ordered wire array filters and tetrameric antibody complexes linked to a dextran iron particle. Prog. Clin. Biol. Res. 389:155–163. [PubMed] [Google Scholar]
- Radbruch, A., B. Mechtold, A. Thiel, S. Miltenyi, and E. Pflüger. 1994. High-gradient magnetic sorting. Methods Cell Biol. 42:387–403. [DOI] [PubMed] [Google Scholar]
- Samaja, M., E. Rovida, R. Motterlini, M. Tarantola, A. Rubinacci, and P. di Prampero. 1990. Human red cell age, oxygen affinity and oxygen transport. Respir. Physiol. 79:69–79. [DOI] [PubMed] [Google Scholar]
- Savicki, J. P., G. Lang, and M. Ikeda-Sato. 1984. Magnetic susceptibility of oxy- and carbonmonoxyhemoglobins. Proc. Natl. Acad. Sci. USA. 81:5417–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, W., D. Boning, and K. Braumann. 1987. Red cell age effects on metabolism and oxygen affinity in humans. Respir. Physiol. 68:215–225. [DOI] [PubMed] [Google Scholar]
- Spees, W. M., D. A. Yablonskiy, M. C. Oswood, and J. J. H. Ackerman. 2001. Water proton MR properties of human blood at 1.5 tesla: magnetic susceptibility, T1, T2, T2*, and non-Lorentzian signal behavior. Magn. Reson. Med. 45:533–542. [DOI] [PubMed] [Google Scholar]
- Svoboda, J. 2000. Separation of red blood cells by magnetic means. Journal of Magnetism and Magnetic Materials. 220:103–105. [Google Scholar]
- Tchikov, V., S. Schütze, and M. Krönke. 1999. Comparison between immunofluorescence and immunomagnetic techniques of cytometry. Journal of Magnetism and Magnetic Materials. 194:242–247. [Google Scholar]
- Weisskoff, R. M., and S. Kiihne. 1992. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn. Reson. Med. 24:375–383. [DOI] [PubMed] [Google Scholar]
- Winoto-Morbach, S., V. Tchikov, and W. Müller-Ruchholtz. 1994. Magnetophoresis: I. Detection of magnetically labeled cells. J. Clin. Lab. Anal. 8:400–406. [DOI] [PubMed] [Google Scholar]
- Williams, P. S., M. Zborowski, and J. J. Chalmers. 1999. Flow rate optimization for the quadrupole magnetic cell sorter. Anal. Chem. 71:3799–3807. [DOI] [PubMed] [Google Scholar]
- Winterbourn, C. C. 1990. Oxidative reactions of hemoglobin. Methods Enzymol. 186:265–272. [DOI] [PubMed] [Google Scholar]
- Zborowski, M., L. R. Moore, P. S. Williams, and J. J. Chalmers. 2002. Separations based on magnetophoretic mobility. Sep. Sci. Technol. 37:3611–3633. [Google Scholar]