Abstract
Human neutrophils are rescued from apoptosis following incubation with once-washed, fibroblast-derived Toxoplasma gondii tachyzoites. Both infected and uninfected neutrophils are rescued, implicating a soluble mediator. In this study we investigated the origin and identity of this soluble mediator. Neutrophils were incubated either with purified tachyzoites or with conditioned medium derived from T. gondii-infected human fibroblasts. Conditioned medium was found to be a potent stimulus that delayed neutrophil apoptosis up to 72 h, whereas purified and extensively washed tachyzoites had no effect. Delayed apoptosis correlated with up-regulation of the neutrophil antiapoptotic protein, Mcl-1, and the neutrophil interleukin 3 receptor α subunit (IL-3Rα), suggesting a role for granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF and granulocyte colony-stimulating factor (G-CSF) were measurable in conditioned medium by enzyme-linked immunosorbent assay. Neutralizing antibodies to GM-CSF and G-CSF were additive in abrogating delayed neutrophil apoptosis induced by conditioned medium. Inhibitors of Src family tyrosine kinases, Gi proteins, phosphatidylinositol 3-kinase, p44erk1 and p42erk2 mitogen-activated protein kinases, and Jak2 kinases partially attenuated the effect of conditioned medium, consistent with a role for G-CSF and/or GM-CSF. Hence, delayed neutrophil apoptosis is mediated by GM-CSF and G-CSF secreted by T. gondii-infected human fibroblasts. This enhanced neutrophil survival may contribute to the robust proinflammatory response elicited in the T. gondii-infected host.
Toxoplasma gondii, the major cause of central nervous system infection in those with AIDS and congenital diseases, is acquired by ingestion of cysts or oocysts or by transplacental transmission. In the United States, approximately 10 to 67% of individuals over 50 years of age have serologic evidence of infection (30, 37). Acute toxoplasmosis is characterized by intracellular growth of the rapidly dividing tachyzoite in a variety of host organs, both hemopoietic and nonhemopoietic. The survival of both the host and the parasite is dependent on some of these tachyzoites becoming encysted as slowly dividing bradyzoites in brain and muscle, while the remaining tachyzoites are eliminated (30). The mechanism that clears the tachyzoites from infected tissues involves both an innate acute inflammatory response and an antigen-specific inflammatory response (20, 60). Neutrophil extravasation from peripheral blood into infected tissue is one of the earliest responses during innate immunity. Neutrophils are the most numerous leukocyte in peripheral blood (>60%) and the most short lived. They are programmed to undergo apoptosis within 24 h of leaving the bone marrow and entering the circulation and the tissues (47). More than 10 billion peripheral blood neutrophils are replaced every day from stores of mature neutrophils in the bone marrow (14). Neutrophil apoptosis is thought to be central to either resolution or persistence of an inflammatory state, since apoptotic neutrophils become nonfunctional and are phagocytosed by tissue macrophages (46, 48, 57). Recent studies have suggested that neutrophils may play an important role in the acute innate response to T. gondii (7, 31, 49, 51). When incubated with tachyzoites in vitro, human neutrophils can lyse many extracellular parasites (20). Nevertheless, as we have reported recently (11), undamaged toxoplasmas enter neutrophils by active penetration, evading phagocytic pathways. Neutrophils are unable to kill these intracellular tachyzoites, although they can retard their division time from the usual rapid 6- to 8-h cycle to a slower 24-h cycle (11). In these studies we observed that neutrophils incubated with fibroblast-derived tachyzoites in vitro survived, whereas neutrophils incubated with medium became apoptotic. The objective of the present study was to determine how neutrophil apoptosis was attenuated. We report that this attenuation is mediated by both granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) secreted by toxoplasma-infected fibroblasts.
MATERIALS AND METHODS
Neutrophil isolation.
Endotoxin-free reagents and disposable plasticware were used in all experiments. Neutrophils were isolated from fresh heparinized blood from healthy human volunteers. Erythrocytes were removed by Dextran sedimentation. Dextran (6% in saline; T500; Amersham Pharmacia Biotech Inc., Piscataway, N.J.) was added to blood at a ratio of 1:9 (vol/vol) at 1 × g for 30 min at room temperature. The leukocyte-rich plasma above the sedimented erythrocytes was removed and overlaid onto a two-step gradient comprised of 1.07 g of Ficoll-Hypaque (Winthrop Laboratories, New York, N.Y.)/ml underlaid with 1.095 g of OptiPrep (Accurate Chemical & Scientific Corp., Westbury, N.Y.)/ml in the ratio 2:1:1 (vol/vol/vol). After centrifugation (500 × g for 20 min), the neutrophil layer was removed from the interphase between the Ficoll-Hypaque and the Optiprep and washed twice in RPMI 1640 containing 25 mM HEPES buffer with l-glutamine (Gibco BRL, Grand Island, N.Y.). Cells were 99% polymorphonuclear (3 to 5% eosinophils) and 1% mononuclear (lymphocytes and monocytes) as determined from Diff-Quik (VWR Scientific Products, Boston, Mass.) stained cytospins (100,000 cells centrifuged for 5 min at 700 rpm using a Shandon Cytospin 3 instrument (Shandon Inc., Pittsburgh, Pa.). Neutrophils were resuspended to 106/ml in RPMI supplemented with gentamicin sulfate (50 μg/ml; United States Biochemical Corp., Cleveland, Ohio) and 10% (vol/vol) heat-inactivated fetal bovine serum (endotoxin-low; HyClone Laboratories, Inc., Logan, Utah). Cell viability was determined for all experiments by fluorescence microscopy of neutrophils stained with fluorescein diacetate (7.5 μg/ml) and propidium iodide (PI) (2.5 μg/ml) and was always >97%. Contaminating erythrocytes that did not pass through the OptiPrep gradient were not lysed.
Parasites.
Human foreskin fibroblasts, maintained for up to 35 generations in minimal essential medium with Earle's salts and l-glutamine (Gibco BRL) supplemented with antibiotic-antimycotic solution (Gibco BRL), were used as a source of T. gondii (PLK strain) tachyzoites and of conditioned medium. Medium from confluent cultures of fibroblasts in 25-cm2 flasks was replaced, and approximately 400,000 tachyzoites were added. Two days later, medium containing egressed parasites was removed and passed through a 3-μm-pore-size Nuclepore polycarbonate filter (Whatman Inc., Clifton, N.J.) to separate fibroblast debris from egressed toxoplasmas. Filtrate was centrifuged at 900 × g for 10 min to pellet parasites. Parasites were resuspended in fresh medium and used in experiments (see Fig. 1 to 3). In the remaining experiments, particulates were removed from the supernatant from this first centrifugation step by passage through a 0.22-μm-pore-size filter, and the resultant filtrate is termed “conditioned medium from infected fibroblasts.” The pelleted tachyzoites from the first centrifugation step were resuspended in 300 times their pelleted volume and then centrifuged at 900 × g for 10 min. This washing step was repeated a total of four times. The final tachyzoite pellet (washed tachyzoites) was resuspended in a volume of fresh medium equivalent to the volume of medium containing egressed parasites that was first removed from the flask of infected fibroblasts. Medium removed from fibroblasts that had been cultured for 2 days without parasites (conditioned medium from uninfected fibroblasts) was processed in a way identical to processing of conditioned medium from infected fibroblasts. Fibroblasts were never removed from flasks by scraping.
FIG. 1.
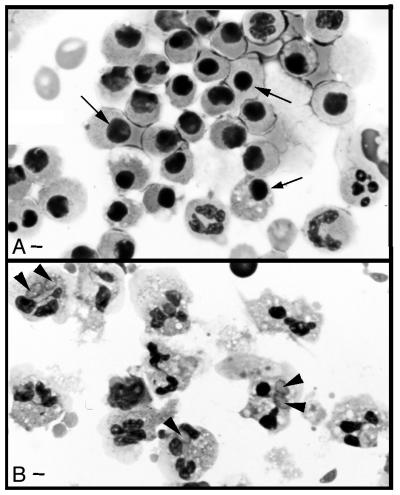
Incubation with once-washed, fibroblast-derived toxoplasmas delays spontaneous neutrophil apoptosis. Neutrophils were incubated overnight with medium (A) or once-washed tachyzoites that were resuspended in fresh medium (1:2 neutrophil-to-parasite ratio) (B), and cytospin preparations were stained with Diff-Quik. Photomicrographs were made using Kodak Elite chrome ASA 100 slide film, and 35-mm slides were scanned into Adobe Photoshop files using a Polaroid SprintScan 35 (Polaroid Corp., Cambridge, Mass.). Apoptotic neutrophils are small cells with condensed, darkly staining nuclei (arrows); nonapoptotic cells have multilobed nuclei; some neutrophils are infected with tachyzoites (arrowheads). Bar = 2 μm. Results are representative of cytospins from eight different donors.
FIG. 3.
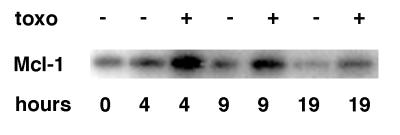
Kinetics of the expression of Mcl-1 in stimulated neutrophils. Neutrophils were incubated for various times with or without once-washed tachyzoites that were resuspended in fresh medium (1:2 neutrophil-to-parasite ratio). Proteins in whole-cell lysates were separated by SDS-PAGE, and Western blots were incubated with anti-Mcl-1. Each lane represents 5 × 105 neutrophil cell equivalents. The results are representative of two experiments.
Assessment of apoptosis.
Neutrophils (5 × 105 in 500 μl) in 24-well tissue culture plates were treated with medium or stimulus for 18 h at 37°C in a CO2 incubator. In some experiments Diff-Quik-stained cytospin preparations of neutrophils were scored for morphological changes characteristic of apoptosis (decreased size, nuclear condensation). At least 300 cells per slide were counted at magnification ×1,000. For other experiments, stimulated cells were stained with green fluorescent protein (GFP)-annexin V and PI as described previously (21). Briefly, 300,000 cells were pelleted and resuspended in 100 μl of GFP-annexin (3.5 μg/ml in RPMI supplemented to a total calcium concentration of 2 mM) and then incubated for 10 min on ice. A further 100 μl of PI (2 μg/ml in RPMI) was added, and cells were analyzed immediately by flow cytometry. Ten thousand events per sample were acquired. GFP-annexin was obtained from J. Ernst (Division of Infectious Diseases, San Francisco General Hospital, and University of California, San Francisco, Calif.). Annexin V binds to phosphatidylserine that is expressed in the outer leaflet of the phospholipid bilayer as a consequence of apoptosis and necrosis. Cells that are late apoptotic or early necrotic will lose membrane integrity and stain with both GFP-annexin and PI. Cells that retain membrane integrity, including viable and early-apoptotic cells, will not take up PI. Therefore, the combined use of GFP-annexin and PI can distinguish between early-apoptotic and late-apoptotic or necrotic cells.
SDS-PAGE and Western blotting.
Neutrophils (2 × 107/3 ml of medium) were incubated in polypropylene tubes at 37°C with or without once-washed, fibroblast-derived tachyzoites for various times. Whole-cell extracts were prepared by boiling pelleted cells for 5 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) reducing sample buffer (62.5 mM Tris-HCl [pH 6.8], 1% SDS, 1% β-mercaptoethanol, 10% glycerol, 0.5% bromophenol blue). Proteins from extracts (5 × 105 cell equivalents per lane) were resolved by electrophoresis on SDS-10% PAGE gels and then transferred (120 V for 1 h) to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, Calif.). Membranes were stained with Ponceau S (0.2% in 3% trichloroacetic acid) and scanned, and protein levels in each lane were compared to ensure equal protein loading per lane. Membranes were rinsed in phosphate-buffered saline (PBS) to remove the stain, and nonspecific binding sites were blocked with 5% nonfat dry milk (Bio-Rad Laboratories) in PBS and then probed with primary antibodies (1:1,000 dilution in PBS; polyclonal antibodies to Mcl-1 [S-19] and Bcl-2 [N-19] were from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.). Detection of antigen-antibody complexes was performed by enhanced chemiluminescence (BM chemiluminescence Western blotting kit [mouse/rabbit]; Boehringer Mannheim, Berkeley, Calif.). For confirmation of specificity of the anti-Mcl-1 antibody, whole-cell extracts of cells that expressed high levels of Mcl-1 were obtained from R. Craig, (Department of Pharmacology, Dartmouth Medical School, Hanover, N.H.) and run on each gel as a positive control for Mcl-1 detection. The anti-Mcl-1 antibody detected the same band (∼40 kDa) in whole-cell extracts of both these control cells and neutrophils.
Flow cytometry and ELISA.
Neutrophils (2.5 × 105/250 μl in 96-well U-bottom polypropylene plates; Costar, Corning, N.Y.) were washed and resuspended in 40 μl of ice-cold human γ-globulin (from Cohn fraction II, III; Sigma Chemical Co., St. Louis, Mo.; 6 mg/ml in PBS containing 1% bovine serum albumin and 0.1% sodium azide) to block nonspecific binding of antibodies to the Fc region of neutrophil FcγRI. Saturating concentrations of antibodies against human interleukin 3 receptor α subunit (IL-3Rα) (phycoerythrin-anti-human CD123; Becton Dickinson, San Jose, Calif.) or isotype control antibodies (phycoerythrin-mouse immunoglobulin G1 [IgG1] isotype control immunoglobulin; BD PharMingen, San Diego, Calif.) were added (volume, 20 μl), and cells were incubated on ice for 45 min and then washed. Stained neutrophils were examined by flow cytometry and were gated for viable cells by forward and side scatter criteria. The OptEIA human GM-CSF ELISA set was from BD Pharmingen. The G-CSF and gamma interferon (IFN-γ) ELISA Duosets were from R&D Systems, Minneapolis, Minn. The G-CSF and GM-CSF ELISA assays were calibrated with human reference GM-CSF and G-CSF obtained from C. W. Reynolds (Biological Response Modifiers Program, National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, Md.).
Neutralization and inhibitor experiments.
Neutralizing or isotype control antibodies were preincubated with 350 pg of G-CSF/ml, 10 pg of GM-CSF/ml, or a 1:80 dilution of conditioned medium from infected fibroblasts, for 1 h at 37°C, and then neutrophils (5 × 105 in 500 μl in a 24-well tissue culture plate) were stimulated for a further 18 h and apoptosis was determined. Human G-CSF (filgrastim neupogen) was obtained from Amgen, Inc., Thousand Oaks, Calif.. Human GM-CSF (sargramostim leukine) was from Immunex Corp., Seattle, Wash. Anti-human GM-CSF and anti-human G-CSF neutralizing antibodies and normal goat IgG (isotype control) were from R&D Systems Inc.. Neutrophils were also incubated with pharmacological inhibitors for 1 h at 37°C before and during stimulation with a 1:80 final dilution of conditioned medium from infected fibroblasts, a mixture of 2 ng of G-CSF/ml and 50 pg of GM-CSF/ml, or medium alone. Apoptosis was measured by GFP-annexin and PI staining 18 h poststimulation. Inhibitors were titrated in preliminary experiments to determine the optimal inhibitory dose. Pertussis toxin (Gi protein inhibitor [1, 42]) was obtained from Sigma Chemical Co.. PP2 (Src family tyrosine kinase inhibitor [45]), PD098059 (ERK inhibitor [18, 36]), wortmannin (phosphatidylinositol 3-kinase inhibitor [19]), SB203580 (p38 MAP kinase inhibitor [54]), and AG490 (Janus kinase inhibitor [38]) were from Calbiochem, La Jolla, Calif. Where necessary, inhibitors were diluted in dimethyl sulfoxide (Sigma Chemical Co.). Diluted dimethyl sulfoxide had no effect on spontaneous neutrophil apoptosis (data not shown).
RESULTS
Incubation of neutrophils with fibroblast-derived toxoplasmas delays spontaneous neutrophil apoptosis, and this effect is dose dependent.
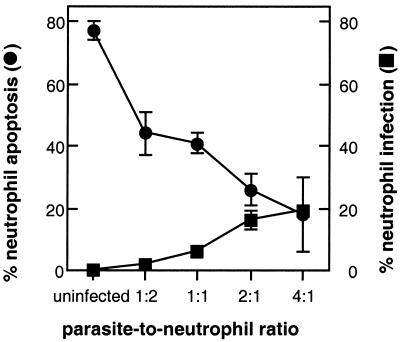
After leaving the bone marrow, peripheral blood neutrophils are programmed to die within 24 h as a result of constitutive and spontaneous apoptosis. Neutrophils maintained in culture in vitro undergo the same fate (48). Apoptosis is characterized by cell shrinkage, blebbing of the plasma membrane, and nuclear condensation (58). When freshly isolated neutrophils were incubated overnight with medium, more than 75% of these cells underwent spontaneous apoptosis (Fig. 1A). To determine the effect of T. gondii on neutrophil apoptosis and intracellular infection, extracellular parasites that had recently egressed from infected human fibroblasts were washed once, resuspended in fresh medium, and coincubated with freshly isolated neutrophils at various multiplicities of infection for 18 h. Cytospin preparations of neutrophils were scored for apoptosis and for intracellular infection. Figure 1B shows that incubation of neutrophils with once-washed, fibroblast-derived tachyzoites attenuated spon-taneous apoptosis. To determine whether apoptosis was completely inhibited, neutrophils were incubated with tachyzoites and apoptosis was determined by annexin and PI staining until 72 h postinfection. Eighty, forty-six, and six percent neutrophils remained viable at 24, 48, or 72 h postinfection, respectively, suggesting that apoptosis was delayed rather than completely inhibited. Both delayed neutrophil apoptosis and neutrophil infection were dose dependent (Fig. 2). Even at a multiplicity of infection of 4:1, only 20% of neutrophils became infected and these neutrophils were rarely apoptotic. Moreover, for each multiplicity of infection, apoptosis was delayed for both infected and uninfected neutrophils. For example, at a parasite-to-neutrophil ratio of 1:1, although only 6% of neutrophils were infected, 36% fewer neutrophils (from 77 to 41%) were apoptotic compared to uninfected neutrophils (Fig. 2).
FIG. 2.
Neutrophil infection and delayed spontaneous neutrophil apoptosis are dose-dependent responses. Neutrophils were incubated overnight with once-washed tachyzoites that were resuspended in fresh medium at various multiplicities of infection, and cytospin preparations were stained with Diff-Quik. At least 300 cells were examined at magnification ×1,000, and the percentages of apoptotic or infected neutrophils were enumerated. Each result is reported as the mean ± standard deviation of triplicate samples and are representative of three donors.
The delay in neutrophil apoptosis following fibroblast-derived toxoplasma infection correlates with transiently increased levels of the antiapoptotic protein Mcl-1.
Neutrophil survival has been shown to be concomitant with the up-regulation of the antiapoptotic protein Mcl-1 but not Bcl-2 (39). mcl-1 is an early response gene whose up-regulation can result in increased Mcl-1 expression 3 to 5 h after stimulation (12, 59). Mcl-1 expression has also been shown to be associated with T. gondii rescue of the human cell lines HL-60 and U937 from apoptosis (28). To examine whether tachyzoites may stimulate Mcl-1 up-regulation in neutrophils, neutrophils were incubated with medium or with once-washed fibroblast-derived tachyzoites for various times. Proteins from whole-cell extracts were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies specific for Mcl-1 or Bcl-2. Immunoblots with anti-Mcl-1 antibody detected strong signals for neutrophils incubated with tachyzoites compared to untreated neutrophils, especially at 4 h postinfection (Fig. 3). The Mcl-1 signal in untreated neutrophils was less apparent after 9 and 19 h in culture, suggesting the down-regulation of Mcl-1 during spontaneous apoptosis. Bcl-2 expression was undetectable in infected or uninfected neutrophils.
Conditioned medium from toxoplasma-infected fibroblasts delays spontaneous neutrophil apoptosis.
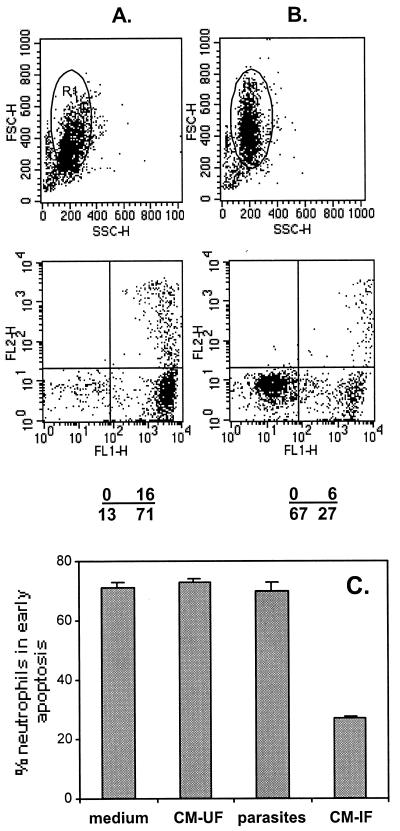
Fig. 1 and 2 show that spontaneous apoptosis was delayed not only in infected neutrophils but also in uninfected neutrophils following incubation with once-washed, fibroblast-derived tachyzoites, suggesting that a soluble factor(s) may trigger the signals for delayed apoptosis. This soluble factor could be derived from contaminating conditioned medium from the infected fibroblasts or from products secreted by the parasites themselves. To determine the source of the soluble factor(s), freshly isolated neutrophils were incubated for 18 h with medium or with conditioned medium from either infected or uninfected fibroblasts and with an equivalent volume of tachyzoites that had been washed four times and then assayed for apoptosis using annexin and PI (Fig. 4A and B). Apoptosis occurred in 71% of the neutrophils incubated overnight with medium, with dilutions of conditioned medium from uninfected fibroblasts, or with dilutions of washed parasites (Fig. 4C). In contrast, when incubated with conditioned medium from infected fibroblasts, only 27% of neutrophils became apoptotic (Fig. 4C). Hence, conditioned medium from infected fibroblasts is the source of the soluble factor that triggers the signals for delayed neutrophil apoptosis. Four washes, each using 300 times the pelleted volume of the parasites, were required to remove contaminating conditioned medium. The supernatant from each of the washing steps was shown to delay neutrophil apoptosis, with each successive supernatant having less activity (data not shown).
FIG. 4.
Conditioned medium from toxoplasma-infected fibroblasts mediates delayed spontaneous neutrophil apoptosis. Neutrophils were incubated with medium (A), with a 1:4 dilution of conditioned medium from uninfected fibroblasts, with a 1:4 dilution of conditioned medium from infected fibroblasts (B), and with a volume of egressed tachyzoites that had been washed four times, equivalent to a 1:4 dilution of conditioned medium from infected fibroblasts (see Materials and Methods). On the next day, washed cells were stained with GFP-annexin and PI and examined by flow cytometry. A gate was set on the neutrophil population of interest (see top, FSC versus SSC plots), and apoptosis was determined by dual color analysis of these cells (see bottom dot plots of GFP-annexin [FL1-H] versus PI [FL2-H]). Numbers show the percentage of cells within each quadrant. Plots of both FSC versus SSC and FL1-H versus FL2-H for conditioned medium from uninfected fibroblasts and washed tachyzoites were identical to that of medium (A). (C) Percent neutrophils in early apoptosis measured as the percentage of neutrophils in the lower right quadrant of the FL1-H versus FL2-H plots. Results in panels A and B are representative of three experiments. Results in panel C are the means ± standard deviations for triplicate samples and are also representative of three experiments.
Conditioned medium from toxoplasma-infected fibroblasts up-regulates the surface expression of neutrophil IL-3Rα, implicating GM-CSF as a mediator of neutrophil survival.
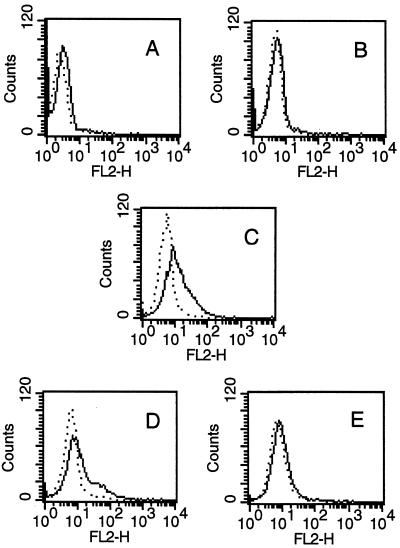
Several antiapoptotic factors, including GM-CSF, G-CSF, and IFN-γ, could rescue neutrophils from apoptosis. GM-CSF is also known to up-regulate Mcl-1 expression in human neutrophils (39). Conditioned medium from uninfected and from toxoplasma-infected fibroblasts was tested by ELISA. The concentration of G-CSF was at least 60-fold that of GM-CSF for each sample of conditioned medium from infected fibroblasts (30 ± 5 ng of G-CSF/ml versus 482 ± 187 pg of GM-CSF/ml), whereas IFN-γ was undetectable. In contrast, conditioned medium from uninfected fibroblasts contained negligible amounts of all three cytokines (1.2 ± 1.0 ng of G-CSF/ml; 22 ± 8 pg of GM-CSF/ml). The presence of functional GM-CSF in conditioned medium was tested by a biological assay: the up-regulation of the surface expression of IL-3Rα on human neutrophils (53). Freshly isolated neutrophils were stained to determine constitutive surface expression of IL-3Rα (Fig. 5A). Conditioned medium from uninfected fibroblasts, GM-CSF, or conditioned medium from infected fibroblasts preincubated for 1 h at 37°C with isotype control antibodies or with anti-GM-CSF neutralizing antibodies was then added to freshly isolated neutrophils. After overnight incubation, neutrophils were stained and examined by flow cytometry to determine whether the surface expression of IL-3Rα was modulated. Neither freshly isolated neutrophils (Fig. 5A) nor neutrophils incubated overnight with conditioned medium from uninfected fibroblasts (Fig. 5B) expressed IL-3Rα. In contrast, after overnight incubation with either GM-CSF or conditioned medium from toxoplasma-infected fibroblasts, 57 and 34% of neutrophils expressed low levels of IL-3Rα (Fig. 5C and D, respectively). Moreover, the increase in IL-3Rα expression induced by conditioned medium from toxoplasma-infected fibroblasts was markedly decreased when anti-GM-CSF neutralizing antibodies were included during the incubation (Fig. 5E) (from 34 to 9%).
FIG. 5.
Cell surface expression of neutrophil IL-3Rα. Freshly isolated neutrophils were stained with anti-IL-3Rα or isotype control antibodies and examined by flow cytometry (A). Neutrophils were also incubated for 18 h with conditioned medium from uninfected fibroblasts (B), 600 pg of GM-CSF/ml (C), or conditioned medium from infected fibroblasts preincubated for 1 h with 1 μg of isotype control antibody/ml (D) or 1 μg of anti-GM-CSF/ml (E) and then stained. Stained neutrophils were gated for viable cells by forward and side scatter criteria. Results are expressed as histograms of the fluorescence intensity of the viable cell population and are representative of two experiments. …., isotype control antibody; ___, anti-IL-3Rα.
G-CSF and GM-CSF in conditioned medium from toxoplasma-infected fibroblasts are additive in mediating delayed spontaneous neutrophil apoptosis.
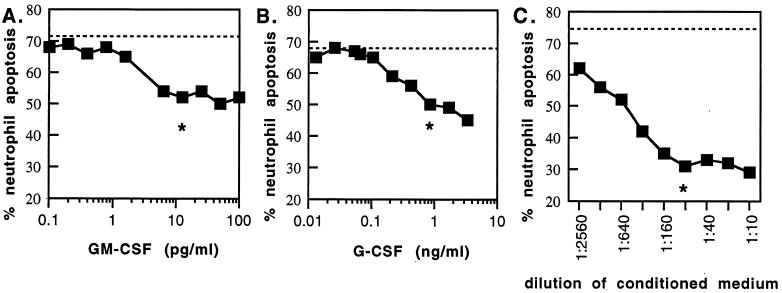
The previous results suggest that G-CSF and/or GM-CSF in conditioned medium from toxoplasma-infected fibroblasts may rescue neutrophils from apoptosis. We then determined the dose response for each cytokine and for conditioned medium on neutrophil apoptosis. Neutrophils were incubated for 18 h with serial dilutions of GM-CSF, G-CSF, or conditioned medium from toxoplasma-infected fibroblasts, and then apoptosis was determined using annexin and PI. Figure 6 shows dose-response curves for each stimulus. The 50% effective dose for G-CSF (300 pg/ml) was 100-fold that for GM-CSF (3 pg/ml). The dilution at the inflection point of each curve was approximately 10 pg/ml for GM-CSF, 850 pg/ml for G-CSF, and a final dilution of between 1:80 and 1:160 for six different samples of conditioned medium from toxoplasma-infected fibroblasts. The cytokine concentration for 1:80 dilutions of conditioned media from toxoplasma-infected fibroblasts was 6.0 ± 2.3 pg of GM-CSF/ml and 375 ± 63 pg of G-CSF/ml (n = 5). These values are consistent with the concentrations of recombinant cytokines that resulted in delayed apoptosis (Fig. 6A, 10 pg/ml; Fig. 6B, 850 pg/ml).
FIG. 6.
Neutrophil apoptosis in response to GM-CSF, G-CSF, and conditioned medium from infected fibroblasts. Neutrophils were incubated with serial dilutions of GM-CSF (A), G-CSF (B), or conditioned medium from infected fibroblasts (C) for 18 h, and then apoptosis was determined using GFP-annexin and PI. Spontaneous apoptosis, seen using similar dilutions of conditioned medium from uninfected fibroblasts, or medium alone, is shown as a dotted line. The asterisk denotes the inflection point for each dose response (the maximal effect for the lowest dilution of the stimulus). Results are representative of two experiments for cytokines and six experiments for conditioned medium from infected fibroblasts.
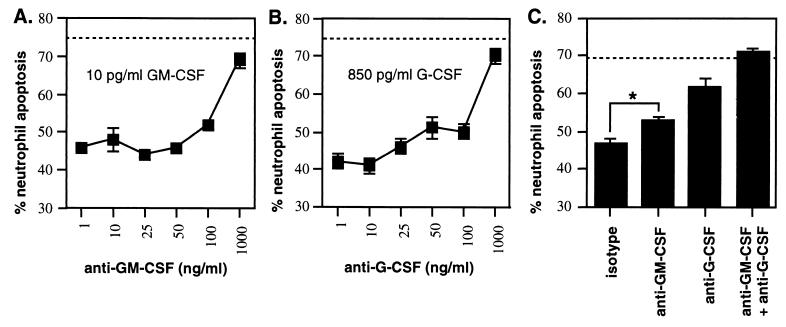
Experiments were then carried out to determine whether neutralizing antibodies were able to abrogate the effects of either 10-pg/ml GM-CSF or 850-pg/ml G-CSF, the maximum cytokine concentrations expected in 1:80 dilutions of conditioned medium from infected fibroblasts. Dilutions of neutralizing antibody or isotype control antibody were preincubated with a saturating dose of cytokines for 1 h and then added to neutrophils. Neutrophils were incubated for a further 18 h, and apoptosis was determined. The GM-CSF- or G-CSF-induced delay in spontaneous neutrophil apoptosis was nearly abrogated with 1 μg of specific neutralizing antibody/ml (Fig. 7A and B), whereas isotype control antibody was without effect. We then determined the effect of neutralizing antibodies on delayed spontaneous neutrophil apoptosis induced by conditioned medium from toxoplasma-infected fibroblasts. Conditioned medium (1:80 dilution) was preincubated with 1 μg of anti-G-CSF and/or anti-GM-CSF or isotype control antibody/ml and incubated with neutrophils overnight, and then neutrophil apoptosis was determined. Anti-G-CSF was more effective than anti-GM-CSF in neutralizing the delay in spontaneous neutrophil apoptosis (Fig. 7C, 71 versus 33% attenuation, respectively). Moreover, a combination of both antibodies was additive and ablated the delay in spontaneous neutrophil apoptosis. These results show that G-CSF and GM-CSF in conditioned medium from toxoplasma-infected fibroblasts mediate delayed spontaneous neutrophil apoptosis.
FIG. 7.
Effect of anti-GM-CSF and anti-G-CSF neutralizing antibodies on delayed spontaneous apoptosis induced by GM-CSF, G-CSF, or conditioned medium from infected fibroblasts. Neutralizing or isotype control antibodies were preincubated for 1 h with 10 pg of GM-CSF/ml (A) or 850 pg of G-CSF/ml (B) and then added to neutrophils. A 1:80 final dilution of conditioned medium from infected fibroblasts (C) was preincubated with 1 μg of neutralizing or isotype control antibodies/ml for 1 h and then added to neutrophils. Neutrophil apoptosis was determined 18 h later by flow-cytometric analysis of staining with GFP-annexin and PI. Spontaneous apoptosis, seen with 1 μg of isotype control antibody/ml, or medium alone, is shown as a dotted line. Asterisk, statistically significant difference (P < 0.001) by a one-way analysis of variance test. Results are reported as the mean ± standard deviation of triplicate samples and are representative of two experiments for cytokines and four experiments for conditioned medium from infected fibroblasts.
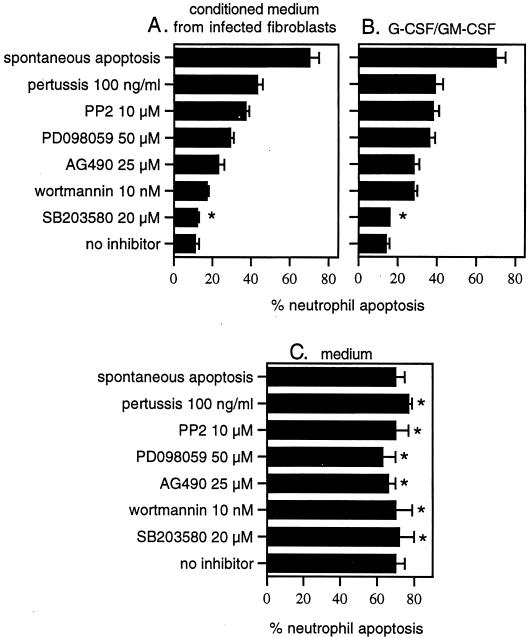
Pharmacological inhibitors of Src family tyrosine kinases, pertussis-sensitive Gi proteins, phosphatidylinositol 3-kinases, and ERKs attenuate the effect of conditioned medium from infected fibroblasts, consistent with a role for GM-CSF and G-CSF.
The signal transduction pathways leading to delayed neutrophil apoptosis were then investigated using pharmacological inhibitors. Neutrophils were incubated with inhibitors before and during exposure to conditioned medium from infected fibroblasts, a mixture of G-CSF and GM-CSF, or medium alone, and apoptosis was measured 18 h later. Neutrophil apoptosis decreased from 70 (spontaneous apoptosis) to 11 or 14% when cells were stimulated with conditioned medium from infected fibroblasts or a mixture of G-CSF and GM-CSF, respectively (Fig. 8A and B, spontaneous apoptosis versus no inhibitor). Whereas each inhibitor had no statistically significant effect on spontaneous apoptosis (Fig. 8C), pertussis toxin, PP2, PD098059, AG490, and wortmannin had a marked effect in attenuating the increased neutrophil survival in response to each stimulus (Fig. 8A and B). For example, in the presence of PP2, neutrophil apoptosis was increased from 11 to 37% and from 14 to 38%, respectively, when stimulated with conditioned medium from infected fibroblasts or by a mixture of G-CSF and GM-CSF. In contrast, SB203580 had no statistically significant effect. Since the patterns of inhibition were similar for the two stimuli, these results suggest that the G-CSF and GM-CSF in conditioned medium from infected fibroblasts could be responsible for delaying neutrophil apoptosis. These results also confirm a role for G protein-coupled receptors (pertussis toxin), Src family tyrosine kinases (PP2), ERKs (PD098059), Jak2 kinases (AG490), and phosphatidylinositol 3-kinases (wortmannin) in pathways that lead to increased neutrophil survival.
FIG. 8.
The effect of pharmacological inhibitors of signal transduction pathways on the response of neutrophils to conditioned medium from infected fibroblasts (A), a mixture of G-CSF and GM-CSF (B), and medium (C). Neutrophils were preincubated with inhibitors for 1 h before and during the addition of a 1:80 final dilution of conditioned medium from infected fibroblasts, a mixture of 2 ng of G-CSF/ml and 50 pg of GM-CSF/ml, or medium. Neutrophil apoptosis was determined 18 h later by flow-cytometric analysis of staining with GFP-annexin and PI. Panel C shows the effect of inhibitors on spontaneous apoptosis (incubation with medium). Spontaneous apoptosis (neutrophils incubated with medium) is included in panels A and B for comparison. Results are reported as the means ± standard deviations of triplicate samples and are representative of three donors. Asterisk, no statistically significant difference from no-inhibitor results by a one-way analysis of variance test (P < 0.02).
DISCUSSION
In this study we have demonstrated that G-CSF and GM-CSF are secreted by toxoplasma-infected human fibroblasts. This is the first report that G-CSF is released from T. gondii-infected cells, in this case, human foreskin fibroblasts. In vivo, circulating G-CSF levels rise promptly as an acute phase response in various infectious diseases. This is accompanied by proliferation of neutrophil precursors in the bone marrow and an accelerated maturation and release of neutrophils into peripheral blood, resulting in neutrophilia (14). GM-CSF is an important regulator of the growth, differentiation, and maturation of neutrophils, monocytes, and dendritic cells (4). That both GM-CSF and G-CSF play an important role during innate immunity is suggested by studies showing that GM-CSF- and G-CSF-deficient gene-targeted knockout mice are each markedly susceptible to infection with Listeria monocytogenes (61). GM-CSF secretion is induced by tachyzoites not only in fibroblasts (this study) but also in human retinal pigment epithelial cells (40), human monocytes (15), and murine astrocytes (22). The human intestinal epithelial cell line, HT-29, also secretes GM-CSF and G-CSF following toxoplasma infection (unpublished observations). T. gondii-infected human fibroblasts also secrete the neutrophil-eliciting chemokines, IL-8 (CXCL8) and Gro-α (CXCL1) (16). Hence, toxoplasma-infected cells secrete chemokines and cytokines that not only elicit an influx of mature neutrophils into infected tissues but also stimulate the release of mature neutrophils from the bone marrow stores to replenish decreased numbers of these cells in peripheral blood.
Spontaneous neutrophil apoptosis occurs when neutrophils are incubated overnight in medium (48). We show that G-CSF and GM-CSF released by tachyzoite-infected fibroblasts delay spontaneous neutrophil apoptosis for up to 72 h, confirming previous reports of enhanced neutrophil survival (6, 9, 13). The concentrations of rhGM-CSF and rhG-CSF that result in enhanced neutrophil survival are similar to those measurable in conditioned medium from toxoplasma-infected fibroblasts. G-CSF and GM-CSF not only rescue neutrophils from apoptosis but have also been reported to prime these cells for enhanced superoxide production, phagocytosis, and antibody-dependent cellular cytotoxicity (3, 5, 23, 35). We know that tachyzoites are lysed extracellularly by neutrophils in vitro, especially in the presence of specific antibody (20). Neutrophils also phagocytose and digest damaged extracellular tachyzoites in vitro (11). We also show that tachyzoites themselves have no effect on neutrophil apoptosis. The persistence of effect shown in Fig. 1 to 3 is most likely due to contaminating fibroblast-derived G-CSF and GM-CSF. Others have investigated the effect of tachyzoites on apoptosis of the human cell lines HL-60 and U937 (28). In these studies apoptosis was induced with actinomycin D and measured by a DNA fragmentation assay. Preincubation of cells with once-washed fibroblast-derived tachyzoites resulted in a 65% reduction of DNA fragmentation and was interpreted as a parasite-mediated effect. One explanation for their observation may be the effect of contaminating fibroblast G-CSF and GM-CSF in their studies. The findings in a previous study (29) from this same group may also be due to fibroblast-derived G-CSF/GM-CSF contamination, e.g., UV-treated parasites show the same response as untreated parasites, and heat-killed parasites lose their response. Human fibroblasts are a frequently used source for in vitro-derived parasites, and inadvertent contamination with host-derived G-CSF and GM-CSF should be considered.
GM-CSF mediates its antiapoptotic effect by rapidly inducing the antiapoptotic proteins Mcl-1 and A1, members of the Bcl-2 family (12, 34). We show that Mcl-1 was rapidly induced following incubation with once-washed fibroblast-derived tachyzoites. Mcl-1 was also up-regulated in HL-60 and U937 cell lines following incubation with fibroblast-derived tachyzoites, and this effect was interpreted to be directly parasite-mediated (28). Up-regulation of A1 has been reported in inflammatory peritoneal neutrophils and macrophages following intraperitoneal infection of mice with T. gondii (43). This study found no correlation between A1 induction and the parasitized state of the neutrophils or macrophages. It is likely that A1 is induced by GM-CSF released in the peritoneum following intraperitoneal infection. Hence, both A1 and Mcl-1 appear to be involved in apoptosis regulation in response to T. gondii infection.
The effects of G-CSF and GM-CSF in our studies appear to be mediated via the Src family kinase/Gi protein/phosphatidylinositol 3-kinase pathway, the Src family kinase/Ras/Raf/ERK pathway, and the Janus kinase/Stat pathway but not via the p38 mitogen-activated kinase pathway. Activation of Src family tyrosine kinases and ERKs have been reported for the delayed apoptotic activity of GM-CSF in human neutrophils (18, 56). In G-CSF-stimulated human neutrophils, phosphatidylinositol 3-kinases and ERKs reportedly activate protein kinase B (17). Phosphatidylinositol 3-kinases may be activated either by Src family tyrosine kinases (17) or by Gi protein βγ subunits (44). Activated protein kinase B is known to mediate delayed apoptosis in GM-CSF-stimulated human neutrophils (33) and to translocate to the nucleus and induce mcl-1 and delayed apoptosis in GM-CSF-stimulated TF-1 cells (55). A variety of toxoplasma-infected cell lines have previously been reported to resist apoptosis, although a mechanism for this activity was not described (41). Goebel et al. found that inhibition of mitochondrial cytochrome c release and subsequent caspase activation, as well as down-regulation of poly(ADP-ribose) polymerase protein levels, was considerably diminished following incubation of myeloid cell lines with fibroblast-derived tachyzoites (28), and they suggest that these effects are T. gondii mediated.
Our studies suggest that neutrophils elicited to infected tissues may not be destroyed within 24 h but may survive for up to 72 h after diapedesis. This can be beneficial and harmful to the host. A number of studies in vivo show the importance of neutrophils in toxoplasmosis. For example, Toxoplasma infection was found to be exacerbated in neutrophil-depleted mice (7, 49), and neutrophils are thought to account for the ability of iNOS knockout animals to control acute Toxoplasma infection (51). Neutrophil depletion in mice at the time of infection led to the development of lesions in multiple organs, including the spleen, lung, liver, and brain, and was associated with a reduction both in absolute numbers of splenocytes and in secretion of IFN-γ, tumor necrosis factor alpha, and IL-12 by splenocytes (7). Furthermore, mice could survive if neutrophils were removed after day 6 of infection. These results suggest that neutrophils play a crucial role in the first few days of infection. Neutrophils provide the first line of defense of the innate immune response against infecting tachyzoites and are elicited to the site of infection by CXC chemokines secreted by infected stromal cells (16). G-CSF and GM-CSF, released in response to tachyzoite infection, stimulate the release of mature neutrophils and monocytes from bone marrow to replace those that have trafficked to infected tissue. That this influx of neutrophils into peripheral blood is important in toxoplasmosis is suggested by a study using CCR1 knockout mice (31). Although these mice could mount a normal innate immune response, they could not immediately replace neutrophils that had trafficked to infected tissue with mature neutrophils stored in the bone marrow. As a consequence, tachyzoites became established and multiplied in many organs, resulting in the death of the host. Hence, it appears that at least two waves of neutrophil recruitment may be necessary during the first few days of infection for a successful immune response against this parasite.
Human neutrophils secrete several chemokines in response to T. gondii antigen, including macrophage inflammatory protein-1 alpha (MIP-1α) (CCL3) and MIP-1β (CCL4), chemokines that elicit monocytes, dendritic cells, NK cells, and T cells (8). Chemokines are crucial regulators of leukocyte trafficking that first bring together antigen-loaded dendritic cells and naïve T and B cells in regional lymph nodes to generate an adaptive immune response and second guide activated T cells back into infected tissues. For example, MIP-1α and MIP-1β are ligands for CCR5, and ligation of these chemokines with their receptors has been shown to activate murine dendritic cells to release IL-12 following T. gondii infection (2). Neutrophils are also known to secrete IP-10 (CXCL10), MIG (CXCL9), and I-TAC (CXCL11), potent chemoattractants for NK cells and Th1 lymphocytes (10, 25), and MIP-3α (CCL20) and MIP-3β (CCL19) (50). MIP-3α is a ligand for CCR6 and attracts immature dendritic cells, memory T cells, and B cells. MIP-3β binds exclusively to CCR7 and elicits mature dendritic cells, naïve and TCR-activated effector/memory T cells, and NK cells. Hence, neutrophils may play a pivotal role in regulating leukocyte recruitment and shaping the immune response that follows in the infected tissue.
The ability of the host to survive an infection with T. gondii is dependent on IL-12 orchestrating the development of a Th1 response, especially IFN-γ secretion (2, 26, 27, 32, 52). Although dendritic cells are thought to be the most important IL-12-secreting cells during an immune response, IL-12p70 secretion by human dendritic cells in response to tachyzoites occurs only in the presence of lymphocytes, especially CD154-expressing lymphocytes (52). In contrast, neutrophils secrete IL-12p70 in response to soluble tachyzoite antigen in the absence of CD154-expressing lymphocytes (8). Dendritic cells are also thought to be the most important antigen-presenting cells during an immune response; however, viable tachyzoites infect human dendritic cells by active penetration and replicate intracellularly rather than being phagocytosed and digested (11). Since neutrophils lyse extracellular tachyzoites (20), they may also provide an extracellular reservoir of tachyzoite antigens for uptake and antigen presentation by dendritic cells. Hence, the advantages to the host of prolonged neutrophil survival during acute toxoplasmosis include increased chemokine secretion that directly or indirectly orchestrates the subsequent antigen-specific immune response, increased IL-12 secretion, increased microbicidal activity providing a pool of extracellular antigen for antigen-presenting cells, and increased phagocytosis of damaged extracellular tachyzoites.
In summary, T. gondii induces the secretion of G-CSF and GM-CSF from human fibroblasts. These cytokines also rescue neutrophils from spontaneous apoptosis. Regulation of neutrophil apoptosis is a key factor for either resolution or persistence of an inflammatory state (24). This enhanced neutrophil survival may contribute to the robust proinflammatory response elicited in the T. gondii-infected host and to shaping the subsequent antigen-specific immune response.
Acknowledgments
This research was supported by grants AI30000 and AI19613 from the National Institutes of Health. Flow cytometry was carried out at Dartmouth Medical School in the Herbert C. Englert Cell Analysis Laboratory, which was established by a grant from the Fannie E. Rippel Foundation and is supported in part by the Core Grant of the Norris Cotton Cancer Center (CA 23108).
We thank R. Craig (Department of Pharmacology, Dartmouth Medical School, Hanover, N.H.) for whole-cell extracts of cells expressing high levels of Mcl-1 and M. Fanger (Department of Microbiology, Dartmouth Medical School, Hanover, N.H.) for gifts of antibodies. We thank Ima-Obong Udom for technical assistance and Dominique Buzoni-Gatel and Sakhina Haque for critical reading of the manuscript.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aas, V., K. Larsen, and J. G. Iversen. 1999. Interferon-gamma elicits a G-protein-dependent Ca2+ signal in human neutrophils after depletion of intracellular Ca2+ stores. Cell Signal. 11:101-110. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shami, A., W. Mahanna, and P. H. Naccache. 1998. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Selective activation of Jak2, Stat3, and Stat5b. J. Biol. Chem. 273:1058-1063. [DOI] [PubMed] [Google Scholar]
- 4.Armitage, J. 1998. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92:4491-4508. [PubMed] [Google Scholar]
- 5.Atkinson, Y. H., A. F. Lopez, W. A. Marasco, C. M. Lucas, G. C. Wong, G. F. Burns, and M. A. Vadas. 1988. Recombinant human granulocyte-macrophage colony-stimulating factor (rH GM-CSF) regulates f Met-Leu-Phe receptors on human neutrophils. Immunology 64:519-525. [PMC free article] [PubMed] [Google Scholar]
- 6.Begley, C. G., A. F. Lopez, N. A. Nicola, D. J. Warren, M. A. Vadas, C. J. Sanderson, and D. Metcalf. 1986. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood 68:162-166. [PubMed] [Google Scholar]
- 7.Bliss, S. K., L. C. Gavrilescu, A. Alcaraz, and E. Y. Denkers. 2001. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliss, S. K., A. J. Marshall, Y. Zhang, and E. Y. Denkers. 1999. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J. Immunol. 162:7369-7375. [PubMed] [Google Scholar]
- 9.Brach, M. A., S. deVos, H. J. Gruss, and F. Herrmann. 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920-2924. [PubMed] [Google Scholar]
- 10.Cassatella, M. A. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 11.Channon, J. Y., R. M. Seguin, and L. H. Kasper. 2000. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect. Immun. 68:4822-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao, J. R., J. M. Wang, S. F. Lee, H. W. Peng, Y. H. Lin, C. H. Chou, J. C. Li, H. M. Huang, C. K. Chou, M. L. Kuo, J. J. Yen, and H. F. Yang-Yen. 1998. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol. Cell. Biol. 18:4883-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 14.Dale, D. C., W. C. Liles, W. R. Summer, and S. Nelson. 1995. Review: granulocyte colony-stimulating factor—role and relationships in infectious diseases. J. Infect. Dis. 172:1061-1075. [DOI] [PubMed] [Google Scholar]
- 15.Delemarre, F. G., A. Stevenhagen, F. P. Kroon, and R. van Furth. 1998. Reduced toxoplasmastatic activity of monocytes from AIDS patients: a role for granulocyte-macrophage colony-stimulating factor. Scand. J. Immunol. 47:163-166. [PubMed] [Google Scholar]
- 16.Denney, C., L. Eckmann, and S. Reed. 1999. Chemokine secretion of human cells in response to Toxoplasma gondii infection. Infect. Immun. 67:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong, F., and A. C. Larner. 2000. Activation of Akt kinase by granulocyte colony-stimulating factor (G-CSF): evidence for the role of a tyrosine kinase activity distinct from the Janus kinases. Blood 95:1656-1662. [PubMed] [Google Scholar]
- 18.Downey, G. P., J. R. Butler, H. Tapper, L. Fialkow, A. R. Saltiel, B. B. Rubin, and S. Grinstein. 1998. Importance of MEK in neutrophil microbicidal responsiveness. J. Immunol. 160:434-443. [PubMed] [Google Scholar]
- 19.Downey, G. P., J. R. Butler, J. Brumell, N. Borregaard, L. Kjeldsen, A. Q. A. K. Sue, and S. Grinstein. 1996. Chemotactic peptide-induced activation of MEK-2, the predominant isoform in human neutrophils. Inhibition by wortmannin. J. Biol. Chem. 271:21005-21011. [DOI] [PubMed] [Google Scholar]
- 20.Erbe, D. V., E. R. Pfefferkorn, and M. W. Fanger. 1991. Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. J. Immunol. 146:3145-3151. [PubMed] [Google Scholar]
- 21.Ernst, J. D., L. Yang, J. L. Rosales, and V. C. Broaddus. 1998. Preparation and characterization of an endogenously fluorescent annexin for detection of apoptotic cells. Anal. Biochem. 260:18-23. [DOI] [PubMed] [Google Scholar]
- 22.Fischer, H. G., B. Nitzgen, G. Reichmann, and U. Hadding. 1997. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur. J. Immunol. 27:1539-1548. [DOI] [PubMed] [Google Scholar]
- 23.Fleischmann, J., D. W. Golde, R. H. Weisbart, and J. C. Gasson. 1986. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood 68:708-711. [PubMed] [Google Scholar]
- 24.Frasch, S. C., J. A. Nick, V. A. Fadok, D. L. Bratton, G. S. Worthen, and P. M. Henson. 1998. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J. Biol. Chem. 273:8389-8397. [DOI] [PubMed] [Google Scholar]
- 25.Gasperini, S., M. Marchi, F. Calzetti, C. Laudanna, L. Vicentini, H. Olsen, M. Murphy, F. Liao, J. Farber, and M. A. Cassatella. 1999. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J. Immunol. 162:4928-4937. [PubMed] [Google Scholar]
- 26.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon-γ by an intracellular parasite and induces resistance in T-cell deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 28.Goebel, S., U. Gross, and C. G. Luder. 2001. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J. Cell Sci. 114:3495-3505. [DOI] [PubMed] [Google Scholar]
- 29.Goebel, S., C. G. Luder, and U. Gross. 1999. Invasion by Toxoplasma gondii protects human-derived HL-60 cells from actinomycin D-induced apoptosis. Med. Microbiol. Immunol. 187:221-226. [DOI] [PubMed] [Google Scholar]
- 30.Kasper, L. H. 2001. Toxoplasma infection, p. 1222-1227. In E. Braunwald, A. Fauci, D. Kasper, S. Hauser, D. Longo, and J. Jameson, (ed.), Harrison's principles of internal medicine, 15th ed. McGraw-Hill, New York, N.Y.
- 31.Khan, I. A., P. M. Murphy, L. Casciotti, J. D. Schwartzman, J. Collins, J. L. Gao, and G. R. Yeaman. 2001. Mice lacking the chemokine receptor CCR1 show increased susceptibility to Toxoplasma gondii infection. J. Immunol. 166:1930-1937. [DOI] [PubMed] [Google Scholar]
- 32.Khan, I. A., T. Matsuura, and L. H. Kasper. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein, J. B., M. J. Rane, J. A. Scherzer, P. Y. Coxon, R. Kettritz, J. M. Mathiesen, A. Buridi, and K. R. McLeish. 2000. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J. Immunol. 164:4286-4291. [DOI] [PubMed] [Google Scholar]
- 34.Lin, E. Y., A. Orlofsky, M. S. Berger, and M. B. Prystowsky. 1993. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J. Immunol. 151:1979-1988. [PubMed] [Google Scholar]
- 35.Lopez, A. F., D. J. Williamson, J. R. Gamble, C. G. Begley, J. M. Harlan, S. J. Klebanoff, A. Waltersdorph, G. Wong, S. C. Clark, and M. A. Vadas. 1986. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J. Clin. Investig. 78:1220-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLeish, K. R., C. Knall, R. A. Ward, P. Gerwins, P. Y. Coxon, J. B. Klein, and G. L. Johnson. 1998. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-alpha and GM-CSF. J. Leukoc. Biol. 64:537-545. [PubMed] [Google Scholar]
- 37.McLeod, R., and J. S. Remington. 1987. Toxoplasmosis, p. 791-797. In E. Braunwald, K. J. Isselbacher, R. G. Petersdorf, J. D. Wilson, J. B. Martin and A. S. Fauci, (ed.), Harrison's principles of internal medicine, 11th ed. McGraw-Hill, New York, N.Y.
- 38.Meydan, N., T. Grunberger, H. Dadi, M. Shahar, E. Arpaia, Z. Lapidot, J. S. Leeder, M. Freedman, A. Cohen, A. Gazit, A. Levitzki, and C. M. Roifman. 1996. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379:645-648. [DOI] [PubMed] [Google Scholar]
- 39.Moulding, D. A., J. A. Quayle, A. Hart, and S. W. Edwards. 1998. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood 92:2495-2502. [PubMed] [Google Scholar]
- 40.Nagineni, C. N., B. Detrick, and J. J. Hooks. 2000. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor, and intercellular adhesion molecule 1 by human retinal pigment epithelial cells. Infect. Immun. 68:407-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nash, P. B., M. B. Purner, R. P. Leon, P. Clarke, R. C. Duke, and T. Curiel. 1998. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 160:1824-1830. [PubMed] [Google Scholar]
- 42.O'Flaherty, J. T., J. S. Taylor, and M. Kuroki. 2000. The coupling of 5-oxo-eicosanoid receptors to heterotrimeric G proteins. J. Immunol. 164:3345-3352. [DOI] [PubMed] [Google Scholar]
- 43.Orlofsky, A., R. D. Somogyi, L. M. Weiss, and M. B. Prystowsky. 1999. The murine antiapoptotic protein A1 is induced in inflammatory macrophages and constitutively expressed in neutrophils. J. Immunol. 163:412-419. [PubMed] [Google Scholar]
- 44.Ptasznik, A., E. R. Prossnitz, D. Yoshikawa, A. Smrcka, A. E. Traynor-Kaplan, and G. M. Bokoch. 1996. A tyrosine kinase signaling pathway accounts for the majority of phosphatidylinositol 3,4,5-trisphosphate formation in chemoattractant-stimulated human neutrophils. J. Biol. Chem. 271:25204-25207. [DOI] [PubMed] [Google Scholar]
- 45.Rizoli, S. B., O. D. Rotstein, and A. Kapus. 1999. Cell volume-dependent regulation of L-selectin shedding in neutrophils. A role for p38 mitogen-activated protein kinase. J. Biol. Chem. 274:22072-22080. [DOI] [PubMed] [Google Scholar]
- 46.Sanui, H., S. Yoshida, K. Nomoto, R. Ohhara, and Y. Adachi. 1982. Peritoneal macrophages which phagocytose autologous polymorphonuclear leucocytes in guinea-pigs. I. induction by irritants and microorganisms and inhibition by colchicine. Br. J. Exp. Pathol. 63:278-284. [PMC free article] [PubMed] [Google Scholar]
- 47.Savill, J., and C. Haslett. 1995. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin. Cell Biol. 6:385-393. [DOI] [PubMed] [Google Scholar]
- 48.Savill, J. S., A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Investig. 83:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sayles, P. C., and L. L. Johnson. 1996-97. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat. Immun. 15:249-258. [PubMed] [Google Scholar]
- 50.Scapini, P., C. Laudanna, C. Pinardi, P. Allavena, A. Mantovani, S. Sozzani, and M. A. Cassatella. 2001. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur. J. Immunol. 31:1981-1988. [DOI] [PubMed] [Google Scholar]
- 51.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seguin, R. M., and L. H. Kasper. 1999. Sensitized lymphocytes and CD40 ligation augment interleukin-12 production by human dendritic cells in response to Toxoplasma gondii. J. Infect. Dis. 179:467-474. [DOI] [PubMed] [Google Scholar]
- 53.Smith, W. B., L. Guida, Q. Sun, E. I. Korpelainen, C. van den Heuvel, D. Gillis, C. M. Hawrylowicz, M. A. Vadas, and A. F. Lopez. 1995. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood 86:3938-3944. [PubMed] [Google Scholar]
- 54.Suzuki, K., M. Hino, F. Hato, N. Tatsumi, and S. Kitagawa. 1999. Cytokine-specific activation of distinct mitogen-activated protein kinase subtype cascades in human neutrophils stimulated by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-alpha. Blood 93:341-349. [PubMed] [Google Scholar]
- 55.Wang, J. M., J. R. Chao, W. Chen, M. L. Kuo, J. J. Yen, and H. F. Yang-Yen. 1999. The anti-apoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol. Cell. Biol. 19:6195-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei, S., J. H. Liu, P. K. Epling-Burnette, A. M. Gamero, D. Ussery, E. W. Pearson, M. E. Elkabani, J. I. Diaz, and J. Y. Djeu. 1996. Critical role of Lyn kinase in inhibition of neutrophil apoptosis by granulocyte-macrophage colony-stimulating factor. J. Immunol. 157:5155-5162. [PubMed] [Google Scholar]
- 57.Whyte, M. K., L. C. Meagher, J. MacDermot, and C. Haslett. 1993. Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 150:5124-5134. [PubMed] [Google Scholar]
- 58.Wyllie, A. H. 1987. Apoptosis: cell death in tissue regulation. J. Pathol. 153:313-316. [DOI] [PubMed] [Google Scholar]
- 59.Yang, T., K. M. Kozopas, and R. W. Craig. 1995. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J. Cell Biol. 128:1173-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yap, G. S., and A. Sher. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 189:1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhan, Y., G. J. Lieschke, D. Grail, A. R. Dunn, and C. Cheers. 1998. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood 91:863-869. [PubMed] [Google Scholar]