Abstract
PDC-109, the major protein of bovine seminal plasma, binds to sperm plasma membranes upon ejaculation and plays a crucial role in the subsequent events leading to fertilization. The binding process is mediated primarily by the specific interaction of PDC-109 with choline-containing phospholipids. In the present study the kinetics and mechanism of the interaction of PDC-109 with phospholipid membranes were investigated by the surface plasmon resonance technique. Binding of PDC-109 to different phospholipid membranes containing 20% cholesterol (wt/wt) indicated that binding occurs by a single-step mechanism. The association rate constant (k1) for the binding of PDC-109 to dimyristoylphosphatidylcholine (DMPC) membranes containing cholesterol was estimated to be 5.7 × 105 M−1 s−1 at 20°C, while the values of k1 estimated at the same temperature for the binding to membranes of negatively charged phospholipids such as dimyristoylphosphatidylglycerol (DMPG) and dimyristoylphosphatidic acid (DMPA) containing 20% cholesterol (wt/wt) were at least three orders of magnitude lower. The dissociation rate constant (k−1) for the DMPC/PDC-109 system was found to be 2.7 × 10−2 s−1 whereas the k−1 values obtained with DMPG and DMPA was about three to four times higher. From the kinetic data, the association constant for the binding of PDC-109 to DMPC was estimated as 2.1 × 107 M−1. The association constants for different phospholipids investigated decrease in the order: DMPC > DMPG > DMPA > DMPE. Thus the higher affinity of PDC-109 for choline phospholipids is reflected in a faster association rate constant and a slower dissociation rate constant for DMPC as compared to the other phospholipids. Binding of PDC-109 to dimyristoylphosphatidylethanolamine and dipalmitoylphosphatidylethanolamine, which are also zwitterionic, was found to be very weak, clearly indicating that the charge on the lipid headgroup is not the determining factor for the binding. Analysis of the activation parameters indicates that the interaction of PDC-109 with DMPC membranes is favored by a strong entropic contribution, whereas negative entropic contribution is primarily responsible for the rather weak interaction of this protein with DMPA and DMPG.
INTRODUCTION
The seminal plasma of mammals is a complex fluid, which serves as a carrier for the spermatozoa in their journey from the male testes to their target, the female uterus. It contains both organic and inorganic molecules of low as well as high molecular weight. While the low molecular weight fraction contains a wide variety of chemical constituents such as metal ions, organic acids, sugars, lipids and amino acids, the only high molecular weight constituents found in seminal plasma are proteins; other biopolymers such as polysaccharides and nucleic acids are not present in it (Shivaji et al., 1990). The spermatozoa at the time of release from the testis do not possess fertilizing capacity, but acquire it during epididymal transit. However, it is suppressed when sperm cells mix with the seminal plasma at the time of ejaculation, and fully regained during residence in the female fallopian tube by a process called capacitation (Shivaji et al., 1990). Though it was discovered approximately 50 years ago that capacitation is a necessary event before fertilization can occur, the underlying molecular basis is not yet clearly understood.
Studies on several mammalian species indicate that seminal plasma contains specific factors that inhibit capacitation and thus prevent inappropriate acrosome reaction as well as proteins that bind to the surface of spermatozoa and potentiate the zona pellucidae-induced acrosome reaction. Thus, together these two groups of factors seem to modulate in vivo sperm capacitation (Harrison, 1996; Visconti et al., 1998). The bovine seminal plasma contains a group of four acidic proteins—designated BSP-A1, BSP-A2, BSP-A3, and BSP-30-kDa—that bind to the spermatozoa. Collectively, these four proteins are referred to as bovine seminal plasma proteins, or as BSP proteins (Manjunath and Sairam, 1987; Manjunath et al., 1987). These proteins have been purified and biochemically well characterized. The molecular weights of the former three are between 12 and 15 kDa, whereas the mass of the BSP-30-kDa protein is ∼30,000 Da (Manjunath et al., 1987). BSP-A1 and BSP-A2 have the same primary structure and differ only in glycosylation, and their mixture is also referred to as PDC-109. PDC-109 is the major protein of bovine seminal plasma and is present at a concentration of 15–25 mg/ml in the seminal plasma (Scheit et al., 1988). It is a polypeptide of 109 amino acids and is composed of an N-terminal 23-residue stretch followed by two tandemly repeating fibronectin type-II (Fn-II) domains (Baker, 1985; Esch et al., 1983; Seidah et al., 1987). The three-dimensional structure of PDC-109 complexed with O-phosphorylcholine, solved by single-crystal x-ray diffraction reveals that the two choline-phospholipid binding sites are on the same face of the PDC-109 protein. Ligand binding is mediated by a cation-π interaction between the quaternary ammonium group of the choline moiety and the indole ring of a core tryptophan residue and hydrogen bonding between the phosphate group and exposed tyrosine residues of the protein (Wah et al., 2002).
The interaction of BSP proteins with spermatozoa is mediated by their interaction with specific phospholipids, particularly with phosphatidylcholine (PC), which is zwitterionic (Desnoyers and Manjunath, 1992). The interaction of PDC-109 with spermatozoa results in the efflux of PC and cholesterol (referred to as cholesterol efflux), which appears to be an important step in the capacitation process, a necessary event before fertilization can occur (Thérien et al., 1998; Moreau and Manjunath, 1999). Each Fn-II domain of PDC-109 contains a choline-phospholipid binding site and both the binding sites are necessary to induce lipid efflux (Desnoyers and Manjunath, 1993; Moreau et al., 1998). Therefore, it is important to understand the interaction of PDC-109 with sperm cell membranes, to understand the molecular events involved in the capacitation process. Most importantly, such an understanding potentially can lead to the development of novel antifertility drugs. Studies on the interaction of PDC-109 with lipid membranes can serve as useful models for its interaction with biological membranes.
Phosphatidylcholine, which is a major phospholipid of mammalian cell membranes, is generally assumed to play a passive structural role as a building block in biological membranes, without any significant specific interactions. Results on lipid-protein interactions with both integral and peripheral membrane proteins also generally indicate a lack of specific interactions with PC (Marsh and Horvath, 1998). In view of the ubiquitous nature of PC in mammalian membranes, the identification of proteins that specifically interact with it is of great interest and potential significance. In addition to the structural and functional significance, studies on these proteins are expected to throw light on the possibility that pathologies might occur due to their specific interactions with PC or with sphingomyelin, another phospholipid containing a choline headgroup. In previous studies the interaction of PDC-109 with DMPC membranes has been investigated using spin-label electron spin resonance (ESR) spectroscopy and the specificity of this protein for spin-labeled phospholipid and sterol probes has been characterized in detail (Ramakrishnan et al., 2001; Greube et al., 2001). These studies have demonstrated that, upon binding, PDC-109 penetrates into the hydrophobic interior of the membrane and that besides PC and sphingomyelin it also recognizes other phospholipids such as phosphatidylglycerol (PG) and phosphatidylserine (PS), albeit with somewhat reduced specificity. In addition, it has been shown that presence of cholesterol in the lipid membranes leads to an increase in the selectivity of PDC-109 for different phospholipid and sterol probes (Swamy et al., 2002).
In the present study, we have investigated the kinetics of interaction of PDC-109 with different phospholipid membranes containing cholesterol by the surface plasmon resonance (SPR) technique. The results indicate that binding of PDC-109 takes place by a single-step mechanism and that the higher affinity of this protein for PC as compared to phospholipids bearing other headgroups, such as PG, phosphatidic acid (PA), and phosphatidylethanolamine (PE) is due to a faster association rate constant and a slower dissociation rate constant. Analysis of the activation parameters shows that binding of this protein to PC/cholesterol membranes is favored by a positive entropic contribution.
MATERIALS AND METHODS
Materials
Phosphorylcholine chloride (Ca2+ salt), choline chloride and tris(hydroxymethyl)aminomethane (Tris) base were purchased from Sigma (St. Louis, MO). Sephadex G-50 (superfine), DEAE Sephadex A-25, and DEAE Sephadex A-50 were obtained from Pharmacia Biotech (Uppsala, Sweden). Dimyristoyl phosphatidylcholine (DMPC), dimyristoyl phosphatidylglycerol (DMPG), dimyristoyl phosphatidylethanolamine (DMPE), dipalmitoyl phosphatidylethanolamine (DPPE), dimyristoylphosphatidic acid (DMPA) and cholesterol were obtained from Avanti Polar Lipids (Alabaster, AL).
Samples of bovine semen and extraction of BSP proteins
Samples of bovine semen, freshly collected from Ongole bulls with the aid of an artificial vagina, were kindly provided by the Department of Animal Reproduction, Acharya N. G. Ranga University of Agricultural Sciences, Hyderabad and Lam Farm, Department of Animal Breeding of the same university at Guntur, Andhra Pradesh, India. The samples were stored on ice for a maximum of 9 h (until they were brought to the laboratory), and then centrifuged at 3000 rpm in a refrigerated centrifuge to separate the sperm cells and the seminal plasma. Total proteins from the seminal plasma were precipitated by adding eight volumes of cold ethanol, and the precipitated proteins were dissolved in distilled water and lyophilized. The lyophilized protein fraction was delipidated by extraction with a mixture of n-butanol/di-isopropylether (40/60 v/v).
Purification of PDC-109
PDC-109 was purified by a modification of the procedure reported by Calvete et al. (1996). In the modified procedure (Ramakrishnan et al., 2001), delipidated BSP protein fraction obtained according to the procedure of Desnoyers and Manjunath (1992, 1993) was used instead of the bovine seminal plasma, used by Calvete and co-workers (Calvete et al., 1996). The delipidated BSP protein fraction was dissolved in 50 mM Tris buffer, 0.15 M NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4 (TBS-I) and subjected to gel filtration on a column of Sephadex G-50 superfine (2.5 × 170 cm), pre-equilibrated with the same buffer. Under these conditions PDC-109 elutes in the void volume, as it exists as a polydisperse aggregate with an average molecular weight of ∼70 kDa (Gasset et al., 1997). The fractions corresponding to this peak were collected, dialyzed against 50 mM Tris, 1.0 M NaCl, 5 mM EDTA, pH 6.4 (TBS-II) and loaded onto a column of DEAE Sephadex A-25, pre-equilibrated with the same buffer. After washing the column with the same buffer until no protein was found in the washings, the bound protein was eluted with 100 mM choline chloride in the same buffer. The eluted protein was dialyzed extensively against TBS-I to remove the choline chloride, concentrated by lyophilization, and then dialyzed again against the same buffer and stored at 4°C. Alternatively, the protein was lyophilized to a powder and stored at −20°C. Both types of sample were found to bind to phosphatidylcholine membranes or to DEAE Sephadex A-25 without any noticeable decrease in the activity over several months of storage. Purity of PDC-109 was assessed by SDS-PAGE (Laemmli, 1970), where the protein moved as two closely spaced bands of Mr ∼13 kDa, corresponding to the glycosylated and unglycosylated forms. Gels of 10% or 12% acrylamide were used.
Preparation of lipid samples
Lipid samples for SPR experiments were prepared as follows. The phospholipid and cholesterol were first weighed to give the desired weight ratio and then dissolved in chloroform:methanol (3:1; v/v). The solvent was removed by a gentle stream of nitrogen gas. The lipid film thus formed was kept under high vacuum for 1 h and then hydrated with TBS-I. The lipid suspension was sonicated for 10 min using a probe sonicator, freeze-thawed thrice, using liquid nitrogen and sonicated again for 10 min to get unilamellar liposomes. The resulting sample was passed through a 0.22 μM syringe filter to remove any suspended particles. Phospholipid and cholesterol in the mixtures were estimated by determining the lipid-bound phosphate (Rouser et al., 1970) and cholesterol (Higgins, 1987).
Surface plasmon resonance experiments
The surface plasmon resonance experiments were performed using a BIAcore 2000 (Amersham Pharmacia Biotech) biosensor system. Experiments were performed at 6, 12, and 20°C. Binding of PDC-109 to lipid membranes was investigated as follows. A four-channel alkanethiol HPA chip was first cleaned by passing a 10 mM solution of n-octyl glucoside at a flow rate of 1 μl/min. The lipid sample, prepared as described above, was then loaded onto the chip and after the chip was saturated with the lipid, it was washed for 30 min with TBS-I, followed by a pulse of 10 mM NaOH to remove the multilamellar structures, yielding a single surface hybrid bilayer of lipid (Thomas et al., 1999). The hybrid bilayer consists of alkanethiol monolayer attached to the chip on which the externally added lipid forms an additional monolayer. Different concentrations of PDC-109 were passed over this lipid surface and the resulting sensograms were recorded. From the sensograms binding parameters were calculated as described below.
SPR data analysis
Association (k1) and dissociation (k−1) rate constants were obtained by nonlinear fitting of the primary sensogram data using the BIA evaluation 3.0 software. The dissociation rate constant is derived using the equation
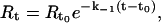 |
(1) |
where Rt is the response at time t, Rt0 is the amplitude of the response at the time of addition of buffer (to dissociate bound PDC-109), and k−1 is the dissociation rate constant. The association rate constant k1 can be derived using Eq. 2 given below, from the measured k−1 values.
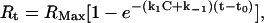 |
(2) |
where Rt is the response time at time t, RMax is the maximum response, C is the concentration of PDC-109 in the solution, k1 is the association rate constant, and k−1 is the dissociation rate constant. The equilibrium constant (Ka) and the dissociation constant (Kd) were obtained using the expressions Ka = k1/k−1 and Kd = k−1/k1. All sensograms were first corrected for bulk drift and analyzed for mass transport-influenced kinetics. All temperature-dependent experiments were performed after both the flow cells and the samples were incubated at the desired temperature for 30 min.
The ligand-binding parameters, namely concentration and response units (RU), obtained from the sensogram data, were analyzed according to the Scatchard method (Rao et al., 1999). Values of RU/[ligand] were plotted against RU, and Ka values were obtained from the slopes of the linear fits (slope = −Ka).
Protein estimation
Protein concentrations were determined by the method of Lowry et al. (1951) using bovine serum albumin as the standard. The concentration of purified PDC-109 was estimated from its extinction coefficient at 280 nm of 2.5 for a 1-mg/ml sample concentration (Calvete et al., 1996).
RESULTS
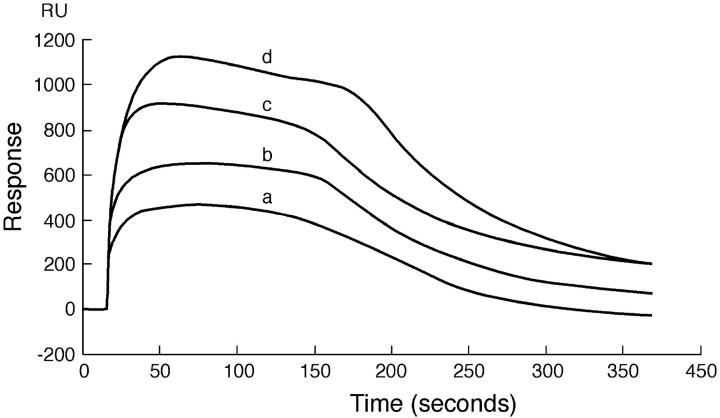
To investigate the binding of PDC-109 to phosphatidylcholine membranes, the initial SPR experiments were carried out with monolayers of pure DMPC using the BIAcore biosensor system and the results obtained are shown in Fig. 1. The initial part of the data (up to ∼15 or 20 s on the x-axis) corresponds to the passage of buffer over the sensor chip (baseline), after which the protein solution is passed over the chip. The rise in the signal with the progression of time reflects binding of protein to the hybrid lipid bilayer. When equilibrium is established, the response reaches a constant value and remains so until the protein solution that is being passed over the sensor chip is replaced with buffer. The decrease in response when buffer is passed over the sensor chip corresponds to the dissociation process. However, all the sensograms shown in Fig. 1 corresponding to different concentrations of the protein show an initial increase of the response, which reaches a high value (corresponding to maximum binding), but then decreases slowly (approximately between 75 and 175 s; the faster decrease after 175 s corresponds to a switch to the buffer). This indicates a loss of mass from the sensor chip surface and most likely suggests a loss of the lipid from the surface of the chip. Gasset et al. (2000) have reported that binding of PDC-109 to phosphatidylcholine vesicles leads to a disruption of the vesicle integrity, resulting in a leakage of the contents. However, they also observed that incorporation of cholesterol in DOPC vesicles stabilized the vesicle structure in a dose-dependent manner and reduced contents leakage. In view of these observations, we tested DMPC membranes containing varying amounts of cholesterol and noted that above 15–18% (wt/wt) cholesterol the hybrid bilayers remain stable, i.e., they do not suffer a loss in RU (mass) due to loss of phospholipid from the bilayers. Hence in all subsequent experiments membranes containing 20% (wt/wt) cholesterol were used. This would be equivalent to ∼28–32% (mol/mol) of cholesterol for the different phospholipids investigated here.
FIGURE 1.
Sensogram depicting the binding of PDC-109 to pure DMPC monolayer at 20°C. PDC-109 in varying concentrations was passed over a monolayer of DMPC: (a) 0.75 μM, (b) 1.0 μM, (c) 1.5 μM, and (d) 3.0 μM. The sensogram obtained shows the distinct removal of the lipid from the monolayer, attesting to the fact that PDC-109 disrupts the membrane structure.
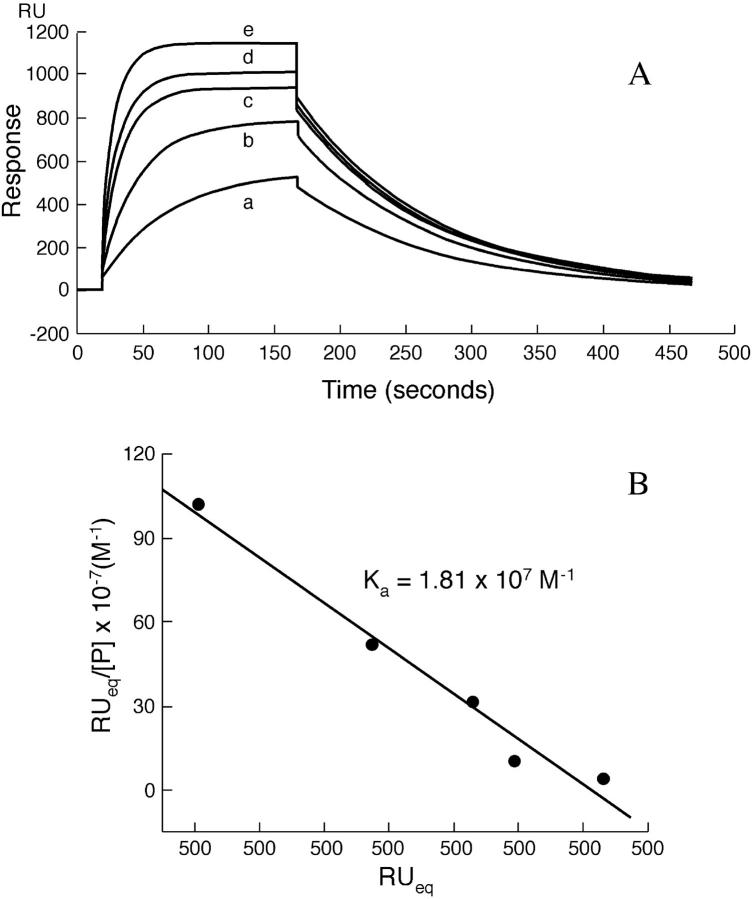
Representative sensograms recorded at 20°C, depicting the interaction of different concentrations of PDC-109 with hybrid bilayers made up of DMPC containing 20% (wt/wt) cholesterol, immobilized on a sensor chip, are shown in Fig. 2 A and a Scatchard analysis of the SPR data is shown in Fig. 2 B. Unlike the profiles shown in Fig. 1, the SPR curves shown here do not show any slow decrease in response during the passage of the PDC-109 solution after equilibrium is attained. This clearly indicates that mixing cholesterol with DMPC stabilizes the hybrid bilayer, and there is no loss of lipid from the surface of the sensor chip.
FIGURE 2.
(A) Sensograms depicting the concentration-dependent binding of PDC-109 to DMPC monolayers containing 20 (wt %) cholesterol at 20°C. PDC-109 in varying concentrations was passed over lipid monolayer of DMPC containing 20% wt/wt cholesterol at a flow rate of 10 μl/min: (a) 0.05 μM, (b) 0.15 μM, (c) 0.3 μM, (d) 1.0 μM, and (e) 3.0 μM. The sensogram was fitted using the linear least-squares method to obtain the association and dissociation rate constants. (B) Scatchard plot depicting the analysis of the sensogram data. From the slope the association constant was determined (slope = −Ka).
The SPR curves shown in Fig. 2 A, fitted by mass transport limited analysis at 20°C, yielded the k1 and k−1 values as 5.7 × 105 M−1 s−1 and 2.7 × 10−2 s−1, respectively. The residuals were found to be distributed equally for the association and dissociation parts, supporting the monoexponential nature of the interaction (data not shown). From the ratio k1/k−1, the equilibrium association constant, Ka, has been estimated to be 2.1 × 107 M−1. Similar analysis was done at 6°C and 12°C also and the values of k1, k−1, and Ka obtained from this analysis are listed in Table 1. The values remain unchanged with increasing salt concentration up to 1 M (data not shown). Scatchard analysis of the data shown in Fig. 2 A yielded a Ka value of 1.81 × 107 M−1 (Fig. 2 B), which is in good agreement with the Ka value of 2.1 × 107 M−1 obtained from the k1/k−1 ratio.
TABLE 1.
Kinetic parameters and association constants obtained from the surface plasmon resonance experiments for the binding of PDC-109 to different phospholipid monolayers
| Lipid layer | Temperature (°C) | k1 (M−1 s−1) | k−1 (s−1) | Ka (M−1) |
|---|---|---|---|---|
| DMPC | 6 | 1.9 (± 0.21) × 105 | 1.1 (± 0.12) × 10−2 | 1.73 (± 0.18) × 107 |
| 12 | 3.9 (± 0.42) × 105 | 1.9 (± 0.19) × 10−2 | 2.05 (± 0.21) × 107 | |
| 20 | 5.7 (± 0.46) × 105 | 2.7 (± 0.21) × 10−2 | 2.11 (± 0.19) × 107 | |
| DMPA | 6 | 0.9 (± 0.11) × 102 | 4.6 (± 0.39) × 10−2 | 1.96 (± 0.16) × 103 |
| 12 | 1.1 (± 0.10) × 102 | 8.1 (± 0.63) × 10−2 | 1.36 (± 0.14) × 103 | |
| 20 | 1.2 (± 0.15) × 102 | 11.0 (± 0.93) × 10−2 | 1.09 (± 0.12) × 103 | |
| DMPG | 6 | 3.6 (± 0.28) × 102 | 4.2 (± 0.33) × 10−2 | 8.57 (± 0.69) × 103 |
| 12 | 4.2 (± 0.39) × 102 | 6.3 (± 0.51) × 10−2 | 6.67 (± 0.63) × 103 | |
| 20 | 5.3 (± 0.42) × 102 | 9.0 (± 0.64) × 10−2 | 5.89 (± 0.43) × 103 |
Number of experiments, n = 4.
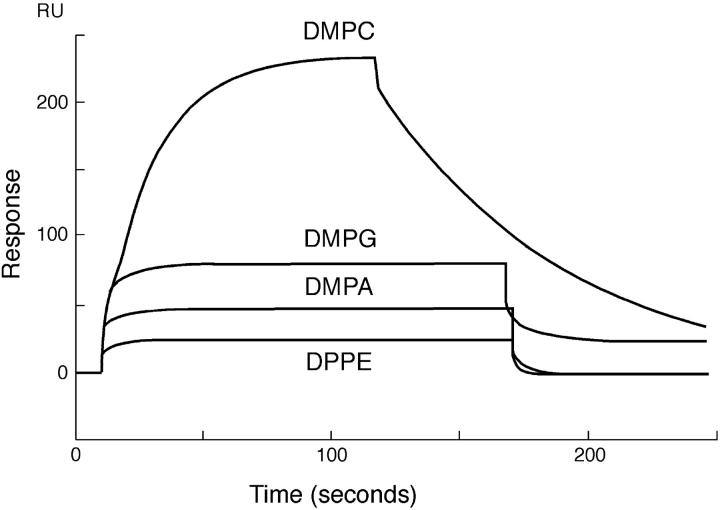
To determine the specificity and strength of binding of PDC-109 toward different phospholipids, SPR experiments were carried out with phospholipids bearing different headgroup structures such as DMPC, DMPG, DMPA, and DPPE, containing 20% (wt/wt) cholesterol. Sensograms depicting the binding of PDC-109 to monolayers of these lipids, recorded at 20°C, are shown in Fig. 3. It is clear from this figure that while binding of PDC-109 to DMPC leads to a large response thereby indicating good binding of the protein to DMPC membranes, its binding to DMPG and DMPA membranes, even at significantly higher concentrations, is associated with considerably smaller changes in the instrument response, suggesting much weaker binding of these two lipids. Binding to DMPE and DPPE yielded very low response and the data were not sufficiently reliable for determining the association and dissociation rate constants from the response curves.
FIGURE 3.
Sensogram depicting the binding of PDC-109 to different lipid monolayers. PDC-109 was passed over different lipid monolayers (DMPC, DMPG, DMPA, and DPPE), containing 20% (wt/wt) cholesterol at 20°C. The concentrations of PDC-109 used were 0.05 μM, 20 μM, 100 μM, and 150 μM, respectively, for DMPC, DMPG, DMPA, and DPPE.
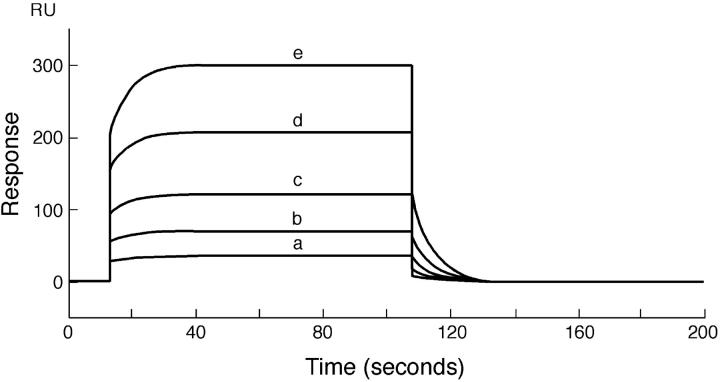
Sensograms obtained at 20°C for the binding of different concentrations of PDC-109 with DMPG monolayers containing 20% (wt/wt) cholesterol are given in Fig. 4. The sensogram data were analyzed as described above for the interaction of PDC-109 with DMPC/cholesterol monolayers. From the analysis, the association (k1) and dissociation (k−1) rate constants were obtained as 1.2 × 102 M−1 s−1 and 0.11 s−1, respectively. Association and dissociation rate constants were also obtained at 6°C and 12°C. The corresponding values of k1, k−1, and the equilibrium association constant, Ka, obtained from their ratio, are listed in Table 1. Similar measurements were also performed with DMPA and the kinetic rate constants and Ka value obtained are also listed in Table 1.
FIGURE 4.
Sensogram depicting the binding of PDC-109 to DMPG monolayer at 20°C. PDC-109 in varying concentrations was passed over a lipid monolayer of DMPG containing 20% wt/wt cholesterol at a flow rate of 10 μl/min: (a)10 μM, (b) 20 μM, (c) 40 μM, (d) 80 μM, and (e) 150 μM. The sensogram was fitted using the linear least-squares method to obtain the association and dissociation constants.
Plots of ln (k/T) versus (1/T) yielded straight lines for both the association and dissociation rate constants (Fig. 5). From the slopes of these plots, the activation enthalpies for the interaction of PDC-109 with different phospholipid/cholesterol membranes were obtained. Activation entropies and free energies were then calculated by the use of the following equations:
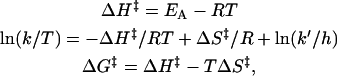 |
where k is the appropriate rate constant, k′ is the Boltzmann constant, and h is Planck's constant. Activation parameters thus obtained, and the thermodynamic parameters, ΔHo, ΔSo, and ΔGo, calculated from them, are listed in Table 2.
FIGURE 5.
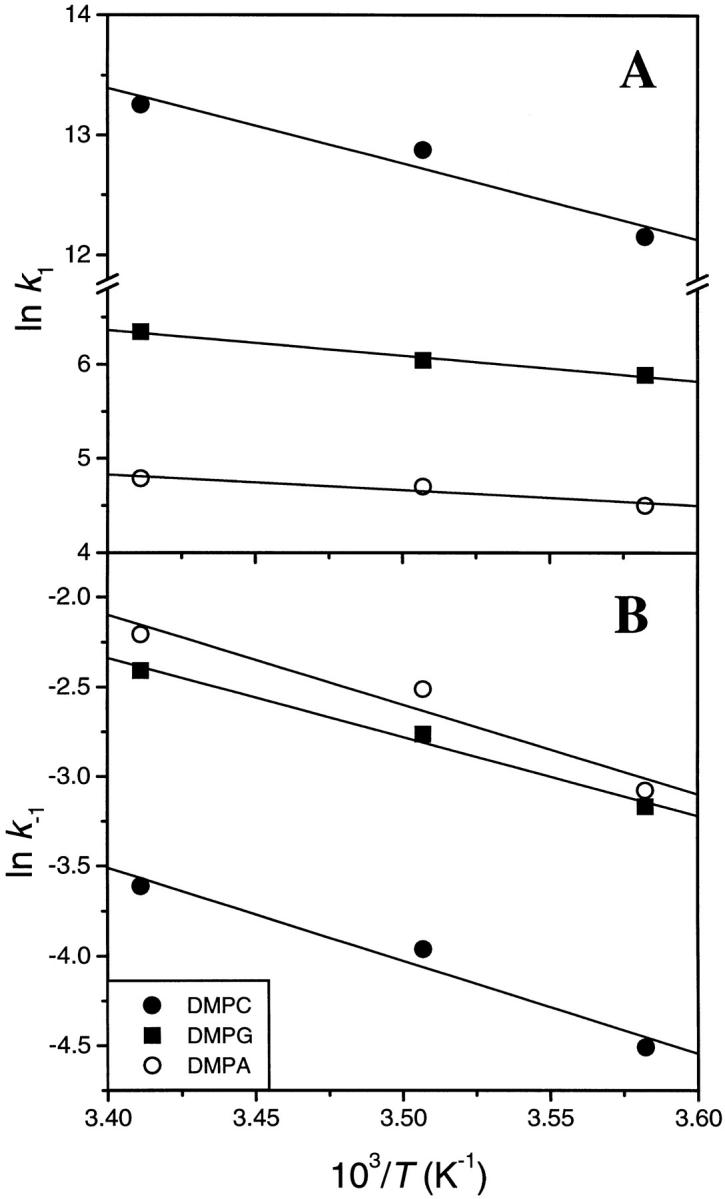
Plots of ln (k/T) versus (1/T) for the association (A) and dissociation (B) rate constants. The data from SPR experiments carried out at different temperatures with DMPC, DMPG, and DMPA containing 20% (wt/wt) cholesterol are shown.
TABLE 2.
Activation parameters and thermodynamic parameters obtained from them for the interaction of PDC-109 with phospholipid/cholesterol membranes
| Lipid | |||
|---|---|---|---|
| Parameter | DMPC | DMPA | DMPG |
| ΔHa‡ | 50.11 | 11.32 | 16.48 |
| ΔHd‡ | 43.03 | 41.64 | 36.74 |
| ΔHo | 7.08 | −30.32 | −20.26 |
| ΔSa‡ | 36.36 | −166.28 | −136.39 |
| ΔSd‡ | −128.0 | −121.06 | −139.46 |
| ΔSo | 164.36 | −45.22 | 3.07 |
| ΔGa‡ | 39.45 | 60.07 | 56.46 |
| ΔGd‡ | 80.55 | 77.13 | 77.62 |
| ΔGo | −41.10 | −17.06 | −21.16 |
Units are: ΔHa‡, ΔHd‡, ΔHo, ΔGa‡, ΔGd‡, and ΔGo: kJ.mol−1; ΔSa‡, ΔSd‡, and ΔSo: J.mol−1 K−1. The standard of deviation of the parameters reported lie within 9% for ΔH and ΔG values whereas for ΔS values they are within 12% of the reported values (n = 4).
DISCUSSION
In view of the role of PDC-109 in the efflux of choline phospholipids and cholesterol, it is of particular interest to investigate its interaction with phospholipid membranes, especially those made up of PC and its mixtures with cholesterol. Though lipid selectivity of this protein has been characterized in considerable detail (Desnoyers and Manjunath, 1992; Ramakrishnan et al., 2001), the kinetics of its interaction with lipid membranes has not been investigated in detail so far. To investigate the kinetics and mechanism of interaction of PDC-109 with phospholipid membranes, SPR experiments were carried out in the present study. SPR is a very sensitive method for the analysis of macromolecule-ligand interactions and can provide information on association and dissociation rate constants as well as association constants from a single experimental run (Johnsson et al., 1991; Chaiken et al., 1992; O'Shannesy et al., 1993). One of the main advantages of this method is that the mass change, taking place during the reaction is the only parameter monitored, obviating the need for employing external labels such as chromophores, which can sometimes alter the reaction process (Schuck, 1997). Also, the ability to form membrane assemblies allows the study, under physiological conditions, of cell membrane/surface-associated phenomena.
The loss of mass observed when PDC-109 was allowed to bind to DMPC monolayers (Fig. 1) is consistent with the fact that binding of this protein to PC vesicles results in a disruption of the vesicle integrity and contents leakage (Gasset et al., 2000). However, addition of cholesterol (at 20% weight ratio) stabilizes the DMPC monolayers (Fig. 2), which is again consistent with the results of Gasset et al. (2000), who reported that cholesterol prevented disruption of DOPC membranes and contents leakage in a dose-dependent manner.
The association rate constant of 5.7 × 105 M−1 s−1 obtained for the binding of PDC-109 to DMPC/cholesterol monolayers at 20°C is approximately three to four orders-of-magnitude slower than the rate constants obtained for diffusion-controlled reactions. The dissociation of PDC-109 is also a single exponential process, and the dissociation rate constant of 2.7 × 10−2 s−1 clearly indicates that, upon binding, the protein is held quite strongly by the DMPC-cholesterol membranes.
The data presented in Fig. 3 clearly demonstrate that PDC-109 exhibits the highest binding affinity to PC membranes among the different phospholipids investigated in the present study. This is consistent with the results of the recent x-ray structure of PDC-109-phosphorylcholine complex, which clearly show that phosphorylcholine binds to each Fn-II domain of the protein via a cation-π interaction between the choline moiety and the indole side chain of a tryptophan residue and H-bond interactions of the phosphate group of the ligand with hydroxyls of exposed tyrosine residues (Wah et al., 2002). However, because another zwitterionic lipid, PE, is very poorly recognized, it is clear that, besides the charge state of the lipid molecules, steric complementarity is also an important factor governing the binding of PDC-109 to the membranes. This is supported by the observations that PDC-109 could be purified by affinity chromatography at high ionic strength on p-aminophenyl phosphorylcholine coupled to Sepharose, or quaternary methylamine coupled to silica or diethylaminoethyl group coupled to Sephadex and eluted by the specific ligand, phosphorylcholine (Desnoyers and Manjunath, 1993).
A comparison of the association and dissociation rate constants for the interaction of PDC-109 with membranes made up of different phospholipids shows that the strength of interaction is determined primarily by the association rate constant (see Table 1). Thus for the binding of PDC-109 to DMPC, DMPA, and DMPG, the k1 values vary by ∼3500 times whereas the variation in the values of k−1 is only ∼3.5 times. This results in a difference in the affinity by more than 104 times. The binding constants (Ka) are determined by the ratio of k1 and k−1. The value of Ka can, therefore, increase with either an enhancement in the values of k1 or a diminution of k−1. In general, for most biologic recognition processes including enzyme-substrate, lectin-sugar interactions, etc., it is usually the reduction in the dissociation rate constants which determines the affinities of cogners (Clegg et al., 1977). In the case of PDC-109-phospholipid interaction, it is the enhanced k1 that dictates the resultant affinities of binding. Hence, one of the striking findings of the present study is the observation that dramatic enhancements in the association rate constants with the change in the phospholipid headgroup underlie the specificities of these recognitions. These observations suggest that binding of PDC-109 to PC membranes require very few changes in the conformation of the protein, whereas binding to membranes made up of other phospholipids such as phosphatidylglycerol or phosphatidic acid probably need considerable changes in the conformation of the protein. This is also in agreement with the crystal structure of PDC-109-phosphorylcholine complex, where the ligand binds quite snugly in the binding pocket on each Fn-II domain of PDC-109 (Wah et al., 2002).
In previous ESR studies we found that besides spin-labeled PC and sphingomyelin, the protein exhibits considerable selectivity for the spin-labeled analog of cholesterol, the cholestane spin-label, CSL, and that the presence of cholesterol in DMPC host matrix leads to an increase in the selectivity of PDC-109 for different spin-labeled phospholipid and sterol probes (Ramakrishnan et al., 2001; Swamy et al., 2002). The data presented in Fig. 3 clearly show that despite the presence of 20 wt % cholesterol, the binding of PDC-109 to membranes bearing different phospholipids is considerably weaker than to those made up of PC. This is contrary to what one would expect if PDC-109 in its native conformation could recognize cholesterol at the membrane interface. One possible explanation for this is that in mixed lipid membranes containing phospholipids and cholesterol, the latter is sterically hindered by the phospholipid headgroups, and therefore is not accessible to PDC-109 present in the aqueous medium. Therefore, it may be expected that only upon binding to the membrane via the headgroup of choline-containing phospholipids would the PDC-109 protein be able to interact with cholesterol, as observed in the ESR experiments (Ramakrishnan et al., 2001; Swamy et al., 2002).
A comparison of the activation parameters given in Table 2 reveals the underlying thermodynamic factors that favor the strong binding of PDC-109 to DMPC as compared to DMPA and DMPG, which are recognized by this protein with considerably weaker affinity. From this Table it is seen that the binding of PDC-109 to DMPA and DMPG is accompanied by smaller activation enthalpies of association than for its binding to DMPC membranes. However, the positive activation entropy for the association with DMPC is contrasted by relatively large negative values of activation entropy for the binding to DMPA and DMPG membranes. These data thus indicate that the interactions of PDC-109 with different phospholipids are discriminated by disparate changes in activation entropies—being favorable for PC and sterically unfavorable for other phospholipids that do not possess the choline moiety in the headgroup structure. As a result, the free energy of activation for DMPC is considerably lower than the corresponding values for DMPA and DMPG. The enthalpies and entropies of activation for the dissociation process for all the three lipids are approximately in the same range, and therefore the relative affinities are primarily governed by the differences in the activation parameters, especially ΔSa‡. The positive entropic contribution associated with the binding of PDC-109 to PC membranes may arise from the disaggregation of the protein, which exists in an aggregated form in the native state, upon binding to the phosphocholine moiety, resulting in an increase in the entropy (see Gasset et al., 1997).
It is interesting to note that only a single exponential binding process could be detected by the SPR studies reported here, whereas in preliminary stopped-flow investigations monitoring changes in the protein fluorescence, Müller et al. (1998) observed that the binding of PDC-109 to DMPC unilamellar vesicles takes place by a biexponential process. FTIR experiments of Gasset et al. (2000) also suggest that PDC-109 undergoes a conformational change upon binding to PC membranes. However, since SPR detects only mass changes as a function of time, it may not be possible to detect subtle conformational changes that do not lead to any changes in the mass. Alternatively, it is also possible that a single step is observed in the present study because the acyl chain dynamics of the lipid molecules in the hybrid bilayer used in the SPR experiments could be considerably different from those in the liposomes used by Müller et al. (1998). As a result of such differences, the partial insertion of PDC-109 into hydrophobic interior of the lipid membrane, which is observed in the earlier studies carried out with liposomes (Müller et al., 1998; Ramakrishnan et al., 2001), may not occur with the planar hybrid bilayers used in the SPR studies.
C-reactive protein, an acute phase protein found in most vertebrates, is another choline phospholipid binding protein that is well characterized. Binding of choline phospholipids to C-reactive protein occurs in a calcium-dependent manner (Volanakis and Wirtz, 1979; Sui et al., 1999) whereas binding to PDC-109 is by the specific interaction of the choline headgroup with the protein and does not require Ca2+ or other factors. Therefore, PDC-109 appears to be quite unique in its ability to recognize choline phospholipids without the need for any other factors.
In summary, in this article the binding of PDC-109 to different phospholipid membranes has been investigated by the surface plasmon resonance technique. Binding of the protein to phosphatidylcholine membranes takes place with highest association rate constant and its dissociation is the slowest among the various phospholipids investigated. Binding of PDC-109 appears to be governed primarily by steric complementarity of the protein-binding site to the choline headgroup. Charge state of the lipid per se is not a significant factor in the recognition process as phosphatidylethanolamine is very poorly recognized by PDC-109.
Acknowledgments
We thank Dr. K. Babu Rao of the Lam Farm, Guntur, Acharya N. G. Ranga University of Agricultural Sciences, for providing samples of bovine semen.
This work was supported in part by a research project from Council of Scientific and Industrial Research (India) to M.J.S. The BIAcore facility is provided by the Department of Biotechnology (India) for program support to Indian Institute of Science in the area of Drug and Molecular Design. M.R. and V.A. are supported by Research Fellowships from the CSIR (India).
Ira Surolia is a summer trainee from Dr. B. R. Ambedkar College, Bangalore 560 045, India.
References
- Baker, M. E. 1985. The PDC-109 protein from bovine seminal plasma is similar to the gelatin-binding domain of bovine fibronectin and a kringle domain of human tissue-type plasminogen activator. Biochem. Biophys. Res. Commun. 130:1010–1014. [DOI] [PubMed] [Google Scholar]
- Calvete, J. J., F. V. Paloma, L. Sanz, and A. Romero. 1996. A procedure for the large-scale isolation of major bovine seminal plasma proteins. Protein Expr. Purif. 8:48–56. [DOI] [PubMed] [Google Scholar]
- Chaiken, I., S. Rose, and R. Karlsson. 1992. Analysis of macromolecular interactions using immobilized ligands. Anal. Biochem. 201:197–210. [DOI] [PubMed] [Google Scholar]
- Clegg, R. M., F. G. Loontiens, and T. M. Jovin. 1977. Binding of 4-methylumbelliferyl α-D-mannopyranoside to dimeric concanavalin A: fluorescence temperature-jump relaxation study. Biochemistry. 16:167–175. [DOI] [PubMed] [Google Scholar]
- Desnoyers, L., and P. Manjunath. 1992. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipids. J. Biol. Chem. 267:10149–10155. [PubMed] [Google Scholar]
- Desnoyers, L., and P. Manjunath. 1993. Interaction of a novel class of phospholipid-binding proteins of bovine seminal fluid with different affinity matrices. Arch. Biochem. Biophys. 305:341–349. [DOI] [PubMed] [Google Scholar]
- Esch, F. S., N. C. Ling, P. Bohlen, S. Y. Ying, and R. Guillemin. 1983. Primary structure of PDC-109, a major protein constituent of bovine seminal plasma. Biochem. Biophys. Res. Commun. 113:861–867. [DOI] [PubMed] [Google Scholar]
- Gasset, M., J. L. Saiz, L. Sanz, M. Gentzel, E. Töpfer-Petersen, and J. J. Calvete. 1997. Conformational features and thermal stability of bovine seminal plasma protein, PDC-109 oligomers and phosphorylcholine-bound complexes. Eur. J. Biochem. 250:735–744. [DOI] [PubMed] [Google Scholar]
- Gasset, M., M. Magdaleno, and J. J. Calvete. 2000. Biophysical study of the perturbation of model membrane structure caused by seminal plasma protein PDC-109. Arch. Biochem. Biophys. 374:241–247. [DOI] [PubMed] [Google Scholar]
- Greube, A., K. Müller, E. Töpfer-Petersen, A. Herrmann, and P. Müller. 2001. Influence of the bovine seminal plasma protein PDC-109 on the physical state of membranes. Biochemistry. 40:8326–8334. [DOI] [PubMed] [Google Scholar]
- Harrison, R. A. P. 1996. Capacitation mechanisms, and the role of capacitation as seen in eutherian mammals. Reprod. Fert. Dev. 8:581–594. [DOI] [PubMed] [Google Scholar]
- Higgins, J. A. 1987. Separation and analysis of membrane lipid components. In Biological Membranes. A Practical Approach. J. B. C. Findlay, and W. H. Evans, editors. IRL Press, Oxford.122.
- Johnsson, B., S. Lofas, and G. Lindquist. 1991. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 198:268–277. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Lowry, O. H., N. J. Rosebrough, L. Farr, and R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- Manjunath, P., and M. R. Sairam. 1987. Purification and biochemical characterization of three major acidic proteins (BSP-A1, BSP- A2, and BSP A3) from bovine seminal plasma. Biochem. J. 241:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath, P., M. R. Sairam, and J. Uma. 1987. Purification of four gelatin-binding proteins from bovine seminal plasma by affinity chromatography. Biosci. Rep. 7:231–238. [DOI] [PubMed] [Google Scholar]
- Marsh, D., and L. I. Horváth. 1998. Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim. Biophys. Acta. 1376:267–296. [DOI] [PubMed] [Google Scholar]
- Moreau, R., and P. Manjunath. 1999. Characterization of lipid efflux particles generated by seminal phospholipid-binding proteins. Biochim. Biophys. Acta. 1438:175–184. [DOI] [PubMed] [Google Scholar]
- Moreau, R., I. Thérien, C. Lazure, and P. Manjunath. 1998. Type II domains of BSP-A1/-A2 proteins: binding properties, lipid efflux, and sperm capacitation potential. Biochem. Biophys. Res. Commun. 246:148–154. [DOI] [PubMed] [Google Scholar]
- Müller, P., K.-R. Erlemann, K. Müller, J. J. Calvete, E. Töpfer-Petersen, K. Marienfeld, and A. Herrmann. 1998. Biophysical characterization of the interaction of bovine seminal plasma protein PDC-109 with phospholipid vesicles. Eur. Biophys. J. 27:33–41. [DOI] [PubMed] [Google Scholar]
- O'Shannesy, D. J., M. Brigham-Burke, K. K. Soneson, P. Hensley, and I. Brooks. 1993. Determination of rate and equilibrium binding constants for macromolecular interactions using surface plasmon resonance: use of nonlinear least-squares analysis methods. Anal. Biochem. 212:457–468. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan, M., V. Anbazhagan, T. V. Pratap, D. Marsh, and M. J. Swamy. 2001. Membrane insertion and lipid-protein interactions of bovine seminal plasma protein, PDC-109 investigated by spin label electron spin resonance spectroscopy. Biophys. J. 81:2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, J., Y. Lin, X. Bing, and G. M. Whitesides. 1999. Using surface plasmon resonance to study the binding of vancomycin and its dimer to self-assembled monolayers presenting D-Ala-D-Ala. J. Am. Chem. Soc. 121:2629–2630. [Google Scholar]
- Rouser, G., S. Fleisher, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5:494–496. [DOI] [PubMed] [Google Scholar]
- Scheit, K. H., M. Kemme, G. Aumüller, J. Seitz, G. Hagendorff, and M. Zimmer. 1988. The major protein of bull seminal plasma: biosynthesis and biological function. Biosci. Rep. 8:589–608. [DOI] [PubMed] [Google Scholar]
- Schuck, P. 1997. Use of surface plasmon resonance to probe the equilibrium and dynamic aspects of interactions between biological macromolecules. Annu. Rev. Biophys. Biomol. Struct. 26:541–566. [DOI] [PubMed] [Google Scholar]
- Seidah, N. G., P. Manjunath, J. Rochemont, M. R. Sairam, and M. Cherétian. 1987. Complete amino acid sequence of BSP-A3 from bovine seminal plasma. Homology to PDC-109 and to the collagen-binding domain of fibronectin. Biochem. J. 243:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaji, S., K.-H. Scheit, and P. M. Bhargava. 1990. Proteins of Seminal Plasma. Wiley, New York.
- Sui, S.-F., Y.-T. Sun, and L.-Z. Mi. 1999. Calcium-dependent binding of rabbit C-reactive protein to supported lipid monolayers containing exposed phosphorylcholine group. Biophys. J. 76:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy, M. J., D. Marsh, V. Anbazhagan, and M. Ramakrishnan. 2002. Effect of cholesterol on the interaction of seminal plasma protein, PDC-109 with phosphatidylcholine membranes. FEBS Lett. 528:230–234. [DOI] [PubMed] [Google Scholar]
- Thérien, I., R. Moreau, and P. Manjunath. 1998. Major proteins of bovine seminal plasma and high-density lipoprotein induce cholesterol efflux from epididymal sperm. Biol. Reprod. 59:768–776. [DOI] [PubMed] [Google Scholar]
- Thomas, C. J., N. Surolia, and A. Surolia. 1999. Surface plasmon resonance studies resolve the enigmatic endotoxin neutralizing activity of polymixin B. J. Biol. Chem. 274:29624–29627. [DOI] [PubMed] [Google Scholar]
- Visconti, P. E., H. Calantino-Hormer, G. D. Moore, J. L. Bailey, X. Ning, M. Fornes, and G. S. Kopf. 1998. The molecular basis of sperm capacitation. J. Androl. 19:242–248. [PubMed] [Google Scholar]
- Volanakis, J. E., and K. W. A. Wirtz. 1979. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers. Nature. 281:155–157. [DOI] [PubMed] [Google Scholar]
- Wah, D. A., C. Fernández-Tornero, L. Sanz, A. Romero, and J. J. Calvete. 2002. Sperm coating mechanism from the 1.8 Å crystal structure of PDC-109-phosphorylcholine complex. Structure. 10:505–514. [DOI] [PubMed] [Google Scholar]