Abstract
Mixing and thermal behavior of hydrated and air-dried mixtures of 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) and 1,2-distearoyl-d70-sn-glycero-3-phosphocholine (DSPCd-70) in the absence and presence of trehalose were investigated by Fourier transform infrared spectroscopy. Mixtures of DLPC:DSPCd-70 (1:1) that were air-dried at 25°C show multiple phase transitions and mixed phases in the dry state. After annealing at high temperatures, however, only one transition is seen during cooling scans. When dried in the presence of trehalose, the DLPC component shows two phase transitions at −22°C and 75°C and is not fully solidified at −22°C. The DSPCd-70 component, however, shows a single phase transition at 78°C. The temperatures of these transitions are dramatically reduced after annealing at high temperatures with trehalose. The data suggest that the sugar has a fluidizing effect on the DLPC component during drying and that this effect becomes stronger for both components with heating. Examination of infrared bands arising from the lipid phosphate and sugar hydroxyl groups suggests that the strong effect of trehalose results from direct interactions between lipid headgroups and the sugar and that these interactions become stronger after heating. The findings are discussed in terms of the protective effect of trehalose on dry membranes.
INTRODUCTION
Removal of water from biological membranes usually results in irreversible structural and functional damage (Crowe et al., 1984). Nevertheless, many organisms can survive almost complete dehydration by entering a state of anhydrobiosis, or life without water. This phenomenon is widespread, occurring in nearly all the major taxa of the plant, animal, and microbial worlds (Crowe et al., 1992). Almost all desiccation-tolerant organisms synthesize large amounts of disaccharides (particularly sucrose and trehalose) during dehydration (Drennan et al., 1993; Gadd et al., 1987; Loomis et al., 1980; Hoekstra et al., 1989; Koster and Leopold, 1988; Clegg, 1965), and their survival is correlated with the presence of these sugars (Madin and Crowe, 1975; Womersley et al., 1986) . Studies in vitro have shown that these sugars are capable of preserving structure and function in biomolecules and molecular assemblages, such as membranes, during drying (Crowe et al., 1987a). Evidence from freeze-fracture suggests that when intact membranes are dried without trehalose, massive and irreversible phase separation of membrane components occurs (Crowe et al., 1983; Crowe and Crowe, 1982). In addition, proteins aggregate, and become redistributed on both membrane faces, while other components such as nonbilayer lipids form separate phases. When the membranes are dried with trehalose, these events are prevented (Crowe et al., 1983; Crowe and Crowe, 1982).
To elucidate the mechanism of membrane stabilization by trehalose, relatively simple models, such as liposomes, have been used (Crowe and Crowe, 1992, 1988; Crowe et al., 1984, 1985; Sun et al., 1996; Tsvetkova et al., 1998; Womersley et al., 1986). Trehalose was found to be particularly effective at preventing fusion and leakage in liposomes during dehydration and rehydration. Trehalose, like most sugars, forms a glass during dehydration (Slade and Levine, 1995; Green and Angell, 1989; Crowe et al., 1996b). The carbohydrate glass is an amorphous, thermodynamically unstable state characterized by high viscosity and low molecular mobility (Franks, 1985; Roos, 1995). From liposome fusion studies, it has been proposed that the trehalose glass has a protective effect during drying (Crowe et al., 1994; Koster et al., 2000). The viscous glass matrix, which surrounds and embeds the liposomes during drying, prevents close approach of lipid bilayers, and thus inhibits fusion of liposomes into multilamellar aggregates. During heating, sugar glasses undergo a transition at a specific temperature (Tg) to a state that exhibits much lower viscosity and higher molecular mobility than the glassy state (Sperling, 1986; Roos, 1995). In previous studies, Crowe and co-workers showed that massive fusion of liposomes occurred in dry sugar/liposome mixtures at temperatures above Tg (Crowe et al., 1994, 1996a). Fourier transform infrared spectroscopy (FTIR) studies have shown that the glass transition is associated with an abrupt change in the sugar's OH hydrogen bonding network (Wolkers et al., 1998).
Further studies with liposomes suggested that direct interactions between trehalose and lipid molecules preserve lipid headgroup spacing in the dry state, and prevent damaging lipid phase transitions that could cause leakage during rehydration (Crowe et al., 1996a,b, 1986). Crowe and others (Crowe et al., 1984, 1996a; Lee et al., 1986; Tsvetkova et al., 1998) have proposed that the sugar acts to ‘replace’ water molecules bound to lipid headgroups in the hydrated state, and maintains headgroup spacing in the dry state similar to that of the hydrated lipid. As a result of these interactions, the lipid hydrocarbon chains are less tightly packed, and the gel-to-liquid crystalline phase transition temperature (Tm) is decreased, compared to lipids dried in the absence of trehalose. The decreased Tm in the presence of trehalose prevents lipids from passing through their transitions upon rehydration, thus preventing leakage in or out of the membrane. In the dry lipid/trehalose mixtures, then, most likely two distinct sugar populations are present—one that is interacting directly with the lipid headgroups and replacing “lost” water, and another population in the bulk glassy state (Crowe et al., 1987b). An alternative, but not mutually exclusive suggestion about the interactions between trehalose and dried lipid has been proposed by Koster and co-workers (Koster et al., 2000).
The effect of trehalose on the phase behavior of bilayers composed of single lipids has been studied extensively, as discussed above. In view of the finding that trehalose can prevent detrimental phase separation in native membranes during drying (Crowe and Crowe, 1982; Wolkers et al., 2001, 2002), it is of interest to investigate the mechanism of the effects of trehalose on the phase behavior of lipid mixtures during drying. Such studies could provide insight into effects that trehalose may have on microdomains during dehydration. Microdomains, which may serve as signaling platforms in biological membranes (Simons and Ikonen, 1997), are formed due to lateral phase separation of lipids (Mouritsen and Jorgensen, 1997, 1995; Leidy et al., 2001; Lehtonen et al., 1996). The effects of dehydration on such domains are not known. A model study is an appropriate starting point for understanding the influence of trehalose on phase separation and microdomain structure of dehydrated membranes.
We have chosen a simple model system, a 1:1 mixture of 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) (Tm = −3°C) and 1,2-distearoyl-d70-sn-glycero-3-phosphocholine (DSPCd-70) (Tm = 56°C), with which to investigate the effects of trehalose on lipid phase behavior during dehydration. The mixing behavior of DLPC and DSPC as a function of lipid composition and temperature is well understood for the hydrated state (Mabrey and Sturtvant, 1976; Ipsen and Mouritsen, 1988). In the present study we show that dehydration of the mixture from a phase separated hydrated state leads to lipid mixing during drying. Further, we show that trehalose has a fluidizing effect on the DLPC component, which results in a phase separated state of the mixture after drying. We then provide evidence concerning the mechanism of these effects.
MATERIALS AND METHODS
Trehalose dihydrate was purchased from Pfanstiehl (Waukegan, IL). 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), 1,2-distearoyl-d70-sn-glycero-3-phosphocholine (DSPCd-70), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-PE), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine Rhodamine B Sulfonyl) (Rh-PE) in chloroform were purchased from Avanti Polar Lipids (Alabaster, AL).
Liposome preparation
Lipid aliquots were dried from chloroform under nitrogen gas, and were placed under vacuum (VirTis Co., New York, NY) for at least 2 h to remove residual chloroform. The dried lipid was then resuspended in either water or sugar solution at a concentration of 10 mg/ml and vortexed at 60°C to form multilamellar vesicles. Liposomes were prepared by extrusion of multilamellar-vesicle suspensions through 100-nm polycarbonate filters (Osmonics, Minnetonka, MN) in a hand-held extruder (LiposoFast, Avestin, Ontario, Canada) that was heated to 60°C. For samples containing trehalose, an aqueous sugar solution of trehalose:lipid, 5:1 by dry weight, was used. After FTIR analysis in the hydrated state, liposomes were air-dried at 25°C for 24 h in a dessicator attached to a Balston (Haverhill, MA) dry air generator. The relative humidity during drying was less than 2%. For FTIR measurements on dry mixtures, 10 μl samples were dried directly on CaF2 windows, and sealed in a dry box before transfer to the spectrometer. For fluorescence measurements, samples were dried in weigh boats, and rehydrated directly in the boats.
Fourier transform infrared spectroscopy
Spectra were obtained with a Perkin-Elmer 2000 FTIR spectrometer and AutoIMAGE microscope, both interfaced to a PC with Spectrum 2000 software (Perkin-Elmer, Norwalk, CT). Both instruments were purged of water vapor with a dry air generator (Balston, Haverhill, MA). The sample temperature was controlled by either a Peltier device or a heating element (FTIR microscope), and monitored with a thermocouple. Temperature was ramped at a rate of 2°C/min for all samples during scanning. It should be noted that the ramp rate did not have a significant effect on the transition temperatures of the lipids, at rates ranging from 0.5°C to 5°C. Spectra were obtained as a function of temperature, with a total of 32 spectra averaged for each temperature point. To monitor each lipid individually in the mixture, deuterated DSPC was used (Mendelsohn and Moore, 1998). The CH2 stretching region, from 3000 cm−1 to 2800 cm−1, CD2 stretching region, from 2200 cm−1 to 2000 cm−1, and OH stretching region, from 3600 cm−1 to 3000 cm−1, were analyzed. Band positions were determined by taking the second derivatives of the original spectra (except for the OH data), and averaging intercepts at 80% peak intensity. Phase transitions were determined by plotting the CH2 symmetric stretching and CD2 asymmetric stretching band positions as a function of temperature. Figures present typical plots. Glass transitions were determined by plotting the OH stretching band at 3350 cm−1, as a function of temperature, as described by Wolkers et al. (1998).
Fluorescence resonance energy transfer
FRET measurements were obtained with a Perkin-Elmer LS 50 B luminescence spectrometer (Norwalk, CT). Fresh liposomes were prepared in the presence of 1 mol % of either NBD-PE or Rh-PE, and were added to the sample cuvette. Initial fluorescence (F0) (ex. 450 nm, em. 530 nm) was then measured. To measure fusion, samples were dried for 24 h, rehydrated, and fluorescence was measured again (Fad). These fluorescence values were compared to those of mock-fused liposomes (Fmf), which contained both NBD-PE and Rh-PE labels. As an indication of fusion between liposomes, percent probe intermixing was calculated using the equation:
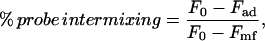 |
based on the method of Uster and Deamer (1981).
RESULTS AND DISCUSSION
Effect of trehalose on hydrated DLPC/DSPCd-70 mixtures
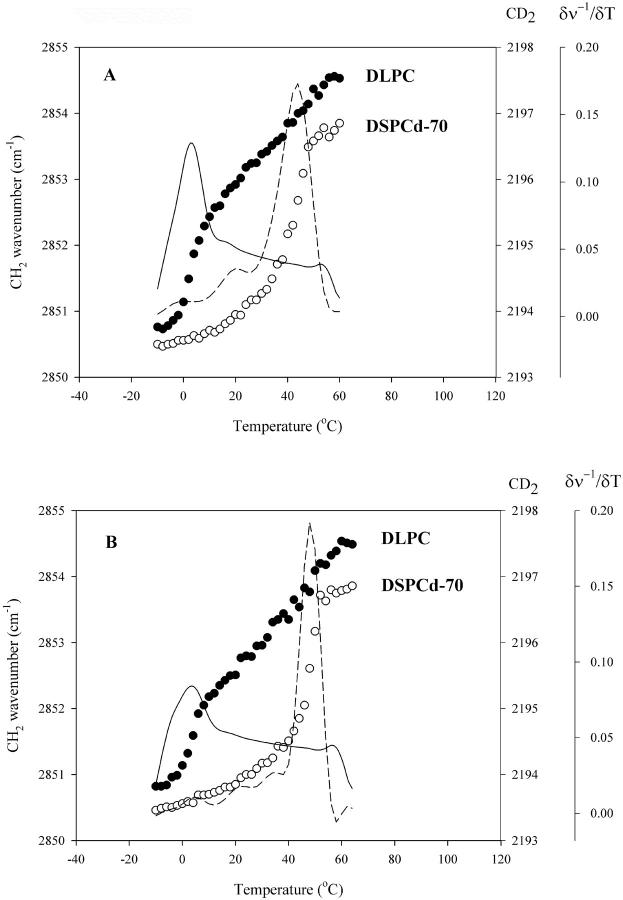
FTIR profiles of hydrated mixtures of 1:1 DLPC/DSPCd-70 show phase transitions at 3°C and 44°C, corresponding to the DLPC and DSPCd-70 components, respectively (Fig. 1 A), in agreement with previous results on the phase behavior of DLPC/DSPC mixtures (Mabrey and Sturtevant, 1976; Ipsen and Mouritsen, 1988). In the presence of trehalose, the transitions occur at slightly higher temperatures, at 5°C and 47°C (Fig. 1 B). Sugars and other membrane cryoprotectants are known to act as water-structuring compounds, or kosmotropes, which increase interfacial tension and, as a result, cause the lipid bilayers to become more closely packed (Collins and Washabaugh, 1985). This change in packing is reflected by an increase in Tm of both components in the presence of trehalose. Trehalose appears to have little effect on the mixing behavior of the lipids in the hydrated state.
FIGURE 1.
Changes in the wavenumber of the CH2 symmetric stretch (•) and CD2 asymmetric stretch (○) as a function of temperature in fully hydrated DLPC:DSPCd-70 1:1 mixtures, in the absence (A) and presence (B) of trehalose (5:1 sugar:lipid); first derivative CH2 (—); and first derivative CD2 (− −).
Effect of trehalose on air-dried lipids and mixtures
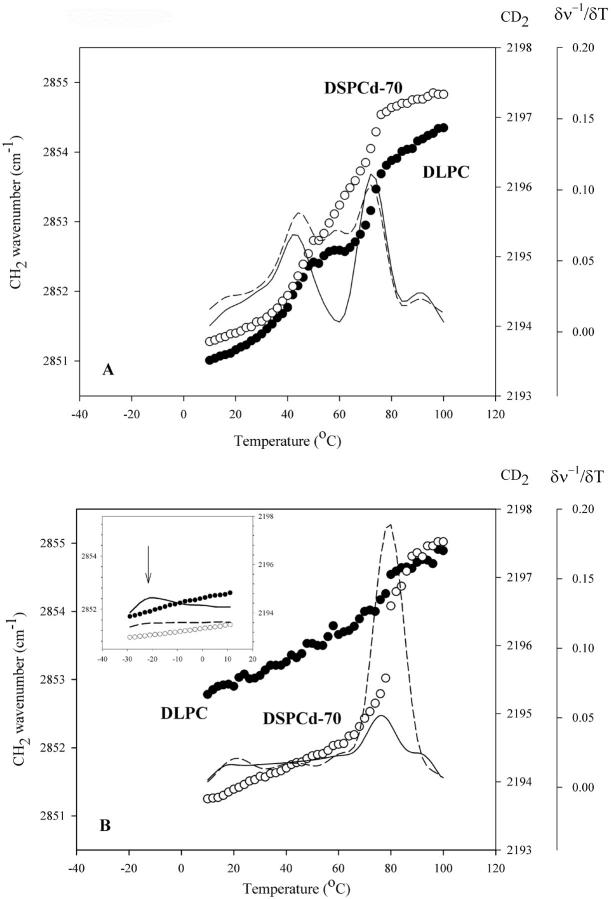
Heating scans of a 1:1 DLPC/DSPCd-70 mixture air dried at 25°C in the absence and presence of trehalose are shown in Fig. 2. After air-drying, samples without sugar contained ∼2% water by weight, and samples with sugar contained ∼3% water by weight. When compared to the hydrated system, air-dried mixtures display much higher phase transition temperatures. This is due to decreased headgroup spacing of the lipids in the dry state, which allows for more van der Waals interactions between lipid hydrocarbon chains, and thus higher onset of the chain melt (Chapman et al., 1967; Crowe et al., 1998). In the mixture, the DLPC component shows two phase transitions at 43°C and 71°C, respectively (Fig. 2 A). DSPCd-70 shows transitions at similar temperatures to DLPC, at 45°C and 71°C, and a transition at 57°C, independent of the DLPC fraction. Thus, after drying, mixed phases of the two lipids are observed. This is in sharp contrast with the situation before drying, in which the lipid components are phase separated at 25°C (the temperature of drying). We conclude that air-drying this binary mixture leads to mixing of the two components.
FIGURE 2.
Changes in the wavenumber of the CH2 symmetric stretch (•) and CD2 asymmetric stretch (○) as a function of temperature during heating of DLPC:DSPCd-70 1:1 mixtures, air-dried in the absence (A) and presence (B) of trehalose (5:1 sugar:lipid); first derivative CH2 (—); and first derivative CD2 (− −). Low temperature wavenumber versus temperature plot of air-dried DLPC/DSPCd-70 in the presence of trehalose (B, inset).
In the presence of trehalose, the mixture exhibits a very different behavior (Fig. 2 B). Within the temperature range of the scan, the DLPC fraction shows a transition at 75°C, with a total change of 0.5 cm−1 in CH2 vibrational frequency. This transition is rather small in magnitude when compared to transitions seen in the mixture without trehalose (see Fig. 2 A). The CH2 vibrational frequency is 2852.7 cm−1 at 10°C, indicating that the lipid acyl chains have a relatively high degree of vibrational freedom, even at this low temperature. This evidence, together with the small frequency change of the 75°C transition, suggests that another transition likely occurs below 10°C. A heating scan at lower temperatures reveals that, indeed, another transition occurs at −22°C, similar in magnitude to the 75°C transition (Fig. 2 B, inset). At −29°C, the CH2 frequency is still high, at 2851.7 cm−1. Therefore, another transition(s) may occur at a temperature(s) lower than −29°C before the DLPC component becomes fully solidified.
DSPCd-70, on the other hand, exhibits a single cooperative phase transition at 78°C, with a total change of 3 cm−1 in CD2 vibrational frequency (Fig. 2 B). The temperature of this transition is slightly higher than those seen in the mixture without sugar, where the highest temperature transition of DSPCd-70 occurs at 71°C (see Fig. 2 A). Unlike DLPC, no additional transitions occur in the DSPCd-70 component below 78°C, as seen in the scan at lower temperatures (Fig. 2 B, inset). Therefore, the data suggest that trehalose interacts with the DLPC component preferentially, strongly fluidizing the lipid. We interpret this behavior in the following way. Samples were dried from full hydration at 25°C, a temperature at which the DLPC component is in liquid crystalline phase and the DSPCd-70 component is in gel phase. DLPC in the liquid crystalline phase has a larger headgroup spacing than the gel phase DSPCd-70, providing trehalose more access to the DLPC headgroups during drying. As water is removed, trehalose interacts strongly with the DLPC headgroups, maintaining headgroup spacing and thus fluidizing the acyl chains. As a result, the transition temperature of a fraction of the DLPC is decreased to low temperatures. This strong fluidizing effect has been observed for single lipid systems where the lipid is in liquid crystalline phase during drying (Crowe and Crowe, 1988), and is reflected in our data on single lipid systems of DLPC and DSPCd-70, shown in Table 1. Taken together, the data on air-dried DLPC/DSPCd-70/trehalose mixtures suggest that the sugar maintains the mixture in a hydrated-like state, with the lipids phase separated.
TABLE 1.
Phase transition temperatures for single lipids air-dried in the absence and presence of trehalose
| Lipid | Tm (− trehalose)heating scan | Tm (+ trehalose)heating scan | Tm (+ trehalose)cooling scan |
|---|---|---|---|
| DLPC | 81°C | 65°C | −30°C |
| DSPCd-70 | 94°C | 90°C | 32°C |
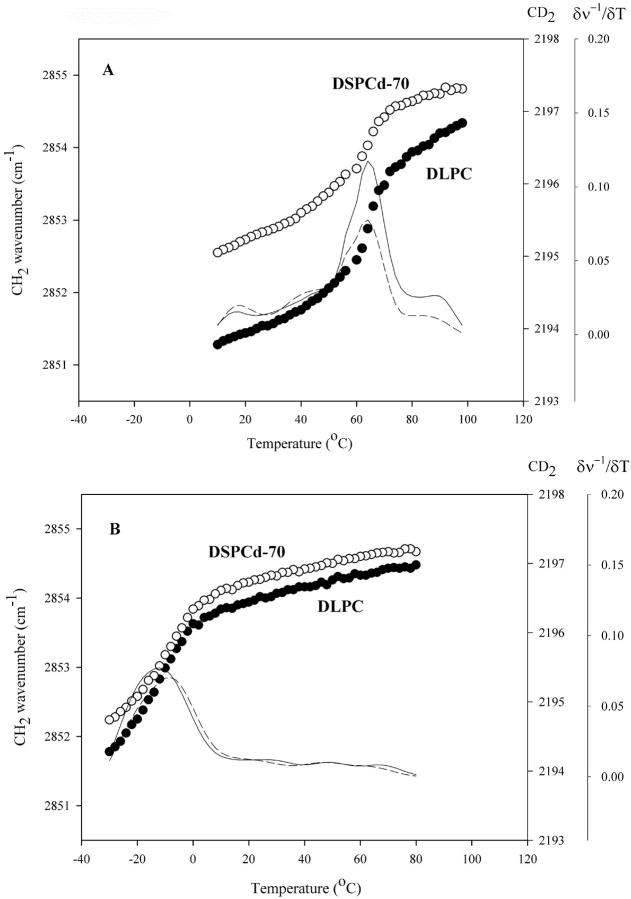
Cooling scans taken after heating the air-dried mixtures to 110°C (above the lipid transition temperatures) are shown in Fig. 3. In the absence of trehalose, both the DLPC and DSPCd-70 components show a single phase transition at 63°C (Fig. 3 A), a temperature intermediate to those seen on the heating scan of this mixture. In the melted (liquid crystalline) phase, dry lipids exhibit considerable molecular motion (Chapman et al., 1967). We suggest that this allows the lipid components to mix during heating, and upon cooling, pass through their main transitions at similar temperatures.
FIGURE 3.
Changes in the wavenumber of the CH2 symmetric stretch (•) and CD2 asymmetric stretch (○) as a function of temperature during cooling of DLPC:DSPCd-70 1:1 mixtures, air-dried in the absence (A) and presence (B) of trehalose (5:1 sugar:lipid); first derivative CH2 (—); and first derivative CD2 (− −).
In the presence of trehalose, the DLPC fraction solidifies at −14°C during the cooling scan, a temperature that is decreased by 89°C relative to the heating scan (Fig. 3 B). Similarly, DSPCd-70 solidifies at −8°C, which is 86°C below the heating scan transition temperature. We interpret these findings as follows. At temperatures above the phase transitions of both components of the mixture, trehalose has increased access to the headgroups of the fluidized lipids. An increase in interaction between sugar and lipid causes a decrease in Tm of both of the components. Similar decreases in Tm have been reported for other dry phospholipids (egg PC, DPPC, POPC-PS) in the presence of trehalose (Crowe et al., 1997, 1986; Tsvetkova et al., 1998, 1988; Crowe and Crowe, 1988), and for the lipids used in this study (see Table 1).
Effect of trehalose on fusion of liposomes
The trehalose glass has been proposed to play a role in prevention of fusion of liposomes during drying (Crowe et al., 1994). Using FRET, we investigated the effect of differing ratios of trehalose:lipid on fusion of DLPC/DSPCd-70 liposomes during drying. The data are summarized in Table 2. When air-dried without trehalose, the DLPC/DSPCd-70 liposomes undergo massive fusion. However, fusion is decreased by more than half with a 5:1 sugar:lipid dry mass ratio. It is further reduced when the sugar to lipid ratio is increased to 8:1. The results suggest that trehalose has a protective effect on the mixture liposomes, most likely by forming a glassy state during dehydration.
TABLE 2.
Fusion of liposomes after air drying in the absence and presence of trehalose
| Mixture | % probe intermixing after air-drying |
|---|---|
| DLPC/DSPCd70 1:1 | 54% |
| DLPC/DSPCd-70/trehalose 1:1:10 | 24% |
| DLPC/DSPCd-70/trehalose 1:1:16 | 16% |
Interactions between trehalose and lipids in the dry state
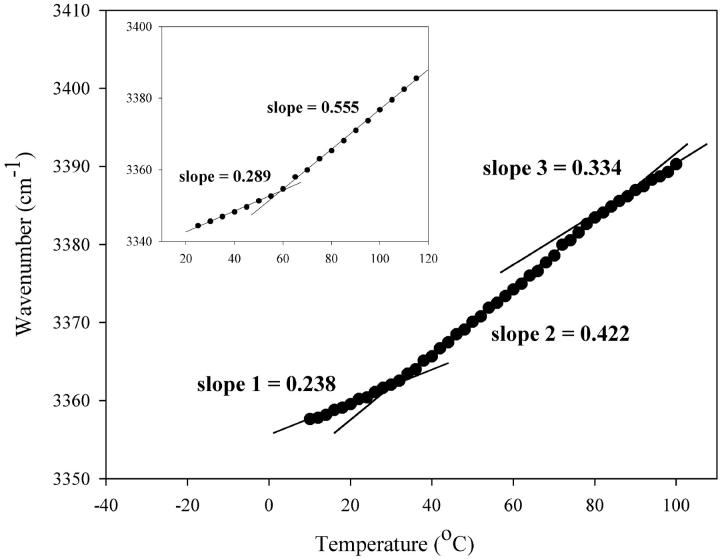
To investigate the thermal behavior of the sugar in the dry mixture, we studied changes in the OH stretching mode of trehalose as a function of temperature with FTIR (Fig. 4). A change in slope of the OH thermal profile can be attributed to a change in the hydrogen bonding network of trehalose hydroxyl groups, which may be caused by a glass transition or interactions with surrounding lipid molecules (Wolkers et al., 1998). Fig. 4 (inset) shows a heating scan of trehalose air-dried without lipid. A sharp increase in slope is observed at temperatures above 59°C, the temperature that corresponds to the glass transition (Tg) of the sugar at 3% water and at a 2°C/min scan rate. Heating scans of trehalose in the presence of the DLPC/DSPCd-70 mixture show a sharp increase in slope at temperatures above 33°C, which can be assigned to Tg of the sugar/lipid mixture (Fig. 4). Numerical values of the slopes are important to consider as well, as they are a measure of the average strength of hydrogen bonding of the network (Wolkers et al., 1998). The slope of the OH stretch at temperatures above Tg is much less steep in the mixture relative to trehalose alone (0.555 for trehalose alone versus 0.422 for the mixture). This suggests that the strength of the hydrogen bonding network of the sugar is increased by the lipid mixture, most likely by interactions between sugar hydroxyl and lipid headgroups. Above 33°C, the slope of the heating scan of the mixture remains constant until the temperature reaches 77°C, above which a slight decrease in slope is observed, coincident with the melting of the lipids (as seen in Fig. 2 B). At this temperature, lipid headgroup spacing increases, allowing for more interactions with sugar OH groups, as discussed above. The increase in sugar-lipid interactions above 77°C is reflected in a decrease in the slope of the OH thermal profile. These interactions result in a large decrease in the Tm of the lipids after heating, by ∼87°C, as seen in the subsequent cooling scan (see Fig. 3 B).
FIGURE 4.
Changes in the wavenumber of the OH stretch as a function of temperature during heating of air-dried DLPC/DSPCd-70/trehalose (5:1 sugar:lipid). Wavenumber versus temperature plot of air-dried trehalose during heating (inset).
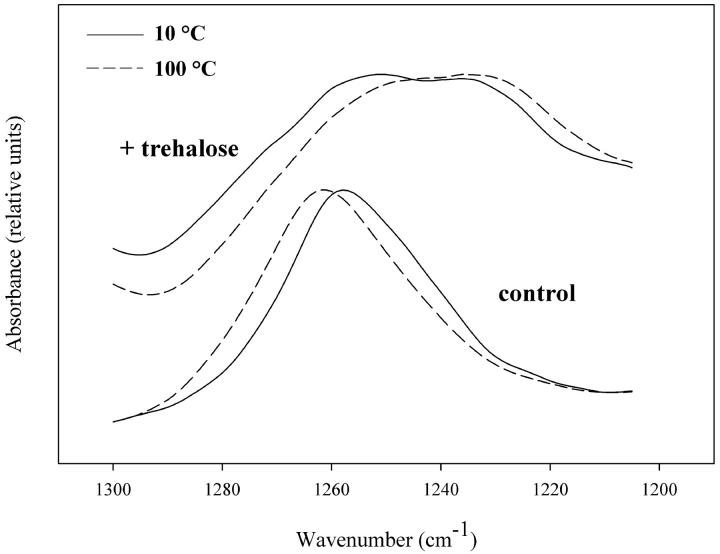
Thermal behavior of the sugar-lipid mixture was further investigated by monitoring the lipid phosphate asymmetric stretching band at 1260 cm−1, which is diagnostic for the microenvironment of the lipid headgroups. Fig. 5 shows FTIR spectra of the phosphate asymmetric stretching vibration for air-dried DLPC/DSPCd-70 with and without trehalose. The band position of the dry lipid mixture shifts to a higher frequency with increasing temperature, indicating an increase in vibrational freedom. In the presence of trehalose there is a change in band shape and frequency, with the appearance of a shoulder at 1230 cm−1, which is at a lower frequency relative to the control (1260 cm−1). Such changes are consistent with interactions between the lipid mixture and sugar after drying, which would restrict lipid phosphate group motion. Examination of the phosphate band of the individual lipids air-dried in the presence of trehalose reveals that the low-frequency shoulder in the spectrum of the mixture arises from the DLPC component (data not shown). As seen in the CH2 and CD2 spectra of the mixture (Fig. 2 B), trehalose interacts much more strongly with the DLPC component during drying, which is reflected in the phosphate spectra as the appearance of a lower frequency band (Fig. 5). In addition, trehalose causes a progressive shift to lower frequency of the phosphate band of the mixture with increasing temperature, suggesting stronger lipid-sugar interactions occur after heating, in agreement with the OH stretch data.
FIGURE 5.
FTIR spectra of the phosphate asymmetric stretch region of DLPC:DSPCd-70 1:1 mixtures air-dried in the absence and presence of trehalose (5:1 sugar:lipid), at 10°C (—), and 100°C (− −).
SUMMARY AND CONCLUSIONS
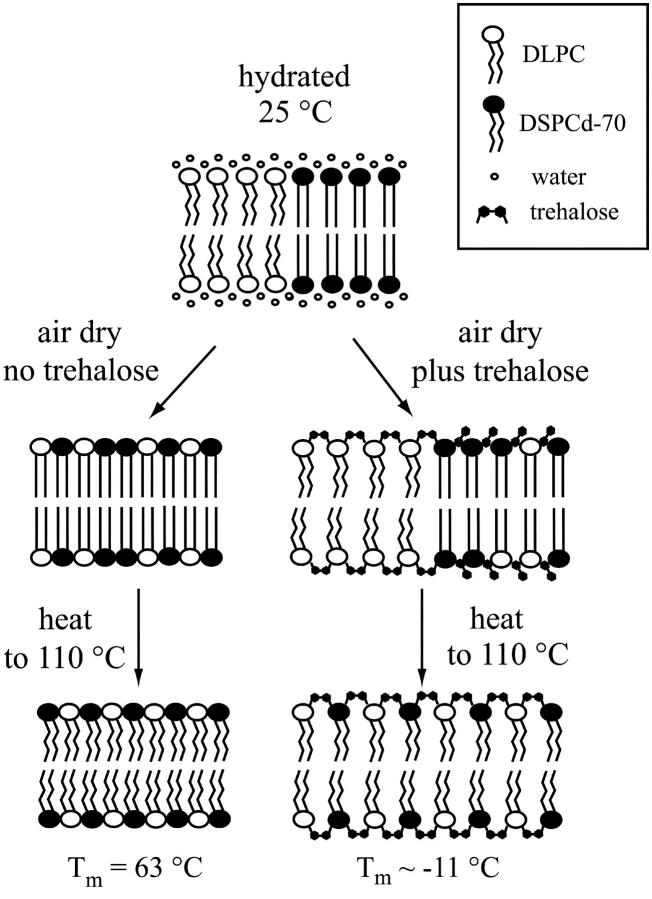
Fig. 6 is a schematic representation of the findings presented in this article. The two lipid components, which are phase separated at 25°C in the hydrated state, form mixed phases when dried without trehalose, which melt at 44°C and 71°C. The mixing may be explained by taking into consideration the dry melting temperatures of the individual components (see Table 1). We propose that the mixed phases in the dry lipid mixture arise due to the proximity of the components' phase transition temperatures, which are 81°C for air-dried DLPC, and 94°C for air-dried DSPCd-70. This hypothesis is currently being investigated in our laboratory.
FIGURE 6.
Schematic representation of the effect of trehalose on air-dried DLPC:DSPCd-70 1:1 mixtures.
When dried in the presence of trehalose, the DLPC component of the mixture is strongly fluidized by the sugar, while the DSPCd-70 component is hardly affected. This results in a phase separated state of the dried mixture. When the dry mixture is heated above the chain melting temperature for the DSPCd-70 component, Tm for DSPCd-70 is decreased to subzero temperatures, and mixing with the DLPC occurs. These drastic decreases in Tm are not observed after heating the mixture that was dried without trehalose.
We conclude that trehalose maintains lipid phase-separation for DLPC/DSPCd-70 mixtures during drying, and in this way preserves a hydrated-like state in the bilayer. The mechanism of this effect is likely to involve strong interaction with one component of the mixture and not the other. Thus, we suggest that trehalose could preserve the structure and composition of phase separated lipid microdomains, which may be of fundamental importance in biological membranes. The protective role of trehalose in biological membranes, therefore, may be a dual one. On one hand, it could maintain the phase separation of ordered microdomains from more fluid lipids during and after drying. On the other, trehalose could protect the structural integrity of the membrane as a whole by preventing leakage, and by inhibiting fusion with surrounding membranes.
Acknowledgments
The authors are very grateful to Dr. Lois Crowe and Dr. Ann Oliver for helpful discussions.
This work was supported by grants HL57810 and HL61204 from the National Institutes of Health, and N66001-02-R-8053 from the Defense Advanced Research Projects Agency, the National Science Foundation Center on Polymer Interfaces and Macromolecular Assemblies, and the Whitaker Foundation.
References
- Chapman, D., R. M. Williams, and B. D. Ladbrooke. 1967. Physical studies of phospholipids. VI. Thermotropic and lyotropic mesomorphism of some 1,2-diacyl-phosphatidylcholines (lecithins). Chem. Phys. Lipids. 1:445–475. [Google Scholar]
- Clegg, J. S. 1965. The origin of trehalose and its significance during the formation of encysted dormant embryos of Artemia salina. Comp. Biochem. Physiol. 14:135–143. [DOI] [PubMed] [Google Scholar]
- Collins, K. D., and M. W. Washabaugh. 1985. The Hofmeister effect and the behavior of water at interfaces. Q. Rev. Biophys. 18:323–422. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., and L. M. Crowe. 1982. Induction of anhydrobiosis: membrane changes during drying. Cryobiology. 19:317–328. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., and L. M. Crowe. 1992. Preservation of liposomes by freeze drying. In Liposome Technology, 2nd Ed. G. Gregoriadis, editor. CRC Press, Boca Raton, FL. pp. 229–252.
- Crowe, J. H., L. M. Crowe, J. F. Carpenter, and C. Aurell Wistrom. 1987a. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 242:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, J. H., L. M. Crowe, and D. Chapman. 1984. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 223:701–703. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., L. M. Crowe, and S. A. Jackson. 1983. Preservation of structural and functional activity in lyophilized sarcoplasmic reticulum. Arch. Biochem. Biophys. 220:477–484. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., F. A. Hoekstra, and L. M. Crowe. 1992. Anhydrobiosis. Annu. Rev. Physiol. 54:579–599. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., F. A. Hoekstra, K. H. N. Nguyen, and L. M. Crowe. 1996a. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1280:187–196. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., S. B. Leslie, and L. M. Crowe. 1994. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology. 31:355–366. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., A. E. Oliver, F. A. Hoekstra, and L. M. Crowe. 1997. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology. 35:20–30. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., B. J. Spargo, and L. M. Crowe. 1987b. Preservation of dry liposomes does not require retention of residual water. Proc. Natl. Acad. Sci. USA. 84:1537–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, J. H., J. F. Carpenter, and L. M. Crowe. 1998. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60:73–103. [DOI] [PubMed] [Google Scholar]
- Crowe, L. M., and J. H. Crowe. 1988. Trehalose and dry dipalmitoylphosphatidylcholine revisited. Biochim. Biophys. Acta. 946:193–201. [DOI] [PubMed] [Google Scholar]
- Crowe, L. M., J. H. Crowe, and D. Chapman. 1985. Interaction of carbohydrates with dry dipalmitoylphosphatidylcholine. Arch. Biochem. Biophys. 236:289–296. [DOI] [PubMed] [Google Scholar]
- Crowe, L. M., D. S. Reid, and J. H. Crowe. 1996b. Is trehalose special for preserving dry biomaterials? Biophys. J. 71:2087–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, L. M., C. Womersley, J. H. Crowe, D. Reid, L. Appel, and A. Rudolph. 1986. Prevention of fusion and leakage in freeze-dried liposomes by carbohydrates. Biochim. Biophys. Acta. 861:131–140. [Google Scholar]
- Drennan, P. M., M. T. Smith, D. Goldsworthy, and J. Van Staden. 1993. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. Plant Physiol. 142:493–496. [Google Scholar]
- Franks, F. 1985. Biophysics and Biochemistry at Low Temperatures. Cambridge University Press, Cambridge.
- Gadd, G. M., K. Chalmers, and R. H. Reed. 1987. The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microb. Letts. 48:249–254. [Google Scholar]
- Green, J. L., and C. A. Angell. 1989. Phase relations and vitrification in saccharide-water solution and the trehalose anomaly. J. Phys. Chem. 93:2880–2882. [Google Scholar]
- Hoekstra, F. A., L. M. Crowe, and J. H. Crowe. 1989. Differential desiccation sensitivity of corn and Pennisetum pollen linked to their sucrose contents. Plant. Cell Env. 12:83–91. [Google Scholar]
- Ipsen, J. H., and O. G. Mouritsen. 1988. Modelling the phase equilibria in two-component membranes of phospholipids with different acyl-chain lengths. Biochim. Biophys. Acta. 944:121–134. [DOI] [PubMed] [Google Scholar]
- Koster, K. L., and A. C. Leopold. 1988. Sugars and desiccation tolerance in seeds. Plant Physiol. 88:829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, K. L., Y. P. Lei, M. Anderson, S. Martin, and G. Bryant. 2000. Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys. J. 78:1932–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. W. B., J. S. Waugh, and R. G. Griffin. 1986. Solid-state NMR study of trehalose/1,2-dipalmitoyl-sn-phosphatidylcholine interactions. Biochemistry. 25:3737–3742. [DOI] [PubMed] [Google Scholar]
- Lehtonen, J. Y., J. M. Holopainen, and P. K. Kinnunen. 1996. Evidence for the formation of microdomains in liquid crystalline large unilamellar vesicles caused by hydrophobic mismatch of the constituent phospholipids. Biophys. J. 70:1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy, C., W. F. Wolkers, K. Jorgensen, O. G. Mouritsen, and J. H. Crowe. 2001. Lateral organization and domain formation in a two-component lipid membrane system. Biophys. J. 80:1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis, S. H., K. A. C. Madin, and J. H. Crowe. 1980. Anhydrobiosis in nematodes: biosynthesis of trehalose. J. Exp. Zool. 211:311–320. [Google Scholar]
- Mabrey, S., and J. M. Sturtevant. 1976. Investigation of phase transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA. 75:3862–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madin, K. A. C., and J. H. Crowe. 1975. Anhydrobiosis in nematodes: carbohydrate and lipid metabolism during dehydration. J. Exp. Zool. 193:335–342. [Google Scholar]
- Mendelsohn, R., and D. J. Moore. 1998. Vibrational spectroscopic studies of lipid domains in biomembranes and model systems. Chem. Phys. Lipids. 96:141–157. [DOI] [PubMed] [Google Scholar]
- Mouritsen, O. G., and K. Jorgensen. 1995. Micro-, nano-, and meso-scale heterogeneity of lipid bilayers and its influence on macroscopic membrane properties. Mol. Membr. Biol. 12:15–20. [DOI] [PubMed] [Google Scholar]
- Mouritsen, O. G., and K. Jorgensen. 1997. Small-scale lipid-membrane structure: simulation versus experiment. Curr. Opin. Struct. Biol. 7:518–527. [DOI] [PubMed] [Google Scholar]
- Roos, Y. H. 1995. Phase Transitions in Foods. Academic Press, San Diego.
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Slade, L., and H. Levine. 1995. Glass transitions and water-food structure interactions. In Advances in Food and Nutrition Research. J. Kinsella, S. Taylor, editors. Academic Press, San Diego. pp. 103–269. [DOI] [PubMed]
- Sperling, L. H. 1986. Introduction to Physical Polymer Science. John Wiley & Sons, New York.
- Sun, W. Q., A. C. Leopold, L. M. Crowe, and J. H. Crowe. 1996. Stability of dry liposomes in sugar glasses. Biophys. J. 70:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova, N., B. Tenchov, L. Tsonev, and T. Tsvetkov. 1988. Dependence of trehalose protective action on the initial phase state of dipalmitoylphosphatidylcholine bilayers. Cryobiology. 25:256–263. [DOI] [PubMed] [Google Scholar]
- Tsvetkova, N. M., B. L. Phillips, L. M. Crowe, J. H. Crowe, and S. H. Risbud. 1998. Effect of sugars on headgroup mobility in freeze-dried dipalmitoylphosphatidylcholine bilayers: solid-state 31P NMR and FTIR studies. Biophys. J. 75:2947–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uster, P. S., and D. W. Deamer. 1981. Fusion competence of phosphatidylserine-containing liposomes quantitatively measured by a fluorescence resonance energy transfer assay. Arch. Biochem. Biophys. 209:385–395. [DOI] [PubMed] [Google Scholar]
- Wolkers, W. F., L. M. Crowe, N. M. Tsvetkova, F. Tablin, and J. H. Crowe. 2002. In situ assessment of erythrocyte membrane properties during cold storage. Mol. Membr. Biol. 19:59–65. [DOI] [PubMed] [Google Scholar]
- Wolkers, W. F., H. Oldenhof, M. Alberda, and F. A. Hoekstra. 1998. A Fourier transform infrared microspectroscopy study of sugar glasses: application to anhydrobiotic higher plant cells. Biochim. Biophys. Acta. 1379:83–96. [DOI] [PubMed] [Google Scholar]
- Wolkers, W. F., N. J. Walker, F. Tablin, and J. H. Crowe. 2001. Human platelets loaded with trehalose survive freeze-drying. Cryobiology. 42:79–87. [DOI] [PubMed] [Google Scholar]
- Womersley, C., P. S. Uster, A. S. Rudolph, and J. H. Crowe. 1986. Inhibition of dehydration-induced fusion between liposomal membranes by carbohydrates as measured by fluorescence energy transfer. Cryobiology. 23:245–255. [DOI] [PubMed] [Google Scholar]