Abstract
The neurotransmitter norepinephrine (NE) stimulates the growth of low inocula of Escherichia coli in a minimal medium (SAPI) supplemented with serum (SAPI+serum) and induces the production of an “autoinducer” (AI) which, in turn, promotes E. coli growth in the absence of NE. Given the importance of NE, epinephrine, and their corresponding adrenergic agonists and antagonists in clinical medicine, we sought to investigate the molecular basis for these observations. Using a variety of NE precursors, metabolites, and therapeutic agents, we demonstrated that their ability to stimulate E. coli growth in SAPI+serum is dependent on the presence of a catechol (1,2-dihydroxybenzene) moiety with maximal activity requiring a two-carbon substituent incorporating a terminal primary amine. Serum contains the iron-binding glycoprotein, transferrin, and when SAPI+serum was supplemented with sufficient Fe3+ to saturate transferrin, growth inhibition was relieved. Other metal cations, including Mg2+, Ca2+, and Zn2+, had no effect. These data suggested that the stimulation of E. coli growth by NE in SAPI+serum may involve the catecholate siderophore, enterobactin, a cyclic triester of 2,3-dihydroxybenzoylserine. Consistent with this hypothesis, E. coli strains with mutations in ferrienterobactin transport (fepA or tonB) or enterobactin biosynthesis (entA) did not respond to NE. Furthermore, NE induced expression of the ferrienterobactin receptor, FepA, during growth in SAPI+serum. The enterobactin degradation product, 2,3-dihydroxybenzoylserine (DBS) was as effective as NE in stimulating the growth of E. coli and mutations in fepA or tonB abolished the DBS-dependent growth stimulation. In contrast to NE, however, DBS stimulated the growth of the entA mutant. Moreover, after growth in an iron-limited M9 medium in the absence of NE, ethyl acetate extracts of the E. coli entA+ parent but not of the entA mutant contained AI, i.e., stimulated the growth of E. coli in SAPI+serum. Taken together, these data show that when low numbers of E. coli are inoculated into SAPI+serum, NE, DBS, and related catecholamines induce the enterobactin iron uptake system. This, in turn, facilitates iron sequestration from transferrin and indicates that the AI present in NE-conditioned SAPI+serum medium is enterobactin and its DBS breakdown products.
Bacterial cells undergo a wide variety of physiological and morphological adaptations in response to chemical and physical changes in their environment. From the prokaryotic perspective, the successful interaction of bacterial cells with mammalian host tissues depends not only on a coordinated response to environmental cues such as nutrient availability, population density, temperature, osmolarity and pH (37, 56) but also on diverse host cell effector molecules. In general, the influence of host signaling molecules on bacteria has received relatively little attention (25). However, hormones such as epinephrine (12) and insulin (57), the neurotransmitter norepinephrine (NE [34]), and cytokines such as interleukin-2 and granulocyte-macrophage colony stimulating factor (9) have all been reported to promote the growth of Escherichia coli and other bacteria. Indeed, the concept of “microbial endocrinology” has been proposed in which pathogens are considered to exploit host effector molecules as environmental signals promoting growth and virulence factor deployment (29, 30). This concept is largely based on observations that a minimal medium supplemented 30% (vol/vol) serum (SAPI+serum) (32) to mimic mammalian extracellular fluids does not promote the growth of low inocula (to mimic the infecting dose) of bacteria such as E. coli unless the medium is supplemented with physiological concentrations of NE (32, 33).
After severe tissue injury, NE is released into the systemic circulation as a consequence of the destruction of adrenergic neurons innervating damaged tissues. The levels of NE released have been reported to be significantly higher in postoperative patients who develop severe sepsis than those who recover without succumbing to infection. Elevated catecholamine levels have also been noted to precede the development of acute infections (5, 16). Using the selective noradrenergic, neurotoxic agent 6-hydroxydopamine in a murine model of trauma-induced NE release, Lyte and Bailey (31) reported a direct correlation between elevated NE levels and the overgrowth and translocation of indigenous cecal bacteria.
NE has also been shown to stimulate the growth of low inocula of diverse gram-negative and gram-positive bacteria in serum-based media (14), indicating that the ability to respond to NE is widely conserved. Furthermore, conditioned medium from E. coli grown in the presence of NE appears to contain a low molecular mass, heat-stable molecule of bacterial origin that Lyte et al. (34) termed an autoinducer (AI) of growth. The addition of such conditioned medium stimulates the growth of a low inoculum of E. coli in SAPI+serum in the absence of NE. Freestone et al. (14) reported that many gram-negative bacteria both produced and responded to AI, whereas a few gram-positive clinical isolates were able to respond, but not produce, AI (14). While the structure of the AI has not been elucidated, NE-treated bacteria have been reported to produce AI during early log phase (34), and on this basis it was suggested to be unrelated to established quorum-sensing AI molecules such as the N-acylhomoserine lactones (AHLs [52, 56]).
The molecular mechanism by which NE and AI stimulate growth in serum-containing media has not been established. NE is capable of facilitating the release of iron, an essential microbial nutrient from the serum iron-binding glycoprotein, transferrin (15). NE may therefore stimulate growth in serum-containing media by facilitating the sequestration and transfer of iron from transferrin to the microbial cell. However, it has been reported that the addition of iron to the growth stimulation assay did not affect bacterial growth (15).
In the present study, we show that among agents that are NE metabolites or biosynthetic precursors and among noradrenergic agonists and antagonists only those containing a catechol moiety can promote the growth of low inocula of E. coli in SAPI+serum. Furthermore, we show here that (i) Fe3+ but not Mg2+, Ca2+, or Zn2+ promotes growth in SAPI+serum; (ii) E. coli strains with mutations in enterobactin synthesis or ferrienterobactin transport do not respond to NE or produce AI; and (iii) NE induces the ferrienterobactin receptor, FepA, in SAPI+serum. We also provide evidence to suggest that AI is an enterobactin- and/or a 2,3-dihydroxybenzoylserine-related breakdown product.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The laboratory and clinical strains used in the present study are listed in Table 1. Bacteria were routinely grown at 37°C on Luria-Bertani agar plates or with shaking in Luria-Bertani broth or in SAPI minimal medium (glucose [2.7 mM], NH4NO3 [6.2 mM], K2HPO4 [1.8 mM], KCl [3.3 mM], MgSO4 · 7H2O [1.01 mM], HEPES [10 mM]; pH 7.5) (32) or in M9 medium (48) supplemented with 0.5 μM Fe3+ to optimize enterobactin production. Where required, SAPI and M9 were modified by supplementation with 0.3% (wt/vol) Casamino Acids and 0.001% (wt/vol) thiamine. In addition, the E. coli entA parent strain 13-6 and the entA mutant AN193 also required 0.003% (wt/vol) guanosine, 0.0015% (wt/vol) adenine hydrochloride, and 0.0015% (wt/vol) hypoxanthine. For the NE growth response assays, SAPI was supplemented with 30% (vol/vol) adult bovine serum (SAPI+serum). For some experiments, serum was replaced with bovine transferrin at 2 or 4 mg/ml (Sigma) as the apo-, 30%, or 100% iron-loaded protein prepared as described below.
TABLE 1.
E. coli laboratory strains used in this study
| Strain | Genotype, serotype, and/or phenotypea | Source or referenceb |
|---|---|---|
| JPN10 | O44:H18; EAEC | S. Knutton (26) |
| PES19 | O126:H27; EAEC | CPHL |
| F444A | DAEC | S. Knutton |
| E47683 | DAEC | CPHL |
| H281A | DAEC | S. Knutton |
| H774B | DAEC | S. Knutton |
| H1288A | DAEC | S. Knutton |
| 14945 | EPEC | S. Knutton |
| 14377 | EPEC | S. Knutton |
| 308 | O125:H6; EPEC | S. Knutton |
| 15544 | EPEC | S. Knutton |
| E21 | EPEC | S. Knutton |
| 226/85 | O142:H34; EPEC | S. Knutton |
| E57 | O119:H6; EPEC | S. Knutton |
| E130 | EPEC | S. Knutton |
| E2348/69 | O127:H6; EPEC | CPHL |
| W3110 | F− λ− | 1 |
| AT2472 | λ−relA1 aroE24 spoT1 thi-1 | CGSC 4511 |
| P1798 | fepA9 λ−relA1 aroE24 spoT1 thi-1 | CGSC 5825 |
| C600 | thr-1 leuB6 fhuA21 lacY1 glnV44(AS) λ− e14−glpR200 thi-1 | CGSC 5394 |
| GUC6 | thr-1 leuB6 fhuA21 lacY1 glnV44(AS) λ− e14−tonB50 rfbD1 glpR200 thi-1 | CGSC 5415 |
| 13-6 | leuB6 secA206(Azir) fhuA23 lacY1 proC14 tsx-67 purE42 glnV44(AS) λ−trpE38 rpsL109(Strr) xylA5. mtl-1 thi-1 | CGSC 1515 |
| AN193 | leuB6 secA206(Azir) fhuA23 lacY1 proC14 tsx-67 entA403 purE42 glnV44(AS) λ−trpE38 rpsL109(Strr) xylA5 mtl-1 thi-1 | CGSC 5131 |
EAEC, enteroaggregative E. coli; DAEC, diffusely adhering E. coli; EPEC, enteropathogenic E. coli.
S. Knutton, Institute of Child Health, University of Birmingham, Birmingham, United Kingdom; CPHL, Central Public Health Laboratories, London, United Kingdom; CGSC. E. coli Genetic Stock Center, Department of Biology, Yale University, New Haven, Conn.
NE and AI growth response assays.
This method was carried out essentially as described by Lyte and Ernst (32). Bacterial inocula were grown overnight at 37°C in SAPI containing any required supplements. After being harvested by centrifugation, the cells were washed once in phosphate-buffered saline and once in SAPI and then resuspended in SAPI to an optical density at 600 nm (OD600) of 1.0 (ca. 109 CFU/ml). This bacterial suspension was then diluted 106-fold in SAPI, and 50 μl was used as an inoculum, providing ca. 50 to 100 CFU/ml. Growth response assays were carried out in 1-ml culture volumes in flat-bottomed 24-well polystyrene plates (Nalgene) in SAPI+serum in the presence or absence of a range of concentrations of NE (0 to 10−3 M). For some experiments, conditioned medium as a source of AI was prepared as described previously (34) by growing E. coli in SAPI+serum supplemented with 50 μM NE or in M9 with 0.5 μM Fe3+ to optimize enterobactin production. After overnight growth, bacteria were removed by centrifugation, and the cell free supernatant was sterilized by filtration through a 0.22 μm (pore-size) membrane filter. As a negative control for the conditioned medium, SAPI+serum supplemented with 50 μM NE was incubated overnight at 37°C without bacteria. A range of dilutions from 0 to 20% (vol/vol) conditioned medium was added to SAPI+serum to assay for the presence of AI. For some experiments, conditioned medium was acidified and extracted with ethyl acetate as described by O'Brien et al. (41). The ethyl acetate layer was removed and evaporated, and the residue was reconstituted to the original volume in modified M9 (mM9) medium. For each growth response experiment, viable counts were determined at the start of the experiment by plating onto MacConkey agar plates to confirm the number of viable bacteria present. Each multiwell plate was then incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. Three 10-μl samples from each well were streaked onto each MacConkey plate such that the limit of detection for each assay was 103 CFU/ml. All assays were conducted in triplicate at least twice.
Structure-activity analysis.
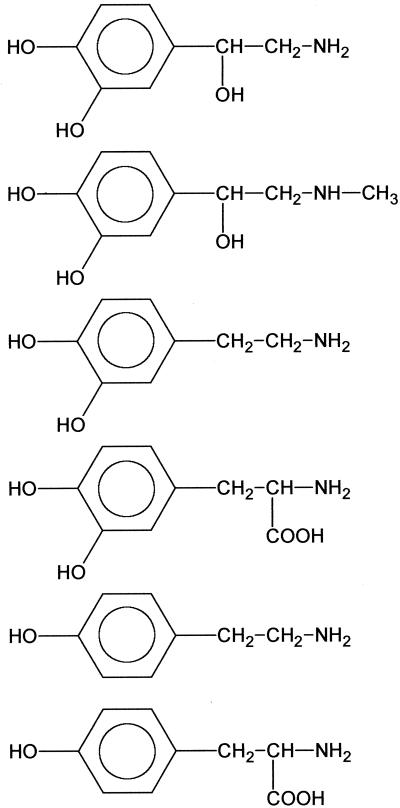
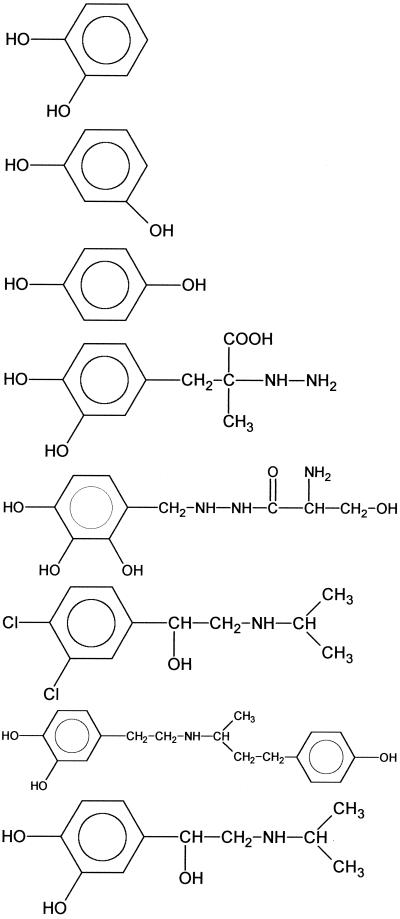
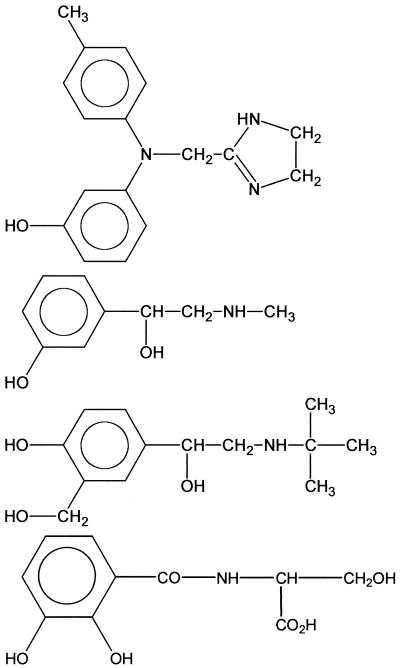
To determine the minimal structural requirements for the growth response assay, NE and a series of related compounds, including several therapeutic agents (see Table 2, were purchased from Sigma. All compounds were dissolved in deionized water apart from tyramine and carbidopa, which required 10 mM HCl followed by neutralization with NaOH (10 mM) and 2,3-dihydroxybenzoic acid (DBA) dissolved in acetonitrile. For these compounds, additional controls of diluent alone were included.
TABLE 2.
Growth response of E. coli EAEC strain JPN10 to NE and related compoundsa
These compounds can be divided into those that are in the mammalian catecholamine biosynthetic pathway (compounds 1 to 6), norepinephrine metabolites (compounds 7 to 11), compounds defining the minimal essential structure for E. coli growth-promoting activity (compounds 12 to 16), therapeutic agents (compounds 17 to 24), and the enterobactin breakdown product DBS (25). The compounds are further divided into subgroups I to IV depending on the concentration required to promote the growth of JPN10 in SAPI+serum to ∼10−8 CFU/ml: group I (10−6 M), group II (10−5 M), group III (10−4 M), and group IV (inactive). NA, no activity.
Influence of metal ions on growth in SAPI+serum.
To determine whether growth inhibition in SAPI+serum could be relieved by metal ions, the growth response assay described above was carried out in SAPI+serum supplemented with iron. Since adult sera contain 1 to 2 mg of transferrin/ml and 1 mg of transferrin binds 1.4 μg of Fe3+, the concentration of iron required to fully saturate the transferrin present in adult bovine serum was estimated to be 7.5 μM. Thus, a range of concentrations (0 to 11.2 μM) of Fe3+, Mg2+, Ca2+, or Zn2+ were assayed in the growth response assay.
Iron-loading of transferrin.
Bovine apotransferrin was iron loaded by using methods adapted from those of Schryvers and Morris (49) and Mazurier and Spik (36). Essentially, two aliquots each of 20 mg of bovine apotransferrin were incubated overnight in 5 ml of buffer (pH 8.6) consisting of 50 mM NaHCO3 and 50 mM sodium citrate, together with sufficient ferric chloride to load to 30 and 100%. Any remaining free iron was removed by dialysis against two changes of citrate-bicarbonate buffer. The percent iron saturation was confirmed by determining the absorbance of the solution at 465 nm (A465) as described by Mazurier and Spik (36).
Synthesis of DBS.
N-(2,3-dihydroxybenzoyl)-l-serine (DBS) was prepared from N-(2,3-dibenzyloxybenzoyl)-l-serine synthesized as described by Tse and Kishi (51). A solution of N-(2,3-dibenzyloxybenzoyl)-l-serine (200 mg, 0.48 mmol) in ethanol (20 ml) was hydrogenated in the presence of 10% Pd/C catalyst (20 mg). The solution was filtered through a celite pad, and the filtrate was concentrated in vacuo. The residue was redissolved in ethyl acetate, and the product was extracted with saturated sodium hydrogen carbonate solution (three times with 20 ml). The combined extracts were carefully acidified with 2 M hydrochloric acid, and the product was extracted with ethyl acetate (five times with 20 ml). The combined extracts were dried over magnesium sulfate, and the solvent was removed to yield DBS (85 mg, 75% yield), the structure of which was confirmed by 1H nuclear magnetic resonance.
Synthesis of AHLs and cyclic dipeptides.
N-(3-oxododecanoyl)homoserine lactone and N-butanoyl homoserine lactone were synthesized as described by Chhabra et al. (3). The following cyclic dipeptides (diketopiperazines), cyclo(l-Phe-l-Phe), cyclo(L-Pro-l-Tyr) cyclo(ΔAla-l-Val), cyclo(l-Ala-l-Val), cyclo(l-Ser-l-Val), and cyclo(l-Leu-l-Val) were prepared as described by Holden et al. (22).
Preparation of outer membranes.
Outer membranes were prepared from E. coli as described previously (53) with 2% (wt/vol) sodium N-lauroyl sarcosinate (Sarkosyl) to remove cytoplasmic membrane material from cell envelopes prepared by sonication of whole bacterial cells.
SDS-polyacrylamide gel electrophoresis and Western blotting.
Outer membrane proteins were separated on 11.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred by electroblotting onto nitrocellulose membranes, and probed with a polyclonal rabbit antibody raised against the purified 81-kDa FepA protein of E. coli (53). Blots were developed with protein A-alkaline phosphatase (Calbiochem) and visualized with the BCIP/NBT liquid substrate system (Sigma).
Detection of catechols in bacterial culture supernatants.
The presence of enterobactin and related catechols in spent bacterial culture supernatants was determined by using the chemical assay described by Rioux et al. (45) and by thin-layer chromatography (TLC). Catechols were extracted from acidified cell-free culture supernatants with ethyl acetate as described by O'Brien et al. (41). Solvent extracts were chromatographed on cellulose TLC plates (PolygramCel300; Macherey-Nagel GMBH) by using a mobile phase of 5% (wt/vol) ammonium formate containing 0.5% (vol/vol) formic acid. Catechols were detected by their fluorescence in UV light at 254 nm and chemically by spraying them with 1% (wt/vol) potassium ferricyanide [K3Fe(CN)6] and 2% (wt/vol) ferric ammonium citrate, followed by washing in 0.2 M HCl.
RESULTS
Growth response of enterovirulent E. coli to NE.
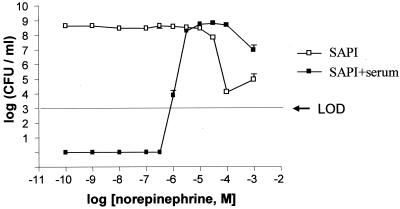
To establish the NE growth response assay, SAPI+serum medium was inoculated with the enteroaggregative (EAEC) E. coli JPN10. Although no growth was apparent in SAPI+serum inoculated with <103 CFU/ml, larger inocula attained a cell population density of 108 CFU/ml after 24 h of incubation (data not shown). Thus, the growth-inhibitory effect of serum is dependent on inoculum size and, for subsequent experiments, SAPI+serum was inoculated with ca. 102 CFU/ml and a range of NE concentrations from 10−3 to 10−10 M. Figure 1 shows that the growth of JPN10 in SAPI+serum is stimulated by NE and that a starting inoculum containing 140 ± 14 CFU/ml reaches 108 CFU/ml when exposed to 3 × 10−6 M NE. In SAPI in the absence of serum, NE concentrations of 10−4 M and above appear to be toxic. To demonstrate that the ability to respond to NE is conserved across all of the E. coli groups, the assay was repeated with a variety of enterovirulent E. coli, including enteroaggregative (EAEC; two strains), enteropathogenic (EPEC; nine strains), and diffuse adherent (DAEC; five strains) strains as well as the laboratory strain W3110. While each strain failed to grow in SAPI+serum when inoculated at 102 CFU/ml, all strains responded to 50 μM NE by reaching a final population density of 108 CFU/ml (data not shown).
FIG. 1.
Response of E. coli JPN10 to NE in SAPI (□) and in SAPI+serum (▪). The initial inoculum of JPN10 used for the assay assay was 144 ± 14 CFU/ml. Bacteria were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. Each point represents the mean ± the standard deviation (SD) of triplicate samples. The dose-response curves are representative of three experiments performed on separate occasions. LOD, limit of detection.
Response of E. coli JPN10 to NE and related catecholamines, NE agonists, and antagonists.
To define the structural basis for the response of E. coli to NE, growth promotion assays were carried out with 24 structurally related compounds, including NE biosynthetic intermediates and breakdown/oxidation products as well as NE agonists and antagonists. Table 2 summarizes the compounds evaluated for their ability to promote growth in SAPI+serum. Based on the lowest concentration that promotes growth to 108 CFU/ml the compounds tested could be separated into four groups. Groups I, II, and III were able to stimulate growth of the test E. coli JPN10 at concentrations of 3 × 10−6 M, 1 × 10−5 M, and 1 × 10−4 M, respectively. Group IV compounds were unable to stimulate growth. From these data, it is clear that the presence of a catechol (1,2-dihydroxybenzene) moiety is essential for activity in this assay, other dihydroxybenzenes (e.g., 1,3- and 1,4-dihydroxybenzenes) such as resorcinol and hydroquinone (Table 2, compounds 15 and 16) are devoid of activity. In addition, both of the phenol groups in catechol must be unsubstituted since alkylation renders the compounds inactive (in Table 2 compare compounds 9, 10, and 11 with compounds 8, 1, and 7, respectively). Furthermore, the activity of catechol is potentiated by the introduction of a two-carbon unit possessing at least two free hydrophilic substituents (from OH, NH2, and CO2H). However, maximum activity resides with the terminal primary amine functionality, as in NE. Amine alkylation reduces activity, and the extent of this reduction is proportional to the steric bulk of the substituent (Table 2 compare compounds 2 and 21).
To determine whether this NE-dependent response involves the bacterial equivalent of α- and β-adrenergic receptors, the ability of phentolamine, an α-adrenergic receptor antagonist, and dichloroisoprenaline, a β-receptor antagonist, to antagonize the NE-stimulated growth of JPN10 in SAPI+serum was evaluated (data not shown). When supplied at concentrations of 10−4 or 10−5 M, neither phentolamine nor dichloroisoprenaline inhibited NE-dependent growth in SAPI+serum. Both compounds were, however, toxic at higher concentrations in that they inhibited the growth of JPN10 in SAPI in the absence of serum (data not shown).
Influence of metal ions on the growth of JPN10 in SAPI+serum.
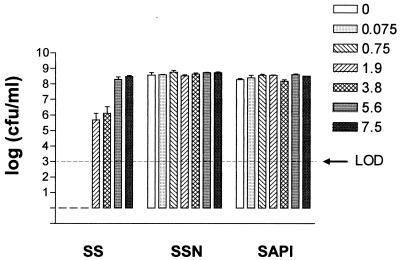
Since serum contains the iron-binding glycoprotein transferrin, which is known to restrict bacterial growth, SAPI+serum was supplemented with a range of iron (as Fe3+) concentrations from 0 to 11.2 μM. Figure 2 shows that the inhibition of JPN10 growth in SAPI+serum is alleviated by 1.9 μM Fe3+ and that the lowest concentration capable of promoting growth to 108 CFU/ml was 5.6 μM. In contrast, neither Mg2+, Ca2+, nor Zn2+ had any effect on JPN10 growth in SAPI+serum (data not shown). Furthermore, the growth of JPN10 could be inhibited by supplementing SAPI with either apo- or 30% iron-loaded bovine transferrin rather than serum. At 100% iron saturation, growth was inhibited to a lesser extent, with cultures reaching between 105 and 106 CFU/ml depending on the transferrin concentration used. However, while NE promoted the growth of JPN10 in SAPI containing 30 or 100% iron-loaded transferrin to 108 CFU/ml, it had no effect in the presence of apotransferrin (data not shown).
FIG. 2.
Influence of iron on growth of E. coli JPN10 in SAPI, SAPI+serum (SS), and SAPI+serum with 50 μM NE (SSN). Bacteria were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. The Fe3+ concentrations in the key are given in micromolar amounts. Each bar represents the mean ± the SD of triplicate readings. LOD, limit of detection.
E. coli strains carrying mutations in enterobactin biosynthesis and transport do not respond to NE.
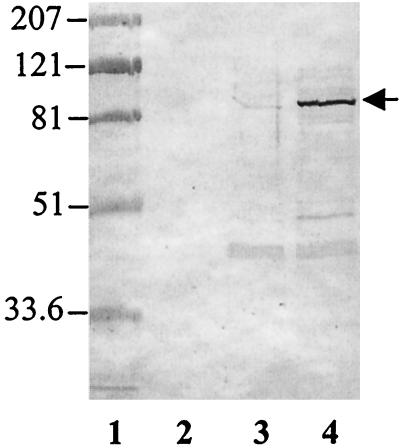
Since E. coli produces the catechol enterobactin, we sought to determine whether the transport or biosynthesis of this siderophore contributes to the NE-dependent stimulation of growth in SAPI+serum. E. coli mutants with defects in enterobactin biosynthesis (entA) or ferrienterobactin transport (fepA and tonB) all failed to respond to NE (Fig. 3). In addition, Fig. 4 shows that the ferrienterobactin receptor, FepA, is induced by NE in JPN10 grown in SAPI+serum providing further support for the role of the enterobactin system in the NE-dependent growth response.
FIG. 3.
Influence of NE on growth of E. coli strains with defects in enterobactin biosynthesis (entA [A]) and transport (fepA [B] and tonB [C]) in modified SAPI (SCTP) or SCTP+serum (SCTP+S), or SCTP+serum plus 50 μM NE (SCTP+SN). (A) JPN10, 13-6 (entA+ parent), and AN193 (entA mutant); (B) JPN10, AT2472 (fepA+ parent), and P1789 (fepA mutant); (C) JPN10, C600 (tonB+ parent), and GUC6 (tonB mutant). Each bar represents the mean ± the SD of triplicate samples. Bacteria were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. The analysis of each mutant and parent strain was performed on at least two separate occasions. LOD, limit of detection.
FIG. 4.
Western blot showing the NE-dependent induction of the ferrienterobactin receptor protein, FepA. Outer membrane proteins were prepared from cultures grown in SAPI+serum (lane 2), SAPI+serum supplemented with 20 μM Fe3+ (lane 3), and SAPI+serum supplemented with 50 μM NE (lane 4). All outer membrane samples were prepared from cultures adjusted to an OD600 of 1.0 prior to the preparation of the outer membranes. After SDS-polyacrylamide gel electrophoresis, proteins were electrophoretically transferred to nitrocellulose and probed with a polyclonal antibody raised against FepA. The positions of the prestained molecular mass markers (Bio-Rad), with sizes given in kilodaltons, are shown in lane 1.
Response of E. coli to DBS.
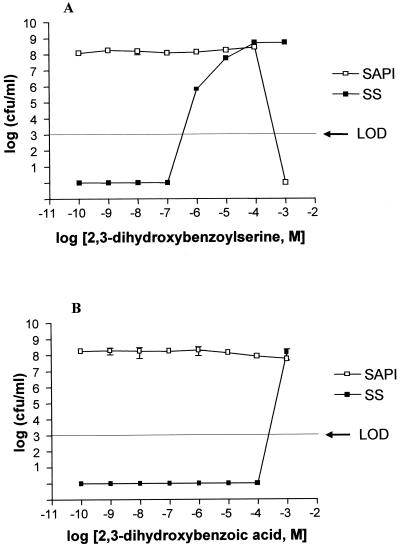
To determine whether an enterobactin biosynthetic precursor (DBA) or a breakdown product (DBS) could mimic the effect of NE in SAPI+serum, we synthesized DBS and examined its activity in the growth promotion assay. Figure 5A shows that ca. 10−5 M DBS is required to stimulate the growth of JPN10 to reach 108 CFU/ml. Interestingly, a much higher concentration of DBA (ca. 10−3 M) is required to promote growth to similar levels (Fig. 5B). However, DBS unlike DBA, is toxic at 10−3 M in the absence of serum.
FIG. 5.
Growth response of E. coli JPN10 in SAPI+serum (SS) to DBS (A) and DBA (B). Bacteria were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. Each point represents the mean ± the SD of triplicate samples. LOD, limit of detection.
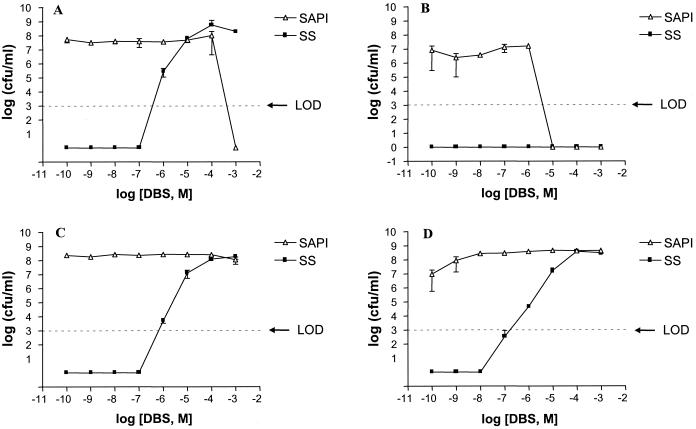
Figure 6 shows that DBS, in common with NE is unable to promote the growth of the E. coli fepA mutant in SAPI+serum. However, in contrast to NE, DBS effectively promoted the growth of the entA mutant which reached 108 CFU/ml when exposed to 10−4 M DBS.
FIG. 6.
Response of (A) fepA+ parent strain, AT2472, (B) fepA mutant strain P1798, (C) entA+ parent strain 13-6, and (D) entA mutant strain AN193 to DBS in modified SAPI+serum. Bacteria were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. Each point represents the mean ± the SD of triplicate samples. LOD, limit of detection.
Quorum-sensing signal molecules, AI, and enterobactin.
Conditioned medium prepared from E. coli grown in the presence of NE has been reported to contain a low-molecular-mass, heat-stable molecule of bacterial origin termed AI that can stimulate bacterial growth in SAPI+serum in the absence of NE (14, 34). To determine whether AI is related to any known bacterial quorum-sensing signal molecules, we examined the activity of N-butanoyl homoserine lactone and N-(3-oxododecanoyl)homoserine lactone in the growth promotion assay. AHL did not possess any growth-promoting activity, nor did any of six different cyclic dipeptides [cyclo(l-Phe-l-Phe), cyclo(l-Pro-l-Tyr) cyclo (ΔAla-l-Val), cyclo(l-Ala-l-Val), cyclo(l-Ser-l-Val), and cyclo(l-Leu-l-Val)], putative quorum-sensing molecules that are capable of activating or inhibiting AHL-dependent signaling (data not shown).
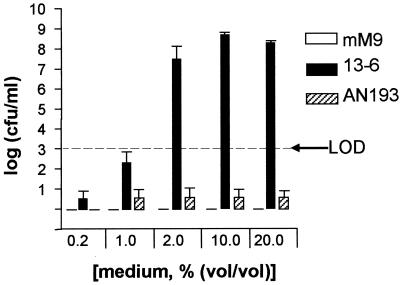
To determine whether AI is related to enterobactin, both the entA+ parent (strain 13-6) and the entA mutant, AN193, were grown to stationary phase in an iron-limited M9 medium modified to support the growth of these E. coli strains. Although 13-6 and AN193 both grew to ∼109 CFU/ml in iron-limited mM9 medium, only cell-free supernatants from strain 13-6 were capable of promoting the growth of JPN10 in SAPI+serum (data not shown). Similar results were obtained with supernatants from JPN10 grown in iron-limited M9 medium. Since enterobactin and its DBS breakdown products can be extracted from culture supernatants with ethyl acetate, spent culture media from strain 13-6 and AN193 were extracted as described in Materials and Methods. Figure 7 shows that as little as 2% (vol/vol) of the strain 13-6 supernatant extract promoted JPN10 growth in SAPI+serum to >107 CFU/ml. Only 0.2% (vol/vol) of the JPN10 extract was required to promote the growth of JPN10 to 108 CFU/ml, a finding that reflects the higher enterobactin levels produced by JPN10 compared to those produced by strain 13-6 (data not shown). Ethyl acetate extracts of the AN193 supernatant failed to promote JPN10 growth in SAPI+serum. The growth-promoting activity present in strain 13-6 and JPN10 but lacking in AN193 culture supernatants contained enterobactin and its DBS breakdown products as determined by the colorimetric method of Rioux et al. (45) and by TLC (data not shown). These results suggest that AI production is dependent on the presence of a functional entA gene and that AI is in fact enterobactin and its DBS breakdown products.
FIG. 7.
Influence of AI from strain 13-6 (entA+ parent) and AN193 (entA mutant) prepared from bacteria grown in iron-limited, mM9 on the growth of JPN10 in SAPI+serum. mM9 was modified as described in the Materials and Methods to support the growth of strain 13-6 and AN193. Uninoculated mM9 medium was used as a control. Spent culture supernatants from strain 13-6, AN193 and uninoculated mM9 medium were acidified and extracted with ethyl acetate, the solvent was removed, and the residue obtained was redissolved in mM9 medium to the original volume. Bacteria were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2, after which each sample was enumerated for viable counts. Each bar represents the mean ± the SD of triplicate samples. LOD, limit of detection.
DISCUSSION
The response of bacteria to diverse self-generated, as well as natural and synthetic, small organic molecules has begun to attract increasing interest, particularly in the context of quorum sensing, growth, and virulence gene expression (15, 24, 25, 38, 52, 55, 56). One such molecule is NE, a catecholamine found in mammals, plants (43), insects (50), fish (19), and protozoa (23). In mammals, NE functions as the major neurotransmitter in the sympathetic branch of the autonomic nervous system (28). The ability of this ubiquitous catecholamine to stimulate the growth of low numbers of bacterial cells in SAPI+serum (14, 32, 33) and to induce the production of AI (34) has raised a number of questions with respect to (i) the mode of action of NE, (ii) the chemical nature of AI, and (iii) the ability of catecholamine drugs used clinically to stimulate bacterial growth and so increase the risk of infection.
In the present study, we have shown that the response of E. coli to NE is conserved in a variety of enterovirulent and laboratory (K-12) strains. In addition to NE, many other naturally occurring catecholamines are capable of stimulating E. coli growth in SAPI+serum. However, irrespective of their structural relationship to NE, only compounds containing a catechol moiety exhibit activity, albeit with different potencies. For example, although the NE metabolites DOMA and DOPEG are active, their 3-methoxy derivatives VMA and MOPEG are inactive, as is normetanephrine (3-O-methylepinephrine), the 3-methoxy derivative of NE. Interestingly, inactivation of catecholamine neurotransmitters in mammals often occurs via methylation of the 3-hydroxyl group of the catechol ring (28). Although the fate and turnover of NE in bacterial cells is not clear, Freestone et al. (15) have shown that 3H-NE is transported into bacterial cells. Although the mechanism by which NE is taken up was not elucidated, it is likely to involve a bacterial surface receptor. Analysis of the prokaryotic genome databases has not revealed any evidence for mammalian type adrenoreceptors (15) and we have shown here that phentolamine and dichloroisoprenaline, which are antagonists, respectively, of α- or β-adrenoreceptors, do not inhibit the ability of NE to stimulate bacterial growth in SAPI+serum. Similar results have been obtained for E. coli ATCC 23723 by Lyte and Ernst (33) with the adrenoreceptor antagonists benextramine and alprenolol.
Since there is no evidence for the existence of bacterial “adrenoreceptors,” we sought to determine whether there was a nutritional basis for the NE-dependent stimulation of growth. Iron is one essential nutrient that is rendered unavailable for bacterial growth in serum by the iron-binding glycoprotein, transferrin (18, 44). Many bacterial pathogens overcome this lack of readily available iron by employing high-affinity iron-scavenging mechanisms that depend on the synthesis and secretion of low-molecular-mass iron chelators (siderophores) (18, 44). E. coli and most other members of the Enterobacteriaceae produce the catecholate siderophore enterobactin, a cyclic trimer of dihydroxybenzoyl serine that has a much greater affinity for iron than does transferrin (18, 44). However, despite producing enterobactin, E. coli strains still exhibit inoculum dependent growth in serum (46). We observed that, in SAPI+serum, the growth of low numbers of E. coli could be stimulated by supplementing serum with sufficient iron to saturate the transferrin present. Other metal ions, including Mg2+, Ca2+, and Zn2+, had no effect on growth. Furthermore, supplementation of SAPI with apotransferrin instead of with serum inhibited bacterial growth. NE was unable to promote the growth of E. coli in this medium unless the transferrin was saturated with iron to the physiological level of 30%. Presumably, the apotransferrin chelates sufficient iron to prevent growth in SAPI but not sufficient to facilitate growth after exposure of the inoculum to NE. This clearly cannot be the case with 30% iron-saturated transferrin, which does promote growth in the presence of NE. Alternatively, it is possible that apotransferrin has a direct effect on bacterial growth unrelated to its iron-binding activity.
These data strongly suggest that the role of NE in serum is to increase the availability of transferrin-bound iron. Freestone et al. (15) have shown that NE forms stable complexes with serum transferrin and is capable of facilitating the release of glycoprotein-bound iron. Thus, the mechanism by which NE promotes bacterial growth in SAPI+serum involves the acquisition of transferrin-bound iron.
Since both the siderophore enterobactin and NE are 2,3-dihydroxy-substituted catechols, we investigated the contribution of the enterobactin iron uptake system to NE-stimulated growth in SAPI+serum. The biosynthesis of enterobactin in E. coli involves the products of six genes (entA to -F) with seven additional genes, (fepA to -G) coding for ferrienterobactin uptake and an additional gene (fes) coding for an esterase capable of hydrolyzing the three enterobactin ester linkages (7, 17, 44). Ferrienterobactin is transported into bacterial cells via the iron-regulated outer membrane protein, FepA in a process that is energized via TonB (27). E. coli strains carrying mutations in either fepA or tonB failed to respond to NE. In addition, an E. coli entA mutant that is unable to synthesize DBA, and thus enterobactin, also fails to respond to NE. Taken together, these data imply a role for both enterobactin biosynthesis and ferrienterobactin transport in the response of E. coli to NE rather than simply a direct role for NE in delivering iron to the bacterial cell. Although the entA mutant did not respond in serum+SAPI to NE, its growth could be promoted by DBS. However, DBS is not a direct precursor of enterobactin (7, 17, 39, 40, 41) and is therefore unlikely to be used as an exogenous substrate for the synthesis of enterobactin. The DBS iron complex can, however, function as a siderophore, which is taken up via the iron-regulated outer membrane proteins FepA and Fiu (20). This suggests that DBS may be more efficient than NE at releasing iron from transferrin and then delivering it to the bacterial cell in the absence of enterobactin biosynthesis.
Supernatants from E. coli strains grown in SAPI+serum and exposed to NE have been reported to contain a heat-stable AI (34), which promotes bacterial growth in SAPI+serum in the absence of NE. To determine whether AI is related to any known or putative quorum-sensing molecules, we exposed E. coli JPN10 to a range of concentrations of AHLs and cyclic dipeptides. However, none of these signal molecules stimulated the growth of E. coli in SAPI+serum.
To evaluate the relationship between AI and enterobactin, JPN10 and the entA parent and mutant strains were each grown in an iron-limited M9 medium and extracted with ethyl acetate. Crude, cell-free culture supernatants and solvent extracts from JPN10 and the entA parent but not from the entA mutant stimulated the growth of JPN10 in SAPI+serum. Since the product of the entA gene is required for the synthesis of DBA, the entA mutant cannot produce DBA, DBS, or enterobactin (7, 17, 44). These data reveal that the AI is enterobactin or a mixture of enterobactin and its breakdown products which, in addition to DBS, include the linear DBS trimer and linear DBS dimer (41). This is because enterobactin is used only once for transporting iron into the bacterial cell such that cyclic triester linkages of ferrienterobactin are cleaved and the DBS derivatives generated are released into the culture supernatant (2, 18). Although DBA is also present in the supernatants of iron-starved E. coli (40; the present study), the relatively weak activity of DBA compared to DBS in the growth promotion assay suggests that the AI consists primarily of enterobactin and its DBS breakdown products. This is further supported by the theoretical study of Chipperfield and Ratledge (4), indicating that DBA is probably not able to function as a siderophore under physiological conditions. In addition, our data are also consistent with previous observations that purified enterobactin can abolish the bacteriostatic effect of serum (46) and can remove iron from transferrin (13).
To account for NE-dependent growth promotion, AI production, and their relationship with enterobactin in E. coli, we propose the following model. When added to SAPI+serum, (i) NE forms a complex with transferrin-bound iron that facilitates the subsequent release of the glycoprotein-bound iron and (ii) NE induces the production of enterobactin, which in turn transports iron from the NE-ferritransferrin complex and into the cell in a TonB-dependent manner via the ferrioenterobactin receptor FepA. This model also accounts for the AI, which consists of enterobactin and its DBS breakdown products. The model assumes that the low bacterial population inoculated into SAPI+serum is unable to overcome the iron deprivation imposed by the presence of transferrin and that NE, DBS, and probably enterobactin itself are capable of inducing the enterobactin system in E. coli in the iron-restricted environment of SAPI+serum. At present, only the iron-dependent repressor protein Fur has been implicated in the control of enterobactin production and transport in E. coli (44). However, for other gram-negative bacteria, the induction of certain ferrisiderophore uptake systems is known to depend on the cell integrating both negative and positive sensory inputs, which include both the lack of readily available iron and the presence of the siderophore. For example, in Pseudomonas aeruginosa, an organism that does not produce enterobactin, the ferrienterobactin receptor gene, pfeA, is expressed only under conditions in which iron is limiting and enterobactin is present. This control mechanism requires the PfeSR two-component system (8). Similar observations have been made with respect to the pyochelin and yersiniabactin siderophore systems of P. aeruginosa and Yersinia pestis, respectively (21, 42). In E. coli, expression of the ferric citrate uptake system depends not only on the derepression of Fur but also on the cytoplasmic membrane sensor, FecR, and the cytoplasmic sigma factor, FecI, which respond to the presence of ferric citrate bound to the outer membrane receptor FecA (11). Thus, E. coli strains may well contain an as-yet-unidentified sensor for catechols. In support of this hypothesis, we have observed that the growth, in SAPI+serum, of an E. coli fur mutant (in which enterobactin production is derepressed) can still be stimulated by NE (unpublished data).
The ability of 23 different species of bacteria (17 gram negative and 6 gram positive) to respond to NE and to synthesize an AI in a serum-supplemented medium has been reported by Freestone et al. (14). While the majority of gram-negative bacterial strains (belonging to the genera Acinetobacter, Citrobacter, Enterobacter, E. coli, Hafnia, Klebsiella, Morganella, Proteus, Salmonella, Yersinia, Pseudomonas, and Xanthomonas) responded to NE and NE-induced AIs, only a few gram-positive genera (Enterococcus, Staphylococcus, and Listeria) responded to NE, and the majority failed to produce an AI. On this basis, Freestone et al. proposed the existence of a novel family of gram-negative bacterial signaling molecules (14). However, the data provided here provide an alternative explanation. Almost all of the organisms shown to respond to NE or the E. coli AI are known to be capable of making and/or transporting ferric enterobactin or related catechol siderophores (6, 8, 10, 18, 35, 47, 53, 54). While gram-positive bacteria such as Listeria monocytogenes do not produce enterobactin, some are capable of using enterobactin and NE as exogenous siderophores (6). Thus, these data can be explained by the model described above, in that bacteria with catechol uptake systems all respond to NE, and those which produce AIs are those capable of sensing catechols as well as synthesizing siderophores that could be enterobactin, related catecholate siderophores, or even chemically unrelated siderophores. Nevertheless, even though the growth response of E. coli to NE can be explained by the production and transport of a well-studied siderophore, the data presented in the present study and by others (31, 33, 38) indicate that the exposure of low numbers of bacteria in tissue fluids to a variety of catecholamines, including many agents used therapeutically, may predispose subjects to infection, a phenomenon first noted for epinephrine, more than half a century ago (12).
Acknowledgments
We thank Chris Harty for assistance with the synthesis of DBS and Catherine Buckley and Keith Bishop for help with the growth promotion assays and FepA induction studies, respectively.
This work was funded via a Medical Research Council U.K. research studentship, programme grant (G9632750), and cooperative group core grant (G0000289).
Editor: V. J. DiRita
REFERENCES
- 1.Bachman, B. 1972. Pedigree of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 165:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brickman, T. J., and M. A. McIntosh. 1992. Overexpression and purification of ferric enterochelin esterase of Escherichia coli. J. Biol. Chem. 267:12350-12355. [PubMed] [Google Scholar]
- 3.Chhabra, S. R., P. Stead, N. J. Bainton, G. P. C. Salmond, G. S. A. B. Stewart, P. Williams, and B. W. Bycroft. 1993. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J. Antibiot. 46:441-454. [DOI] [PubMed] [Google Scholar]
- 4.Chipperfield, J. R., and C. Ratledge. 2000. Salicylic acid is not a bacterial siderophore: a theoretical study. Biometals 13:165-168. [DOI] [PubMed] [Google Scholar]
- 5.Costa, M., and S. J. H. Brookes. 1994. The enteric nervous system. Am. J. Gastroenterol. 89:S129-S137. [PubMed] [Google Scholar]
- 6.Coulanges, V., P. Andre, O. Ziegler, L. Buchheit, and J.-M. Vidon. 1997. Utilization of iron-catecholamine complexes involving ferric reductase activity in Listeria monocytogenes. Infect. Immun. 65:2778-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosa, J. H., and C. T. Walsh. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 66:223-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean, C. R., S. Neshat, and K. Poole. 1996. PfeR, an enterobactin-responsive activator of ferric enterobactin receptor gene expression in Pseudomonas aeruginosa. J. Bacteriol. 178:5361-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis, M., D. Campbell, and E. O. Gregg. 1991. Interleukin-2 and granulocyte-macrophage colony-stimulating factor stimulate growth of a virulent strain of Escherichia coli. Infect. Immun. 59:1853-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echenique, J. R., H. Arienti, M. E. Tolmasky, R. R. Read, R. J. Staneloni, J. H. Crosa, and L. A. Actis. 1992. Characterization of a high-affinity iron transport-system in Acinetobacter baumannii. J. Bacteriol. 174:7670-7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signalling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, D. G., A. A. Miles, and J. S. F. Niven. 1948. The enhancement of bacterial infections by adrenaline. Br. J. Exp. Pathol. 29:20-39. [PMC free article] [PubMed] [Google Scholar]
- 13.Ford, S., R. A. Cooper, R. W. Evans, R. C. Hider, and P. H. Williams. 1988. Domain preference in iron removal from human transferrin by the bacterial siderophores aeobactin and enterobactin. Eur. J. Biochem. 178:477-481. [DOI] [PubMed] [Google Scholar]
- 14.Freestone, P. E., R. D. Haigh, P. H. Williams, and M. Lyte. 1999. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol. Lett. 172:53-60. [DOI] [PubMed] [Google Scholar]
- 15.Freestone, P. E., M. Lyte, C. P. Neal, A. F. Maggs, R. D. Haigh, and P. H. Williams. 2000. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J. Bacteriol. 182:6091-6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furness, J. B., and M. Costa. 1974. The adrenergic innervation of the gastrointestinal tract. Ergeb. Physiol. 69:2-51. [PubMed] [Google Scholar]
- 17.Gehring, A. M., I. Mori, and C. T. Walsh. 1998. Reconstitution and characterization of the Escherichia coli enterobactin synthetase from EntB, EntE and EntF. Biochemistry 37:2648-2659. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths, E., and P. Williams. 1999. Bacterial iron and haem-sequestering mechanisms, p. 87-212. In J. J. Bullen and E. Griffiths (ed.), Iron and infection, molecular, physiological, and clinical aspects, 2nd ed. John Wiley & Sons, Ltd., Chichester, England.
- 19.Guerrero, H. Y., G. Caceres, C. L. Paiva, and D. Marcano. 1990. Hypothalamic and telencephalic content in the brain of the teleost fish, Pygocentrus notatus, during the annual reproductive cycle. Gen. Comp. Endocrinol. 80:257-263. [DOI] [PubMed] [Google Scholar]
- 20.Hantke, K. 1990. Dihydroxybenzoylserine: a siderophore for E. coli. FEMS Microbiol. Lett. 67:5-8. [DOI] [PubMed] [Google Scholar]
- 21.Heinrichs, D. E., and K. Poole. 1996. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa functions both as an activator and as a repressor. J. Bacteriol. 178:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holden, M. T. G., S. R. Chhabra, R. de Nys, P. Stead, N. J. Bainton, P. J. Hill, M. Manefield, N. Kumar, M. Labatte, D. England, S. Rice, M. Givskov, G. P. C. Salmond, G. S. A. B. Stewart, B. W. Bycroft, S. Kjelleberg, and P. Williams. 1999. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol. Microbiol. 33:1254-1266. [DOI] [PubMed] [Google Scholar]
- 23.Janakidevi, K., V. C. Dewey, and G. W. Kidder. 1966. The biosynthesis of catecholamines in two genera of protozoa. J. Biol. Chem. 241:2576-2578. [PubMed] [Google Scholar]
- 24.Kaprelyants, A. S., and D. B. Kell. 1996. Do bacteria need to communicate with each other for growth? Trends Microbiol. 4:237-242. [DOI] [PubMed] [Google Scholar]
- 25.Kaprelyants, A. S., G. V. Mukamolova, S. S. Kormer, D. H. Weichart, M. Young, and D. B. Kell. 1999. Intercellular signalling and the multiplication of prokaryotes: bacterial cytokines, p. 33-69. In R. England, G. Hobbs, N Bainton, and D. M. Roberts (ed.), Microbial signalling and communication. SGM Symposium vol. 57. Cambridge University Press, Cambridge, England.
- 26.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen, R. A., M. G. Thomas, and K. Postle. 1999. Proton-motive force, ExbB, and ligand-bound FepA drive conformational changes in TonB. Mol. Microbiol. 31:1809-1824. [DOI] [PubMed] [Google Scholar]
- 28.Laurence, D. R., P. N. Bennett, and M. J. Brown. 1997. Clinical pharmacology, 8th ed. Churchill Livingstone, London, England.
- 29.Lyte, M. 1993. The role of microbial endocrinology in infectious disease. J. Endocrinol. 137:343-345. [DOI] [PubMed] [Google Scholar]
- 30.Lyte, M., B. Arulanandam, K. Nguyen, C. Frank, A. Erickson, and D. Francis. 1997. Norepinephrine induced growth and expression of virulence associated factors in enterotoxigenic and enterohemorrhagic strains of Escherichia coli. Adv. Exp. Med. Biol. 412:331-339. [DOI] [PubMed] [Google Scholar]
- 31.Lyte, M., and M. T. Bailey. 1997. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J. Surg. Res. 70:195-201. [DOI] [PubMed] [Google Scholar]
- 32.Lyte, M., and S. Ernst. 1992. Catecholamine induced growth of gram-negative bacteria. Life Sci. 50:203-212. [DOI] [PubMed] [Google Scholar]
- 33.Lyte, M., and S. Ernst. 1993. Alpha- and beta-adrenergic receptor involvement in catecholamine-induced growth of gram-negative bacteria. Biochem. Biophys. Res. Commun. 190:447-452. [DOI] [PubMed] [Google Scholar]
- 34.Lyte, M., C. D. Frank, and B. T. Green. 1996. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol. Lett. 139:155-159. [DOI] [PubMed] [Google Scholar]
- 35.Maskell, J. P. 1980. The functional interchangeability of enterobacterial and staphylococcal iron chelators. Antonie Leeuwenhoek 46:343-351. [DOI] [PubMed] [Google Scholar]
- 36.Mazurier, J., and G. Spik. 1980. Comparative study of the iron-binding properties of human transferrins. Biochim. Biophys. Acta 629:399-408. [DOI] [PubMed] [Google Scholar]
- 37.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neal, C. P., P. P. E. Freestone, A. F. Maggs, R. D. Haigh, P. H. Williams, and M. Lyte. 2001. Catecholamine inotropes as growth factors for Staphylococcus epidermidis and other coagulase-negative staphylococci. FEMS Microbiol. Lett. 194:163-169. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien, I. G., G. B. Cox, and F. Gibson. 1969. 2,3-Dihydroxy-N-benzoylserine: chemical synthesis and comparison with the natural product. Biochim. Biophys. Acta 177:321-328. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien, I. G., G. B. Cox, and F. Gibson. 1970. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim. Biophys. Acta 201:453-460. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien, I. G., and F. Gibson. 1970. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim. Biophys. Acta 215:393-402. [DOI] [PubMed] [Google Scholar]
- 42.Perry, R. D., P. B. Balbo, H. A. Jones, J. D. Fetherson, and E. DeMoll. 1999. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 145:1181-1190. [DOI] [PubMed] [Google Scholar]
- 43.Pitman, R. M. 1971. Transmitter substances in insects: a review. Comp. Gen. Pharmacol. 2:347-371. [DOI] [PubMed] [Google Scholar]
- 44.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 45.Rioux, C., D. C. Jordan, and J. B. M. Rattray. 1983. Colorimetric determination of catechol siderophores in microbial cultures. Anal. Biochem. 133:163-169. [DOI] [PubMed] [Google Scholar]
- 46.Rogers, H. J. 1973. Iron binding catechols and virulence in Escherichia coli. Infect. Immun. 7:445-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutz, J. M., T. Abdullah, S. P. Singh, V. I. Kalve, and P. E. Klebba. 1991. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J. Bacteriol. 173:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schryvers, A. B., and L. J. Morris. 1988. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol. Microbiol. 2:281-288. [DOI] [PubMed] [Google Scholar]
- 50.Smith, T. A. 1971. The occurrence, metabolism, and function of amines in plants. Biol. Rev. Cambridge Phil. Soc. 46:201-241. [DOI] [PubMed] [Google Scholar]
- 51.Tse, B., and Y. Kishi. 1994. Conformationally rigid tricyclic tripods: synthesis and application to preparation of enterobactin analogs. J. Org. Chem. 59:7807-7814. [Google Scholar]
- 52.Williams, P., M. Camara, A. Hardman, S. Swift, D. Milton, V. J. Hope, K. Winzer, B. Middleton, D. I. Pritchard, and B. W. Bycroft. 2000. Quorum sensing and the population-dependent control of virulence. Phil. Trans. R. Soc. Sect. B 355:667-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams, P., H. Chart, E. Griffiths, and P. Stevenson. 1987. Expression of high-affinity iron-uptake systems by clinical isolates of Klebsiella. FEMS Microbiol. Lett. 44:407-412. [Google Scholar]
- 54.Williams, P., D. J. Morton, K. J. Towner, P. Stevenson, and E. Griffiths. 1990. Utilisation of enterobactin and other exogenous iron sources by Haemophilus influenzae, H. parainfluenzae, and H. paraphrophilus. J. Gen. Microbiol. 136:2343-2350. [DOI] [PubMed] [Google Scholar]
- 55.Winzer, K., and P. Williams. 2001. Quorum sensing and the regulation of gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291:131-143. [DOI] [PubMed] [Google Scholar]
- 56.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]
- 57.Woods, D. E., A. L. Jones, and P. J. Hill. 1993. Interaction of insulin with Pseudomonas pseudomallei. Infect. Immun. 61:4045-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]