Abstract
Membranes of the lens of the eye of mammals have two particular characteristics, high concentrations of sphingomyelin, and dihydrosphingomyelin and cholesterol. We have studied the miscibility of cholesterol with both egg sphingomyelin and with dihydrosphingomyelin made by hydrogenation of egg sphingomyelin. At a cholesterol mol fraction of 0.5 and lower, crystallites of cholesterol are not present with either form of sphingomyelin, as observed by differential scanning calorimetry and by 13C CP/MAS NMR. However, in the range of 0.6 to 0.8 mol fraction of cholesterol increasing amounts of crystallites form, with the amount of anhydrous cholesterol crystals formed being somewhat greater with dihyrosphingomyelin compared with sphingomyelin. Interestingly, cholesterol monohydrate crystallites formed in these two phospholipids exhibit a temperature of dehydration higher than that of pure cholesterol monohydrate crystals. These cholesterol monohydrate crystals form more rapidly and in greater amounts with the unmodified form of sphingomyelin. This difference is likely a consequence of differences at the membrane interface. The chemical shift of the 13C of the carbonyl group, as measured by CP/MAS NMR, shows that there are differences between the two phospholipids in both the presence and absence of cholesterol. The bilayers with dihydrosphingomyelin are more hydrogen bonded. Cholesterol crystallites are known to be present in the lens of the eye. Our studies show that the ratio of sphingomyelin to dihydrosphingomyelin can affect the rate of formation of these cholesterol crystallites and thus play a role in the membrane of cells of the lens, affecting ocular function.
INTRODUCTION
The lens of the eye is composed largely of rigid elongated cells known as fiber cells or lens fibers. The lipid composition of these cells is unusual compared with other mammalian cells in that they have a high mol fraction of cholesterol and of HSM. Most mammalian cell membranes have cholesterol mol fractions between 0.3 and 0.5. In comparison, fiber cell membranes contain cholesterol mol fractions of 0.5 to 0.67 in the cortex of the lens (lens periphery) and up to between 0.75 and 0.8 in the nucleus (center) of the lens (Li et al., 1985, 1987). The cholesterol-to-phospholipid ratio varies somewhat among mammalian species and also with the age of the animal (Zelenka, 1984; Li et al., 1987; Iwata, 1985). Some of the cholesterol in plasma membranes isolated from this tissue is present as crystallites that are sufficiently organized to exhibit diffraction (Jacob et al., 1999, 2001). These crystallites may play a functional role in vision (Mason et al., 2003). It has been suggested that the ordered cholesterol is intercalated within the membrane bilayer (Jacob et al., 1999, 2001), but it may also be separated from the membrane as cholesterol crystals. The functional importance of the high cholesterol concentration is suggested by the fact that inhibition of cholesterol biosynthesis is associated with cataract formation (Cenedella, 1996). The other unusual property of the lipid composition of the lens is the high content of SM and HSM that reaches half of the total phospholipid (Byrdwell et al., 1994). The phospholipid composition of the lens, unlike cholesterol, is uniform throughout the lens (Borchman et al., 1994). In the human lens, the fraction of SM that is in the dihydro form was found to be 77% (Byrdwell and Borchman, 1997), with HSM comprising approximately half of the phospholipids of the lens (Ferguson et al., 1996). Curiously, in other mammalian species, although the total SM and HSM remains high, the fraction of SM in the lens that is in the dihydro form is much smaller (Greiner et al., 1994; Iwata et al., 1995). For example, HSM is only 14.4% in the porcine lens (Byrdwell, 1998). This is still somewhat higher than the fraction of dihydro form found in whole bovine brain SM (11.5%) and much higher than the fraction in human plasma (below 1%) (Byrdwell, 1998). However, some poikilothermal animals do not have a high concentration of SM and HSM in their lens (Iwata et al., 1995; Iwata, 1985). The high mol fraction of HSM in the human lens may contribute to a greater resistance to oxidation compared with SM, because of the lack of the unsaturation in the sphingosine moiety. Another aspect of the unusual lipid composition of the lens is that the phospholipids of the lens are largely devoid of polyunsaturated acyl chains (Byrdwell and Borchman, 1997; Broekhuyse and Soeting, 1976) as are the lipids used in the present work. Oxidation of lipids in the lens may contribute to increased nuclear light scattering (Borchman et al., 2000). In addition, vitamins A and E reduce the risk of cataract formation (Trevithick and Mitton, 2000). The loss of functional integrity of the lens plasma membrane is likely an early step in the formation of many types of cataracts (Kinoshita, 1974). Oxidation of cholesterol, catalyzed by the enzyme cholesterol oxidase, is reduced by the presence of SM compared with phosphatidylcholine (Slotte, 1992).
There are other differences in the properties of SM and HSM. HSM has a higher phase transition temperature than SM (Borchman et al., 1996; Kuikka et al., 2001), and it is more resistant to partitioning into detergent (Ollila and Slotte, 2002). Monolayers of HSM undergo an expanded to condensed phase transition at lower surface pressures than SM (Kuikka et al., 2001). It is also suggested that HSM forms stronger intermolecular H-bonds than SM and that water molecules are an integral part of the H-bonding network in the interfacial region of HSM (Ferguson-Yankey et al., 2000; Talbott et al., 2000). This is also in accord with the finding that calcium induces a greater dehydration of SM than of HSM, resulting in increased intermolecular interactions (Rujoi et al., 2002).
Cholesterol formed more condensed domains with HSM than with SM (Kuikka et al., 2001). Cholesterol also had a slower rate of desorption from HSM to cyclodextrin than from SM (Kuikka et al., 2001). These findings suggest that cholesterol interacts more favorably with HSM than with SM. In the present work we study the properties of mixtures of cholesterol with SM and HSM at high mol fractions of cholesterol, comparable to those found in the lens of the eye. We have used SM from egg and HSM that was prepared by hydrogenation of this SM. Palmitate is the most prevalent acyl group in egg SM (Karlsson et al., 1998). Palmitate is also the most abundant acyl component of both SM (30.6%) and HSM (41.6%) in the porcine lens (Byrdwell, 1998).
Clusters of cholesterol appear in monolayers and vesicles of SM at mol fractions above 0.67, higher than the 0.5 mol fraction of cholesterol required for cluster formation with phosphatidylcholine (Bittman et al., 1994; Slotte, 1992). The clustering of cholesterol will lead to the formation of cholesterol crystallites. We have used DSC and 13C CP/MAS NMR (Guo and Hamilton, 1996, 1993) to assess the mol fraction of cholesterol in crystalline form. Recently CP/MAS NMR was used to demonstrate the similarity between mixtures of cholesterol with SM and with dipalmitoylphosphatidylcholine (Guo et al., 2002).
EXPERIMENTAL PROCEDURES
Materials
Cholesterol and phospholipids were purchased from Avanti Polar Lipids (Alabaster, AL). The SM was extracted from chicken eggs and the HSM prepared by hydrogenation of this lipid. Egg sphingomyelin was hydrogenated over 10% palladium on charcoal in toluene/methanol (8:2, v/v) at room temperature and 50 psi hydrogen for 16 to 24 h. The product was isolated by silica gel column chromatography utilizing a gradient system of increasing polarity. Mass spectroscopy showed the shift of the primary species from a mass of 704 before hydrogenation to 706 after hydrogenation. It should be noted that no unsaturated N-acyl chains are detected by fatty acid analysis of egg SM, and 84% of the acyl chains are palmitate. In addition, as described below, DSC and 13C CP/MAS NMR confirm the identity of the HSM and the essential absence of SM in the product.
Preparation of hydrated mixtures of SM or HSM and cholesterol
SM or HSM and cholesterol were codissolved in chloroform/methanol (2:1, v/v). The solvent was evaporated under a stream of nitrogen with constant rotation of a test tube so as to deposit a uniform film of lipid over the bottom third of the tube. Last traces of solvent were removed by placing the tube under high vacuum for at least two hours. The lipid film was then hydrated with 20 mM PIPES, 1 mM EDTA, 150 mM NaCl with 0.002% NaN3, pH 7.40. The lipid film was suspended and hydrated by intermittent vortexing and heating to 60°C over a period of 30 min under argon.
Differential scanning calorimetry
Measurements were made using an N-DSC II calorimeter (Calorimetry Sciences, American Fork, UT). Unless otherwise stated, the scan rate was 2 K/min and there was a delay of 5 min between sequential scans in a series to allow for thermal equilibration. DSC curves were analyzed by using the fitting program, DA-2, provided by Microcal (Northampton, MA) and plotted with Origin, version 5.0.
13C CP/MAS NMR
Lipid suspensions in buffer were spun in an Eppendorf centrifuge at room temperature. The resulting hydrated pellet was transferred to an 18 × 4 mm ZrO2 rotor, attempting to pack the maximal amount of lipid into the rotor while maintaining it wet. There was always some excess buffer present in these samples so that they, like the lipid samples used for DSC, are suspended in excess water. The sample was maintained at room temperature for 24 h to ensure conversion of any anhydrous cholesterol crystals to the monohydrate form.
The rotor was placed in a Bruker AVANCE 300 spectrometer operating at 75.48 MHz for 13C and equipped with CP-MAS capabilities. The spectra were referenced to an external standard of glycine crystals, assigning a chemical shift of 176.14 ppm for the carbonyl carbon. Samples were generally spun at 5 kHz and at a temperature of 25°C, but spinning at rates between 2 and 10 kHz had little effect on the spectrum, except for some changes in the resolution. The power levels used for cross-polarization were not ramped during the contact and corresponded to a 4 μs π/2 pulse. The Hartmann-Hahn match was established on the sample of glycine. Continuous-wave decoupling at an increased power level was used during acquisition. Some experiments were repeated to verify the stability and reproducibility of the cross-polarization.
The temperature inside the rotor, controlled by the variable temperature unit of the instrument, was calibrated by measuring the chemical shift of ethylene glycol as a function of spinning speed between 0 and 10 kHz. At 5 kHz the temperature of the sample was ∼1 degree warmer than the set temperature. Contact times between 0.02 and 4 ms were used with a recycle time of 5 s. Generally each spectrum was obtained with 12,000 scans and processed with a 1 Hz line broadening. Resonances were assigned based on reports of cholesterol (Guo and Hamilton, 1996) and SM (Bruzik et al., 1990b; Guo et al., 2002).
RESULTS
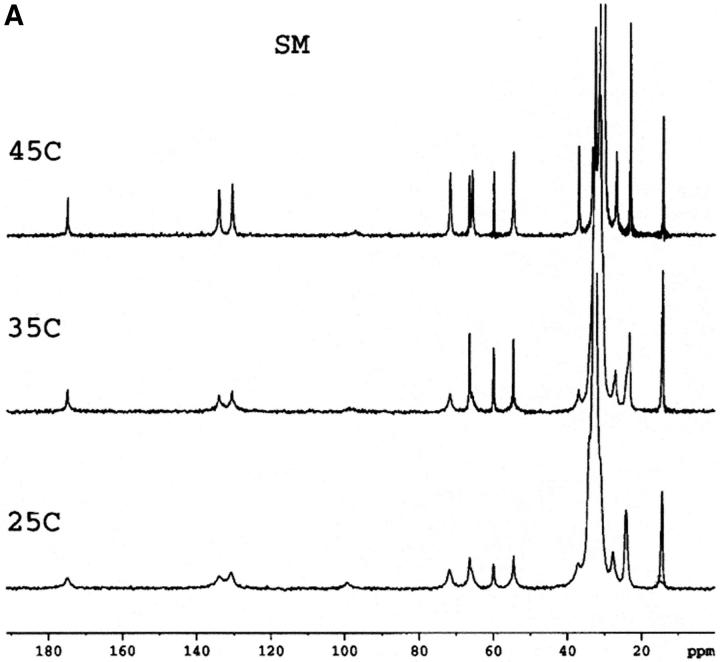
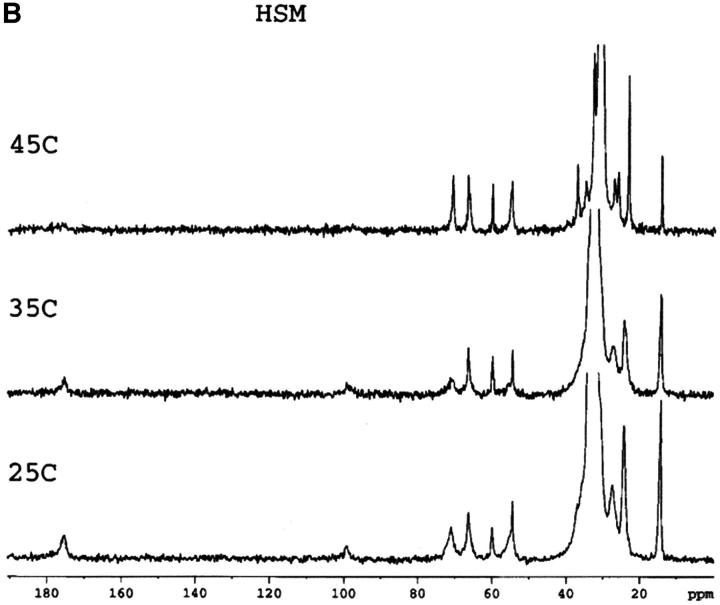
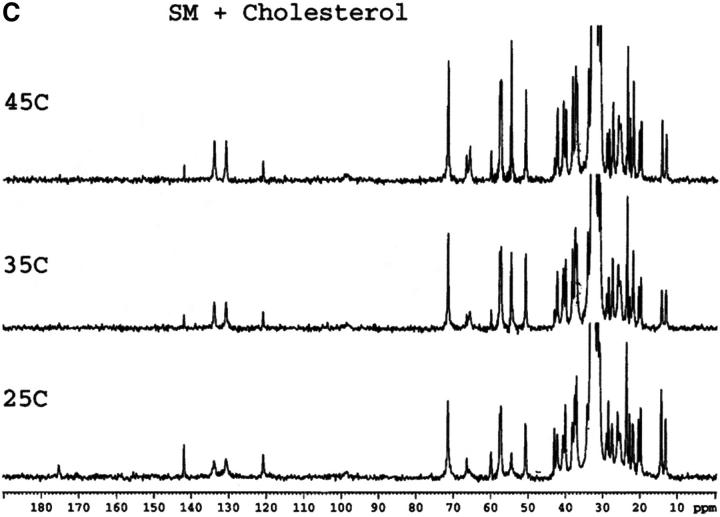
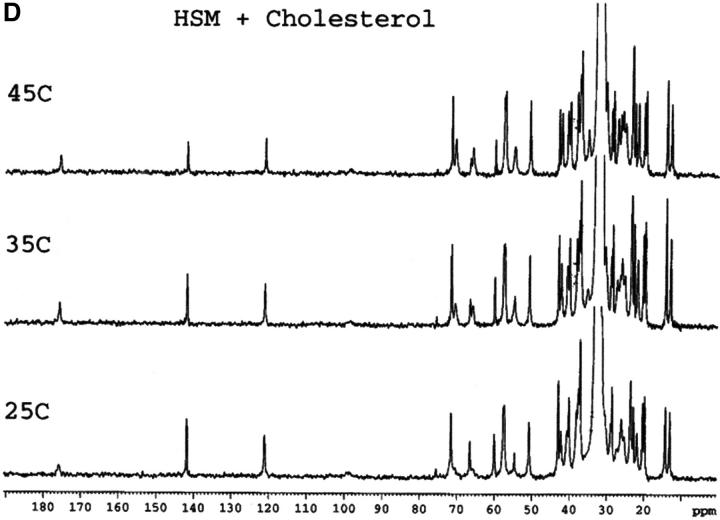
Mixtures of either SM or HSM with an equimolar amount of cholesterol do not exhibit any transition by DSC indicating the essential absence of cholesterol crystals, either in the form of the monohydrate or anhydrous cholesterol. This is in agreement with results recently reported with an equimolar mixture of egg SM and cholesterol (Mannock et al., 2003). The absence of cholesterol crystals is confirmed by the 13C CP/MAS NMR of samples with 0.5 mol fraction cholesterol that show a single resonance for the C18 of cholesterol at 13.1 ppm (Fig. 1), corresponding to the chemical shift of cholesterol dissolved in a membrane (Guo and Hamilton, 1996; Epand et al., 2002). These spectra also confirm that the HSM contains very little, if any, SM because of the disappearance of the signals from the C4 and C5 carbons of SM. These carbon resonances are still present in HSM but at a different chemical shift where they are less well resolved from other resonances. The assignments of some of the better resolved resonances are summarized in Table 1.
FIGURE 1.
13C CP/MAS NMR of SM (A), HSM (B), an equimolar mixture of SM and cholesterol (C), an equimolar mixture of HSM and cholesterol (D) as a function of temperature. Measurement made at 75.48 MHz with sample spinning at 5 kHz at 25°C, 35°C, and 45°C as indicated. The contact time was 1 ms. The assignments of several of the better resolved resonances are indicated in Table 1.
TABLE 1.
Chemical shifts
| Lipid | Group | Approximate chemical shift (ppm) |
|---|---|---|
| SM | C |
176 |
| Cholesterol | C5 | 142 |
| SM | C5 | 134 |
| SM | C4 | 131 |
| Cholesterol | C6 | 121 |
| SM | C3 | 71 |
| SM | Quaternary ammonium N(CH3)3 | 55 |
| Cholesterol | C9 | 51 |
| Cholesterol | C19 | 20 |
| SM | Terminal CH3 | 14 |
| Cholesterol | C18 | 13 |
In general, except for the missing C4 and C5 resonances in HSM, the 13C CP/MAS spectra of SM and HSM are quite similar. This is the case at all three of the temperatures shown as well as for the pure phospholipids and the 1:1 molar ratio of phospholipid to cholesterol. In all of the spectra, including SM at 45°C, the quaternary ammonium peak at 54.5 to 54.6 ppm is prominent. This is true despite the fact that SM is in the Lα phase at this temperature and HSM is in the mixed phase region. Dipalmitoylphosphatidylcholine, with a comparable phase transition temperature, shows essentially no intensity for this peak in the Lα phase and even in the Pβ′ phase, the intensity of this peak is very weak in CP/MAS (Bruzik et al., 1990a). This peak is also not observed in the CP/MAS spectra of 1-palmitoyl-2-oleoyl-phosphatidylcholine (Epand et al., 2002). This indicates that in the liquid crystalline phase the quaternary ammonium headgroup is more rigid in SM and HSM than it is in phosphatidylcholine. This is further shown by our observation (data not presented) that the resonance for this group is also observed in all of the spectra using a shorter contact time of 0.1 ms, instead of 1 ms.
A detailed NMR study of the properties of bovine brain SM as a function of temperature and mol fraction of cholesterol up to 0.6 has recently been published (Guo et al., 2002). The present work shows that there is a consistent and significant difference in the chemical shift of the carbonyl group of ∼0.4 to 0.5 ppm between HSM and SM, either in pure form or in a 1:1 mixture with cholesterol (Table 2). Our values for SM are in very good agreement with those reported by Guo et al. using bovine brain SM (Guo et al., 2002). The chemical shifts we report are ∼0.2 ppm higher, which may reflect the lower degree of acyl chain unsaturation of the egg SM used in the present work. The chemical shift of the carbonyl group is a measure of the degree of hydrogen bonding, with anhydrous lipid monomers having resonances at particularly low chemical shifts (Schmidt et al., 1977). For example, the chemical shift of anhydrous SM in solid form is 173.75 ppm (Bruzik et al., 1990b), lower than any of the values we report for our hydrated samples. The effect of temperature is relatively small, but cholesterol has a greater effect in increasing the chemical shift. Our results demonstrate that HSM forms stronger hydrogen bonds than SM in agreement with previous NMR studies using different criteria (Ferguson-Yankey et al., 2000; Talbott et al., 2000).
TABLE 2.
13C carbonyl chemical shifts from CP/MAS
| Temperature (°C)
|
|||
|---|---|---|---|
| Lipid | 25 | 35 | 45 |
| SM | 174.81 | 174.95 | 175.01 |
| HSM | 175.26 | 175.41 | Peak not well resolved |
| SM + cholesterol* | 175.27 | 175.21 | 175.15 |
| HSM + cholesterol* | 175.85 | 175.73 | 175.70 |
Phospholipid to cholesterol, 1:1 molar ratio.
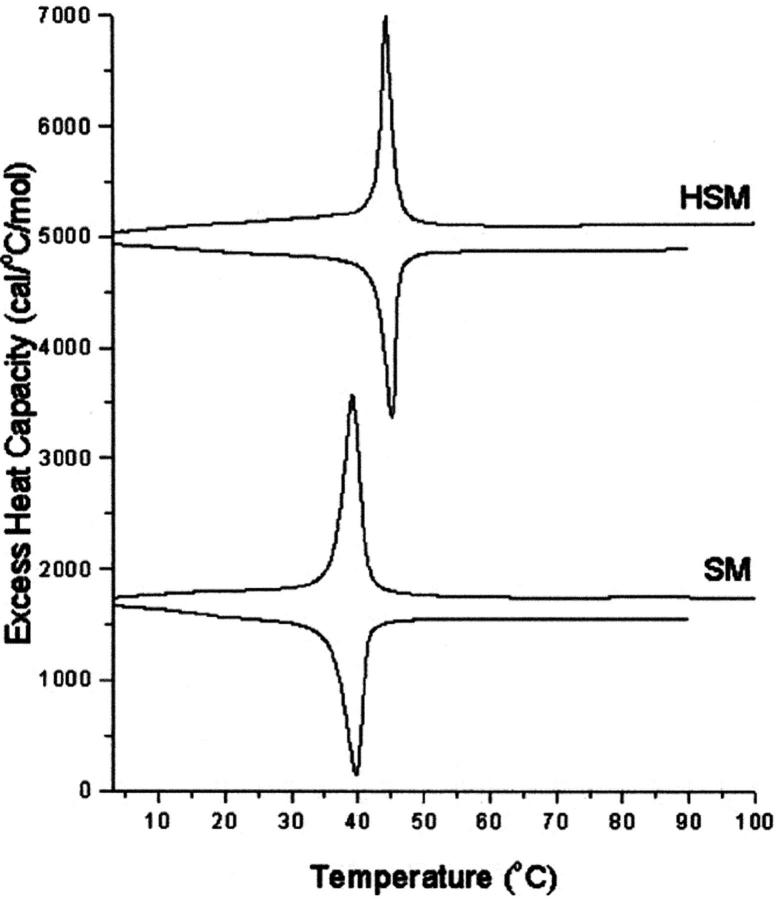
We have used DSC to further quantify the amount and nature of crystalline cholesterol that may form at higher mol fractions of cholesterol. The pure phospholipids give phase transitions at 39.0°C for SM and 44.5°C for HSM (Fig. 2). Several successive heating and cooling scans were performed (not shown). There was a progressive increase in the transition enthalpy for each sequential heating scan and a progressive decrease in the transition enthalpy for each sequential cooling scan. This phenomenon was observed for both SM and HSM, but the effect was considerably larger for SM. It was not investigated further but may relate to the known gel state polymorphism exhibited by SM (Bruzik et al., 1990b). The transition of SM occurs at about the same temperature as that of the polymorphic transition of anhydrous cholesterol. However, because of the large hysteresis in the polymorphic transition of anhydrous cholesterol (Epand et al., 2000), this transition occurs at much lower temperatures than that of the phospholipid in cooling scans.
FIGURE 2.
DSC heating (positive peaks) and cooling (negative peaks) for pure HSM and SM. Scan rate 2 K/min. Relative heat capacity given per mol sphingolipid.
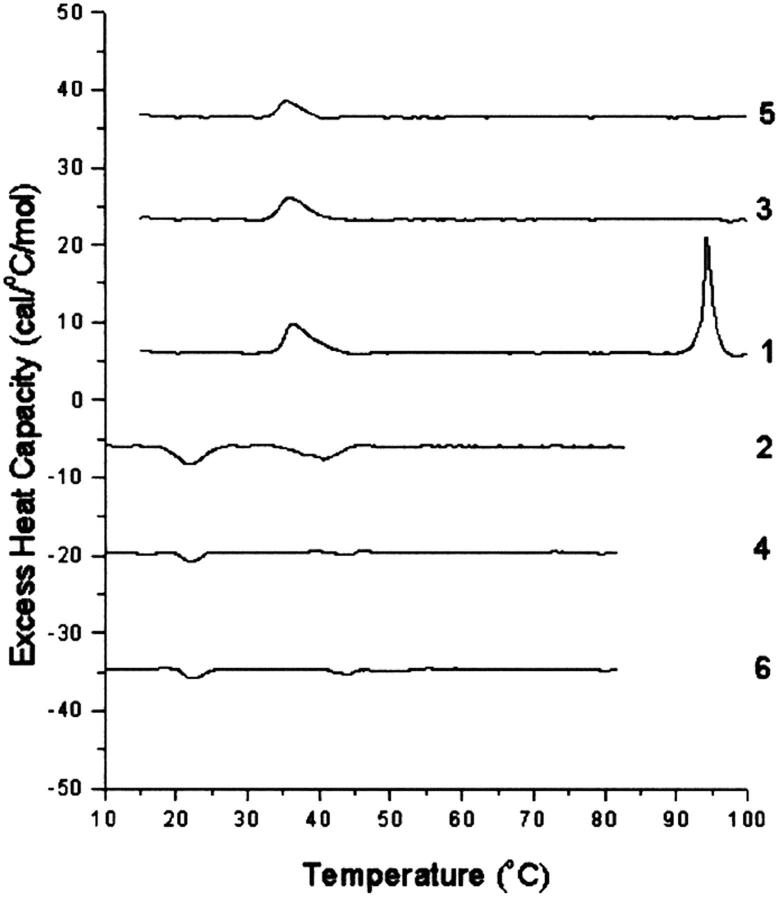
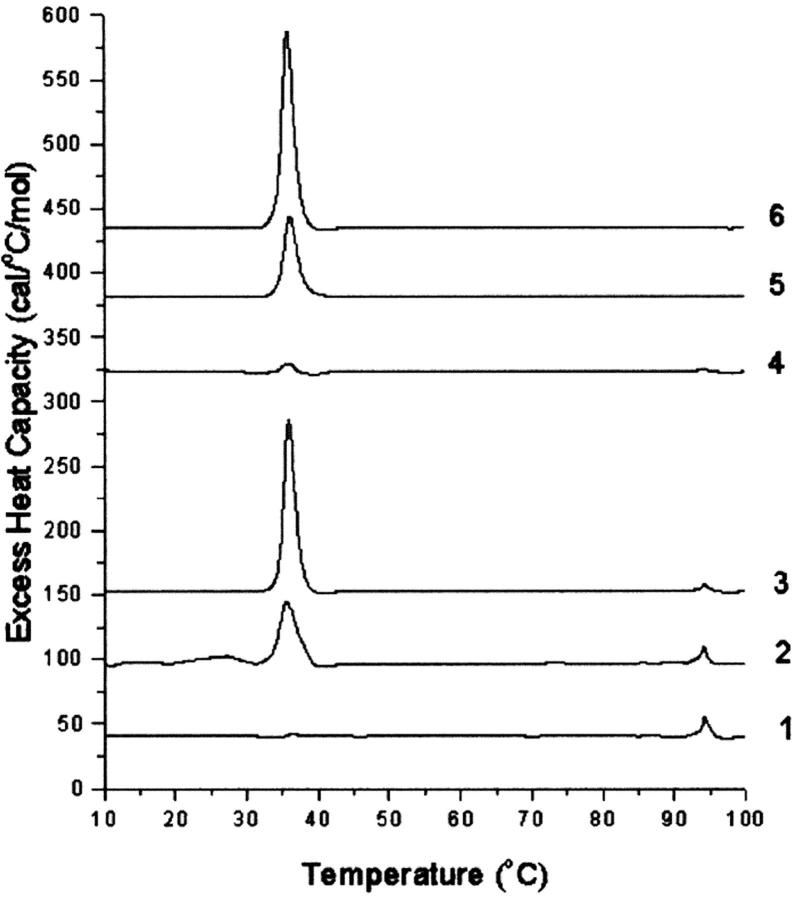
In the presence of mol fractions of cholesterol of 0.6 and higher, transitions corresponding to crystalline cholesterol were observed. Each lipid mixture was scanned six times with three pairs of alternative heating and cooling scans. The transition appearing at 94°C, when observed on the first scan, was not seen on the second heating scan nor was an exotherm, corresponding to the reversal of this transition, observed on cooling. This is shown, for example, in the scans for SM with 0.6 mol fraction cholesterol (Fig. 3). The lack of, or only very partial, reversibility of the transition corresponding to the dehydration of cholesterol is a consequence of the very slow rate of rehydration of anhydrous cholesterol, on the order of many hours, even in the presence of excess water (Loomis et al., 1979; Epand et al., 2001a). In addition to the 94°C transition, there is also a transition at 38°C in the first scan. This later transition arises from both the polymorphic transition of anhydrous cholesterol, as well as a small residual transition from the SM. It should be noted that the SM transition at 38°C has an enthalpy in the order of 10,000 times larger than that from the anhydrous cholesterol crystals present in this sample. It therefore represents a very small fraction of the SM. On cooling, the transitions are separated and the transition from SM is seen as an exotherm at ∼38°C in the first cooling scan (scan 2), whereas the reversal of the polymorphic transition of anhydrous cholesterol crystals exhibits hysteresis (Epand et al., 2000) and is observed at ∼22°C on cooling in these samples. In subsequent heating scans (scans 3 and 5) as well as cooling scans (scans 4 and 6), only the polymorphic transition of cholesterol is observed. There are qualitative differences between samples with cholesterol and SM and those with cholesterol and HSM. To illustrate this, for simplicity we show only the first heating scan for a series of samples with increasing mol fractions of cholesterol (Fig. 4). It is clear that SM has more cholesterol monohydrate crystals. The lower amount of cholesterol monohydrate in the samples with HSM is not a consequence of insufficient water being present, as there is a huge excess of water, but rather because of a kinetic barrier to hydration. In quantifying the amount of anhydrous cholesterol, for mixtures with SM, the enthalpy of the transition in the cooling scans at ∼20°C was used to avoid overlap with any residual transition from the phospholipid (Table 3). For samples at 0.6 or 0.7 mol fraction of cholesterol, the magnitude of this peak decreased to some extent in each subsequent scan. This is noted in Table 3 for the cases in which this decrease was relatively large. We believe this is a consequence of the sample coming to equilibrium from the state that is initially formed on hydration. It has been suggested that some cholesterol may separate during preparation of the lipid film by solvent evaporation (Buboltz and Feigenson, 1999; McMullen et al., 2000). It is interesting to note that SM has more cholesterol monohydrate crystals, whereas HSM has more anhydrous cholesterol crystals.
FIGURE 3.
Sequential heating and cooling scans of SM with 0.6 mol fraction cholesterol. Numbers refer to sequence in which the scans were measured. The top three scans (1, 3, and 5) are heating scans and the bottom three scans (2, 4, and 6) are cooling scans. Each cooling scan was done subsequent to a heating scan after a delay of 5 min. Scan rate 2 K/min. Relative heat capacity given per mol cholesterol.
FIGURE 4.
First heating scan for mixtures of SM (curves 1, 2, and 3) or HSM (curves 4, 5, and 6). The mol fractions of cholesterol in these samples are 0.6 (curves 1 and 4), 0.7 (curves 2 and 5), and 0.8 (curves 3 and 6). Scan rate 2 K/min. Relative heat capacity given per mol cholesterol.
TABLE 3.
Enthalpy of cholesterol crystalline transitions of freshly prepared samples
| Sample composition
|
Transition enthalpy (cal/mol cholesterol)
|
||
|---|---|---|---|
| Phospholipid | Mol fraction cholesterol | Anhydrous cholesterol | Cholesterol monohydrate |
| SM | 0.6 | 10 ± 1 | 156 ± 5 |
| SM | 0.7 | 70 ± 5* | 110 ± 20 |
| SM | 0.8 | 320 ± 20 | 60 ± 10 |
| HSM | 0.6 | 25 ± 5 | 45 ± 10 |
| HSM | 0.7 | 140 ± 10 | 0 |
| HSM | 0.8 | 380 ± 5 | 0 |
Average final two cooling scans. Progressive decrease in size of transition with each subsequent scan.
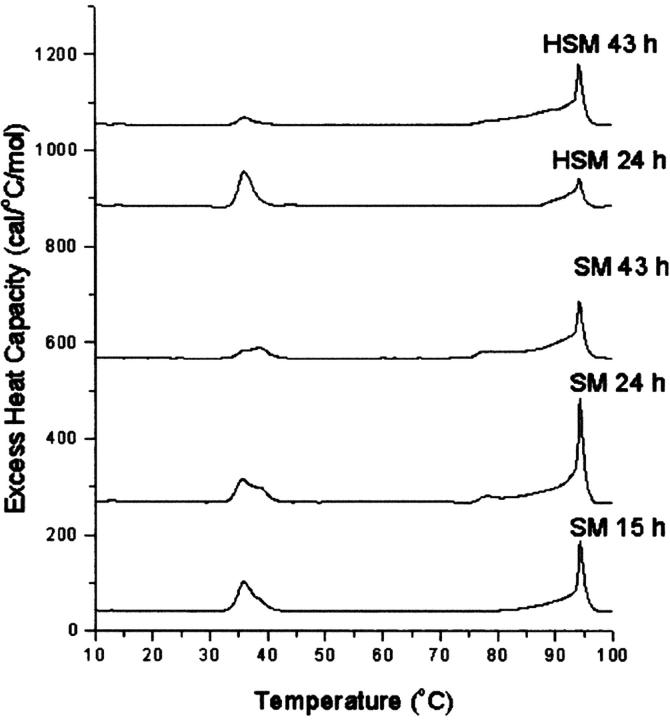
Anhydrous cholesterol crystals slowly convert to cholesterol monohydrate upon incubation in excess water (Loomis et al., 1979). In certain conditions, the dehydration of cholesterol monohydrate crystals is shifted to higher temperatures with increasing time of incubation of the sample (Epand et al., 2001a). We evaluated how the time of incubation at 37°C of SM or HSM with 0.7 mol fraction cholesterol affected the first heating DSC scans (Fig. 5). In the scans of SM, particularly at earlier times, the residual transition of the SM can be seen at ∼38°C, overlapping the polymorphic transition of anhydrous cholesterol. However, the enthalpy of the residual SM transition is small. There is also some residual HSM appearing at 44.5°C in scans with this lipid. In addition, there is clearly more cholesterol monohydrate in the samples with SM than those with HSM. The calculated enthalpy values for the peak at 94°C and its low temperature shoulder are summarized in Table 4. In addition, the dehydration of cholesterol monohydrate at the commonly observed temperature of ∼80°C can be clearly identified in the scan with SM after 24 h, but not in the 24-hour scan with HSM. At longer times of incubation it is difficult to distinguish between a transition at ∼80°C and a low temperature shoulder of the 94°C transition. The shift of the dehydration transition to higher temperatures demonstrates that there is some interaction of the cholesterol crystals with the membrane since pure crystals of cholesterol monohydrate undergo transformation to the anhydrous form at ∼80°C.
FIGURE 5.
Effect of time of incubation at 37°C on the DSC properties of SM or HSM with 0.7 mol fraction cholesterol. Time in hours is indicated in the curve. Scan rate 2 K/min. Relative heat capacity given per mol cholesterol.
TABLE 4.
Time dependence of the formation of cholesterol monohydrate
| Transition enthalpy (cal/mol cholesterol) (94°C transition)
|
||
|---|---|---|
| Time (hours) | SM | HSM |
| 15 | 375 | 0 |
| 24 | 970 | 45 |
| 43 | 710 | 849 |
Cholesterol mol fraction 0.7, incubation temperature 37°C.
DISCUSSION
On the basis of the 13C chemical shift of the carbonyl group (Table 2), we conclude that HSM forms stronger hydrogen bonds than SM, in agreement with previous studies (Ferguson-Yankey et al., 2000; Talbott et al., 2000). We find that the chemical shift of the carbonyl group increases with cholesterol, indicating greater hydrogen bonding. This is also consistent with the finding that HSM monolayers undergo an expanded to condensed transition at lower surface pressures (Kuikka et al., 2001). Diffraction studies of the hydration of sphingomyelin bilayers with and without cholesterol showed that more energy is required to remove water when cholesterol is added to the membrane (McIntosh et al., 1992). This suggests that the increased hydrogen bonding seen with cholesterol is caused by greater hydrogen bonding to water. This could be a consequence of cholesterol weakening bonding between phospholipids, leaving the polar groups more free to be hydrated. Transfer of more tightly bound water from the bilayer interface to form cholesterol monohydrate is therefore slower in the case of HSM where the hydrogen bonding is greater (Table 2). This greater hydration of HSM may also be responsible for the observation that anhydrous cholesterol is less miscible with HSM than with SM and therefore phase segregates in HSM as anhydrous cholesterol crystallites. There will be a greater difference in polarity between the hydrated HSM and the poorly hydrated cholesterol resulting in greater phase separation. SM plays an important role in domain formation in biological membranes (Ramstedt and Slotte, 2002). At low mol fractions of cholesterol (15%), HSM forms more condensed domains with cholesterol than does SM (Kuikka et al., 2001). The greater interaction of cholesterol with HSM compared with SM (Kuikka et al., 2001) is consistent with our findings that fewer total cholesterol crystals (monohydrate and anhydrous) form with HSM, i.e., the cholesterol is more miscible with HSM.
A shift of the temperature of dehydration of cholesterol monohydrate to exhibit a sharp transition at ∼95°C has been previously observed. It does not occur with pure cholesterol monohydrate crystals and is evidence for the crystallites being in close contact with the phospholipid. The phenomenon has been found to occur in the presence of the protein NAP-22 (Epand et al., 2001b) and also after incubation of mixtures of phosphatidylserine and cholesterol for many hours (Epand et al., 2001a). In the case of SM mixtures with cholesterol, this shift is observed in freshly prepared samples (Figs. 3 and 4). With phosphatidylserine (Epand et al., 2001a), as with fresh samples of sphingomyelin (Table 3), the amount of cholesterol monohydrate undergoing dehydration at this temperature decreased between 0.6 and 0.7 mol fraction cholesterol. It also has been observed with phosphatidylserine that the amount of cholesterol monohydrate crystals formed decreases with higher mol fractions of cholesterol (Epand et al., 2001a). The molar enthalpy for the dehydration of cholesterol is 2.35 kcal/mol (Loomis et al., 1979), and there is evidence that this enthalpy is unchanged when the dehydration transition temperature is shifted to higher values (Epand et al., 2002). Therefore, at a cholesterol mol fraction of 0.7, ∼35% of the cholesterol is in the form of cholesterol monohydrate crystals after prolonged incubation for both SM and HSM (Table 4).
Cholesterol is more soluble in SM or HSM than in many other phospholipids (Bach and Wachtel, 2003). Only at mol fractions of 0.7 and 0.8 are substantial amounts of anhydrous cholesterol crystals found (Table 3). At a cholesterol mol fraction of 0.5 there is no cholesterol present that is phase separated as crystals. This is true both for anhydrous cholesterol crystals that would be observed by DSC in fresh samples as well as cholesterol monohydrate crystals that could have been observed in the incubated samples used for CP/MAS. This agrees with the results of Hamilton and co-workers who showed by different criteria that cholesterol was miscible with SM up to a mol fraction of 0.5 (Guo et al., 2002). At 0.6 mol fraction of cholesterol, the final amount of anhydrous cholesterol crystals present after heating and cooling is only 1% and 3% of the total cholesterol for SM and HSM, respectively. Even at the high cholesterol mol fraction of 0.7, only 9% of the cholesterol is present as anhydrous crystals with SM. There is only one biological membrane in which such high cholesterol mol fractions are found; that is the membranes of the lens of the eye of mammals. SM and HSM are major phospholipid components of the mammalian lens membrane. HSM is particularly abundant in the lens of the human eye. This study shows that cholesterol crystals can form in lipid mixtures of the major lipid components of lens membranes. Cholesterol crystals have been found in plasma membranes isolated from the lens of the eye (Jacob et al., 1999, 2001), and these crystals may play a functional role in the normal lens (Mason et al., 2003) as well as being associated with certain pathological conditions. The appearance of these crystals can be explained simply on the basis of the nature of the lipids in this membrane.
Acknowledgments
We are grateful to Mr. Brian Sayer for assistance with the NMR measurements and to Raquel Epand for helpful discussions. The hydrogenated egg sphingomyelin was prepared by Avanti Polar Lipids. I also thank Dr. David A. Mannock and Ronald N. McElhaney for making available to me their manuscript before publication and Joseph Costello for interesting discussions.
This work was supported by a grant from the Canadian Institutes of Health Research (MT-7654). Richard M. Epand is a Senior Investigator of the Canadian Institutes of Health Research.
Abbreviations used: HSM, hydrogenated egg sphingomyelin (dihydrosphingomyelin); SM, sphingomyelin; CP, cross-polarization; MAS, magic angle spinning; DSC, differential scanning calorimetry.
References
- Bach, D., and E. Wachtel. 2003. Phospholipid-cholesterol model membranes: formation of cholesterol crystallites. Biochim. Biophys. Acta. 1610:187–197. [DOI] [PubMed] [Google Scholar]
- Bittman, R., C. R. Kasireddy, P. Mattjus, and J. P. Slotte. 1994. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 33:11776–11781. [DOI] [PubMed] [Google Scholar]
- Borchman, D., W. C. Byrdwell, and M. C. Yappert. 1994. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest. Ophthalmol. Vis. Sci. 35:3938–3942. [PubMed] [Google Scholar]
- Borchman, D., W. C. Byrdwell, and M. C. Yappert. 1996. Thermodynamic phase transition parameters of human lens dihydrosphingomyelin. Ophthalmic Res. 28(Suppl 1):81–85. [DOI] [PubMed] [Google Scholar]
- Borchman, D., F. J. Giblin, V. R. Leverenz, V. N. Reddy, L. R. Lin, M. C. Yappert, D. Tang, and L. Li. 2000. Impact of aging and hyperbaric oxygen in vivo on guinea pig lenslipids and nuclear light scatter. Invest. Ophthalmol. Vis. Sci. 41:3061–3073. [PubMed] [Google Scholar]
- Broekhuyse, R. M., and W. J. Soeting. 1976. Lipids in tissues of the eye. XV. Essential fatty acids in lens lipids. Exp. Eye Res. 22:653–657. [DOI] [PubMed] [Google Scholar]
- Bruzik, K. S., G. M. Salamonczyk, and B. Sobon. 1990a. 13C CP-MAS study of the gel phases of 1,2-dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta. 1023:143–146. [DOI] [PubMed] [Google Scholar]
- Bruzik, K. S., B. Sobon, and G. M. Salamonczyk. 1990b. Nuclear magnetic resonance study of sphingomyelin bilayers. Biochemistry. 29:4017–4021. [DOI] [PubMed] [Google Scholar]
- Buboltz, J. T., and G. W. Feigenson. 1999. A novel strategy for the preparation of liposomes: rapid solvent exchange. Biochim. Biophys. Acta. 1417:232–245. [DOI] [PubMed] [Google Scholar]
- Byrdwell, W. C. 1998. Dual parallel mass spectrometers for analysis of sphingolipid, glycerophospholipid and plasmalogen molecular species. Rapid Commun. Mass Spectrom. 12:256–272. [DOI] [PubMed] [Google Scholar]
- Byrdwell, W. C., and D. Borchman. 1997. Liquid chromatography/mass-spectrometric characterization of sphingomyelin and dihydrosphingomyelin of human lens membranes. Ophthalmic Res. 29:191–206. [DOI] [PubMed] [Google Scholar]
- Byrdwell, W. C., D. Borchman, R. A. Porter, K. G. Taylor, and M. C. Yappert. 1994. Separation and characterization of the unknown phospholipid in human lens membranes. Invest. Ophthalmol. Vis. Sci. 35:4333–4343. [PubMed] [Google Scholar]
- Cenedella, R. J. 1996. Cholesterol and cataracts. Surv. Ophthalmol. 40:320–337. [DOI] [PubMed] [Google Scholar]
- Epand, R. M., D. Bach, N. Borochov, and E. Wachtel. 2000. Cholesterol crystalline polymorphism and the solubility of cholesterol in phosphatidylserine. Biophys. J. 78:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand, R. M., D. Bach, R. F. Epand, N. Borochov, and E. Wachtel. 2001a. A new high-temperature transition of crystalline cholesterol in mixtures with phosphatidylserine. Biophys. J. 81:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand, R. M., A. D. Bain, B. G. Sayer, D. Bach, and E. Wachtel. 2002. Properties of mixtures of cholesterol with phosphatidylcholine or with phosphatidylserine studied by 13C magic angle spinning nuclear magnetic resonance. Biophys. J. 83:2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand, R. M., S. Maekawa, C. M. Yip, and R. F. Epand. 2001b. Protein-induced formation of cholesterol-rich domains. Biochemistry. 40:10514–10521. [DOI] [PubMed] [Google Scholar]
- Ferguson, S. R., D. Borchman, and M. C. Yappert. 1996. Confirmation of the identity of the major phospholipid in human lens membranes. Invest. Ophthalmol. Vis. Sci. 37:1703–1706. [PubMed] [Google Scholar]
- Ferguson-Yankey, S. R., D. Borchman, K. G. Taylor, D. B. DuPre, and M. C. Yappert. 2000. Conformational studies of sphingolipids by NMR spectroscopy. I. Dihydrosphingomyelin. Biochim. Biophys. Acta. 1467:307–325. [DOI] [PubMed] [Google Scholar]
- Greiner, J. V., D. B. Auerbach, C. D. Leahy, and T. Glonek. 1994. Distribution of membrane phospholipids in the crystalline lens. Invest. Ophthalmol. Vis. Sci. 35:3739–3746. [PubMed] [Google Scholar]
- Guo, W., and J. A. Hamilton. 1993. Molecular organization and motions of cholesteryl esters in crystalline and liquid crystalline phases: a 13C and 1H magic angle spinning NMR study. Biochemistry. 32:9038–9052. [DOI] [PubMed] [Google Scholar]
- Guo, W., and J. A. Hamilton. 1996. 13C MAS NMR studies of crystalline cholesterol and lipid mixtures modeling atherosclerotic plaques. Biophys. J. 71:2857–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., V. Kurze, T. Huber, N. H. Afdhal, K. Beyer, and J. A. Hamilton. 2002. A solid-state NMR study of phospholipid-cholesterol interactions: sphingomyelin-cholesterol binary systems. Biophys. J. 83:1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata, J. L., L. G. Bardygula-Nonn, T. Glonek, and J. V. Greiner. 1995. Interspecies comparisons of lens phospholipids. Curr. Eye Res. 14:937–941. [DOI] [PubMed] [Google Scholar]
- Iwata, S. 1985. Effect of temperature on the rainbow trout lens. Curr. Eye Res. 4:441–446. [DOI] [PubMed] [Google Scholar]
- Jacob, R. F., R. J. Cenedella, and R. P. Mason. 1999. Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. J. Biol. Chem. 274:31613–31618. [DOI] [PubMed] [Google Scholar]
- Jacob, R. F., R. J. Cenedella, and R. P. Mason. 2001. Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. J. Biol. Chem. 276:13573–13578. [DOI] [PubMed] [Google Scholar]
- Karlsson, A. A., P. Michelsen, and G. Odham. 1998. Molecular species of sphingomyelin: determination by high-performance liquid chromatography/mass spectrometry with electrospray and high-performance liquid chromatography/tandem mass spectrometry with atmospheric pressure chemical ionization. J. Mass Spectrom. 33:1192–1198. [DOI] [PubMed] [Google Scholar]
- Kinoshita, J. H. 1974. Mechanisms initiating cataract formation. Proctor Lecture. Invest. Ophthalmol. 13:713–724. [PubMed] [Google Scholar]
- Kuikka, M., B. Ramstedt, H. Ohvo-Rekila, J. Tuuf, and J. P. Slotte. 2001. Membrane properties of D-erythro-N-acyl sphingomyelins and their corresponding dihydro species. Biophys. J. 80:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. K., L. So, and A. Spector. 1985. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 26:600–609. [PubMed] [Google Scholar]
- Li, L. K., L. So, and A. Spector. 1987. Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim. Biophys. Acta. 917:112–120. [DOI] [PubMed] [Google Scholar]
- Loomis, C. R., G. G. Shipley, and D. M. Small. 1979. The phase behavior of hydrated cholesterol. J. Lipid Res. 20:525–535. [PubMed] [Google Scholar]
- Mannock, D. A., T. J. McIntosh, X. Jiang, D. F. Covey, and R. N. McElhaney. 2003. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys. J. 84:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, R. P., T. N. Tulenko, and R. F. Jacob. 2003. Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim. Biophys. Acta. 1610:198–207. [DOI] [PubMed] [Google Scholar]
- McIntosh, T. J., S. A. Simon, D. Needham, and C. H. Huang. 1992. Interbilayer interactions between sphingomyelin and sphingomyelin/cholesterol bilayers. Biochemistry. 31:2020–2024. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P., R. N. Lewis, and R. N. McElhaney. 2000. Differential scanning calorimetric and Fourier transform infrared spectroscopic studies of the effects of cholesterol on the thermotropic phase behavior and organization of a homologous series of linear saturated phosphatidylserine bilayer membranes. Biophys. J. 79:2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollila, F., and J. P. Slotte. 2002. Partitioning of Triton X-100, deoxycholate and C(10)EO(8) into bilayers composed of native and hydrogenated egg yolk sphingomyelin. Biochim. Biophys. Acta. 1564:281–288. [DOI] [PubMed] [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 2002. Membrane properties of sphingomyelins. FEBS Lett. 531:33–37. [DOI] [PubMed] [Google Scholar]
- Rujoi, M., D. Borchman, D. B. DuPre, and M. C. Yappert. 2002. Interactions of Ca(2+) with sphingomyelin and dihydrosphingomyelin. Biophys. J. 82:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. F., Y. Barenholz, C. Huang, and T. E. Thompson. 1977. Phosphatidylcholine 13C-labeled carbonyls as a probe of bilayer structure. Biochemistry. 16:3948–3954. [DOI] [PubMed] [Google Scholar]
- Slotte, J. P. 1992. Enzyme-catalyzed oxidation of cholesterol in mixed phospholipid monolayers reveals the stoichiometry at which free cholesterol clusters disappear. Biochemistry. 31:5472–5477. [DOI] [PubMed] [Google Scholar]
- Talbott, C. M., I. Vorobyov, D. Borchman, K. G. Taylor, D. B. DuPre, and M. C. Yappert. 2000. Conformational studies of sphingolipids by NMR spectroscopy. II. Sphingomyelin. Biochim. Biophys. Acta. 1467:326–337. [DOI] [PubMed] [Google Scholar]
- Trevithick, J. R., and K. P. Mitton. 2000. Vitamins C and E in cataract risk reduction. Int. Ophthalmol. Clin. 40:59–69. [DOI] [PubMed] [Google Scholar]
- Zelenka, P. S. 1984. Lens lipids. Curr. Eye Res. 3:1337–1359. [DOI] [PubMed] [Google Scholar]