Abstract
The thermotropic properties of binary mixtures of D-erythro-n-palmitoyl-dihydrosphingomyelin (16:0-DHSM), D-erythro-n-palmitoyl-sphingomyelin (16:0-SM), cholesterol, lathosterol, and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were studied by differential scanning calorimetry. Addition of sterol to 16:0-DHSM and 16:0-SM bilayers resulted in a progressive decrease in both the Tm and the enthalpy of the main transition. The sterol-induced broad components in 16:0-DHSM endotherms had markedly lower enthalpies than those induced in 16:0-SM. Pretransitions recorded in 16:0-DHSM and 16:0-SM membranes responded differently to low concentrations of cholesterol. The presence of 5 mol % cholesterol increased the pretransition temperature in 16:0-SM bilayers, whereas it decreased the temperature in 16:0-DHSM membranes. Lathosterol behaved in general as cholesterol with regard to its effects on the thermotropic behavior of both sphingolipids, but it appeared to form more stable sterol-rich domains, as seen from the higher Tm of the broad component, in comparison to cholesterol. Thermograms recorded on binary mixtures of 16:0-SM:16:0-DHSM and DPPC:16:0-DHSM showed that 16:0-SM mixed nearly ideally with 16:0-DHSM, whereas DPPC mixing was less ideal in a 16:0-DHSM membrane. In conclusion, we observed that 16:0-DHSM interactions with sterols differed from that seen with 16:0-SM, and that 16:0-DHSM mixed better with 16:0-SM than DPPC, which indicates that DHSM could function as a membrane organizer within laterally condensed domains.
INTRODUCTION
Mammalian cell membranes are composed of a complex array of various glycerophospholipids, sphingolipids, and cholesterol. Sphingomyelin (SM) is the major sphingolipid class present in the external leaflet of cellular plasma membranes. SM and phosphatidylcholine (PC) both contain phosphocholine as the polar headgroup, but their hydrophobic backbone is different. This leads to differences in the interfacial properties resulting from hydrogen bonding differences, since SM contains both hydrogen bond donating and accepting groups, while PC only has hydrogen bond accepting groups (Barenholz, 1984). Because of these different properties it is thought that SMs have stronger intermolecular interactions between neighboring molecules and with cholesterol than PCs (Slotte, 1999; Ohvo-Rekila et al., 2002). It has been suggested that the favorable interactions between SM and cholesterol gives rise to cholesterol and SM-rich domains or rafts. The rafts are believed to be important in cellular processes such as protein and lipid transport and sorting, as well as in signal transduction (Simons and Ikonen, 1997; Brown and London, 2000; Simons and Toomre, 2000). The nature of the interactions between SM and cholesterol remains unclear. However, it seems that the interactions are stabilized by hydrogen bonding (Sankaram and Thompson, 1990; Bittman et al., 1994; Veiga et al., 2001).
Most of the naturally occurring SMs have the phosphocholine headgroup linked to the hydroxyl group on carbon one of a long-chain base (most often an 18-carbon amine diol), and have a long and highly saturated acyl chain linked to the amide group on carbon two of the long-chain base (for a review, see Barenholz, 1984). These SMs have the D-erythro-(2S,3R) configuration of the long-chain base (Sarmientos et al., 1985). In cultured cells (i.e., human skin fibroblast and baby hamster kidney cells) ∼90–95% of the SMs contain sphingosine (1,3-dihydroxy-2-amino-4-octadecene) as the long-chain base, whereas the remainder has sphinganine (1,3-dihydroxy-2-amino-4-octadecane) as the base (Ramstedt et al., 1999). The latter SMs are also called dihydrosphingomyelins (DHSM). Although it is currently not known why cells need both SMs and DHSMs, it is remarkable that DHSM accounts for 50% of all phospholipids in human lens membranes (Byrdwell and Borchman, 1997). Human eye lens membranes are known to be highly enriched in both DHSM and cholesterol (Li et al., 1985; Byrdwell and Borchman, 1997). This could indicate that cholesterol interacts especially well with DHSM, and that DHSM possibly could function as an efficient solubilizer of cholesterol in the human lens cells.
The physico-chemical properties of DHSM are not as well documented as those of SM, but it is known that the lack of the trans-double bond between carbons 4 and 5 in DHSM leads to the formation of more ordered membranes with a higher melting temperature (Tm) compared to acyl-chain-matched SM (Barenholz et al., 1976; Kuikka et al., 2001; Nyholm et al., 2003). As a consequence, DHSMs are even more likely than SM to undergo lateral phase separation in bilayer membranes, and may thus contribute to the formation of laterally condensed domains in biomembranes (Brown, 1998; Kuikka et al., 2001).
Sphingolipid and cholesterol-rich cell membranes are known to be resistant to Triton X-100-induced solubilization at low temperatures (Brown and Rose, 1992; Schroeder et al., 1994; London and Brown, 2000). Model membrane studies have shown that pure SM membranes are less resistant to Triton X-100 solubilization compared to DPPC membranes, but that addition of cholesterol increased the resistance markedly (Patra et al., 1999; Nyholm and Slotte, 2001). How cholesterol inclusion affects the resistance to detergent-induced solubilization in DHSM membranes is not known, but it is likely that detergent resistance is linked to the presence of a liquid-ordered phase in cholesterol-rich membranes also containing mostly saturated (sphingo)lipids. In the present work we have studied how the thermotropic phase behavior of 16:0-DHSM and 16:0-SM was affected by the presence of cholesterol and lathosterol, and compared the miscibility of 16:0-DHSM in DPPC and 16:0-SM bilayer membranes. Lathosterol that differs from cholesterol by having a double bond between carbons 7 and 8 (in cholesterol the double bond is positioned between carbons 5 and 6) has been shown to have dramatically different membrane properties than cholesterol (Leppimaki et al., 2000), and was therefore included in the study.
EXPERIMENTAL PROCEDURES
Material
D-erythro-n-palmitoyl-sphingomyelin (16:0-SM) was purified from egg yolk sphingomyelin (Avanti Polar Lipids, Alabaster, AL) by reverse-phase HPLC (LiChrospher 100 RP-18 column, 5 μm particle size, 250 × 4 mm column dimension) using 5 vol % water in methanol as eluent (at 1 ml/min, column temperature 40°C). The fatty acid and long-chain base composition of the product was verified by mass spectroscopy (Micromass Quattro II, Manchester, UK). D-erythro-n-palmitoyl-dihydrosphingomyelin (16:0-DHSM) was prepared from 16:0-SM by hydrogenation using palladium oxide (Aldrich Chemical Co., Milwaukee, WI) as catalyst (Schneider and Kennedy, 1967), and purified as described for 16:0-SM. Its identity was also verified by mass spectroscopy. Cholesterol and lathosterol were obtained from Sigma Chemicals (St. Louis, MO), whereas 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was from Avanti Polar Lipids. Stock solutions of lipids were prepared in hexane/2-propanol (3/2, v/v), stored in the dark at −20°C, and warmed to ambient temperature before use. The water used was purified by reverse osmosis followed by passage through a Millipore UF Plus water purification system, to yield a product with a resistivity of 18.2 MΩcm.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) measurements were performed in a Calorimetry Sciences Corporation Nano II differential scanning calorimeter (Provo, UT). The samples were prepared from lipid stock solutions, which were evaporated under a constant flow of N2, after which they were placed in vacuum for 1 h. The dry lipids were hydrated in 60°C water and sonicated for 1 min at a 60°C in a Bransonic 2510 bath sonicator (Branson Ultrasonics Corporation, CT). The final concentration of phospholipid in the solutions was 1.4 mM, whereas the final sterol concentration varied between 0 and 0.035 mM. The resulting lipid solutions contained 0, 5, 10, 15, 20, or 25 mol % sterol. The suspensions were degassed under vacuum before being loaded into the DSC. Heating scans were run with a rate of 0.5°C/min. Data analysis was performed using the software provided by the DSC manufacturer (CPcalc 2.1) and Microcal Origin 6.0.
The ideality of the binary phospholipid mixtures was assessed according to Mabrey and Sturtevant (1976). The ideal phase diagram was calculated according to
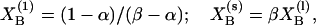 |
(1) |
where the subscripts refer to the liquidus (l) and the solidus (s) curve, and the quantities α and β are defined as
 |
(2) |
where ΔHA and ΔHB are the transition enthalpies of the pure lipids, and TA and TB are their absolute transition temperatures. The onset and completion temperatures of the measured data were corrected for the contributions to the total transition widths of the pure phospholipids, as described (Mabrey and Sturtevant, 1976).
RESULTS
Sterol interactions with 16:0-SM and 16:0-DHSM
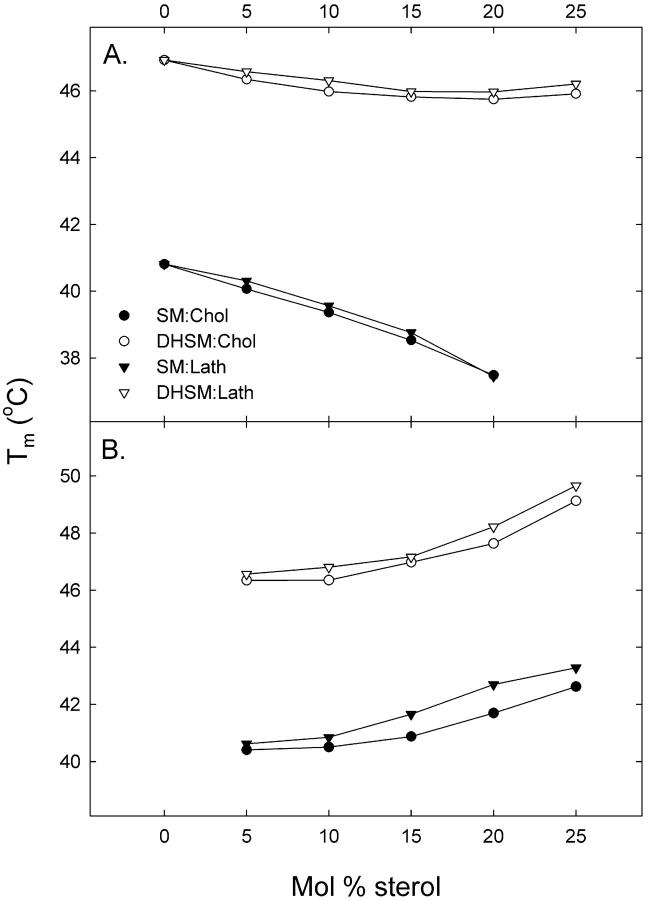
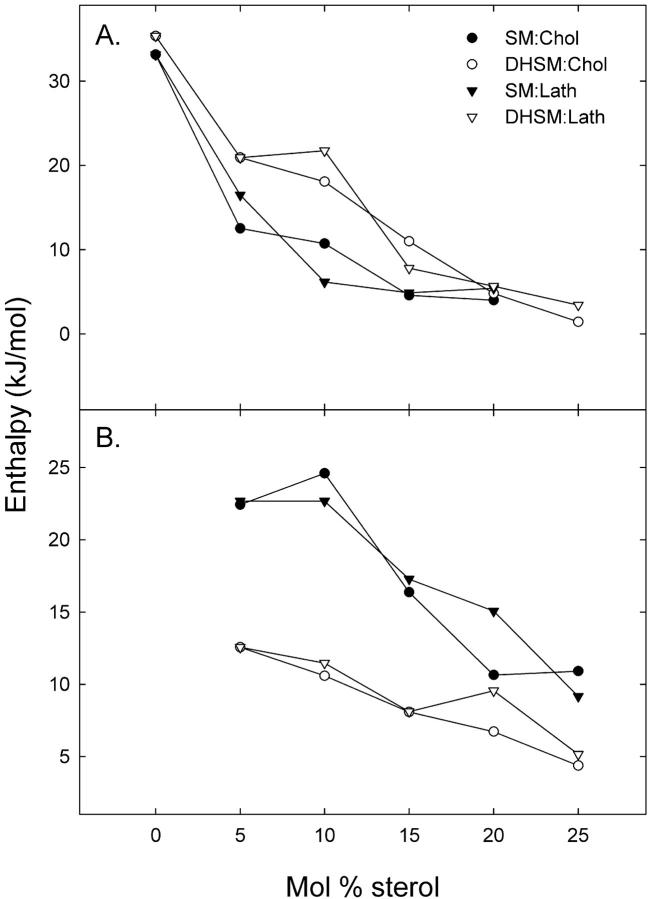
Binary mixtures of sterol (cholesterol or lathosterol) and 16:0-DHSM or 16:0-SM were studied using DSC. Thermograms of sphingomyelin bilayers containing 0–25 mol % sterol were recorded and are shown in Fig. 1. The derived results are summarized in Table 1. Inclusion of low concentrations of cholesterol into PC and SM membranes have been shown to induce bimodal gel-to-liquid-crystalline phase transitions, which can be deconvoluted into a sharp and a broad component (Estep et al., 1979, 1981; McMullen et al., 1993, 1995; McMullen and McElhaney, 1995; Maulik and Shipley, 1996a). The sharp component results from the melting of cholesterol-poor, and the broad component from the melting of cholesterol-rich, membrane domains. Based on this we will therefore be referring to the higher-melting transition as the sterol-induced transition. Both the enthalpy and the Tm of the transition were affected by an increasing sterol concentration, as shown in Figs. 2 and 3. In 16:0-SM bilayers sterol inclusion decreased the Tm from ∼41°C in a pure 16:0-SM bilayer to ∼37.5°C at 20 mol % sterol. The enthalpy of the main transition was decreased almost linearly with the sterol concentration in 16:0-SM bilayers. This finding is in accordance with results published in Maulik and Shipley (1996a). It has been extensively reported that increasing cholesterol concentrations in bilayer membranes leads to diminished phase transition enthalpies (Ladbrooke et al., 1968; McMullen et al., 1993; McMullen and McElhaney, 1995; Mannock et al., 2003). At 25 mol % sterol the main transition disappeared and only the sterol-induced transition remained. Sterol inclusion in 16:0-DHSM bilayers had similar effects on Tm and the enthalpy of the main transition, but the Tm decreased only by ∼1°C, in contrast to the almost 3.5°C decrease in Tm seen for 16:0-SM bilayers. Furthermore, the main transition was still clearly observable at 25 mol % sterol in 16:0-DHSM, in contrast to the 16:0-SM system. The sterol-induced transition was first detectable at 5 mol % sterol both in 16:0-SM and 16:0-DHSM bilayers. As the sterol concentration in the sphingomyelin bilayers increased, the sterol-induced transition shifted to higher temperatures. Qualitatively similar results have been reported for binary mixtures of egg SM and cholesterol (Mannock et al., 2003). The pretransitions in pure 16:0-SM and 16:0-DHSM bilayers were at ∼27.5 and 41.5°C, respectively (Kuikka et al., 2001). As the sterol concentration in the bilayers was increased the enthalpy of the pretransitions decreased. At 10 mol % sterol the pretransitions were no longer measurable (data not shown). The pretransitions in 16:0-SM and 16:0-DHSM responded differently to an increased sterol concentration (Fig. 4). In 16:0-SM bilayers the pretransition occurred at higher temperatures as the sterol concentration was increased from 0 to 5 mol %. In 16:0-DHSM bilayers, on the other hand, the pretransition was observed at lower temperatures as the sterol concentration was increased. Inclusion of lathosterol had similar effects on the phase transitions of both the sphingolipid bilayers as had cholesterol. However, in bilayers containing lathosterol the sterol-induced phase transitions were shifted to higher temperatures than in bilayers containing the corresponding concentrations of cholesterol.
FIGURE 1.
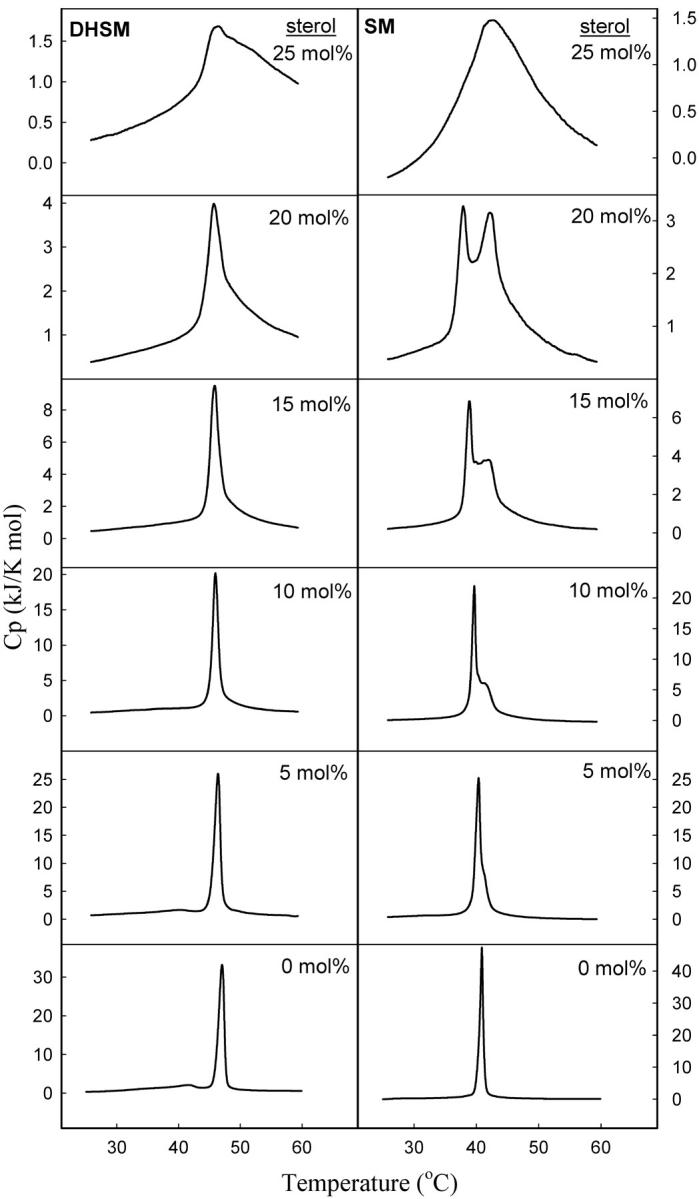
Representative DSC heating thermograms of 16:0-DHSM and 16:0-SM bilayers containing cholesterol. Binary cholesterol:phospholipid mixtures containing 0–25 mol % cholesterol were heated at a rate of 0.5°C/min. The sterol mol % is indicated in the figure.
TABLE 1.
Thermodynamic data for the thermal transitions of phospholipid/sterol mixtures
| Main transition
|
Sterol-induced transition
|
||||||
|---|---|---|---|---|---|---|---|
| Lipids | Sterol Mol % | Tm (°C) | ΔH (kJ/mol) | T1/2 (°C) | Tm (°C) | ΔH (kJ/mol) | T1/2 (°C) |
| SM:cholesterol | 0 | 40.8 | 33.1 | 0.8 | – | – | – |
| 5 | 40.1 | 12.5 | 0.7 | 40.4 | 22.4 | 2.5 | |
| 10 | 39.4 | 10.7 | 0.6 | 40.5 | 24.6 | 3.9 | |
| 15 | 38.5 | 4.6 | 1.0 | 40.9 | 16.4 | 4.9 | |
| 20 | 37.5 | 4.0 | 1.9 | 41.7 | 10.6 | 5.1 | |
| 25* | – | – | – | 42.6 | 10.9 | 10.2 | |
| DHSM:cholesterol | 0 | 46.9 | 35.4 | 1.1 | – | – | – |
| 5 | 46.3 | 20.9 | 1.0 | 46.4 | 12.6 | 2.4 | |
| 10 | 46.0 | 18.1 | 1.1 | 46.4 | 10.6 | 3.9 | |
| 15 | 45.8 | 11.0 | 1.5 | 46.0 | 8.1 | 4.9 | |
| 20 | 45.7 | 4.8 | 2.2 | 47.6 | 6.7 | 7.1 | |
| 25* | 45.9 | 1.4 | 3.1 | 49.1 | 4.4 | 9.0 | |
| SM:lathosterol | 0 | 40.8 | 33.3 | 0.8 | – | – | – |
| 5 | 40.3 | 16.5 | 0.7 | 41.5 | 7.7 | 2.5 | |
| 10 | 39.6 | 6.2 | 0.7 | 40.6 | 22.7 | 2.5 | |
| 15 | 38.8 | 4.9 | 0.9 | 41.7 | 17.3 | 5.3 | |
| 20 | 37.5 | 5.4 | 1.9 | 42.7 | 15.1 | 5.5 | |
| 25* | – | – | – | 43.3 | 9.2 | 9.3 | |
| DHSM:lathosterol | 0 | 46.9 | 35.3 | 1.1 | – | – | – |
| 5 | 46.6 | 20.9 | 0.9 | 46.6 | 12.6 | 2.2 | |
| 10 | 46.3 | 21.7 | 1.0 | 46.8 | 11.5 | 4.1 | |
| 15 | 45.0 | 7.8 | 1.4 | 47.2 | 8.1 | 4.0 | |
| 20 | 45.0 | 5.7 | 2.8 | 48.2 | 9.5 | 7.7 | |
| 25* | 46.2 | 3.4 | 3.8 | 49.7 | 5.1 | 8.1 | |
Binary mixtures of 16:0-DHSM or 16:0-SM and cholesterol or lathosterol (1.4 mM) were studied using DSC. The lipids were rehydrated at 60°C and vesicles prepared by bath sonication.
The results from the experiments with membranes containing 25 mol % sterol have a error margin that is larger than with lower sterol concentrations, due to broader transitions and a smaller area (see Fig. 1).
FIGURE 2.
Transition temperatures of binary mixtures of sterol and sphingomyelins as a function of sterol content. Phospholipid membranes containing 0–25 mol % cholesterol or lathosterol were heated at a rate of 0.5°C/min. The bimodal transition was deconvoluted into a phospholipid main transition component (A), and a sterol-induced broad transition component (B).
FIGURE 3.
Transition enthalpies of binary mixtures of sterol and sphingomyelins as a function of sterol content. Phospholipid membranes containing 0–25 mol % cholesterol or lathosterol were heated at a rate of 0.5°C/min. The enthalpy of the phospholipid main transition is shown in A, and the sterol-induced broad transition in B.
FIGURE 4.
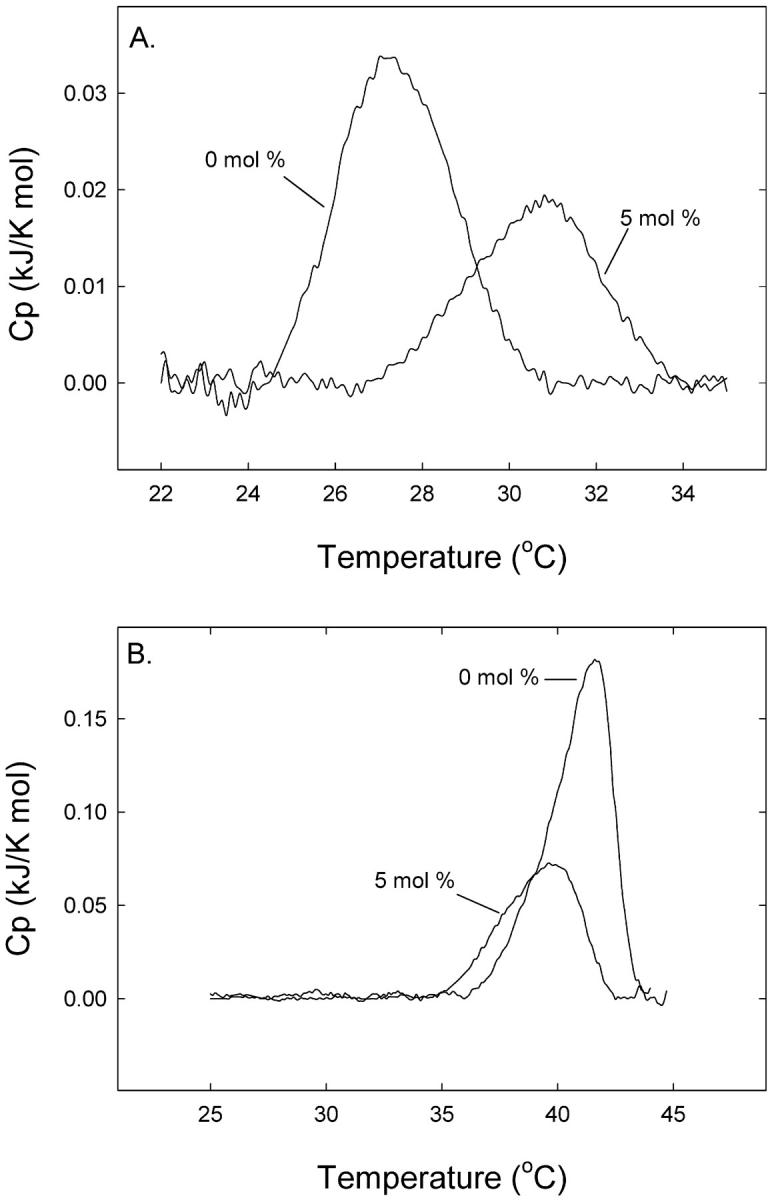
Effect of cholesterol on the pretransitions of 16:0-DHSM and 16:0-SM bilayers. 16:0-SM (A) and 16:0-DHSM (B) membranes containing 0 or 5 mol % cholesterol were heated at a rate of 0.5°C/min.
Thermotropic properties of binary mixtures of DPPC and 16:0-SM or 16:0-DHSM
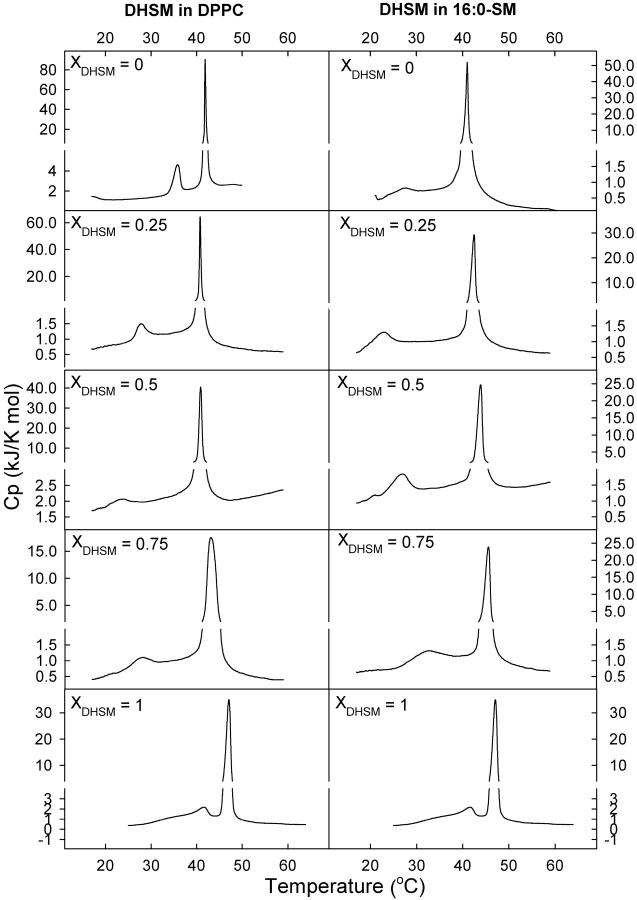
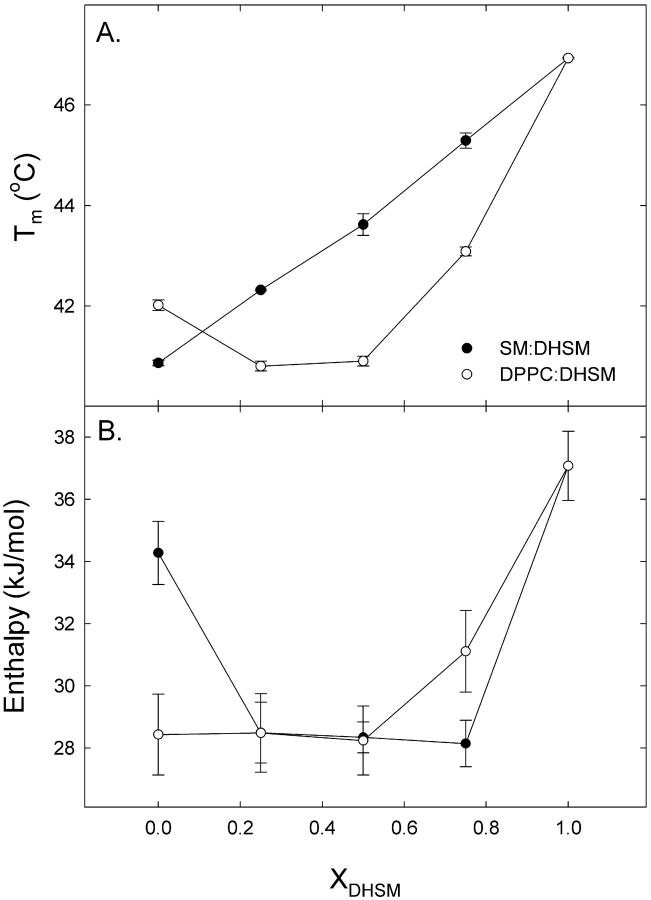
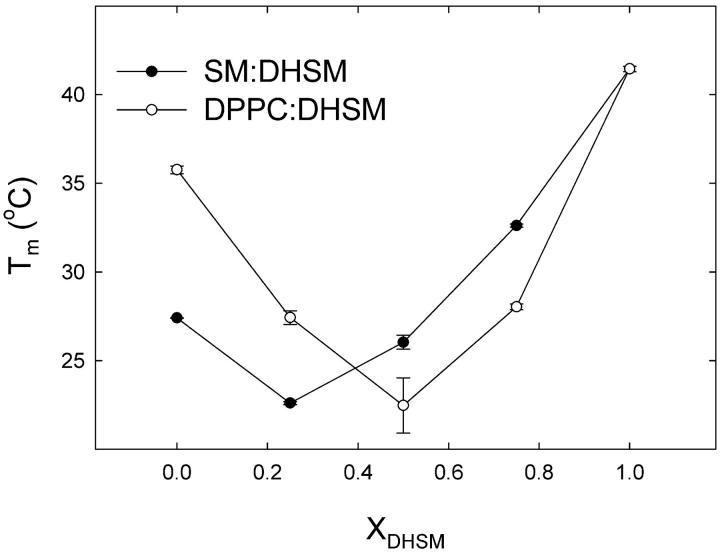
Natural membranes are composed of a variety of lipids, among others PCs, SMs, and DHSMs. Here we have studied binary mixtures of 16:0-DHSM with either DPPC or 16:0-SM (XDHSM = 0, 0.25, 0.5, 0.75, or 1). Fig. 5 shows representative thermograms from these experiments, and Table 2 lists the thermodynamic parameters for the transitions. Inclusion of 16:0-DHSM into 16:0-SM bilayers raised the main transition as a linear function of XDHSM (Fig. 6 B). The enthalpy of the main transition in 16:0-SM:16:0-DHSM bilayers varied between ∼37 kJ/mol (XDHSM = 1) and ∼28 kJ/mol (XDHSM = 0.5). The main transition temperatures of DPPC and 16:0-DHSM mixtures were lower than that for pure DPPC bilayers until XDHSM >0.5, after which the transition temperature was raised. The enthalpy of the main transition was ∼27 kJ/mol until XDHSM = 0.5, above which it increased toward ∼37 kJ/mol in pure DHSM membranes. No low-temperature subtransitions could be observed for pure 16:0-DHSM or 16:0-SM bilayers with the experimental conditions used in this study (data not shown). However, this was seen for pure DPPC (data not shown), as has been reported previously (McMullen and McElhaney, 1995).
FIGURE 5.
Representative DSC heating thermograms of binary mixtures of 16:0-DHSM and 16:0-SM or DPPC. The phospholipid mixtures containing 0, 25, 50, 75, or 100 mol % 16:0-DHSM were heated at a rate of 0.5°C/min. The molar ratios of the mixtures are indicated in the figure as XDHSM.
TABLE 2.
Thermodynamic data for the pre and main transition of binary phospholipid mixtures
| Main transition
|
Pretransition
|
||||
|---|---|---|---|---|---|
| Sample (XDHSM) | Tm (°C) | ΔH (kJ/mol) | T1/2 (°C) | Tp (°C)* | ΔH (kJ/mol) |
| DHSM:SM | |||||
| 0 | 40.9 | 34.3 | 0.76 | 27.4 | 0.59 |
| 0.25 | 42.3 | 28.5 | 0.97 | 22.6 | 1.42 |
| 0.5 | 43.6 | 28.3 | 1.14 | 26.0 | 1.84 |
| 0.75 | 45.3 | 28.2 | 1.27 | 32.6 | 2.93 |
| 1 | 46.9 | 37.1 | 1.08 | 41.5 | 1.80 |
| DHSM:DPPC | |||||
| 0 | 42.0 | 28.4 | 0.34 | 35.8 | 3.39 |
| 0.25 | 40.8 | 27.5 | 0.42 | 27.4 | 1.42 |
| 0.5 | 40.9 | 26.0 | 0.65 | 22.5 | 0.67 |
| 0.75 | 43.1 | 32.6 | 1.99 | 28.0 | 1.67 |
| 1 | 46.9 | 37.1 | 1.08 | 41.5 | 1.80 |
Binary mixtures of 16:0-DHSM and DPPC or 16:0-SM (1.4 mM) were studied using DSC. The lipids were rehydrated at 60°C and vesicles prepared by bath sonication.
Tp denotes the midpoint of the pretransition.
FIGURE 6.
Effect of DHSM inclusion into SM and PC membranes. Transition temperature (A) and enthalpy (B) of 16:0-DHSM membranes containing 0, 25, 50, 75, or 100 mol % 16:0-SM or DPPC. Phospholipid membranes were heated at a rate of 0.5°C/min. The data are the average values from three experiments mean ± SD.
The miscibility of 16:0-SM and DPPC in 16:0-DHSM was further analyzed according to Mabrey and Sturtevant (1976), with the results being shown in Fig. 7. For comparison we also included a phase diagram of binary mixtures of 16:0-SM and DPPC. The phase diagrams for 16:0-SM:DPPC and DPPC:16:0-DHSM mixtures deviated clearly from the simulated ideal curves, while the 16:0-SM:16:0-DHSM phase diagram seemed to mix fairly ideally. Taken together, the results show that 16:0-DHSM mixed better with 16:0-SM than with DPPC.
FIGURE 7.
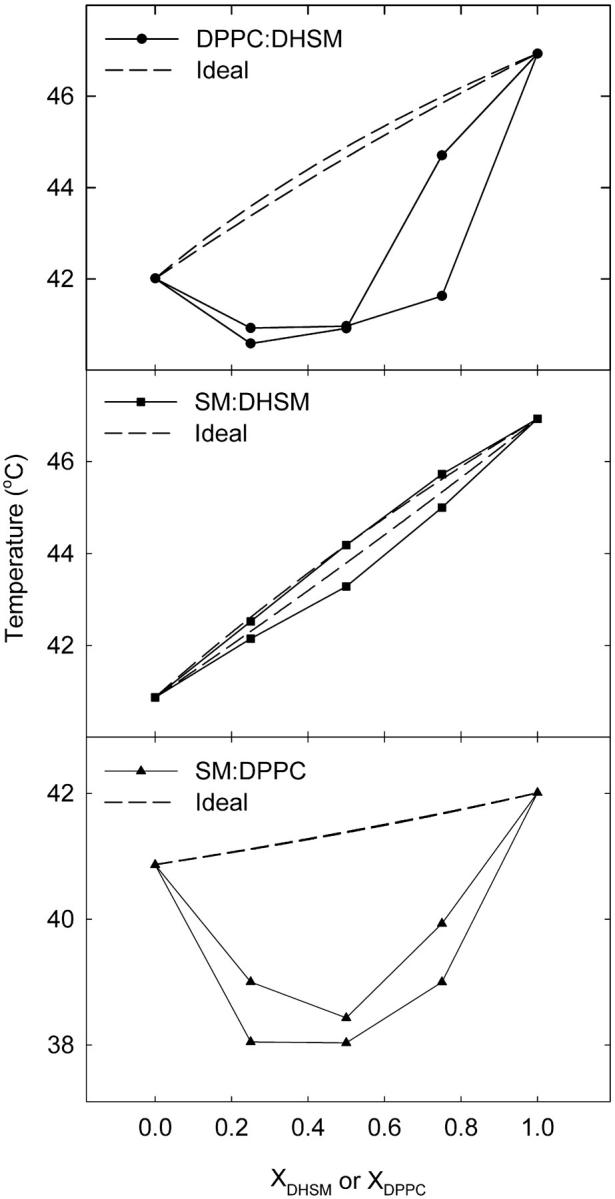
Binary phase diagram of phospholipid mixtures. Phase diagrams of DPPC:16:0-DHSM, 16:0-SM:16:0-DHSM, and 16:0-SM:DPPC mixtures constructed from DSC data. Solid lines represent observed data that was corrected as described in the text. The dashed lines are ideal phase diagrams calculated according to Eqs. 1 and 2.
All the phospholipid mixtures that were analyzed had clear pretransitions (Fig. 8). In DPPC:16:0-DHSM bilayers the pretransition was lowered by the presence of 16:0-DHSM at concentrations XDHSM <0.5. At XDHSM >0.5, the pretransition temperature was again increased. Inclusion of 16:0-DHSM into 16:0-SM bilayers lowered the pretransition temperature up to XDHSM 0.25 after which it was increased by addition of further 16:0-DHSM. It has previously been observed that the pretransition in 16:0-DHSM membranes occur at a markedly lower temperature in the cooling thermogram than in the heating thermogram (Kuikka et al., 2001). On cooling the bilayers, the pretransition temperatures were at a markedly lower temperature (as compared to the heating thermogram), but the temperature shifts induced by mixing two components had very similar trends as shown in Fig. 8 for the heating thermograms (data for cooling thermograms are not shown).
FIGURE 8.
Pretransitions of SM and PC membranes as a function of 16:0-DHSM content. 16:0-SM or DPPC membranes containing 0, 25, 50, 75, or 100 mol % 16:0-DHSM were heated at a rate of 0.5°C/min. All data points are the average value from three experiments mean ± SD.
DISCUSSION
In this study, we have used enantiomerically pure and acyl-chain-defined sphingomyelins and one glycerophosholipid, and studied their thermal properties and mixing behavior and interactions with sterols using high-sensitivity differential scanning calorimetry (DSC). Our objective was to gather information about the interactions between cholesterol and 16:0-DHSM and to study the miscibility of 16:0-DHSM in DPPC and 16:0-SM, to evaluate how the presence of 16:0-DHSM in membranes might affect the lateral organization of other lipids in membranes.
Sterol interactions with 16:0-DHSM and 16:0-SM
Using DSC we compared how sterol inclusion affected the thermotropic behavior of 16:0-DHSM and 16:0-SM bilayers. The literature already contains a number of publications where the thermal behavior of sphingomyelin and binary mixtures of sphingomyelin and cholesterol have been studied (e.g., Barenholz et al., 1976; Calhoun and Shipley, 1979; Estep et al., 1979, 1981; Maulik and Shipley, 1996a,b; Mannock et al., 2003). The thermotropic behavior of DHSM is not as well documented, and only pure DHSM membranes have been studied up to now (Barenholz et al., 1976; Kuikka et al., 2001).
As expected, the thermograms recorded for the binary cholesterol and 16:0-DHSM or 16:0-SM were bimodal, and the total enthalpy of the transition decreases with an increasing cholesterol concentration. The results with binary cholesterol:16:0-SM mixtures were similar to previously published results (Calhoun and Shipley, 1979; Estep et al., 1979; Maulik and Shipley, 1996b), but with regard to the thermograms recorded on 16:0-DHSM:cholesterol mixtures the results were different. Inclusion of cholesterol affected the Tm of the main transition less in DHSM membrane than in SM membranes. This indicates that the gel phase in 16:0-DHSM membranes was less destabilized by the presence of cholesterol than in 16:0-SM membranes. A similar observation was made in a study of membranes composed of DPPC and sterols with different side chains (McMullen et al., 1995). In that study it was shown that the decrease in Tm upon sterol incorporation was larger when the hydrophobic mismatch between the sterol and DPPC increased. Hence, an interpretation of our results would be that 16:0-DHSM would have a better hydrophobic fit with cholesterol than 16:0-SM, and that the affinity between cholesterol and 16:0-DHSM consequently was greater compared with the 16:0-SM system. This interpretation agrees with the findings in our previous 16:0-DHSM paper (Kuikka et al., 2001). The Tm and cooperativity of the broad component, or the sterol-induced transition, changed in a similar fashion in both SM and DHSM membrane as a function of an increasing cholesterol concentration. However, the enthalpy of the broad transition was markedly lower in 16:0-DHSM membranes than in 16:0-SM membranes.
Thermograms recorded on pure phospholipid membranes showed a pretransition at ∼41.5°C in DHSM and ∼27.5°C in SM membranes, which is in fairly good agreement with previous results (Bar et al., 1997; Ramstedt and Slotte, 1999; Kuikka et al., 2001). The slightly lower transition temperatures in both the main and the pretransition observed in this study could be due to a slightly higher degree of impurity in the samples used in the present study. An interesting notion concerning the pretransitions is that inclusion of low cholesterol concentrations shifted the pretransition toward higher temperatures in SM membranes, but toward lower temperatures in DHSM membranes. The cause of this effect remains unclear. In phosphatidylcholine membranes the pretransition has been shown to decrease with increasing cholesterol (McMullen et al., 1993). Previous reports have shown that the pretransition is abolished upon incorporation of ∼5 mol % cholesterol (McElhaney, 1982; McMullen et al., 1993; McMullen and McElhaney, 1995), which agreed well with what was observed in this work.
An earlier study has shown that lathosterol-phospholipid interactions differ from cholesterol-phospholipid interactions (Leppimaki et al., 2000). In that study it was shown that lathosterol does not stabilize the lamellar-to-hexagonal phase transition of POPE membranes to the same degree as cholesterol. In cells, lathosterol also failed to activate CTP:phosphocholine cytidylyltransferase (in contrast to cholesterol; see Leppimaki et al., 2000), an enzyme which is known to be sensitive to lipid-induced membrane torque tension (Attard et al., 1998). In this study, therefore, it was somewhat surprising that the thermotropic phase behavior of lathosterol-containing DHSM and SM membranes was so similar to that of corresponding cholesterol-containing membranes. From these experiments the only observable difference was that lathosterol affected the Tm of the main transition less than cholesterol, and that the broad, sterol-induced transition occurred at slightly higher temperatures in membranes containing lathosterol than in membranes containing cholesterol. The latter observation would indicate that the sterol-rich domains formed by lathosterol were more stable than domains formed by cholesterol, which also have been observed in other types of experiments (unpublished observation).
16:0-DHSM interactions with 16:0-SM and DPPC
DHSMs have been found both in cultured cells and in commercially available natural sphingomyelin mixtures (e.g., bovine brain SM and egg SM; see Barenholz et al., 1976; Byrdwell and Borchman, 1997; Karlsson et al., 1998; Ramstedt et al., 1999). What role DHSM fulfills in membranes remains unclear. Recent studies have, however, shown that DHSM forms more stable domains with cholesterol than acyl-chain-matched SM, and that Triton X-100 partitioning into egg DHSM is less favorable than into egg SM (Kuikka et al., 2001; Ollila and Slotte, 2002). In a recent study in which the membrane properties of DHSM membranes were examined using fluorescent probes it was observed that 16:0-DSHM formed more ordered membranes than both 16:0-SM and DPPC (Nyholm et al., 2003). Further, the fluorophores used in the aforementioned study reported differences in the interfacial properties of DHSM and SM. In conclusion, the known properties of DSHM suggest that it is an order-making lipid in membranes and could possibly play a role in lateral domain formation in cell membranes. The thermograms of binary mixtures of 16:0-DHSM and DPPC or 16:0-SM showed that 16:0-DHSM formed almost ideal mixtures with SM while the DPPC:DHSM mixtures were less ideal. Analysis of SM:DPPC mixtures showed that these had similar properties as the DPPC:DHSM mixtures. Since sphingomyelins in general have different hydrogen bonding properties than phosphatidylcholines, the nonideal mixing of DPPC in DHSM or SM bilayers could in principle be partly due to changes in the hydrogen bond network at the membrane-water interface. Previously published data on SM:PC mixtures have, however, shown that SM mixes more ideally with DMPC than with DPPC (Calhoun and Shipley, 1979; Lentz et al., 1981; Maulik and Shipley, 1996b; Bar et al., 1997). Hence, the low ideality of DPPC:DHSM mixtures more likely derives from differences in the hydrophobic length of the two lipids. It has been suggested that the PC that most closely resembles 16:0-SM structurally would be a 1-myristoyl-2-palmitoyl-sn-glycero-3-phosphocholine (14:0/16:0-PC) (Maulik and Shipley, 1996b; Holopainen et al., 2000; Li et al., 2001). Fourier transform infrared spectroscopy studies of DPPC:Egg-SM mixtures suggests that mixing of Egg-SM and DPPC do not alter the hydrogen bond network, but that conformational changes in the phospholipids occur (Villalain et al., 1988).
Pretransitions arising from the conversion of the lamellar gel phase to the rippled gel phase have previously been observed in pure 16:0-SM, 16:0-DHSM, and DPPC bilayers (McMullen et al., 1993; McMullen and McElhaney, 1995; Bar et al., 1997; Ramstedt and Slotte, 1999; Kuikka et al., 2001). The origin of the pretransition observed in 16:0-DHSM membranes has not been characterized by other means, but it seems reasonable to assume that it has a similar origin as those observed for DPPC and 16:0-SM membranes.
CONCLUSIONS
In this work we have studied the thermotropic phase behavior of binary mixtures of 16:0-DHSM and sterols (cholesterol and lathosterol), DPPC, or 16:0-SM. Cholesterol inclusion into DHSM membranes was observed to have similar, but clearly different, effects on the gel-to-liquid-crystalline phase transition as compared to acyl-chain-matched SM membranes. The results suggest that cholesterol disrupts the gel phase in DHSM membranes to a lower degree than the SM membranes. Lathosterol had similar effects on the thermotropic behavior of DHSM and SM membranes as cholesterol, but formed more stable sterol-rich domains than corresponding cholesterol concentrations. Miscibility studies of binary SM:DHSM and DPPC:DHSM mixtures showed that DHSM mixed almost ideally with acyl-chain-matched SM, and less favorably with DPPC. This supports the working hypothesis that DHSM might reside in the ordered sphingomyelin and cholesterol-rich domains, where favorable interactions with SM and cholesterol together with the high Tm indicate that DHSM possibly could function as a membrane organizer within these laterally condensed domains.
Acknowledgments
We thank Professor Ronald N. McElhaney for sending a preprint of his recent article.
This work was supported by generous grants from the Sigrid Juselius Foundation, the Academy of Finland, the Magnus Ehrnrooth Foundation, and Medicinska Understödsföreningen Liv och Hälsa.
References
- Attard, G. S., W. S. Smith, R. H. Templer, A. N. Hunt, and S. Jackowski. 1998. Modulation of CTP: phosphocholine cytidylyltransferase by membrane torque tension. Biochem. Soc. Trans. 26:S230. [DOI] [PubMed] [Google Scholar]
- Bar, L. K., Y. Barenholz, and T. E. Thompson. 1997. Effect of sphingomyelin composition on the phase structure of phosphatidylcholine-sphingomyelin bilayers. Biochemistry. 36:2507–2516. [DOI] [PubMed] [Google Scholar]
- Barenholz, Y. 1984. Sphingomyelin-lecithin balance in membranes: composition, structure, and function relationships. Phys. Membr. Fluidity. 1:131–173. [Google Scholar]
- Barenholz, Y., J. Suurkuusk, D. B. Mountcastle, T. E. Thompson, and R. L. Biltonen. 1976. A calorimetric study of the thermotropic behavior of aqueous dispersions of natural and synthetic sphingomyelins. Biochemistry. 15:2441–2447. [DOI] [PubMed] [Google Scholar]
- Bittman, R., C. R. Kasireddy, P. Mattjus, and J. P. Slotte. 1994. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 33:11776–11781. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68:533–544. [DOI] [PubMed] [Google Scholar]
- Brown, R. E. 1998. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 111:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrdwell, W. C., and D. Borchman. 1997. Liquid chromatography/mass-spectrometric characterization of sphingomyelin and dihydrosphingomyelin of human lens membranes. Ophthalmic Res. 29:191–206. [DOI] [PubMed] [Google Scholar]
- Calhoun, W. I., and G. G. Shipley. 1979. Sphingomyelin-lecithin bilayers and their interaction with cholesterol. Biochemistry. 18:1717–1722. [DOI] [PubMed] [Google Scholar]
- Estep, T. N., E. Freire, K. F. Anthony, Y. Barenholz, R. L. Biltonen, and T. E. Thompson. 1981. Thermal behavior of stearoylsphingomyelin-cholesterol dispersions. Biochemistry. 20:7115–7118. [DOI] [PubMed] [Google Scholar]
- Estep, T. N., D. B. Mountcastle, Y. Barenholz, R. L. Biltonen, and T. E. Thompson. 1979. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 18:2112–2117. [DOI] [PubMed] [Google Scholar]
- Holopainen, J. M., J. Lemmich, F. Richter, O. G. Mouritsen, G. Rapp, and P. K. Kinnunen. 2000. Dimyristoylphosphatidylcholine/C16:0-ceramide binary liposomes studied by differential scanning calorimetry and wide- and small-angle x-ray scattering. Biophys. J. 78:2459–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, A. A., P. Michelsen, and G. Odham. 1998. Molecular species of sphingomyelin: determination by high-performance liquid chromatography/mass spectrometry with electrospray and high-performance liquid chromatography/tandem mass spectrometry with atmospheric pressure chemical ionization. J. Mass Spectrom. 33:1192–1198. [DOI] [PubMed] [Google Scholar]
- Kuikka, M., B. Ramstedt, H. Ohvo-Rekila, J. Tuuf, and J. P. Slotte. 2001. Membrane properties of d-erythro-n-acyl sphingomyelins and their corresponding dihydro species. Biophys. J. 80:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbrooke, B. D., R. M. Williams, and D. Chapman. 1968. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and x-ray diffraction. Biochim. Biophys. Acta. 150:333–340. [DOI] [PubMed] [Google Scholar]
- Lentz, B. R., M. Hoechli, and Y. Barenholz. 1981. Acyl chain order and lateral domain formation in mixed phosphatidylcholine-sphingomyelin multilamellar and unilamellar vesicles. Biochemistry. 20:6803–6809. [DOI] [PubMed] [Google Scholar]
- Leppimaki, P., J. Mattinen, and J. P. Slotte. 2000. Sterol-induced upregulation of phosphatidylcholine synthesis in cultured fibroblasts is affected by the double-bond position in the sterol tetracyclic ring structure. Eur. J. Biochem. 267:6385–6394. [DOI] [PubMed] [Google Scholar]
- Li, L. K., L. So, and A. Spector. 1985. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 26:600–609. [PubMed] [Google Scholar]
- Li, X. M., M. M. Momsen, J. M. Smaby, H. L. Brockman, and R. E. Brown. 2001. Cholesterol decreases the interfacial elasticity and detergent solubility of sphingomyelins. Biochemistry. 40:5954–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London, E., and D. A. Brown. 2000. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta. 1508:182–195. [DOI] [PubMed] [Google Scholar]
- Mabrey, S., and J. M. Sturtevant. 1976. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA. 73:3862–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannock, D. A., T. J. McIntosh, X. Jiang, D. F. Covey, and R. N. McElhaney. 2003. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys. J. 84:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik, P. R., and G. G. Shipley. 1996a. Interactions of n-stearoyl sphingomyelin with cholesterol and dipalmitoylphosphatidylcholine in bilayer membranes. Biophys. J. 70:2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik, P. R., and G. G. Shipley. 1996b. N-palmitoyl sphingomyelin bilayers: structure and interactions with cholesterol and dipalmitoylphosphatidylcholine. Biochemistry. 35:8025–8034. [DOI] [PubMed] [Google Scholar]
- McElhaney, R. N. 1982. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem. Phys. Lipids. 30:229–259. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 1993. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 32:516–522. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P. W., and R. N. McElhaney. 1995. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim. Biophys. Acta. 1234:90–98. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P. W., C. Vilcheze, R. N. McElhaney, and R. Bittman. 1995. Differential scanning calorimetric study of the effect of sterol side chain length and structure on dipalmitoylphosphatidylcholine thermotropic phase behavior. Biophys. J. 69:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm, T., M. Nylund, A. Söderlund, and J. P. Slotte. 2003. Properties of palmitoyl phosphatidylcholine, sphingomyelin, and dihydrosphingomyelin bilayer membranes as reported by different fluorescent reporter molecules. Biophys. J. 84:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm, T., and J. P. Slotte. 2001. Comparison of Triton X-100 penetration into phosphatidylcholine and sphingomyelin mono- and bilayers. Langmuir. 17:4724–4730. [Google Scholar]
- Ohvo-Rekila, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- Ollila, F., and J. P. Slotte. 2002. Partitioning of Triton X-100, deoxycholate and C(10)EO(8) into bilayers composed of native and hydrogenated egg yolk sphingomyelin. Biochim. Biophys. Acta. 1564:281–288. [DOI] [PubMed] [Google Scholar]
- Patra, S. K., A. Alonso, J. L. R. Arrondo, and F. M. Goni. 1999. Liposomes containing sphingomyelin and cholesterol: detergent solubilisation and infrared spectroscopic studies. J. Liposome Res. 9:247–260. [Google Scholar]
- Ramstedt, B., P. Leppimaki, M. Axberg, and J. P. Slotte. 1999. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur. J. Biochem. 266:997–1002. [DOI] [PubMed] [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 1999. Comparison of the biophysical properties of racemic and d-erythro-n-acyl sphingomyelins. Biophys. J. 77:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram, M. B., and T. E. Thompson. 1990. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 29:10670–10675. [DOI] [PubMed] [Google Scholar]
- Sarmientos, F., G. Schwarzmann, and K. Sandhoff. 1985. Direct evidence by carbon-13 NMR spectroscopy for the erythro configuration of the sphingoid moiety in Gaucher cerebroside and other natural sphingolipids. Eur. J. Biochem. 146:59–64. [DOI] [PubMed] [Google Scholar]
- Schneider, P. B., and E. P. Kennedy. 1967. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid Res. 8:202–209. [PubMed] [Google Scholar]
- Schroeder, R., E. London, and D. Brown. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA. 91:12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- Slotte, J. P. 1999. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem. Phys. Lipids. 102:13–27. [DOI] [PubMed] [Google Scholar]
- Veiga, M. P., J. L. Arrondo, F. M. Goni, A. Alonso, and D. Marsh. 2001. Interaction of cholesterol with sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry. 40:2614–2622. [DOI] [PubMed] [Google Scholar]
- Villalain, J., A. Ortiz, and J. C. Gomez-Fernandez. 1988. Molecular interactions between sphingomyelin and phosphatidycholine in phospholipid vesicles. Biochim. Biophys. Acta. 941:55–62. [DOI] [PubMed] [Google Scholar]