Abstract
Atomic force microscopy was used to image Bdellovibrio bacteriovorus 109J, a gram-negative bacterial predator that consumes a variety of other gram-negative bacteria. In predator-prey communities grown on filters at hydrated air-solid interfaces, repeated cycles of hunting, invasion, growth, and lysis occurred readily even though the cells were limited to near two-dimensional movement. This system allowed us to image the bacteria directly without extensive preparation or modification, and many of the cells remained alive during imaging. Presented are images of the life cycle in two species of prey organisms, both Escherichia coli (a small prey bacterium that grows two-dimensionally on a surface) and Aquaspirillum serpens (a large prey bacterium that grows three-dimensionally on a surface), including high-resolution images of invaded prey cells called bdelloplasts. We obtained evidence for multiple invasions per prey cell, as well as significant heterogeneity in morphology of bdellovibrios. Mutant host-independent bdellovibrios were observed to have flagella and to excrete a coating that causes the predators to clump together on a surface. Most interestingly, changes in the texture of the cell surface membranes were measured during the course of the invasion cycle. Thus, coupled with our preparation method, atomic force microscopy allowed new observations to be made about Bdellovibrio at an interface. These studies raise important questions about the ways in which bacterial predation at interfaces (air-solid or liquid-solid) may be similar to or different from predation in solution.
INTRODUCTION
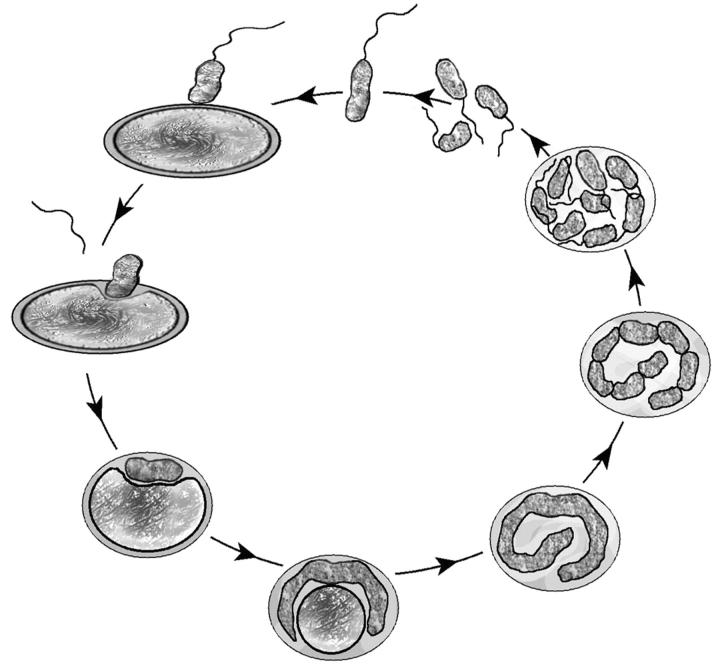
Bdellovibrio bacteriovorus is a gram-negative, obligately aerobic bacterium that preys upon a wide variety of other gram-negative bacteria, including Escherichia coli and Aquaspirillum serpens (Diedrich, 1988; Martin, 2002; Rittenberg, 1983; Ruby, 1991). Its life cycle has two major stages: a free-swimming stage spent searching for prey in water or soil (the “attack phase”) and a growth stage spent inside the periplasm of the prey bacterium (Fig. 1) (Seidler and Starr, 1969a). After violently colliding with a prey cell and irreversibly attaching to its surface, the predator breaches the outer membrane, kills the prey cell by halting its respiration and growth, and takes up residence in the prey's periplasm between its inner and outer membranes (Rittenberg and Shilo, 1970). Here the bdellovibrio cell grows and divides, utilizing the prey's macromolecules for fuel and essential building blocks. At this stage the bdellovibrio predator and the killed prey cell in which it is growing are together termed a bdelloplast. Once the resources of the prey have been consumed, the bdellovibrio divides into progeny bdellovibrios, which then lyse the remains of the cell and swim away to pursue new hosts (Kessel and Shilo, 1976). Depending on the prey and environment, this life cycle takes roughly 3–4 h.
FIGURE 1.
Diagram of the two-stage life cycle of the bacterial predator Bdellovibrio bacteriovorus, which consists of a free-swimming stage spent living in water or soil, and a growth stage spent inside its prey bacterium. A Bdellovibrio predator and the killed prey cell in which it is growing are together termed a bdelloplast.
In the course of the invasion and digestion cycle, the bdellovibrio makes significant changes in the structure of the prey cell, not all of which are fully understood. The predator modifies the prey's exterior membrane and peptidoglycan layer without destroying them, using a variety of enzymes including glycanases, deacetylases, peptidases, and lipopolysaccharidases (Diedrich, 1988; Martin, 2002; Rittenberg, 1983; Ruby, 1991). This process of modification and invasion changes the shape and other characteristics of the invaded cell, so that it “rounds up” and swells (Tudor et al., 1990). The bdellovibrio also modifies the prey's inner (cytoplasmic) membrane so that degradative enzymes and degraded cellular material can be transferred between the prey's cytoplasm and the predator (Cover et al., 1984; Odelson et al., 1982; Pritchard et al., 1975; Rittenberg and Langley, 1975; Saier, 1994).
We used atomic force microscopy to investigate Bdellovibrio structure and life cycle at an interface. Atomic force microscopy (AFM) is a type of scanning probe microscopy that can resolve structures that are tens or hundreds of nanometers in size with soft biological samples such as macromolecules and cells (Dufrene, 2001; Hansma, 2001; Kumar and Hoh, 2001; Stolz et al., 2000). As optical microscopy is to vision, AFM is to Braille: because the instrument tip moves across the sample surface, the technique can potentially reveal not only information about shape and size, but also about tip-sample interactions involving texture, adhesion, and elasticity of the sample (Hansma et al., 1997). The major benefit of AFM is that it provides better resolution than light microscopy, and samples need not be fixed or stained in any way nor imaged under vacuum as needed for electron microscopy. In fact, living eukaryotic cells, functioning macromolecular systems, and bacterial biofilms have been imaged and investigated in real time in fluid using AFM (Beech et al., 2002; Dufrene, 2001; Hansma, 2001; Kumar and Hoh, 2001; Stolz et al., 2000; Surman et al., 1996). This study provides the first use of atomic force microscopy in the examination of Bdellovibrio.
Because with AFM the object to be imaged must be supported by a solid surface, we have developed a system for growing and imaging predator/prey populations at an interface that may be generally applicable to many bacterial systems. On a surface where prey cells can grow and reproduce, bdellovibrios are able to carry out their full life cycle. Not only does this system allow us to image bdellovibrios and bdelloplasts at different stages of invasion, but it allows us to start thinking about bacterial predation and other processes at interfaces (air-solid or liquid-solid) that can in fact be quite different that those that take place in solution (Costerton et al., 1995; O'Toole et al., 2000; Stoodley et al., 2002). In the complex and heterogeneous milieu of a biofilm, intricate mechanisms for hunting, invasion, and growth might emerge.
METHODS
Bacterial Cultures
Bdellovibrio bacteriovorus strain 109J was obtained from the American Type Culture Collection (Manassas, VA). Two member liquid growth cultures of Bdellovibrio on E. coli (strains ML35 or DH5α) or other host strains were carried out as described by Ruby (1991) in HM buffer (10 mM HEPES, 0.1 mM MgCl2, 1 mM CaCl2). A. serpens was obtained from Carolina Biological Supply (Burlington, NC) and propagated as recommended by the supplier. Frozen bdelloplasts were prepared in glycerol as described by Ruby (1991).
Host independent B. bacteriovorus strain 109J-KAI was obtained from E. G. Ruby, and was propagated on PYE medium (10 g peptone, 3 g yeast extract per liter) at 30°C.
Preparing bacterial communities and imaging by AFM
For the samples imaged on mica, frozen bdelloplast stocks were thawed and bdelloplasts were isolated by centrifugation. The supernatant containing medium and glycerol was discarded, and the pellet was resuspended in 10 mM MgSO4; ∼8 μL of this solution was deposited onto freshly cleaved mica and allowed to dry before imaging.
For all other samples, 8–15 μL of a solution containing bacteria, suspended in either culture medium or 10 mM MgSO4, were deposited onto filters over dilute nutrient broth (DNB) plates (0.08% Difco (Detroit, MI) nutrient broth, 0.05% Difco casamino acids, 0.01% Difco yeast extract, pH 7.6; and 1 mM CaCl2 and 0.1 mM MgCl2 added after autoclaving). Either 0.2 μm or 1.2 μm Isopore polycarbonate filters (Millipore, Billerica, MA) were used after sterilization by autoclaving. The solution soaked through the filters into the plates within ∼30 min and the bacteria remained on top. Samples were incubated at room temperature for periods ranging from 1 h up to several days. Filters containing bacterial cells were removed from the plates and fastened to stainless-steel pucks with double-sided adhesive tape, and the microorganisms were imaged immediately without any further treatment.
Bacteria were imaged for up to several hours after removal from the agar by either contact or tapping-mode AFM in air using a Digital Instruments Multimode SPM with a NanoScope IIIa controller. Although tapping-mode is generally suggested for imaging soft samples, we found that contact mode also worked quite well. Oxide-sharpened silicon nitride tips were used for contact mode, and silicon tips with cantilever resonance frequencies around 300 kHz were used for tapping mode (both from Digital Instruments, Santa Barbara, CA). Only one modification to the images was performed as provided by the image software: the vertical offset between scan lines was removed, termed flattening. For a given image, this was accomplished by subtraction of the average vertical value of the scan line from each point in the scan line. Images were then exported to a graphics program (Adobe Photoshop 6.0) for final adjustment of dimensions and contrast. The difference between the highest and lowest points within a given area was calculated after flattening using the roughness analysis in the NanoScope software (Digital Instruments).
RESULTS
Bdellovibrio-E. coli predation imaged on mica
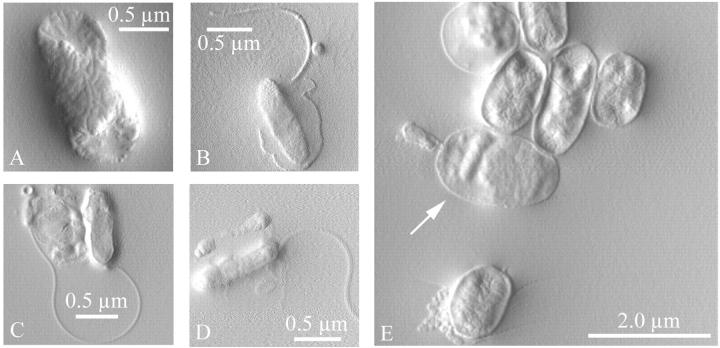
We obtained high-resolution images of B. bacteriovorus and one of its potential prey organisms, E. coli, by atomic force microscopy, after the bacteria were grown in liquid, rinsed, and dried on a smooth mica surface (Fig. 2). Panel A shows a prey E. coli (DH5α). The bacterium was roughly 0.75 μm × 2 μm in size and oblong in shape. Due to the drying process, it appeared wrinkled and dried-out like a raisin, and the ends were sunken. Panels B–D show the predator B. bacteriovorus. These cells were ∼800 nm–1 μm in length and oblong in shape. Their slightly curved, vibrioid character was not evident from these images. Each bacterium possessed a single polar flagellum. A significant amount of dried salt, cell debris, and other material was seen around the cells, making the fine structure of the flagella and edges difficult to resolve. Panel E shows a group of unmodified prey cells and two invaded prey cells, or bdelloplasts. In the transformation from live, healthy prey bacteria to bdelloplasts, several alterations in the appearance of the cells occurred. As documented by electron microscopy (EM) and confirmed here by AFM, the invaded cells lost their oblong shape and became round (Starr and Baigent, 1966). Furthermore, we observed that when viewed from the outside, “lumps” in the shape of the interior predator were often observed, and the surface texture changed. In the images, bdelloplasts were identified by their roundness, bumpy shape, and smooth surface texture, like the cell at the top left of Fig. 2 E. The bdelloplast at the center (arrow) contained a visible predator in its periplasm, consuming the cytoplasm of the prey cell, while a second predator was seen attached to the external surface of the cell with its long axis oriented perpendicular to the bdelloplast's outer surface.
FIGURE 2.
Bdellovibrio bacteriovorus and invaded and uninvaded prey E. coli were imaged by atomic force microscopy in tapping mode (amplitude image). Bacteria were rinsed and dried on a smooth mica surface before imaging. (A) The prey E. coli, (B–D) the predator Bdellovibrio, and (E) invaded prey cells (bdelloplasts) were all observed. The arrow in E indicates a bdelloplast inside of which the predator was visible; a second predator was seen attached to the external surface of the cell. Another bdelloplast was visible at the top left, identified by its roundness, bumpy shape, and smooth surface texture.
Bdellovibrio predation of E. coli at an interface
Because of drying artifacts and cell debris, we desired to image the bacteria in as native a state as possible. Growing, healthy bacteria are very difficult to image well in fluid without drying or chemical or physical modification (vide infra). Instead, we grew them on smooth, small-pore filters placed on top of DNB agar plates, and then imaged the bacteria in air. Several different kinds of bacteria grew well on the filters over plates, indicating that the bacteria had access to liquid and nutrients from the plate (Auerbach et al., 2000; Steinberger et al., 2002, and unpublished results). When placed on the filter, E. coli grew and divided immediately, formed a two-dimensional layer, spread outward across the filter, and divided into smaller cells ∼1 μm in diameter (Fig. 3 A).
FIGURE 3.
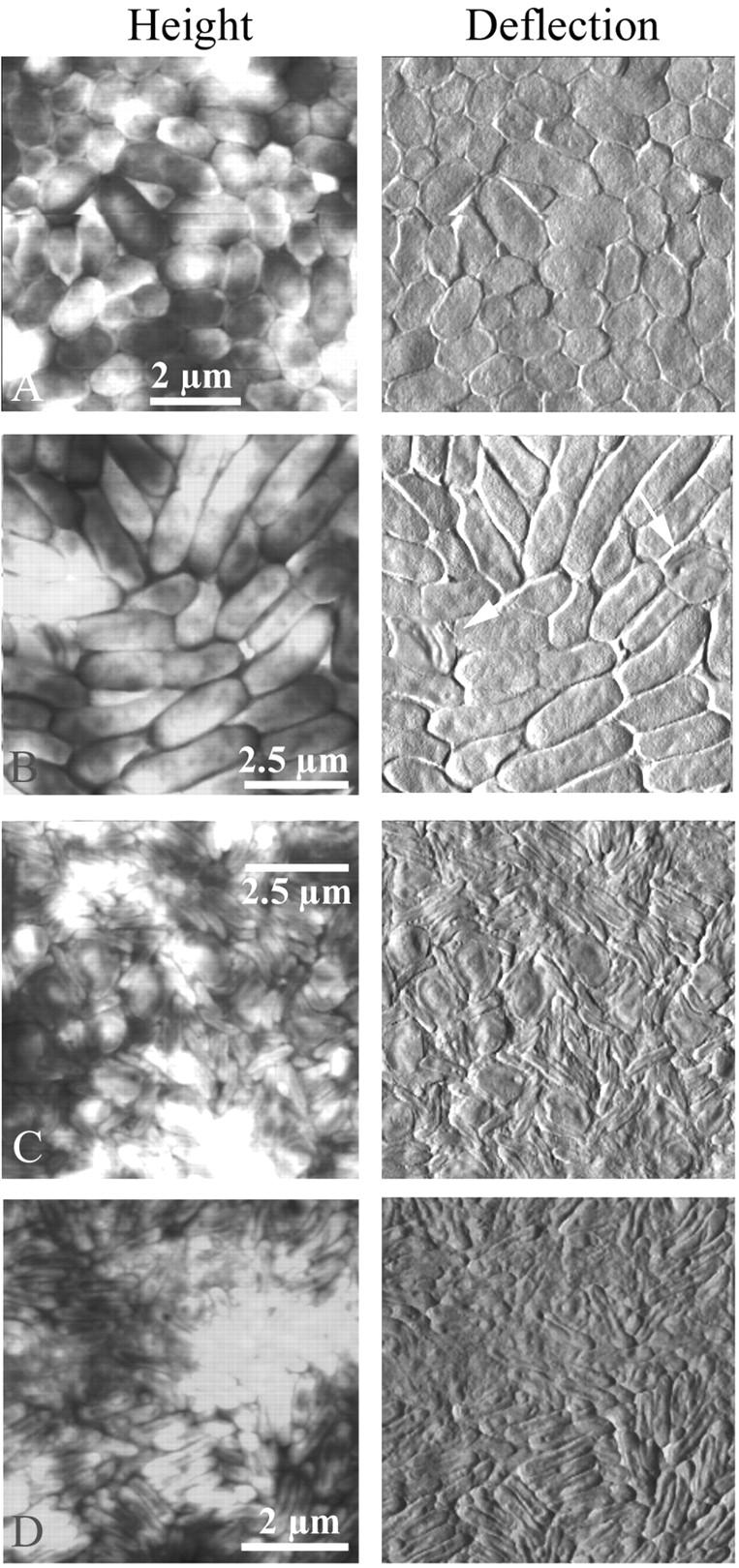
The life cycle of Bdellovibrio in a population of E. coli was imaged by atomic force microscopy in contact mode (height and deflection images). Solutions of predator and prey were mixed and deposited on smooth, small-pore filters over DNB plates. At various times over several days, filters were removed to the AFM and imaged. (A) Prey E. coli grew quickly on the filters to form a continuous layer of cells. The total depth range of the height image was only ≈200 nm, compared to a cell height of ∼400 nm, which indicated that the cells were all roughly the same height and were therefore growing next to but not on top of each other. (B) A few invaded cells, or bdelloplasts, were initially present in the population of healthy cells. (C) Given a few days to hunt in the monolayer of prey, Bdellovibrio invaded every cell, and only bdelloplasts and predators were seen. (D) Eventually only the predator bacteria and some cell debris remained. The total depth range of the height image was ≈150 nm. The exact time elapsed between different stages depended upon the concentration and vigor of the two initial bacterial cultures, as well as the location on the filter that was imaged.
E. coli and Bdellovibrio were mixed before placing onto a filter and the life cycle of Bdellovibrio was imaged by AFM over the course of several days (Fig. 3). The exact time elapsed between different stages depended upon the concentration and vigor of the two initial bacterial cultures and the location on the filter being imaged, but the general course of the invasion was always the same. Within 1–2 h of mixing, a few bdelloplasts became apparent (Fig. 3 B). Given several hours or a few days to hunt in the monolayer of prey bacteria, bdellovibrios invaded every cell, and only round, smooth bdelloplasts and smaller oblong, vibrioid predators were seen (Fig. 3 C). Eventually only the voracious predator bacteria and some cell debris remained (Fig. 3 D). It was difficult to see the predators' flagella because of the tight cell packing and the debris of the digested prey cells. The predators seen here averaged around ∼1.5 μm in length, longer than those seen in Fig. 2 or than the predators initially mixed with the prey, which averaged around 700 nm–1 μm.
Bdelloplasts and predation
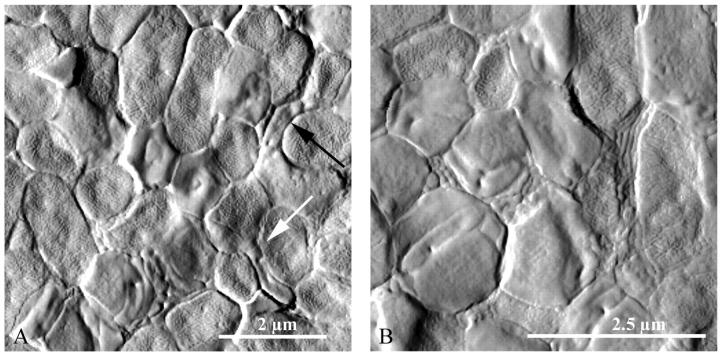
We obtained higher-resolution AFM images of a mixed E. coli-Bdellovibrio system (Fig. 4). Several bdelloplasts were interspersed with healthy cells. Again we saw that the bdelloplasts were round or irregular in shape and had a smooth surface texture, whereas the uninvaded cells generally had a more oblong shape and a rough surface texture. Furthermore, the growing Bdellovibrio were visible inside of many of the bdelloplasts as smaller oblong or longer coiled shapes. In Fig. 4 A, two bdellovibrio predators were observed attacking or leaving prey cells (arrows). Both newly attacked prey cells appeared to have rough texture and fairly intact shape, except in the immediate vicinity of the bdellovibrio attack, where the cell texture more closely resembled that of a bdelloplast. One bdellovibrio (black arrow) seemed to have just left a lysed cell behind, which appeared smoother and more irregular in shape than neighboring uninvaded cells. Alternatively, the bdellovibrio may have been moving in the opposite direction, and this image of a smooth, irregular cell was captured later in the attack phase when the outer surface had been extensively modified but the bdellovibrio had not fully entered the prey to make a bdelloplast. The unusual surface appearance of the cell behind it would then have been due to an interaction of the prey cell or AFM tip with the predator's flagellum.
FIGURE 4.
High-resolution AFM images of a mixed E. coli-Bdellovibrio system, taken in contact mode (deflection). Several bdelloplasts were visibly mixed in with healthy prey cells. The bdelloplasts were round or irregular in shape and had a smooth surface texture, whereas the uninvaded cells generally had a more oblong shape and a rough surface texture. Furthermore, the growing Bdellovibrio were visible inside of many of the bdelloplasts as smaller, oblong, or coiled shapes. Arrows indicate bdellovibrios that were in the process of attacking prey cells.
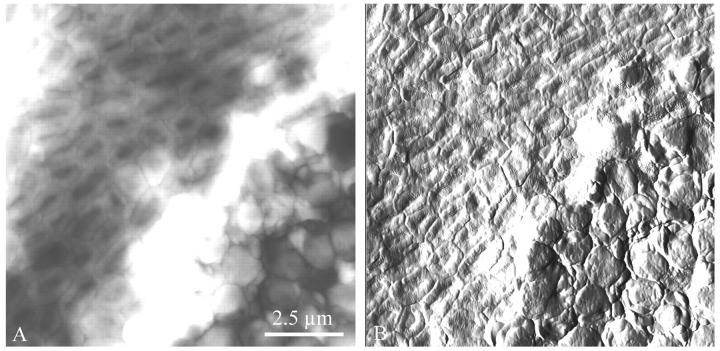
A hint of the population dynamics is seen in Fig. 5, which shows an advancing “tide” of bdellovibrios and bdelloplasts encroaching on a flat expanse of uninvaded prey cells. It was common for the prey bacteria (which have a life cycle with a much shorter period than the predator) to multiply at the edges of the circular community of cells, away from the predator bacteria at the center. On any one filter, multiple stages in the invasion cycle were seen, with bdellovibrios and bdelloplasts toward the center, and normal prey near the edge. In this field of view, the uninvaded cells, at top left, were slightly wrinkled in texture, whereas the bdelloplasts, at bottom right, were smoother and more rounded. Notably, the interface between the two areas, presumably where the attacking predators and newly invaded bdelloplasts are located, was raised compared to the cells on both sides. This height difference was possibly due to pressure by the swelling of the new bdelloplasts and/or by the movement of the bdellovibrios toward their prey.
FIGURE 5.
An advancing “tide” of bdellovibrios and bdelloplasts overtaking a flat expanse of uninvaded prey cells. In the deflection picture at right (B), it is clear that the uninvaded E. coli (top left) were slightly wrinkled in texture, whereas the bdelloplasts (bottom right) were smoother and more rounded. Notably, in the height image at left (A), the interface between the invaded and uninvaded cells, where the attacking predators and newly invaded bdelloplasts are located, was raised compared to the cells on either side by 150–250 nm. The prey bacteria, which have a life cycle with a much shorter period, multiplied at the edges of the circular community away from the predator bacteria at the center, and thus a gradient of predation stages was present on the filter.
Bdellovibrio predation of Aquaspirillum at an interface
The life cycle of Bdellovibrio in a population of A. serpens was also imaged by atomic force microscopy to explore whether the morphology of bdelloplasts or progeny predators is noticeably different in another prey organism. Again, solutions of predator and prey were mixed and deposited on smooth, small-pore filters over DNB plates, and at various times over several days, filters were removed to the AFM and imaged. Healthy Aquaspirillum, the prey bacteria, were very long and lumpy, and unlike E. coli grew in multiple layers on top of the filter (Fig. 6 A). The presence of “lumps” in the uninvaded cells made bdelloplasts difficult to identify. In panel B showing a very early-stage bdelloplast, it appeared that two bdellovibrios invaded the same aquaspirillum. Although it is possible that one or both of the predators have been trapped next to the prey but are not inside of it, we observed that the predators often appeared to be lying next to the prey with their long axes parallel to the prey outer membrane. In the initial stages of invasion, the available space in the periplasm for the predator is very small, and so the large indentations in the cytoplasm and distended periplasm characteristic of later bdelloplasts have not yet been formed. In panel C, a long “coil” of growing Bdellovibrio was visible inside of the Aquaspirillum bdelloplast. The remainder of the cytoplasm was also visible between the coils. These very long, spiraled nascent bdellovibrio were proposed earlier from electron microscope images to be common in spirilla, which are long and coiled themselves (Abram et al., 1974). As a result, these prey bacteria with large volumes yield more progeny cells (Gray and Ruby, 1989; Kessel and Shilo, 1976). Panel D shows that given a few days to hunt for prey on the filter, bdellovibrios invaded and consumed every prey cell, as with E. coli mixed monolayers. However, because the Aquaspirillum prey cells grew three-dimensionally on top of the filter, the predator cells were also piled on top of each other and the cell debris. The aquaspirillum at the left had many bdellovibrio nestled up next to it, some of which may actually be inside the periplasm of the host cell.
FIGURE 6.
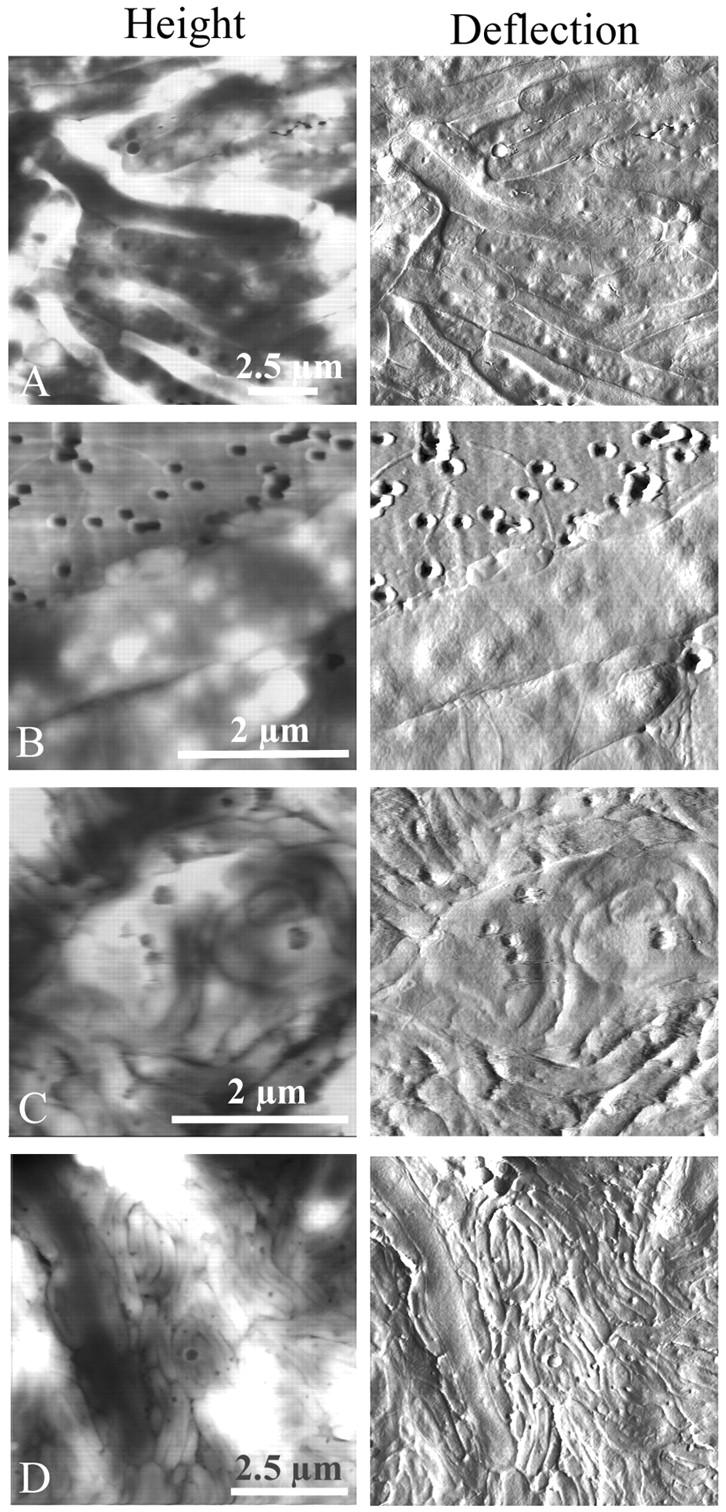
The life cycle of Bdellovibrio in a population of Aquaspirillum serpens imaged by atomic force microscopy in contact mode (height and deflection). Again, solutions of predator and prey were mixed and deposited on smooth, small-pore filters over DNB plates, and at various times over several days, filters were removed to the AFM and imaged. (A) Healthy prey Aquaspirillum were very long and lumpy, and unlike E. coli many grew in multiple layers on top of the filter. The total depth range of this height image was ≈400 nm, and in other fields was >600 nm, which indicated that the cells grew on top of each other. The presence of “lumps” in the uninvaded cells made bdelloplasts difficult to identify. (B) Two Bdellovibrio invaded the same Aquaspirillum. (C) A long coil of growing Bdellovibrio was visible inside of the Aquaspirillum bdelloplast, as proposed earlier from electron microscope images. (D) As with E. coli mixed monolayers, given a few days to “graze” on the prey, Bdellovibrio invaded and consumed every cell. One remaining spirillum was being attacked and consumed at the left side of the figure. Note that because the Aquaspirillum prey cells grew three-dimensionally on top of the filter, the predator cells were also piled on top of each other and the cell debris. The total depth range of this height image was ≈1.2 μm, and in other fields was at least 800 nm.
In addition to examining bdelloplasts and the life cycle of Bdellovibrio in different prey communities, we also compared the wild-type and host-independent mutant Bdellovibrio grown under different conditions (Fig. 7). The most interesting observation was that the morphology, more particularly the length, varied widely. Slight differences in texture between samples were due to differences in individual AFM tips. Panel A shows wild-type bdellovibrios that were cocultured with E. coli on filters over DNB plates for several days and have consumed all of the prey. They were fairly long (∼1.5 μm) and slender, and despite the fact that they grew on a flat surface, many appear to have flagella. Panel B shows wild-type bdellovibrios that were grown in solution with E. coli, and then after clearing the solution of prey were spotted onto a filter and imaged. Many of these predators were also very long, although the size varied widely. On the bottom right, four bdellovibrios in a row septated from a ∼3 μm proto-bdellovibrio, most likely after they were spotted onto the filter. Panels C–F are images of host-independent mutant Bdellovibrio. These bdellovibrios stuck together on filters in clumps and strings, even when washed by centrifugation and resuspension. These mutants had flagella (panels D and E) and varied in size from ∼800 nm long (C and D) when spotted onto dilute nutrient broth plates and imaged the same day, to 4 μm long (E and F) when spotted onto regular nutrient broth plates and imaged one day later.
FIGURE 7.
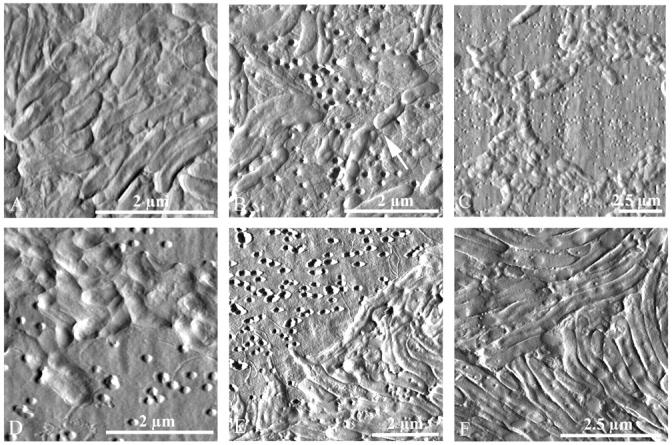
Images of wild-type and host-independent mutant Bdellovibrio taken in contact mode (deflection). The morphology varied widely. (A) Cocultured with E. coli on filters over DNB plates, the wild-type Bdellovibrio consumed all of the prey. (B) These wild-type Bdellovibrio was grown in solution with E. coli, and then after clearing, the solution of prey were spotted onto a filter and imaged. (C and D) The host-independent mutant Bdellovibrio stuck together on filters, even when pelleted, rinsed, and deposited on plates with minimal nutrients. (E and F) These mutants had flagella and, when left to grow on nutrient media plates overnight, were very long.
DISCUSSION
Images of the Bdellovibrio life cycle with E. coli prey
We obtained detailed images of the Bdellovibrio life cycle in E. coli, and in large part, our images of Bdellovibrio obtained by AFM agreed with the extensive electron microscope studies of the 1960s and '70s (Abram and Davis, 1970; Abram et al., 1974; Burnham et al., 1968; Seidler and Starr, 1968; Snellen and Starr, 1974; Starr and Baigent, 1966). The general size and shape of the bdellovibrios and the presence of a single polar flagellum were consistent with earlier images (Figs. 2 and 7; vide infra), as was the characteristic “rounding” of the bdelloplasts after invasion as seen by EM (Figs. 2–4) (Starr and Baigent, 1966). Although AFM is a technique that gives information about shape and topology of the surfaces of cells, we were also able to image features of the interior of the bdelloplast, resolving the shape of the growing bdellovibrio and the remaining cytoplasm. We found that the distended periplasm of late bdelloplasts was not an artifact of the EM sample preparation, and that the prey's cytoplasmic envelope remained relatively intact through the early attachment-invasion-digestion cycle.
Changes in the outer membrane of invaded cells
We also were able to examine the surface properties of Bdellovibrio using AFM. Although the electron microscopy studies revealed important information about the size, shape, and ultrastructure of the predators and bdelloplasts, these structural studies were not able to answer many pressing questions about cell surfaces and membranes that are too thin, fragile, or highly modified by the sample preparation or imaging technique. Because AFM is a scanning probe technique, it very effectively revealed differences in properties of bdelloplast surfaces. Consistent with what is known about how the invading predator modifies the surface of the prey cell (Diedrich, 1988; Martin, 2002; Rittenberg, 1983; Ruby, 1991, and references therein), we detected clear differences in the surface of invaded and native prey cells using the deflection data in contact mode and amplitude data in tapping mode. These data gave us information about structure and texture of the surface (Figs. 2–4). Bdelloplasts, unambiguously identified by the rounding of the prey cell and the distinctive shape of the coiled predator inside, were seen to additionally have a distinctively smooth texture when compared to normal uninvaded E. coli, the surfaces of which are slightly bumpy or wrinkled. The wrinkled appearance is thought to be due to shrinkage of periplasm on drying, causing the outer membrane and peptidoglycan to shrink down over the cytoplasmic membrane (Umeda et al., 1998). The different appearance of the bdelloplasts could have occurred for a variety of reasons: because the outer membrane was stretched out around the bdellovibrio cell in the periplasm; because the prey cell's inner membrane potential or ion balance has been breached; because the predator had broken down the outer membrane and peptidoglycan sufficiently that they shrank more evenly; or because the bdellovibrio cell had removed molecules from or added molecules to the outer membrane that made it “appear” different to the tip.
To address these possibilities, we turned to the AFM's force measurement mode, which can be used to quantitatively measure the adhesion or elasticity of a sample by measuring the forces exerted on the tip when extended toward or retracted from a surface. However, when AFM was used to measure the surface properties of bdellovibrios, bdelloplasts, and uninvaded prey cells side-by-side on the same filter, a significant difference in adhesion or elasticity of the bacteria and bdelloplasts was not observed. We believe that small changes and differences in the cell surfaces were masked by meniscus forces between the tip and the hydrated surface. Thus, we will continue to pursue the issue by measuring surface properties in a liquid environment.
Bdellovibrio attacks in progress have been imaged here by AFM at high resolution. A “swelling of cell envelope at the site of attachment” (Burnham et al., 1968) had been identified by EM, but it was unclear whether this phenomenon was native or an artifact of preparation. Using AFM, we observed that the texture of the cell near the site of attachment of the attacking bdellovibrio may be different than the texture of the rest of the cell (Fig. 4 A). This unique image captured the modification of the cell membrane and peptidoglycan that is necessary to provide the bdellovibrio with an entryway into the periplasm. This alteration of the prey cell would then appear at the attack site and spread from there to the rest of the cell. Alternatively, the difference in the texture of the cell surface at the site of attachment may merely have been due to localized stretching of the membrane caused by entry of the predator cell.
Images of the Bdellovibrio life cycle with Aquaspirillum prey
The progress of Bdellovibrio predation in Aquaspirillum provides new and different information about the life cycle of this predator. Unlike E. coli bdelloplasts and previous images of Aquaspirillum bdelloplasts (Abram et al., 1974), the Aquaspirillum bdelloplasts shown here did not “round up” after invasion. Clearly the change in shape of the bdelloplast is not necessary for the predator to grow inside, but is merely a side effect of modification of the prey structures. Consistent with this observation, bdellovibrio strain W that does not form spherical bdelloplasts still invades, multiplies, and lyses prey cells (Tudor et al., 1990). The retention of the normal shape that we observed may be caused by steric restrictions on a major change in shape and size by neighboring spirilla and bdellovibrios. Interestingly, the surface texture of the aquaspirillum bdelloplasts shown here also did not change noticeably upon invasion, which makes these bdelloplasts difficult to differentiate from uninvaded cells. The retention of normal shape and surface texture may have been due to the presence of S-layers, which are common in Aquaspirillum. However, if the shape and texture of these aquaspirillum cells were retained due to S-layers, it is notable that this S-layer did not protect the cells from invasion and lysis by the predator (Koval and Hynes, 1991).
Contrary to the hypothesis in the published literature that the first predator to invade excludes invasion by multiple predators, we observed two invasion events in the same aquaspirillum cell (Fig. 6 B). In the bdelloplast thin slices prepared for electron microscopy, it was sometimes difficult to tell how many bdellovibrios are inside, or if the presence of more than one bdellovibrio was due to invasion by multiple predators or early division of the first predator (Starr and Baigent, 1966). Thus it is possible that multiple invasions had been seen before and not recognized. However, in this case, because there was no sectioning of the bdelloplast involved in the preparation, it is clear that the two predators were separate and independent. Given the initially localized changes in membrane structure that may accompany invasion and the large size of an aquaspirillum cell, it is reasonable to imagine that two bdellovibrios could invade distal parts of the same cell independently and simultaneously, and still modify the membrane to exclude subsequent invasions. Simultaneous irreversible attachment of more than one predator to a single prey cell (but not invasion) has even been captured by EM (Burnham et al., 1968).
Bdellovibrio pleomorphism
The morphology of Bdellovibrio imaged here by AFM was strikingly heterogeneous, or pleomorphic (Fig. 7), consistent with reports of significant variation in the observed sizes of bdellovibrios (Barel and Jurkevitch, 2001; Burnham et al., 1968; Eksztejn and Varon, 1977; Seidler and Starr, 1969b). After grown on surfaces with prey bacteria, well-fed bdellovibrios were much longer than before feeding (∼1500 nm vs. ∼800–1000 nm) and were quite uniform in shape and size. On the filters, wild-type bdellovibrios might have “grazed” on the remaining debris of lysed cells, permitting them to stay long and healthy even after the local prey cells were all killed. Additionally, the bdellovibrios that grew on the surfaces may have spread themselves out along the surface to increase their surface area and thus their access to water and/or nutrients (Steinberger et al., 2002).
In contrast, when imaged after feeding on prey bacteria in solution, bdellovibrios were heterogeneous in size, with some long ones and others that were in the process of dividing to the pre-feeding size. Wild-type bdellovibrios have been proposed to divide and grow flagella before exiting the bdelloplast, but here a wild-type bdellovibrio outside of a bdelloplast was observed in the process of septation (Fig. 7 B). The presence of other very long bdellovibrios in the same field of view hinted that perhaps cell division and flagellar growth outside of the bdelloplast may also occur (Ruby and Rittenberg, 1983).
Although the host-independent bdellovibrios were quite short and rounded when initially placed on filters, strikingly long host-independent bdellovibrios (∼4–5 μm in length) were observed at high predator densities after incubation on filters over nutrient plates (compare Fig. 7, C and D, and Fig. 7, E and F). Clearly the host-independent bdellovibrios could grow well on top of the filters, much like the prey bacteria. These actively growing host-independent bdellovibrios also had flagella. Flagella had been observed previously on a different strain of host-independent bdellovibrios by EM (Seidler and Starr, 1968), but are frequently not present after long-term growth in the absence of prey (Seidler and Starr, 1969b). The presence of flagella on this particular mutant strain was especially interesting given that it has no motility as assayed by inoculation into motility agar (M. Martin, unpublished results), consistent with Seidler and Star (1969b), but in contrast to Barel and Jurkevitch (2001). The differences reported in the literature may be due to the fact that different isolates of bdellovibrios were used for these studies.
We also observed a layer of some extracellular material that encourages cell clumping in mutant host-independent bdellovibrios. The presence of this coating was not observed by AFM with wild-type bdellovibrios. Truly host-independent bdellovibrios are thought to arise from at least two mutations, whereas host-independent bdellovibrios arising from a single mutation grow only in the presence of other host-independent bdellovibrios or cell extracts (Thomashow and Cotter, 1992). It may be that this coating serves as an adhesive agent, helping host-independent bdellovibrios to clump together and grow better than they would grow alone.
Bacterial predation at an interface
The clear benefit of this system for growing Bdellovibrio is its direct use with atomic force microscopy. Visualization of native bacteria is severely hampered by the fact that bacteria grown in solution are not easily adhered to a surface for imaging by AFM (Dufrene, 2002; Robichon et al., 1999). Bacteria can be dried from a liquid culture onto a solid surface such as mica, but we find that the choice of media can dramatically affect the appearance of the cells, and salts and cell debris can obscure the sample. Alternatively, bacterial cells can be chemically fixed to make them more sticky, but this method has the potential to change the surface of the cells (Razatos, 2001). Finally, some spherical cells, especially spores, have been trapped in membranes by suction filtration and imaged successfully in liquid (Dufrene, 2002), but for our more heterogeneous, dynamic system, we find that the suction and drying process distorts or damages many cells and results in very few intact, trapped bacteria for imaging. Clearly no one method yet described will be useful for every bacterial system being studied, but we believe that growing bacteria at an interface provides a good alternative for preparing many different bacteria. A variety of bacteria, including Pseudomonas putida, Pseudomonas aeruginosa, Micrococcus luteus, E. coli, and A. serpens grow, divide, and spread out on these filters and can be imaged at various stages by AFM (Auerbach et al., 2000; Steinberger et al., 2002, and unpublished results). The bacteria grown this way can be imaged directly without treatment of any kind, even washing by centrifugation and resuspension. Cells are not dried before imaging, and indeed appear to be still moist for some time; although there is some loss of viability, a large fraction remain alive and can be cultured even after removal of the filter from the plate, transfer to the puck, and imaging by AFM (data not shown). It may be that cells grown in an interfacial environment are adapted to be more resistant to desiccation than solution-grown cells. A retention of viability after drying and imaging is consistent with previous studies of more harshly treated bacteria imaged by AFM (Yao et al., 2002).
Moreover, this predator/prey system raises important questions about the dynamics and processes of bacterial populations at interfaces, which may be more relevant than these same processes in solution. It has emerged in the last few years that biofilms are probably the native state of many bacteria (Costerton et al., 1995; O'Toole et al., 2000; Stoodley et al., 2002; Watnick and Kolter, 2000). Thus bacteria are not free-swimming, as we have traditionally thought of them, but are clumped and often attached to a surface. They can live at an interface, generally solid-liquid but sometimes solid-air or liquid-air. The bacteria, phage, and other microbes live together like a multicellular organism, with layers and channels between the cells, gradients in cell activity and nutrients, intercellular communication via small molecules, and excreted materials to cement and shield cells. AFM has previously been successfully used to visualize simple biofilms, although not with predator bacteria (Beech et al., 2002; Surman et al., 1996). In the system described here, the bacteria grew at hydrated solid-air interface. These E. coli monolayers thus shared some similarity to early stages of biofilm development. Aquaspirillum communities more closely resembled biofilms, in that they were dense and multilayered, and appeared to excrete some extracellular substances that made them sticky and hard to image after approximately one week on a filter.
Despite taking place at an interface between air and a well-hydrated surface, the Bdellovibrio-E. coli and Bdellovibrio-Aquaspirillum predator-prey systems resembled their related systems in solution. Further work remains to explore what differences may exist between the population dynamics of these interfacial cultures and the more traditional liquid cultures. The dynamics of predation in monolayer (E. coli) versus multilayer (Aquaspirillum; also Pseudomonas) systems should be examined, as well as environmental factors such as nutrient concentrations and temperature. The recent evidence that Bdellovibrio can associate with other bacteria on surfaces (Markelova and Colwell, 1999; Williams et al., 1995), as well as the prevalence and complexity of bacterial biofilms in nature indicate that understanding predation at an interface is critical to understanding biofilms in the environment. The heterogeneous diversified structure, morphology, and prey composition of mixed-species biofilms, as well as the role of slime layers and the possibility of cell-cell communication, present challenges to the bacterial predator and fascinating new research directions for us.
CONCLUSIONS
Atomic force microscopy is an excellent and novel tool for the examination of bacterial morphology and interactions. We have successfully explored the growth of complex bacterial communities on filters, and demonstrated the use of AFM to image cells and study predation in these communities at an interface without modification or staining. These studies will be extended to an exploration of predation in biofilms, a much more biologically relevant yet complicated state of prokaryotes in nature.
Acknowledgments
We thank Sarah Studer for her assistance in growing bacteria and Professor J. Quinn for her artistic talent in drawing Fig. 1.
The authors gratefully acknowledge the National Science Foundation (Leveraged Starter Grant, Research Planning Grant, and Presidential Early Career Award for Scientists and Engineers), the Dreyfus Foundation, Research Corporation, ACS Petroleum Research Fund, the James Irvine Foundation, Occidental College, and the Howard Hughes Medical Institute for generous financial support.
References
- Abram, D., and B. K. Davis. 1970. Structural properties and features of the parasitic Bdellovibrio bacteriovorus. J. Bacteriol. 104:948–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram, D., J. Castro e Melo, and D. Chou. 1974. Penetration of Bdellovibrio bacteriovorus into host cells. J. Bacteriol. 118:663–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach, I. D., C. Sorensen, H. G. Hansma, and P. A. Holden. 2000. Physical morphology and surface properties of unsaturated Pseudomonas putida biofilms. J. Bacteriol. 182:3809–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel, G., and E. Jurkevitch. 2001. Analysis of phenotypic diversity among host-independent mutants of Bdellovibrio bacteriovorus 109J. Arch. Microbiol. 176:211–216. [DOI] [PubMed] [Google Scholar]
- Beech, I. B., J. R. Smith, A. A. Steele, I. Penegar, and S. A. Campbell. 2002. The use of atomic force microscopy for studying interactions of bacterial biofilms with surfaces. Colloids Surf. B. 23:231–247. [Google Scholar]
- Burnham, J. C., T. Hashimoto, and S. F. Conti. 1968. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J. Bacteriol. 96:1366–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton, J. W., Z. Lewandowski, and D. E. Caldwell. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745. [DOI] [PubMed] [Google Scholar]
- Cover, W. H., R. J. Martinez, and S. C. Rittenberg. 1984. Permeability of the boundary layers of Bdellovibrio bacteriovorus 109J and its bdelloplasts to small hydrophilic molecules. J. Bacteriol. 157:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich, D. L. 1988. Bdellovibrios: recycling, remodelling and relocalizing components from their prey. Microbiol. Sci. 5:100–103. [PubMed] [Google Scholar]
- Dufrene, Y. F. 2001. Application of atomic force microscopy to microbial surfaces: from reconstituted cell surface layers to living cells. Micron. 32:153–165. [DOI] [PubMed] [Google Scholar]
- Dufrene, Y. F. 2002. Atomic force microscopy, a powerful tool in microbiology. J. Bacteriol. 184:5205–5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksztejn, M., and M. Varon. 1977. Elongation and cell division in Bdellovibrio bacteriovorus. Arch. Microbiol. 114:175–181. [DOI] [PubMed] [Google Scholar]
- Gray, K. M., and E. G. Ruby. 1989. Unbalanced growth as a normal feature of development of Bdellovibrio bacteriovorus. Arch. Microbiol. 152:420–424. [DOI] [PubMed] [Google Scholar]
- Hansma, H. G. 2001. Surface biology of DNA by atomic force microscopy. Annu. Rev. Phys. Chem. 52:71–92. [DOI] [PubMed] [Google Scholar]
- Hansma, H. G., K. J. Kim, D. E. Laney, R. A. Garcia, M. Argaman, M. J. Allen, and S. M. Parons. 1997. Properties of biomolecules measured from atomic force microscope images: a review. J. Struct. Biol. 119:99–108. [DOI] [PubMed] [Google Scholar]
- Kessel, M., and M. Shilo. 1976. Relationship of Bdellovibrio elongation and fission to host cell size. J. Bacteriol. 128:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval, S. F., and S. H. Hynes. 1991. Effect of paracrystalline protein surface layers on predation by Bdellovibrio bacteriovorus. J. Bacteriol. 173:2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., and J. H. Hoh. 2001. Probing the machinery of intracellular trafficking with the atomic force microscope. Traffic. 2:746–758. [DOI] [PubMed] [Google Scholar]
- Markelova, N. Y., and R. R. Colwell. 1999. Two-component bacterial predator-prey system immobilized on the surface of a transparent plastic substrate is a promising model for investigation of the role of bacterial predators in ecosystems. Microbiology (translated from Mikrobiologiya) 68:328–331 (383–386). [Google Scholar]
- Martin, M. 2002. Predatory prokaryotes: an emerging research opportunity. J. Mol. Microbiol. Biotechnol. 4:5–16. [PubMed] [Google Scholar]
- O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79. [DOI] [PubMed] [Google Scholar]
- Odelson, D. A., M. A. Patterson, and R. B. Hespell. 1982. Periplasmic enzymes in Bdellovibrio bacteriovorus and Bdellovibrio stolpii. J. Bacteriol. 151:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard, M. A., D. Langley, and S. C. Rittenberg. 1975. Effects of methotrexate on intraperiplasmic and axenic growth of Bdellovibrio bacteriovorus. J. Bacteriol. 121:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razatos, A. 2001. Application of atomic force microscopy to study initial events in bacterial adhesion. Methods Enzymol. 337:276–285. [DOI] [PubMed] [Google Scholar]
- Rittenberg, S. C. 1983. Bdellovibrio: attack, penetration, and growth on its prey. ASM News. 49:435–439. [Google Scholar]
- Rittenberg, S. C., and D. Langley. 1975. Utilization of nucleoside monophosphates per se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J. Bacteriol. 121:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg, S. C., and M. Shilo. 1970. Early host damage in the infection cycle of Bdellovibrio bacteriovorus. J. Bacteriol. 102:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon, D., J.-C. Girard, Y. Cenatiempo, and J.-F. Cavellier. 1999. Atomic force microscopy imaging of dried or living bacteria. C. R. Acad. Sci. III. 322:687–693. [DOI] [PubMed] [Google Scholar]
- Ruby, E. G. 1991. The genus Bdellovibrio. In The Prokaryotes: A Handbook on The Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications, 2nd ed. A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer, editors. Springer-Verlag, New York. 3400–3415.
- Ruby, E. G., and S. C. Rittenberg. 1983. Differentiation after premature release of intraperiplasmically growing Bdellovibrio bacteriovorus. J. Bacteriol. 154:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier, M. H., Jr. 1994. Protein uptake into E. coli during Bdellovibrio infection. A process of reverse secretion? FEBS Lett. 337:14–17. [DOI] [PubMed] [Google Scholar]
- Seidler, R. J., and M. P. Starr. 1968. Structure of the flagellum of Bdellovibrio bacteriovorus. J. Bacteriol. 95:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler, R. J., and M. P. Starr. 1969a. Factors affecting the intracellular parasitic growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J. Bacteriol. 97:912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler, R. J., and M. P. Starr. 1969b. Isolation and characterization of host-independent Bdellovibrios. J. Bacteriol. 100:769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellen, J. E., and M. P. Starr. 1974. Ultrastructural aspects of localized membrane damage in Spirillum serpens VHL early in its association with Bdellovibrio bacteriovorus 109J. Arch. Microbiol. 100:179–195. [DOI] [PubMed] [Google Scholar]
- Starr, M. P., and N. L. Baigent. 1966. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J. Bacteriol. 91:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger, R., A. Allen, H. G. Hansma, and P. A. Holden. 2002. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa-unsaturated biofilms. Microb. Ecol. 43:416–423. [DOI] [PubMed] [Google Scholar]
- Stolz, M., D. Stoffler, U. Aebi, and C. Goldsbury. 2000. Monitoring biomolecular interactions by time-lapse atomic force microscopy. J. Struct. Biol. 131:171–180. [DOI] [PubMed] [Google Scholar]
- Stoodley, P., D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187–209. [DOI] [PubMed] [Google Scholar]
- Surman, S. B., J. T. Walker, D. T. Goddard, L. H. G. Morton, C. W. Keevil, W. Weaver, A. Skinner, K. Hanson, D. Caldwell, and J. Kurtz. 1996. Comparison of microscope techniques for the examination of biofilms. J. Microbiol. Meth. 25:57–70. [Google Scholar]
- Thomashow, M. F., and T. W. Cotter. 1992. Bdellovibrio host dependence: the search for signal molecules and genes that regulate the intraperiplasmic growth cycle. J. Bacteriol. 174:5767–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor, J. J., M. P. McCann, and I. A. Acrich. 1990. A new model for the penetration of prey cells by bdellovibrios. J. Bacteriol. 172:2421–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda, A., M. Saito, and K. Amako. 1998. Surface characteristics of gram-negative and gram-positive bacteria in an atomic force microscope image. Microbiol. Immunol. 42:159–164. [DOI] [PubMed] [Google Scholar]
- Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, H. N., J. I. Kelley, M. L. Baer, and B.-F. Turng. 1995. The association of bdellovibrios with surfaces in the aquatic environment. Can. J. Microbiol. 41:1142–1147. [Google Scholar]
- Yao, X., J. Walter, S. Burke, S. Stewart, M. H. Jericho, D. Pink, R. Hunter, and T. J. Beveridge. 2002. Atomic force microscopy and theoretical considerations of surface properties and turgor pressures of bacteria. Colloids Surf B. 23:213–230. [Google Scholar]