Abstract
Helminth infections are among the most potent stimulators of Th2-type immune responses and have been widely demonstrated to modify responsiveness to both nonparasite antigens and other infectious agents in a nonspecific manner in infected animals. We investigated the immunomodulatory properties of pseudocoelomic body fluid from adult Ascaris suum gastrointestinal helminths (ABF) and its defined allergen (ABA-1) by examining their effects on the immune response to a heterologous antigen, ovalbumin. Our results indicate that ABF has potent immunomodulatory activity and that the effects observed are consistent with skewing towards a Th2-type response rather than induction of anergy. Our findings show that the immunomodulatory activities of ABF are associated with components other than the major constituent and putative allergen, ABA-1. Furthermore, the allergic responses to ABA-1 are not a result of an intrinsic allergenicity of the protein but are more a reflection of the wider induction of a Th2 response by the infection. Importantly, the induction of interleukin-10 by ABF also suggests that T regulatory cells may play a role in immunomodulation of immune responses by parasitic helminths.
Infections with gastrointestinal nematode parasites are extremely widespread and contribute significantly to both morbidity and mortality among humans and livestock in developing countries (60). The most prevalent parasitic helminth in humans, Ascaris lumbricoides, is estimated to infect 1.5 billion people globally (9), and the associated induction of an immunoglobulin E (IgE) response can cause potentially lethal hypersensitivity responses, especially in infected children (41). A number of parasitic nematodes have also been reported to exert potent nonspecific immunomodulatory effects on the host immune system (2, 3, 32, 51). However, parasite infections may also promote pathological responses; pinworm infection promotes the development of autoimmune ovarian disease, and eradication of the infestation results in amelioration of the condition (3). In contrast, such infections may also prevent deleterious pathological responses associated with coinfections. For example, concurrent infection with A. lumbricoides appears to alleviate the pathology of cerebral malaria infections (43). Helminth infections also have the capacity to promote allograft survival through induction of type 2 immunity, development of regulatory Tc2 cells, and inhibition of allospecific cytotoxic T-lymphocyte activity (34). While this presumably promotes parasite survival, it may markedly impair protective immune responses to vaccine antigens, such as BCG of Mycobacterium tuberculosis infections or the cholera toxin B subunit (11, 20, 46, 55). Therefore, understanding how parasites can affect concomitant immune responses has obvious consequences for the design of vaccines against both parasites and other pathogens, as well as for the potential therapeutic application of parasite-derived molecules.
The balance of Th1 and Th2 immune responses is known to be crucial for determining both the protective and pathological responses to infections with a variety of pathogens (1). In the case of a number of gastrointestinal nematode infections, Th2 responses are generally associated with protection, while Th1 responses are associated with susceptibility (16, 58). The features of helminth infections that promote Th2 immune responses remain undefined, but induction of IgE and development of host hypersensitivity responses are common features of both infection and immunization protocols (44). As both infection and immunization result in induction of Th2 responses, it has been suggested that parasite products act to divert Th1 responses to a Th2 phenotype. For instance, products of Nippostrongylus brasiliensis induce Th2 responses to bystander antigens in uninfected mice (14, 15, 25). A major product of Ascaris suum, ABA-1, is a 14-kDa fatty acid-binding protein and is the target of major histocompatibility complex (MHC)-restricted IgE responses in both infected humans and animals (29). Furthermore, ABA-1 is a major constituent of allergen preparations used for investigating allergic reactions to the parasite (61). However, it remains to be determined whether the allergic response to this molecule is intrinsic or is a reflection of the wider induction of Th2 responses induced by the parasite infection or other molecules produced by the parasite.
In this study we evaluated the potentials of pseudocoelomic body fluid of the adult stage of A. suum (ABF) and the parasite allergen, ABA-1, for modulating the immune response to a heterologous protein, ovalbumin (OVA), and the role that this response plays in the associated allergenicity of ABA-1. Our findings revealed that the ability of ABF to modulate the effector response against OVA was independent of ABA-1 and that, contrary to expectations, ABA-1 itself was not intrinsically allergenic as it did not induce the production of interleukin-4 (IL-4) or IgE in immunized mice. Furthermore, the modulation of the immune response to OVA by ABF was not the result of induction of anergy in the local T-cell population but required the presence of the Th2-associated cytokine IL-4. Moreover, the induction of IL-10 by ABF also suggests that T regulatory cells may play a role in modulation of immune responses by helminths.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were bred in the Joint Animal Facility, University of Glasgow, and female B10.S and B10.BR mice were purchased from Harlan Olac (Oxford, United Kingdom). IL-4-deficient mice (129 × C57BL/6 background) were a gift from A. Mowat, Department of Immunology, University of Glasgow. All mice were maintained under standard animal house conditions until they were used at 6 to 8 weeks of age. All animal experiments were conducted under the regulations of the Home Office Scientific Procedures Act (1986).
Parasite antigen preparation.
ABF was collected from parasites freshly recovered from pigs at a local abattoir as described previously (57) and was centrifuged at 13,000 × g for 5 min to remove particulate debris. The supernatant was removed, and the total protein concentration was quantified by the Coomassie blue protein assay (Pierce, Rockford, Ill.). ABF was stored at −70°C until it was used. Recombinant ABA-1 (rABA-1) was produced as described previously (61). Briefly, it was produced as a glutathione S-transferase fusion protein in NovaBlue cells (CN Biosciences Ltd., Nottingham, United Kingdom) which had been transformed with a pGEX-1λT expression vector (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) encoding the ABA-1A1 unit of the ABA-1 polyprotein allergen from A. suum. The fusion protein was purified and processed by using standard techniques to obtain rABA-1. Parasite-derived proteins were fractionated by using a Pharmacia Superose 12 gel filtration column fitted to an isocratic fast protein liquid chromatography system (Bio-Rad). The column was equilibrated with phosphate-buffered saline (pH 7.2) prior to elution at a flow rate of 0.3 ml/min and monitoring for absorbance at 280 nm.
Induction and assessment of DTH.
Mice were each primed in the footpad with 100 μg of OVA (fraction V; Sigma, Poole, United Kingdom) alone or mixed with ABF or ABA-1 emulsified with complete Freund's adjuvant (Sigma) in 50 μl. Seven days after systemic immunization, each mouse was challenged with 100 μg of heat-aggregated OVA (HAO) in 50 μl of saline in the opposite footpad. Delayed-type hypersensitivity (DTH) responses were assessed by measuring the thickness of the challenged footpad prior to and 24 h after challenge by using dial gauge microcalipers (Röhn GB Ltd., Kingston-Upon-Thames, United Kingdom). The difference between the means of the two measurements gave an index of footpad swelling in millimeters, which was used for group comparisons. Naive animals that were injected with HAO in both footpads were included as controls for nonspecific swelling.
Neutralization of IL-10 in vivo.
Mice were injected intraperitoneally with either 200 μg of a neutralizing rat anti-mouse IL-10 monoclonal antibody (clone JES5-2A5) or 200 μg of a rat IgG isotype control 2 h prior to antigen sensitization as indicated below. The two antibodies were generous gifts from Tim Mitchell, Division of Infection and Immunity, Institute of Biomedical and Life Sciences, University of Glasgow.
Lymphocyte culture and cytokine responses.
All tissue culture reagents were obtained from Gibco-BRL (Paisley, United Kingdom). Single-cell suspensions of popliteal lymph nodes (PLN) were prepared 7 days postchallenge in RPMI 1640 containing 10% fetal calf serum, 5 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 25 mM HEPES, and 0.05 mM 2-mercaptoethanol. Cells (1 × 107 cells/ml) were incubated either alone or in the presence of 50 μg of ABF per ml, 50 μg of rABA-1 per ml, 50 μg of OVA per ml, or 5 μg of concanavalin A per ml at 37°C in 5% CO2 either in triplicate 200-μl cultures for proliferation assays or in 1.5-ml mixtures for cytokine production assays. Cells from each group were pooled as insufficient numbers of cells could be obtained from individual animals to measure all of the cytokines. Proliferation of cells was assessed after 72 h by adding 0.5 μCi of [3H]thymidine for the last 18 h of culture. Cells were then harvested with an automated cell harvester, and the activity incorporated was estimated by scintillation counting. Culture supernatants for cytokine production assays were harvested after 24 h and stored at −20°C until cytokine levels were determined.
Cytokine assays.
Gamma interferon (IFN-γ), IL-4, IL-5, and IL-10 production was quantified by a sandwich enzyme-linked immunosorbent assay technique by using antibody pairs purchased from PharMingen, Cambridge, United Kingdom. Capture antibodies were used at a concentration of 2 μg/ml, and detecting antibodies were used at a concentration of 1 μg/ml. Results were expressed in units per milliliter after comparison to the results obtained for commercially produced standards of recombinant IFN-γ, recombinant IL-4, IL-5, or recombinant IL-10 (PharMingen).
Measurement of antibody responses.
OVA-specific IgG1 and IgG2a levels were determined as described previously. OVA was used as a target antigen at a concentration of 5 μg/ml. Sera were diluted 1/50. IgG1 and IgG2a were detected by using biotinylated anti-mouse IgG1 and IgG2a (clones A85-1 and R19-15, respectively; PharMingen) at a concentration of 2 μg/ml, followed by streptavidin peroxidase (Immunodiagnostics Scotland, Carluke, United Kingdom). Total serum IgE levels were measured by a sandwich enzyme-linked immunosorbent assay technique as described previously (33). Anti-mouse IgE was used as a capture antibody, and IgE was detected by using biotinylated anti-mouse IgE (PharMingen). An IgE monoclonal antibody specific for trinitrophenol was used as the standard (PharMingen).
Statistics.
Results are presented below as means + standard errors of the means for groups of animals undergoing identical treatments. Differences between groups were analyzed by using a Student's t test with a standard Bonferonni correction factor. A P value of 0.05 was used as the upper limit for significance unless otherwise indicated.
RESULTS
Parasite products suppress DTH to heterologous antigens in a dose-dependent manner.
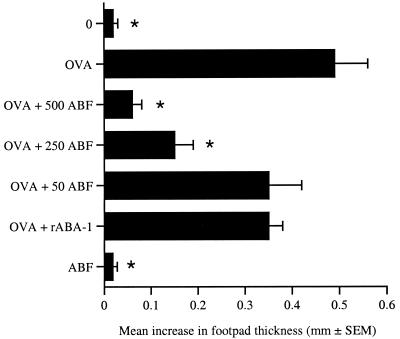
To examine whether the products of Ascaris, in particular the allergen ABA-1, could modulate a DTH response against the unrelated protein OVA, BALB/c mice were each sensitized in a rear footpad with OVA alone or mixed with ABF or rABA-1 emulsified in complete Freund's adjuvant. Seven days later each animal was challenged in the opposite footpad with HAO. By monitoring the ensuing swelling in the challenged footpads (Fig. 1), it was found that sensitization and challenge with OVA resulted in a pronounced thickening of the footpad that was indicative of a DTH response. Priming in the presence of ABF resulted in significant suppression of the DTH response (P ≤ 0.001). This effect was dose dependent, and ABF doses of 250 μg of protein or more prevented the generation of DTH. In contrast, sensitization with OVA combined with rABA-1 had no significant effect on the development of the OVA-specific DTH response. Immunization with ABF followed by challenge with OVA did not induce a DTH response.
FIG. 1.
Suppression of DTH responses to a heterologous antigen by ABF. BALB/c mice were sensitized with OVA alone or in combination with various amounts of ABF (in micrograms, as indicated) or 100 μg of rABA-1. Challenge occurred 7 days later with HAO, and the DTH responses were assessed over the subsequent 24 h. The members of the unimmunized control group (group 0) were challenged with HAO. The DTH responses are expressed as the mean + standard error of the mean increase in footpad thickness for five mice per group. An asterisk indicates that there was a significant difference compared to the value for the OVA-sensitized group (P ≤ 0.001).
Suppression, unlike the IgE response, is MHC independent.
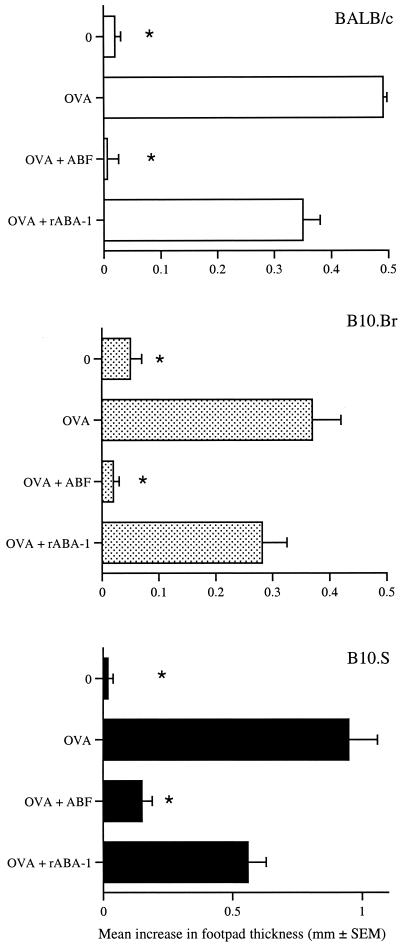
Previous work has shown that the IgE response to ABA-1 following infection with A. suum is MHC restricted to H-2s (57). As the previous experiment was performed with BALB/c mice (H-2d), we investigated whether the DTH suppression was restricted in a similar manner. Groups of BALB/c (H-2d), B10.BR (H-2k), and B10.S (H-2s) mice were immunized with OVA alone or mixed with 250 μg of ABF or rABA-1 (Fig. 2). In all cases a DTH response was observed in response to OVA. While ABF significantly depressed the DTH response to OVA in each strain of mice (P ≤ 0.001), addition of rABA-1 did not have a significant effect. This indicates that the immunomodulatory properties of ABF were not MHC restricted as the strains of mice used had disparate haplotypes. Furthermore, rABA-1 was unable to modulate the DTH response even in mice with a responder MHC haplotype (B10.S mice).
FIG. 2.
Suppression of OVA DTH responses by ABF is not MHC restricted. Mice were sensitized with OVA alone or in combination with 250 μg of ABF or 100 μg of rABA-1. Challenge occurred 7 days later with HAO, and DTH responses were assessed over the subsequent 24 h. The members of the unimmunized control group (group 0) were challenged with only HAO. The DTH responses are expressed as the mean + standard error of the mean increase in footpad thickness for five mice per group. An asterisk indicates that there was a significant difference compared to the value for the OVA-sensitized group (P ≤ 0.001).
Suppression by ABF is independent of the major constituent and IgE target, ABA-1.
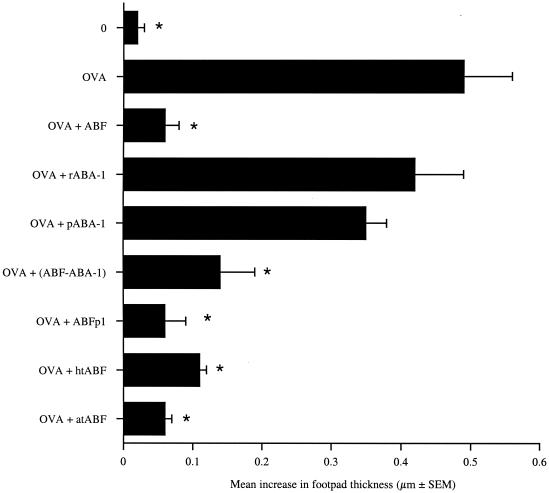
Although rABA-1 did not alter the OVA-specific DTH response, it was not known if this was also true for parasite-derived ABA-1 (pABA-1). This was an important consideration as pABA-1, which constitutes 40 to 50% of the total protein in ABF, may be posttranscriptionally modified, have a different tertiary structure than the recombinant protein, or be associated with another immunomodulatory factor (30). To address this point, we fractionated ABF by fast protein liquid chromatography, and we obtained four main peaks. Peaks 1 and 2 represented high-molecular-mass molecules (>150 kDa) (ABFp1), whereas peak 3 comprised the 14.4-kDa molecule pABA-1, as described previously (18, 40). Low-molecular-mass degraded proteins made up peak 4.
BALB/c mice were immunized with OVA alone or in combination with unfractionated ABF, ABF with pABA-1 removed (ABF-pABA-1), ABF protein peak 1 (ABFp1), rABA-1, or pABA-1. All groups were challenged 7 days later with HAO. As shown in Fig. 3, the ability of ABF to modulate the response was not dependent upon the presence of the allergen, ABA-1 (P = 0.001), as ABF-pABA-1 suppressed the DTH response to OVA in a fashion similar to unfractionated ABF. Sensitization of BALB/c mice with OVA mixed with ABFp1 resulted in significant depression of the DTH response (P ≤ 0.005), demonstrating that immunomodulatory activity was present in high-molecular-mass peak 1. Furthermore, pABA-1, like rABA-1, had no significant effect on the development of the OVA-specific DTH response. This further demonstrated that posttranscriptional modification of ABA-1 has no effect on the ability of ABA-1 to suppress the DTH response and therefore that ABA-1 is not required for the effects of ABF observed.
FIG. 3.
BALB/c mice were sensitized with OVA alone or mixed with ABF, ABF with ABA-1 removed (ABF-ABA-1), ABFp1, heat-treated ABF (htABF), acid-treated ABF (atABF), rABA-1, or pABA-1. Mice received OVA alone or in combination with 250 μg of treated ABF or 100 μg of ABA-1. Challenge occurred 7 days later with HAO, and DTH responses were assessed over the subsequent 24 h. The members of the unimmunized control group (group 0) were challenged with only HAO. The DTH responses are expressed as the mean + standard error of the mean increase in footpad thickness for five mice per group. An asterisk indicates that there was a significant difference compared to the value for the OVA-sensitized group (P ≤ 0.005).
Other models of parasite-mediated immunomodulation have investigated the biochemical nature and stability of the products involved. In order to denature any proteinaceous material, we heated ABF to 100°C for 30 min or acid treated it by lowering the pH to pH 2 and then neutralizing the preparation (pH 7). BALB/c mice were immunized with OVA alone or with OVA mixed with the acid-treated ABF or heat-treated ABF. Figure 3 shows that treating ABF with heat or a low pH did not alter the ability of ABF to modulate the DTH response. This suggests that the immunomodulatory activity may not be mediated by protein, unless denatured protein is active, or that a remarkably stable protein is present.
Immunomodulation affects induction of a response and not an effector response.
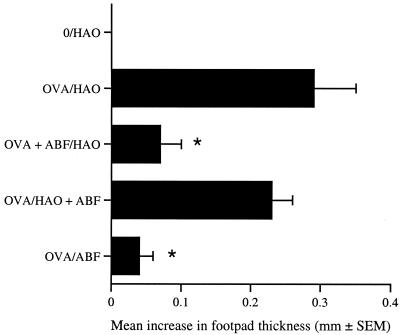
Exposure to helminths or their antigens can divert the immune response generated against heterologous antigens or concurrent infections (2, 46, 55). Our results demonstrate that exposure to ABF during priming with OVA decreases the OVA-specific DTH response. Therefore, we examined whether ABF could modulate the DTH response at the effector phase of the response (i.e., antigen challenge) rather than at the induction phase (immunization). BALB/c mice were immunized with OVA alone or combined with ABF and challenged 1 week later with HAO as described above. Another group was immunized with OVA alone and challenged with HAO combined with ABF. Figure 4 shows that exposure to ABF at the time of antigen priming significantly depressed (P ≤ 0.01) the OVA DTH response, whereas no effect was observed when ABF was administered during antigenic challenge. Therefore, immunomodulation resulting from exposure to ABF is restricted to the induction phase of a response and not the effector response.
FIG. 4.
Suppression of OVA DTH by ABF occurs during priming only. BALB/c mice were immunized with OVA alone or mixed with 250 μg of ABF. Challenge occurred 7 days later with HAO alone or mixed with 250 μg of ABF. DTH responses were assessed over the subsequent 24 h. The members of the unimmunized control group (group 0) were challenged with only HAO. The DTH responses are expressed as the mean + standard error of the mean increase in footpad thickness for five mice per group. An asterisk indicates that there was a significant difference compared to the value for the OVA-sensitized group (P ≤ 0.01).
The effect of ABF may be mediated by immunoregulatory cytokines.
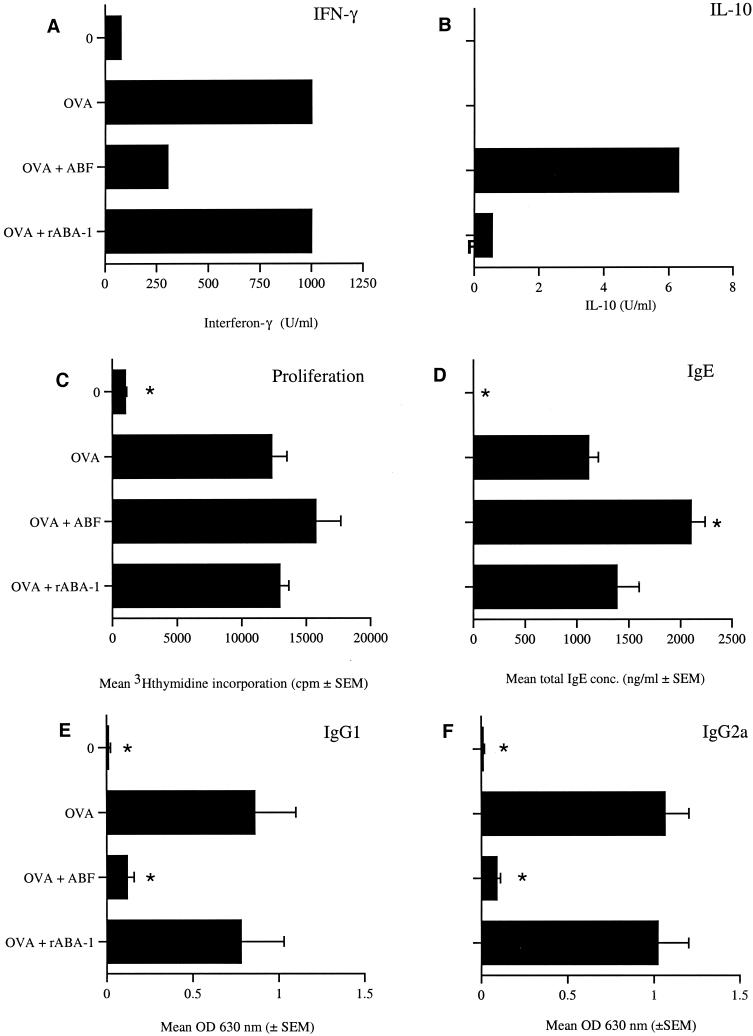
Infections with helminths or exposure to their products have been shown to promote the production of cytokines associated with a Th2-type response (39, 54). Conversely, the DTH response is an inflammatory response associated with the production of Th1-type cytokines, such as IFN-γ (10). To examine whether exposure to ABF skewed the immune response towards a Th2 response, cytokine production by cells from the draining PLN following immunization with OVA, OVA combined with ABF, or OVA combined with rABA-1 was analyzed. The levels of OVA-elicited IFN-γ (Fig. 5A) were higher following exposure to OVA alone, but sensitization in the presence of ABF resulted in a 70% decrease in production of the cytokine. The responses of PLN cells from animals immunized with OVA combined with rABA-1 were similar to the responses of PLN cells from animals immunized with OVA alone. The decrease in OVA-specific IFN-γ in the OVA and ABF-sensitized animals may have been due to the Ascaris antigens promoting the production of Th2 cytokines and thus skewing the OVA-specific response away from a Th1 response. However, neither IL-4 nor IL-5 was detected following restimulation with OVA in vitro. Interestingly, differences were found with IL-10, a cytokine that has been suggested to mediate the balance between inflammation and humoral immunity (21). Minimal OVA-specific IL-10 production occurred following immunization with OVA plus rABA-1; however, production was substantially increased when animals were primed with OVA plus ABF (Fig. 5B). No OVA-specific IL-10 was detected as a result of exposure to OVA alone.
FIG. 5.
Suppression of OVA DTH by ABF is associated with induction of a Th2 response and suppression of Th1 responses. BALB/c mice were sensitized with OVA with or without Ascaris products and challenged with HAO 7 days later. The members of the unimmunized control group (group 0) were challenged with only HAO. Serum and PLN cells were harvested 7 days postchallenge and stimulated with 50 μg of OVA per ml. (A and B) Levels of IFN-γ (A) and IL-10 (B) production were determined after 24 h of in vitro culture. (C) OVA-specific proliferation was measured after 72 h of in vitro culture. (D) Total serum IgE levels. (E) OVA-specific serum IgG1 levels. (F) OVA-specific serum IgG2a levels. The data in panels A to C were obtained with pooled cells as insufficient numbers of cells could be obtained from individual animals. The data in panels D to F are means + standard errors of the means for five mice per group. An asterisk indicates that there was a significant difference compared to the value for the OVA-sensitized group (P ≤ 0.05). OD 630 nm, optical density at 630 nm.
Loss of T-lymphocyte proliferation concomitant with the development of Th2 responses is characteristic of infection with other parasitic nematodes (5, 32). In order to determine whether the suppression of DTH was the result of T-cell hyporesponsiveness, the ability of PLN cell cultures to proliferate following antigen restimulation in vitro was examined. All groups proliferated strongly following stimulation with concanavalin A (data not shown). While no OVA-specific proliferation was observed in unimmunized animals, significant proliferation was observed in all groups immunized with OVA alone or in combination with parasite antigen (Fig. 5C). The responses of animals primed with OVA and ABF appeared to be enhanced, but the differences were not statistically significant.
Total IgE and OVA-specific IgG1 and IgG2a titers were measured in serum samples collected 7 days postchallenge. Exposure to OVA in combination with rABA-1 did not significantly alter the IgG1 or IgG2a responses compared to the responses of animals immunized with OVA alone (Fig. 5E and F). However, the OVA-specific IgG1 and IgG2a levels in animals immunized with OVA plus ABF were both significantly reduced. Similar to the pattern of IgG1 and IgG2a production described above, the levels of total IgE were not significantly different in animals immunized with OVA alone and in animals immunized with OVA in combination with rABA-1 (Fig. 5D). In contrast, significantly more total serum IgE was produced in the group sensitized with OVA plus ABF than in the group immunized with OVA alone (P ≤ 0.008).
Together, these results indicated that while ABF downregulated the DTH response, this was not due to the development of anergy in the OVA- and ABF-primed mice as the draining PLN population retained the ability to proliferate in vitro. Moreover, the elevation of the IgE level in the animals immunized with OVA and ABF suggests that there was a shift towards a Th2 response. However, the depression of the IgG1 response and the unaltered level of IL-4 coupled with the increase in the IL-10 level indicate a possible role for an alternative regulatory mechanism involving a population associated with, but distinct from, Th2-type T cells, such as the recently described Th3 or T-regulatory-cell subset (22, 47).
IL-4 and/or IL-10 mediates the immunomodulatory effect.
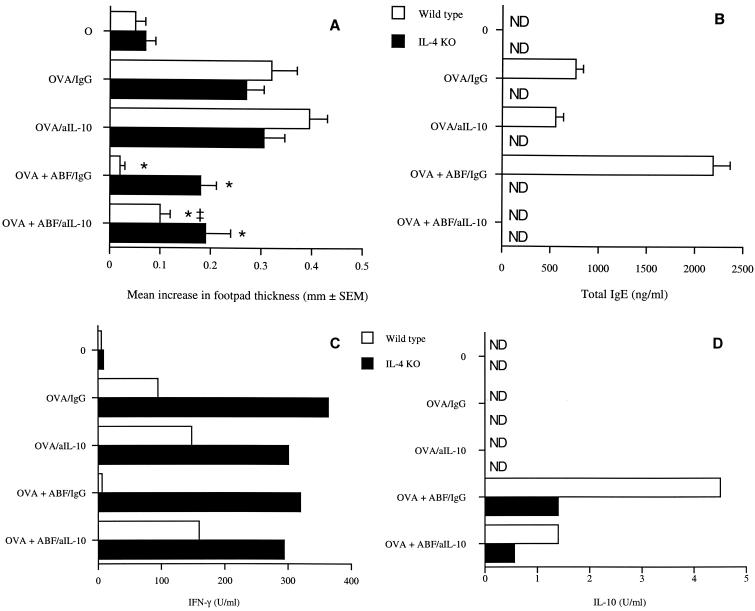
To determine whether the immunomodulatory effects of ABF were mediated through perturbations in IL-4 and/or IL-10 production, the DTH response in IL-4-deficient mice (IL-4 KO) was assessed following treatment with either 200 μg of rat IgG or 200 μg of anti-IL-10 (Fig. 6A). Anti-IL-10 treatment of wild-type mice immunized with OVA did not affect the development of DTH (data not shown), and treatment of mice immunized with OVA plus ABF ameliorated the inhibition of DTH by ABF, although the response was lower than that seen in mice immunized with OVA alone. Immunization of IL-4 KO mice with OVA alone resulted in the development of a strong DTH response similar to the responses observed in the wild-type controls. Addition of ABF to OVA did not significantly affect the DTH elicited in IL-4 KO animals, although the DTH was reduced by about 25% compared to that of animals immunized with OVA alone. Treatment of IL-4 KO animals that were immunized with OVA plus ABF with anti-IL-10 did not significantly alter the DTH induced compared to that of animals treated with rat IgG.
FIG. 6.
Suppression of DTH by ABF is mediated by IL-4. IL-4-deficient (IL-4 KO) mice were used. (A) IL-4 KO mice and wild-type controls were immunized with OVA alone or in combination with 250 μg of ABF. Challenge occurred 7 days later with HAO, and the DTH responses were assessed over the subsequent 24 h. The members of the unimmunized control group (group 0) were challenged with only HAO. The DTH responses are expressed as the mean + standard error of the mean increase in footpad thickness for five mice per group. Serum and PLN cells were harvested 7 days postchallenge and stimulated with 50 μg of OVA per ml. (B to D) Total serum IgE levels (B) and IFN-γ (C) and IL-10 (D) production were determined after 24 h of in vitro culture. The data in panels C and D were obtained with pooled cells as insufficient numbers of cells could be obtained from individual animals. The data in panels A and B are means + standard errors of the means for five mice per group. An asterisk indicates that there was a significant difference compared to the value for the unsensitized group (P ≤ 0.001). ND, none detected.
The levels of serum IgE were markedly enhanced in wild-type animals immunized with OVA plus ABF, but no IgE was detected in animals treated with anti-IL-10 or in IL-4 KO mice (Fig. 6B). No significant differences in IgG2a levels between IL-10-treated mice and untreated wild-type or IL-4 KO mice were detected, nor were significant differences in IgG1 levels detected in wild-type animals. IgG1 was undetectable in IL-4 KO mice (data not shown). Examination of the cytokines produced in vitro in response to OVA restimulation indicated that as shown previously, OVA plus ABF suppressed the induction of IFN-γ and induced IL-10 production in wild-type animals (Fig. 6C and D). Treatment with anti-IL-10 resulted in an increase in IFN-γ production and a corresponding decrease in IL-10 production. The IFN-γ production in IL-4 KO mice was substantially higher than that in wild-type mice, as might be expected; however, immunization with OVA plus ABF or treatment with anti-IL-10 did not alter production of this cytokine. Correspondingly, IL-10 levels were lower in IL-4 KO mice than in wild-type animals, and treatment with anti-IL-10 only marginally reduced the levels of this cytokine.
Together, our initial results suggest that the immunomodulation observed may be mediated by skewing the response to a Th2 and/or Th3 phenotype. Depletion of IL-10 in IL-4 KO mice could not completely restore the DTH response to OVA. It is possible that IL-10 was not fully neutralized by the reagents used; however, no IL-10 was detected following in vitro restimulation. It is also possible that some other factor, possibly transforming growth factor β (TGF-β), may also be involved. However, further experiments with IL-10 knockout mice and determining the levels of TGF-β in the immunomodulation mediated by ABF are necessary before a firm conclusion concerning the role of Th3 cells can be drawn.
DISCUSSION
Infections with parasitic helminths are characterized by the initiation of both allergic and immunomodulatory responses (5, 27, 32). Protective responses to these parasites are associated with the production of the Th2 cytokines IL-3, IL-4, and IL-5, and the resulting mastocytosis, IgE response, and eosinophilia are responses that are characteristic of allergic reactions (16, 58). Nevertheless, the precise Th2-controlled effector mechanisms responsible for parasite expulsion remain to be determined (33). In addition to promoting Th2 responses, helminth infections have been shown to modulate immune responses to nonparasite or heterologous antigens and are able either to potentiate or to suppress such responses (4, 32). This has serious implications for the host because these infections have been shown to be involved in breaking T-cell tolerance, triggering autoimmune disease, interfering with oral tolerance induction, increasing susceptibility to secondary infections, and decreasing vaccine efficacy (2, 51, 53). The mechanisms responsible for these phenomena have not been elucidated as yet. The ability to modulate immune responsiveness is not dependent on the presence of a live infection, as antigens derived from the parasites have similar properties (5, 13-15, 25).
In this study we demonstrated that ABF of A. suum changes the immune response normally generated against the heterologous antigen OVA. Furthermore, ABF modulated the immune response in an MHC-independent manner, and while a similar effect has been observed with other parasite products, it is in contrast to tight MHC control of immune specificity in helminth infections (25, 57). While the results which we obtained are in general agreement with studies of other workers (18, 19), we demonstrated that the immunomodulatory effects of the extract are independent of ABA-1, the principal target of a substantial IgE response (31, 57).
Nematode allergens have been identified as possible candidate molecules for the generation of allergic Th2 responses (29). The pulmonary phase of A. lumbricoides infection of humans causes a lethal IgE-mediated hypersensitivity (44). Moreover, naturally immune children produce significantly more IgE antibody to the Ascaris allergen, ABA-1, than children susceptible to the infection produce (41). Other allergens capable of direct immunomodulation have also been described; for instance, the Der p 1 allergen of the house dust mite Dermatophagoides pteronyssinus proteolytically cleaves CD25 on T cells, which promotes IL-4 expression at the expense of IFN-γ, resulting in enhanced IgE production (52). A calreticulin-like molecule secreted by Nector americanus is also an allergen and target of IgE responses in infected humans (49). Its activity appears to be mediated by inhibition of the biological function of human C1q and binding to the cytoplasmic signaling domains of a number of integrins (28). In the case of the Ascaris allergen, ABA-1, priming mice with OVA in the presence of either recombinant or parasite-derived allergen revealed that neither form had any influence on the development of the Th1-type DTH response. Additionally, removal of ABA-1 from ABF had no effect on the ability of ABF to eliminate the DTH response to OVA, implying that the immunomodulation by ABF observed is not mediated by ABA-1. Moreover, ABA-1 is not innately allergenic, as neither a Th2 response nor an IgE response was detected following coadministration of OVA and ABA-1. Similarly, ABF has been shown to contain a neutrophil chemotactic factor which was not present in the ABA-1 fraction (17). Together, these results suggest that the allergic response to ABA-1 is a reflection of the wider induction of Th2 responses by other molecules present in ABF. The pulmonary pathology associated with ascariasis may be a result of the Th2 adjuvanticity of ABF driving an allergic IgE response against ABA-1. However, the ability of ABF to modulate the DTH response was observed only if exposure occurred at the time of priming, not if it occurred at the time of challenge with heterologous antigen. This suggests that the active parasite factors act on early activation events in the developing immune response, unlike the activities of other parasite proteins in modulating ongoing responses (15, 25).
Intestinal nematodes have been shown to produce a number of molecules with potential anti-inflammatory activities. These include a superoxide dismutase and a neutrophil inhibitory factor glycoprotein which binds with high affinity to the integrin CD11b/CD18 and results in a block of adhesion of neutrophils to vascular endothelium and their generation of H2O2 (42, 56). Moreover, inhibition of eosinophil recruitment in vivo in response to eotaxin by hookworm excretory and secretory products is mediated by cleavage of eotaxin by a parasite-derived metalloprotease, while N. brasiliensis secretes a platelet-activating factor acetylhydrolase that cleaves platelet-activating factor to an inactive form, thus preventing eosinophil and neutrophil recruitment and activation (7, 12). Furthermore, N. brasiliensis products have also been shown to inhibit lipopolysaccharide-stimulated B-cell proliferation, possibly by modulation of accessory cell function (14, 15). In addition, serine protease inhibitor purified from Trichuris suis potently inhibits chymotrypsin, pancreatic elastase, neutrophil elastase, and mast cell chymase (50). However, the immunosuppressive factor present in ABF is unlikely to be proteinaceous like other nematode parasite products (25, 48), as the activity was retained following heating to 100°C for 30 min and was highly resistant to extremes of pH. Putative candidate molecules with similar biological activities include glycosphingolipids, polylactosamine sugars, and phosphorylcholine (PC) (24, 36, 59). PC has been detected in many parasite molecules bound to both glycolipids and glycoproteins. Indeed, the immunomodulatory glycolipid present in ABF contains PC molecules, and the lack of activity present in either rABA-1 or pABA-1 may reflect the absence of PC on the molecule (35). The immunoregulatory properties of the PC-containing ES-62 glycoprotein from the rodent filarial nematode Acanthocheilonema viteae are related to its capacity to inhibit B- and T-cell activation and its capacity to promote a Th2 response (23). This effect was further shown to be mediated by induction of the anti-inflammatory cytokine IL-10 (26). Similarly, PC on its own has the ability to induce the production of IL-10 by B1 cells (45), and thus the induction of IL-10 by ABF may be related to the presence of PC molecules.
We have shown that cytokines have a direct involvement in the ABF-mediated suppression of the OVA-specific DTH response manifested by a marked decrease in IFN-γ production and an increase in IL-10 production. Furthermore, both IL-4 and IL-10 appear to be crucial components of the immunomodulatory response induced by ABF, as the immunomodulatory activity was blocked in IL-4-deficient mice or by treatment with neutralizing anti-IL-10. As the suppression of the DTH response could not be fully restored by treating IL-4 KO mice with anti-IL-10, the data suggest that induction of the Th2 response by ABF results in production of both IL-4 and IL-10. Also, they imply that some other factor may also be responsible for the immunomodulatory activities of ABF. This is in contrast to what has been observed in filarial infections, where the induction of proliferative suppression was shown to be dependent on IL-4 but not dependent on IL-10 (37).
Previous studies have shown that injection of an A. suum extract resulted in a pan suppression of the responses to OVA (18, 19, 38). This suppression was characterized by decreases in DTH; proliferation; IL-2, IFN-γ, IL-4, and IL-10 production; and IgG1, IgG2a, and IgE levels in response to OVA. These results are in contrast to the results of our study, in which we showed that not only was IgE production in response to OVA enhanced by the presence of ABF but the production of IL-10 was also increased and proliferative responses were slightly elevated. The previous report showed that neutralization of IL-4 and IL-10 could restore the DTH response despite neutralization of IL-4 and IL-10 with monoclonal antibodies and that there was still substantial production of these cytokines following in vitro restimulation, suggesting that effective neutralization was not achieved (38). In our case we used IL-4 KO mice treated with an antibody to IL-10 and were unable to detect either IL-4 or IL-10 following in vitro restimulation. As we could not fully restore suppression of the DTH response by treating IL-4 KO mice with anti-IL-10, we suggest that some other factor may be involved in the suppression of the response observed. One explanation for the apparent differences between our study and the previous studies may involve the parasite preparations used. Macedo et al. used a homogenized, dialyzed, and lyophilized extract of A. suum (18, 19, 38). Not only may the use of homogenized parasites lead to inclusion of bacteria and hence lipopolysaccharide present on and in the parasites, but dialysis and lyophilization may lead to the loss of low-molecular-weight molecules and/or breakdown of molecules. In contrast, in our preparations we used sterile A. suum pseudocoelemic fluid divided into aliquots and stored at −70°C. Also, the strain of mice used in the previous study (DBA/2) is different from that used in this study (BALB/c). Furthermore, in order to define the role of ABA-1 in the induction of immunosuppression, we compared responses in mice with the H-2s haplotype (required for an IgE response to ABA-1) (31, 57). Our results demonstrated that although this molecule is the major target of IgE responses in this strain of mice, it is not required for the mediation of immunosuppression. We further extended the studies of Macedo et al. (18, 19, 38) by showing that it is the inductive phase, not the effector phase, of the response that is suppressed by ABF and that the factor is both heat and acid stable.
The induction of IL-10 production by ABF may not be merely a consequence of a Th2 response; it may be the consequence of a regulatory T-cell response as elimination of both IL-4 and IL-10 could not restore the DTH response. The recently described regulatory T cells (Tr1) inhibit responses of bystander T cells and prevent colitis induction through the secretion of TGF-β and IL-10 (6, 22). IL-10, which has previously been described primarily as a Th2-specific cytokine that inhibits Th1 responses, has displayed more general immune suppression of both types of effector T-cell responses and can suppress antigen-specific immune responses and actively downregulate pathological immune responses. Moreover, it has been demonstrated that Tr1 can also prevent Th2-induced autoimmunity and cannot be equated with Th2 cells (8). Therefore, the immune response to parasite infections may be a reflection of an immunomodulatory Tr1 response induced to limit immunopathology elicited by the protective Th2 response in response to parasitic helminths. Further studies are thus required to examine the role of this cell subset in the immunomodulation induced by parasitic helminths.
Together, the data clearly demonstrate that ABA-1, the target of the IgE response in hypersensitive individuals, is not intrinsically allergenic. The IgE response generated against ABA-1 in the context of Ascaris infection is therefore likely to be a result of a combination of route of exposure, dose of antigen, and the presence other molecules promoting a Th2 response to ABA-1 as a bystander antigen. Moreover, this study shows that immunosuppression mediated by ABF is biased towards a Th2-type response and may be mediated by the immunoregulatory cytokine IL-10. The isolation of such a component(s) and studies of effects both in vitro and in vivo have wide-ranging and long-term implications for vaccine design in developing countries and also increase our understanding of the mechanisms of allergic disease. Furthermore, identification of parasite-derived molecules with immunomodulatory properties may have potential therapeutic applications for the treatment of inflammatory disorders.
Acknowledgments
We thank Allan Mowat, University of Glasgow, for advice and for providing IL-4-deficient mice. We are also indebted to Lindsay McDermott, University of Glasgow, for invaluable assistance with purification of pABA-1.
This work was supported by BBSRC grant 17/S11031 to C.E.L., by Wellcome Trust grant 042679 to M.W.K., and by Wellcome Trust grant 055503 to C.E.L.
Editor: J. M. Mansfield
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agersborg, S. S., K. M. Garza, and K. S. Tung. 2001. Intestinal parasitism terminates self tolerance and enhances neonatal induction of autoimmune disease and memory. Eur. J. Immunol. 31:851-859. [DOI] [PubMed] [Google Scholar]
- 4.Allen, J. E., R. A. Lawrence, and R. M. Maizels. 1996. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int. Immunol. 8:143-151. [DOI] [PubMed] [Google Scholar]
- 5.Allen, J. E., and A. S. MacDonald. 1998. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 20:241-247. [DOI] [PubMed] [Google Scholar]
- 6.Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackburn, C. C., and M. E. Selkirk. 1992. Inactivation of platelet-activating factor by a putative acetylhydrolase from the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunology 75:41-46. [PMC free article] [PubMed] [Google Scholar]
- 8.Bridoux, F., A. Badou, A. Saoudi, I. Bernard, E. Druet, R. Pasquier, P. Druet, and L. Pelletier. 1997. Transforming growth factor beta (TGF-beta)-dependent inhibition of T helper cell 2 (Th2)-induced autoimmunity by self-major histocompatibility complex (MHC) class II-specific, regulatory CD4(+) T cell lines. J. Exp. Med. 185:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundy, D. A. 1994. Immunoepidemiology of intestinal helminthic infections. 1. The global burden of intestinal nematode disease. Trans. R. Soc. Trop. Med. Hyg. 88:259-261. [DOI] [PubMed] [Google Scholar]
- 10.Cher, D. J., and T. R. Mosmann. 1987. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J. Immunol. 138:3688-3694. [PubMed] [Google Scholar]
- 11.Cooper, P. J., M. Chico, C. Sandoval, I. Espinel, A. Guevara, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 69:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culley, F. J., A. Brown, D. M. Conroy, I. Sabroe, D. I. Pritchard, and T. J. Williams. 2000. Eotaxin is specifically cleaved by hookworm metalloproteases, preventing its action in vitro and in vivo. J. Immunol. 165:6447-6453. [DOI] [PubMed] [Google Scholar]
- 13.Deehan, M. R., M. M. Harnett, and W. Harnett. 1997. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J. Immunol. 159:6105-6111. [PubMed] [Google Scholar]
- 14.Ehigiator, H. N., A. W. Stadnyk, and T. D. Lee. 2000. Extract of Nippostrongylus brasiliensis stimulates polyclonal type-2 immunoglobulin response by inducing de novo class switch. Infect. Immun. 68:4913-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehigiator, H. N., A. W. Stadnyk, and T. D. Lee. 2000. Modulation of B-cell proliferative response by a soluble extract of Nippostrongylus brasiliensis. Infect. Immun. 68:6154-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Else, K. J., F. D. Finkelman, C. R. Maliszewski, and R. K. Grencis. 1994. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 179:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falcone, F. H., A. G. Rossi, R. Sharkey, A. P. Brown, D. I. Pritchard, and R. M. Maizels. 2001. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect. Immun. 69:4007-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faquim-Mauro, E. L., and M. S. Macedo. 1998. The immunosuppressive activity of Ascaris suum is due to high molecular weight components. Clin. Exp. Immunol. 114:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira, A. P., E. S. Faquim, I. A. Abrahamsohn, and M. S. Macedo. 1995. Immunization with Ascaris suum extract impairs T cell functions in mice. Cell. Immunol. 162:202-210. [DOI] [PubMed] [Google Scholar]
- 20.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 21.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 22.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J. E. de Vries, and M. G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389:737-742. [DOI] [PubMed] [Google Scholar]
- 23.Harnett, W., and M. M. Harnett. 1993. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J. Immunol. 151:4829-4837. [PubMed] [Google Scholar]
- 24.Harnett, W., and M. M. Harnett. 2001. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim. Biophys. Acta 1539:7-15. [DOI] [PubMed] [Google Scholar]
- 25.Holland, M. J., Y. M. Harcus, P. L. Riches, and R. M. Maizels. 2000. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30:1977-1987. [DOI] [PubMed] [Google Scholar]
- 26.Houston, K. M., E. H. Wilson, L. Eyres, F. Brombacher, M. M. Harnett, J. Alexander, and W. Harnett. 2000. Presence of phosphorylcholine on a filarial nematode protein influences immunoglobulin G subclass response to the molecule by an interleukin-10-dependent mechanism. Infect. Immun. 68:5466-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrett, E. E., and H. R. Miller. 1982. Production and activities of IgE in helminth infection. Prog. Allergy 31:178-233. [PubMed] [Google Scholar]
- 28.Kasper, G., A. Brown, M. Eberl, L. Vallar, N. Kieffer, C. Berry, K. Girdwood, P. Eggleton, R. Quinnell, and D. I. Pritchard. 2001. A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunol. 23:141-152. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy, M. W. 2000. The nematode polyprotein allergens/antigens. Parasitol. Today 16:373-380. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy, M. W., A. Brass, A. B. McCruden, N. C. Price, S. M. Kelly, and A. Cooper. 1995. The ABA-1 allergen of the parasitic nematode Ascaris suum: fatty acid and retinoid binding function and structural characterization. Biochemistry 34:6700-6710. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy, M. W., L. A. Tomlinson, E. M. Fraser, and J. F. Christie. 1990. The specificity of the antibody response to internal antigens of Ascaris: heterogeneity in infected humans, and MHC (H-2) control of the repertoire in mice. Clin. Exp. Immunol. 80:219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King, C. L., V. Kumaraswami, R. W. Poindexter, S. Kumari, K. Jayaraman, D. W. Alling, E. A. Ottesen, and T. B. Nutman. 1992. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J. Clin. Investig. 89:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence, C. E., J. C. Paterson, L. M. Higgins, T. T. MacDonald, M. W. Kennedy, and P. Garside. 1998. IL-4-regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 28:2672-2684. [DOI] [PubMed] [Google Scholar]
- 34.Liwski, R., J. Zhou, V. McAlister, and T. D. Lee. 2000. Prolongation of allograft survival by Nippostrongylus brasiliensis is associated with decreased allospecific cytotoxic T lymphocyte activity and development of T cytotoxic cell type 2 cells. Transplantation 69:1912-1922. [DOI] [PubMed] [Google Scholar]
- 35.Lochnit, G., R. D. Dennis, H. Muntefehr, S. Nispel, and R. Geyer. 2001. Immunohistochemical localization and differentiation of phosphocholine-containing antigens of the porcine, parasitic nematode Ascaris suum. Parasitology 122:359-370. [DOI] [PubMed] [Google Scholar]
- 36.Lochnit, G., R. D. Dennis, A. J. Ulmer, and R. Geyer. 1998. Structural elucidation and monokine-inducing activity of two biologically active zwitterionic glycosphingolipids derived from the porcine parasitic nematode Ascaris suum. J. Biol. Chem. 273:466-474. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald, A. S., R. M. Maizels, R. A. Lawrence, I. Dransfield, and J. E. Allen. 1998. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J. Immunol. 160:1304-1312. [PubMed] [Google Scholar]
- 38.Macedo, M. S., E. Faquim-Mauro, A. P. Ferreira, and I. A. Abrahamsohn. 1998. Immunomodulation induced by Ascaris suum extract in mice: effect of anti-interleukin-4 and anti-interleukin-10 antibodies. Scand. J. Immunol. 47:10-18. [DOI] [PubMed] [Google Scholar]
- 39.Mahanty, S., C. L. King, V. Kumaraswami, J. Regunathan, A. Maya, K. Jayaraman, J. S. Abrams, E. A. Ottesen, and T. B. Nutman. 1993. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J. Immunol. 151:3704-3711. [PubMed] [Google Scholar]
- 40.McGibbon, A. M., J. F. Christie, M. W. Kennedy, and T. D. Lee. 1990. Identification of the major Ascaris allergen and its purification to homogeneity by high-performance liquid chromatography. Mol. Biochem. Parasitol. 39:163-171. [DOI] [PubMed] [Google Scholar]
- 41.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyle, M., D. L. Foster, D. E. McGrath, S. M. Brown, Y. Laroche, J. De Meutter, P. Stanssens, C. A. Bogowitz, V. A. Fried, J. A. Ely, et al. 1994. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J. Biol. Chem. 269:10008-10015. [PubMed] [Google Scholar]
- 43.Nacher, M., F. Gay, P. Singhasivanon, S. Krudsood, S. Treeprasertsuk, D. Mazier, I. Vouldoukis, and S. Looareesuwan. 2000. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 22:107-113. [DOI] [PubMed] [Google Scholar]
- 44.Ogilvie, B. M., and D. de Savigny (ed.). 1982. Immune responses to nematodes. Blackwell Scientific Publications, London, United Kingdom.
- 45.Palanivel, V., C. Posey, A. M. Horauf, W. Solbach, W. F. Piessens, and D. A. Harn. 1996. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp. Parasitol. 84:168-177. [DOI] [PubMed] [Google Scholar]
- 46.Pearlman, E., J. W. Kazura, F. E. Hazlett, Jr., and W. H. Boom. 1993. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J. Immunol. 151:4857-4864. [PubMed] [Google Scholar]
- 47.Powrie, F., S. Menon, and R. L. Coffman. 1993. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur. J. Immunol. 23:3043-3049. [DOI] [PubMed] [Google Scholar]
- 48.Pritchard, D. I., N. M. Ali, and J. M. Behnke. 1984. Analysis of the mechanism of immunodepression following heterologous antigenic stimulation during concurrent infection with Nematospiroides dubius. Immunology 51:633-642. [PMC free article] [PubMed] [Google Scholar]
- 49.Pritchard, D. I., A. Brown, G. Kasper, P. McElroy, A. Loukas, C. Hewitt, C. Berry, R. Fullkrug, and E. Beck. 1999. A hookworm allergen which strongly resembles calreticulin. Parasite Immunol. 21:439-450. [DOI] [PubMed] [Google Scholar]
- 50.Rhoads, M. L., R. H. Fetterer, D. E. Hill, and J. F. Urban, Jr. 2000. Trichuris suis: a secretory chymotrypsin/elastase inhibitor with potential as an immunomodulator. Exp. Parasitol. 95:36-44. [DOI] [PubMed] [Google Scholar]
- 51.Rocken, M., J. F. Urban, and E. M. Shevach. 1992. Infection breaks T-cell tolerance. Nature 359:79-82. [DOI] [PubMed] [Google Scholar]
- 52.Schulz, O., H. F. Sewell, and F. Shakib. 1998. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J. Exp. Med. 187:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi, H. N., C. J. Ingui, I. Dodge, and C. Nagler-Anderson. 1998. A helminth-induced mucosal Th2 response alters nonresponsiveness to oral administration of a soluble antigen. J. Immunol. 160:2449-2455. [PubMed] [Google Scholar]
- 54.Steel, C., and T. B. Nutman. 1998. Helminth antigens selectively differentiate unsensitized CD45RA+ CD4+ human T cells in vitro. J. Immunol. 160:351-360. [PubMed] [Google Scholar]
- 55.Stewart, G. R., M. Boussinesq, T. Coulson, L. Elson, T. Nutman, and J. E. Bradley. 1999. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin. Exp. Immunol. 117:517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taiwo, F. A., P. M. Brophy, D. I. Pritchard, A. Brown, A. Wardlaw, and L. H. Patterson. 1999. Cu/Zn superoxide dismutase in excretory-secretory products of the human hookworm Necator americanus. An electron paramagnetic spectrometry study. Eur. J. Biochem. 264:434-438. [DOI] [PubMed] [Google Scholar]
- 57.Tomlinson, L. A., J. F. Christie, E. M. Fraser, D. McLaughlin, A. E. McIntosh, and M. W. Kennedy. 1989. MHC restriction of the antibody repertoire to secretory antigens, and a major allergen, of the nematode parasite Ascaris. J. Immunol. 143:2349-2356. [PubMed] [Google Scholar]
- 58.Urban, J. F., Jr., K. B. Madden, A. W. Cheever, P. P. Trotta, I. M. Katona, and F. D. Finkelman. 1993. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite Nippostrongylus brasiliensis. J. Immunol. 151:7086-7094. [PubMed] [Google Scholar]
- 59.Velupillai, P., W. E. Secor, A. M. Horauf, and D. A. Harn. 1997. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J. Immunol. 158:338-344. [PubMed] [Google Scholar]
- 60.World Health Organization. 1981. Intestinal protozoan and helminthic infections, vol. 666. World Health Organization, Geneva, Switzerland. [PubMed]
- 61.Xia, Y., H. J. Spence, J. Moore, N. Heaney, L. McDermott, A. Cooper, D. G. Watson, B. Mei, R. Komuniecki, and M. W. Kennedy. 2000. The ABA-1 allergen of Ascaris lumbricoides: sequence polymorphism, stage- and tissue-specific expression, lipid binding function, and protein biophysical properties. Parasitology 120:211-224. [DOI] [PubMed] [Google Scholar]