Abstract
Vibrio vulnificus is a gram-negative bacterium that contaminates filter-feeding shellfish such as oysters. After ingestion of contaminated oysters, predisposed people may experience highly lethal septicemia. Contamination of wounds with the bacteria can result in devastating necrotizing fasciitis, which can progress to septicemia. The extremely rapid progression of these diseases can render antibiotic treatment ineffective, and death is a frequent outcome. In this study, we examined the potential use of bacteriophages as therapeutic agents against V. vulnificus in an iron-dextran-treated mouse model of V. vulnificus infection. Mice were injected subcutaneously with 10 times the lethal dose of V. vulnificus and injected intravenously, either simultaneously or at various times after infection, with phages. Treatment of mice with phages could prevent death; systemic disease, as measured by CFU per gram of liver and body temperature; and local disease, as measured by CFU per gram of lesion material and histopathologic analysis. Two different phages were effective against three different V. vulnificus strains with various degrees of virulence, while a third phage that required the presence of seawater to lyse bacteria in vitro was ineffective at treating mice. Optimum protection required that the phages be administered within 3 h of bacterial inoculation at doses as high as 108 PFU. One of the protective phages had a half-life in blood of over 2 h. These results demonstrate that bacteriophages have therapeutic potential for both localized and systemic infections caused by V. vulnificus in animals. This model should be useful in answering basic questions regarding phage therapy.
Although research regarding the therapeutic use of bacteriophages has gone on without interruption since 1926 in Eastern Europe, this field of study has been, until very recently, somewhat neglected in the West (for reviews, see references 10 and 39). Within the past two decades, however, there has been a renewed interest in phage therapy due primarily to the increasing incidence of antibiotic-resistant bacteria and the lack of development of new types of antibiotics to control infections caused by these antibiotic-resistant organisms. The efficacy of phages in treating bacterial disease has been demonstrated by using animal models for Escherichia coli (21, 31-34), Salmonella enterica serovar Typhimurium (2), and Pseudomonas aeruginosa (35, 36). Phages have also been used successfully to prevent bacterial disease in fish (26) and to control pathogens of tomatoes (12). Although it is unlikely that phages will ever replace antibiotics, they may be of some use when no effective antibiotics are available or in conjunction with antibiotics for better treatment of disease. To examine the potential usefulness of phages, either alone or in conjunction with antibiotics, phages will have to be studied in a variety of animal models against bacteria with different mechanisms of virulence. To contribute to this understanding of where and how phage therapy may be appropriate, we have begun studying the therapeutic effect of phages against V. vulnificus.
V. vulnificus is an opportunistic pathogen of humans that causes septicemia after ingestion of contaminated oysters and necrotizing fasciitis after contamination of wounds (reviewed in references 19 and 38). Septicemia occurs primarily in people with high levels of iron saturation caused by genetic mutation, such as primary hemochromatosis, or by liver damage (cirrhosis). Immunosuppressed individuals and people with diabetes are also at risk (4, 6, 18, 43). Septicemia is characterized by fever, chills, and bullous skin lesions on the lower extremities and has a mortality rate of greater than 50% (14). Wound infection leads to necrotizing fasciitis, which is characterized by extensive tissue damage down to, but not usually including, the musculature and can necessitate surgical intervention for debridement or amputation. Wound infection can occur in the absence of predisposing conditions but progresses more frequently to septicemia and has a higher mortality rate in predisposed people.
V. vulnificus is a halophilic, gram-negative, curved rod that thrives in tropical and temperate estuarine environments throughout the world. The bacteria are found in filter-feeding shellfish, primarily oysters. Estimates of the prevalence of V. vulnificus in oysters from the Gulf of Mexico during the summer months have been as high as nearly 100% (23). Bacteriophages for V. vulnificus also are frequently found in oysters and estuarine waters (8, 9, 27). V. vulnificus is highly genetically diverse, and single oysters can contain over 100 different strains (5). Despite the diversity of strains present in oysters, one study demonstrated that only single strains of V. vulnificus were recovered from the blood of patients who had lethal infections and who had consumed oysters contaminated with numerous strains (15), suggesting that not all strains possess equal potential for human disease.
Little is known about the virulence mechanisms of V. vulnificus. The primary virulence factor is the polysaccharide capsule, which prevents phagocytosis and activation of complement (1, 30, 41, 42, 45, 46), classifying V. vulnificus as an extracellular pathogen. The ability to acquire iron from the host via siderophore production is also an essential virulence attribute (20). The production of a prepilin peptidase of a type 2 secretion system, which exerts pleiotropic effects on numerous secreted proteins, is required for full virulence in mice (24). Two other putative virulence factors, hemolysin and metalloprotease, have failed to be confirmed as virulence factors by genetic analysis (11, 16, 29, 44), despite the fact that injection of the purified proteins into laboratory animals induces several symptoms of V. vulnificus infection (13, 17, 22, 25). An iron-dextran-treated mouse model of V. vulnificus disease was previously used to compare the virulence of three clinical isolates with that of three environmental strains isolated from oysters or seawater (37). It appeared that the environmental strains either grew more slowly in or were killed more effectively by the host.
The availability of a useful animal model to examine virulence, the existence of bacteriophages for V. vulnificus, and the extracellular nature of the disease process led us to use V. vulnificus as a model for testing the effectiveness of phage therapy for human disease. We show here that phage treatment of iron-dextran-treated mice infected subcutaneously (s.c.) with V. vulnificus can prevent both local and systemic disease.
(These results were presented in preliminary form at the 100th General Meeting of the American Society for Microbiology in Los Angeles, Calif. [K. E. Cerveny, T. J. Doyle, G. M. Escudero, D. H. Duckworth, and P. A. Gulig, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. D230, p. 279, 2000] and the 13th Annual International Phage Biology Meeting in Montreal, Quebec, Canada [K. Cerveny, T. J. Doyle, A. DePaola, P. Gulig, and D. Duckworth, Abstr. Millennial Phage Biol. Meet., 2000]).
MATERIALS AND METHODS
Bacterial strains and growth media.
We used four clinical isolates of V. vulnificus, M06-24/0, VV1009, 2400112, and NSV-5829, obtained from patients who died from sepsis following ingestion of contaminated oysters, and environmental strains MLT403, MLT365, MLT367,and 99-796DP-E7, isolated from either oysters or seawater (7, 15, 37, 40, 45). The four clinical isolates and strain 99-796DP-E7 were highly virulent in s.c. inoculated, iron-dextran-treated mice, while the environmental strains were less virulent, although still able to cause disease at higher doses (37; A. DePaola, J. L. Nordstrom, A. Dalsgaard, J. Oliver, T. Baytes, K. L. Bourdage, and P. A. Gulig, unpublished data). V. vulnificus strains were grown in Luria-Bertani broth containing 0.85% (wt/vol) NaCl (LB-N) or on LB-N plates containing 1.5% (wt/vol) agar. Luria-Bertani broth containing seawater (Sigma) (LB-SW) or sea salts (Sigma) adjusted to a total salt concentration of 0.85% (LB-SS) was used when sea salts were required for phage activity. Soft-agar overlays of each medium contained 0.75% (wt/vol) agar. Bacteria were grown by diluting a static overnight culture into prewarmed medium, 1:10 for phage preparations or 1:20 for the animal infections, and then shaking the mixture at 37°C until the bacteria reached approximately 108 CFU/ml, as determined by measuring the optical density at 600 nm. For titration of phages, bacteria were grown to a density of between 1.5 × 108 and 2.5 × 108 CFU/ml, as determined by measuring the optical density at 600 nm. Preliminary experiments demonstrated that this stage of growth allowed maximum plating efficiency of the phage. For infection of mice, cultures were grown in LB-N, harvested, and diluted in phosphate-buffered saline containing 0.01% (wt/vol) gelatin (BSG) (28) exactly as described previously (37).
Phage isolation, growth, and titration.
Phage CK-2 was isolated from estuarine sediments from Cedar Key, Fla. A slurry of 50 ml of sediment and 50 ml of LB-N was mixed with 5 ml each of LB-N cultures of V. vulnificus strains VV1009, LL728, 2400112, MLT403, MLT365, and MLT367. The mixture was shaken overnight at 30°C. On the next day, the mud and bacteria were removed by centrifugation at 10,000 × g for 10 min, and the supernatant was filtered through a 0.2-μm-pore-size filter. Phage activity in the supernatant was amplified by mixing with an equal volume of a static overnight culture of each of the six V. vulnificus strains separately and shaking the mixtures overnight at 30°C. On the next day, the cultures were centrifuged and filtered as described above, and 10 μl of the filtrates was dropped onto LB-N agar plates seeded with a lawn of each of the bacterial strains. Plaques appeared on plates seeded with MLT403 but not with any of the other V. vulnificus strains. The phage from the MLT403 lysate was then plaque purified and amplified. This phage was designated CK-2. It produced very clear plaques on MLT403 grown in LB-N but had no activity on any of the other V. vulnificus strains that we examined.
Phages 153A-5 and 153A-7 were isolated from oysters as described previously (8, 9) and formed plaques on several of the virulent clinical strains, but phage 153A-7 formed plaques only when the vibrios were suspended in seawater-containing medium, either LB-SS or LB-SW. Broth and soft-agar lysates of these phages were produced by using strain MO6/24-0 as the host and standard procedures (28). Phages 153A-5 and 153A-7 had relatively broad host ranges, forming plaques on five of seven and seven of seven V. vulnificus strains tested, respectively.
Phage titers were determined in two ways: by dropping 10 μl of a phage dilution onto a soft-agar overlay containing 106 CFU/ml of bacteria or by mixing a phage dilution with bacteria, adding soft agar, and pouring the mixture onto an agar plate.
Infection of mice.
Seven- to 10-week-old female ICR mice (Harlan Sprague-Dawley, Indianapolis, Ind.) housed under specific-pathogen-free conditions were used for all infections. Infections were performed as described previously (37). Briefly, mice were injected intraperitoneally (i.p.) with 250 μg of iron dextran (Sigma)/g of body weight 2 h to 30 min prior to inoculation with bacteria. Mice were injected s.c. with 0.1 ml of bacteria suspended in BSG in the lower portion of the back. Bacteriophages suspended in BSG (0.1 ml) were injected intravenously (i.v.) into the lateral tail vein at various times after bacterial inoculation. Control mice not receiving phages were injected with sterile BSG. Mice were monitored for evidence of disease by visual inspection as well as rectal temperature. Mice with temperatures of <33°C were considered moribund and were euthanized. Euthanasia was done by carbon dioxide asphyxiation. We measured four quantitative criteria for disease: temperature, lesion score, number of bacteria in tissues, and histologic features. Differences in quantitative analyses of infection were examined by using a two-tailed Student t test. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Florida.
Temperature.
The rectal temperature of the mice was recorded at the time of euthanasia to objectively quantify the state of disease. It was previously shown that the body temperature of V. vulnificus-infected mice significantly drops in the later stages of disease (37). If a mouse died before it could be euthanized, the temperature assigned was the lowest temperature in the surviving infected mice for the given experiment or 33°C, whichever was lowest, for the purpose of statistical analysis. For analysis of survival, a temperature of 33°C or less was scored as a death.
Lesion score.
The skin was peeled back from head to tail, revealing the s.c. tissues of the back. The s.c. lesion was photographed along with a 2-cm2 standard. Photographs of lesions were scored for severity in a blinded manner by using the following scale: 0, no visible change; 1, lesion with discoloration but no hemorrhage; 2, lesion with hemorrhage but smaller than 2 cm2; 3, lesion larger than 2 cm2. Because lesion scores are not normally distributed, only the mean is shown, with no statistical analysis.
Quantitative analysis of bacteria in tissues.
Tissue samples from s.c. lesions and from livers were removed from the mice, weighed, homogenized in 5 ml of BSG by using glass tissue homogenizers, diluted, and plated to enumerate CFU per gram of tissue. If a mouse died before it could be euthanized and examined, the presence of a typical skin lesion was visually confirmed and photographed, and the levels of tissue infection assigned were the highest levels among the surviving mice in the same group. When no bacteria were found in an undiluted sample, the minimum detectable level of infection was assigned for the purpose of statistical analysis.
Survival of mice.
Survival rates for mice were calculated as a combination of actual deaths and a rectal temperature of <33°C at the time of euthanasia. Survival rates were examined for significance by using χ2 analysis.
Histologic analysis.
We examined the histopathologic features of a subset of the infected mice to determine whether phage therapy affected microscopic damage. Tissues of s.c. lesions were collected immediately after euthanasia, fixed in 10% (vol/vol) buffered formalin, embedded in paraffin, sectioned, mounted, and stained with hematoxylin and eosin as described previously (37).
Determination of phage half-life in mice.
Uninfected mice were injected i.v. with108 PFU of phage 153A-5. At 1, 6, and 12 h postinjection, mice were euthanized, and the peritoneal cavity was subjected to lavage with 4 ml of phosphate-buffered saline. The lavage fluid was collected by needle and syringe. A sample of liver was then removed, weighed, and homogenized in BSG. Finally, 10 μl of cardiac blood was collected in 0.05 M EDTA. All of the samples were examined for PFU on V. vulnificus MO6/24-0 as described above.
RESULTS
Analysis of V. vulnificus MLT403 and phage CK-2.
To examine the possible usefulness of bacteriophages in treating disease caused by V. vulnificus, we isolated phages which exhibited lytic activity against V. vulnificus. Phage CK-2 was isolated from estuarine sediments of Cedar Key, Fla., and was highly lytic, forming clear plaques on host V. vulnificus strain MLT403. MLT403 was the most virulent of the previously described environmental strains; however, its minimum lethal dose for iron-dextran-treated mice was 500-fold higher than that of clinically derived strains such as MO6-24/0 (37).
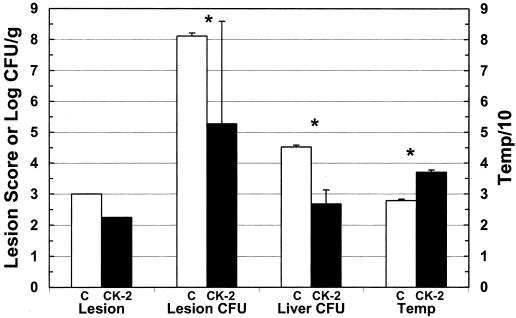
To determine whether phage CK-2 could protect against V. vulnificus infection, mice were injected i.p. with iron dextran, inoculated s.c. with 106 CFU (100 times the lethal dose) of MLT403, and immediately injected i.v. with 108 PFU of phage. Control mice were treated with iron dextran and infected with MLT403 but received BSG instead of phage. After 14 h of infection, all four of the untreated (control) mice were visibly ill, lethargic, and scruffy, with rectal temperatures ranging from 27.5 to 28.2°C (Fig. 1). All control mice had large s.c. lesions with a score of 3 (out of a maximum of 3) and with characteristic hemorrhage and edema at the site of inoculation. The control mice had a mean of 108 CFU/g of lesion tissue, and their livers contained a mean of nearly 105 CFU/g of tissue. In contrast, the four mice in the phage-treated group appeared to be only slightly ill and had normal rectal temperatures of 36.5 to 37.6°C. Phage-treated mice euthanized at 18 h postinfection had lesions with a mean score of 2.3 and with some discoloration but little edema. Two of these mice had undetectable bacteria in their lesion samples and were assigned a minimum detectable level of log 3.2 and log 2.9 CFU/g (these numbers are different because different amounts of tissue were sampled), while the other two mice had log 7.6 and log 4.8 CFU/g of lesion tissue. For all three criteria used to measure disease, the phage-treated mice were significantly protected compared with the control mice. The survival, determined by counting either actual deaths or a temperature of <33°C, of control mice was none of four at 18 h; in comparison, four of four phage-treated mice survived (χ2 analysis; P < 0.005).
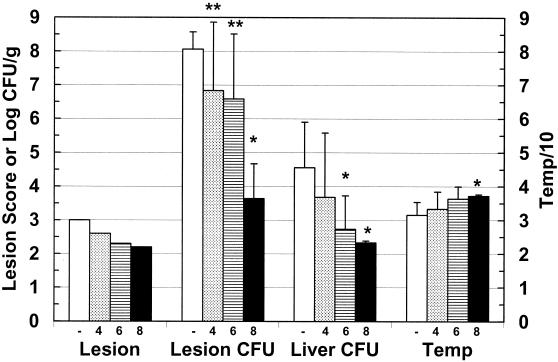
FIG. 1.
Short-term protection of V. vulnificus MLT403-infected mice by phage CK-2. Iron-dextran-treated mice were inoculated s.c. with 106 CFU of V. vulnificus MLT403. Mice were immediately injected i.v. with BSG (C) or phage CK-2 (CK-2), and samples were harvested 14 to 18 h postinfection. Data are means and standard deviations for log CFU per gram of skin lesion (Lesion CFU), log CFU per gram of liver (Liver CFU), and rectal temperature (Temp). The temperature was divided by 10 and then plotted to fit the same scale as the other values. For lesion score (Lesion), the mean is shown. All groups contained five animals. An asterisk indicates that the difference between control and phage-treated groups was statistically significant, as determined by the Student t test, as follows: lesion CFU, P = 0.016; liver CFU, P = 1.6 × 10−4; temperature, P = 7.6 × 10−8.
To determine whether the protection that we observed in the first experiment was only a delay in the onset of disease or whether the mice were destined to remain healthy, a long-term protection experiment was conducted. As in the first experiment, after 1 day of infection, control mice had severe lesions with high numbers of CFU in the skin and liver and depressed rectal temperatures, while phage CK-2-treated mice were significantly protected from CFU in the skin and liver (data not shown). A second set of six phage-treated mice was observed for 8 days. One of these mice died on day 5 of an unknown cause, and the remaining mice continued to appear healthy and were euthanized on day 8; no detectable bacteria were found in their skin or liver. Therefore, mice treated with CK-2 appeared to be protected from disease for the long term.
In a final set of experiments with phage CK-2, we examined how long phage treatment could be delayed without affecting its efficacy by injecting the phage 6 and 12 h after bacterial inoculation. As described above, V. vulnificus-infected mice were either treated with buffer (control) or 108 PFU of phage CK-2 at various times postinfection. Of the 10 mice in the control groups, only 1 survived, with a temperature of >33°C. Only mice treated with phage immediately after bacterial inoculation had a 1-day survival rate significantly higher than that of their control group counterparts (four of four mice) (P = 0.0027). The survival rates for the 6- and 12-h treatment delay groups were three of five and one of five mice, respectively. Results for the 0-h treatment delay group were similar to those in the initial experiment; mice in the group were significantly protected in terms of all quantitative criteria (P < 0.015), except for liver CFU (P = 0.07; although there was a greater than 1,000-fold difference in CFU, the high standard deviation in the control group reduced significance). Furthermore, the 0-h treatment delay group was significantly protected in terms of all criteria compared with the 12-h treatment delay group (P ≤ 0.03) and was significantly protected in terms of temperature compared with the 6-h treatment delay group (P ≤ 0.025). Therefore, delays of as little as 6 h in phage treatment rendered such treatment ineffective in this model with this particular phage-host pair.
Analysis of V. vulnificus MO6/24-0 with two different V. vulnificus-specific phage.
V. vulnificus MLT403, used in the initial experiment, is a less virulent environmental strain which requires a relatively high inoculum to cause disease (37). We therefore tested phages 153A-5 and 153A-7, which had lytic activity against V. vulnificus MO6/24-0, a widely studied, highly virulent clinical isolate (16, 45). As few as 100 CFU of MO6/24-0 can cause lethal infection in s.c. inoculated, iron-dextran-treated mice (16). Phage 153A-5 was lytic for MO6/24-0 in LB-N or LB-SS (20 ppt sea salts), while phage 153A-7 required sea salts (LB-SS). We examined phage 153A-5 for its ability to protect mice from V. vulnificus infection and examined whether the requirement of phage 153A-7 for sea salts to lyse V. vulnificus would be an impediment to its effectiveness in preventing V. vulnificus infection in mice.
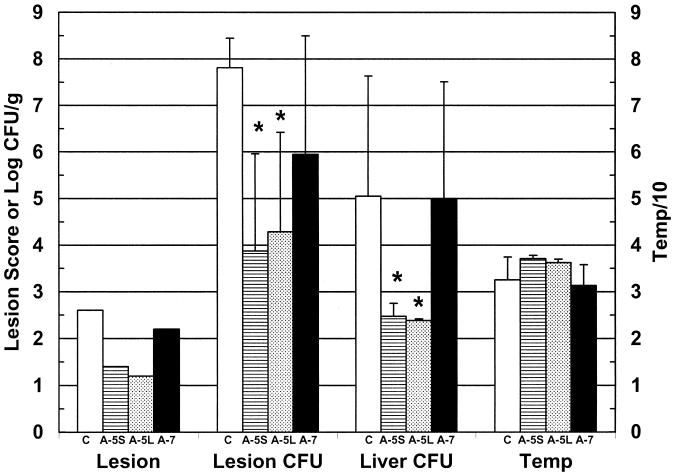
When mice were injected s.c. with 418 CFU of V. vulnificus MO6/24-0 and given a simultaneous i.v. injection with 108 PFU of phage 153A-5, significant protection was observed over a 1-day period in terms of CFU per gram of lesion and CFU per gram of liver (Fig. 2). Images of lesions from control (infected but not phage treated), phage 153A-5-treated, and phage 153A-7-treated mice are shown in Fig. 3. Phage 153A-5-treated mice had minor s.c. skin lesions at 19 h postinfection, with a mean lesion score of less than 1.5. Although body temperatures were more normal for phage 153A-5-treated mice, the differences were not statistically significant in comparison with the values for control mice. The survival rate for the phage 153A-5 group examined at 18 h (five of five mice) was significantly higher than that for the control group (two of five mice) (P = 0.038). In contrast, sea salts-requiring phage 153A-7 did not cause significant protection in terms of any of these criteria (Fig. 2), including survival rate (two of five mice). s.c. lesions typical of untreated V. vulnificus infection were observed (Fig. 3).
FIG. 2.
Protection of V. vulnificus MO6-24/0-infected mice by phage 153A-5 but not phage 153A-7. Iron-dextran-treated mice were inoculated s.c. with 417 CFU of V. vulnificus MO6-24/0 and immediately injected i.v. with BSG (control) (C), phage 153A-5 (A-5S and A-5L), or phage 153A-7 (A-7S). Samples were harvested at 14 h (C, A-5S, and A-7S) or 6 days (A-5L) after infection. Data are means and standard deviations for log CFU per gram of skin lesion (Lesion CFU), log CFU per gram of liver lesion (Liver CFU), and rectal temperature (Temp). For lesion score (Lesion), the mean is shown. For the A-5L liver data, the bar represents the minimum detectable level, since no CFU were recovered. All groups contained five animals. Protection was demonstrated for A-5S Lesion CFU and Temp (P < 0.01) and for A-5L Lesion CFU (P < 0.01); marginal protection was observed for Liver CFU (P = 0.05). Asterisks indicate significant differences from control. Mice treated with phage 153A-7 did not show any significant protection.
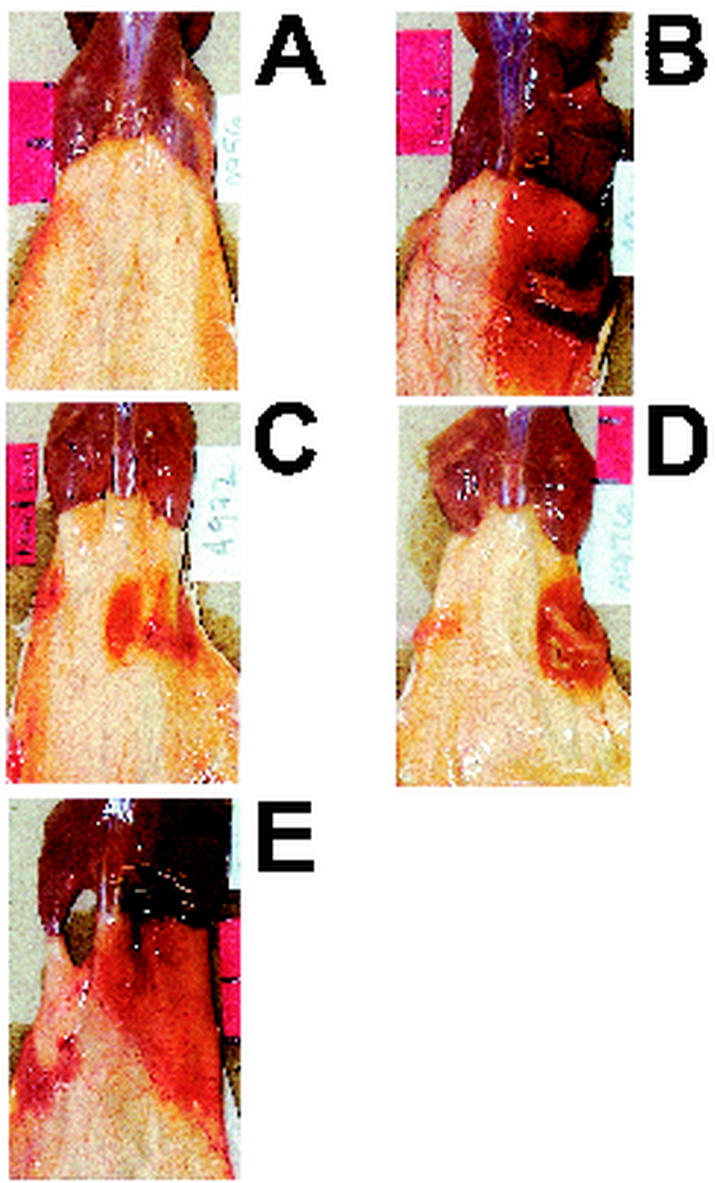
FIG. 3.
Gross pathologic features of V. vulnificus MO6-24/0-infected mice treated with phages 153A-5 and 153A-7. Dorsal views of gross pathologic features of s.c. lesions caused by V. vulnificus MO6-24/0 in iron-dextran-treated mice from the experiment shown in Fig. 2 are presented.(A) Iron-dextran-treated mouse that was not infected. (B to E) Mice injected i.p. with iron dextran and then immediately injected s.c. in the lower right dorsal quadrant with 417 CFU of MO6-24/0. Immediately after s.c. infection, mice received an i.v. injection of sterile BSG (B), 108 PFU of phage 153A-7 (C and D), or 108 PFU of phage 153A-5 (E). Mice in panels B, C, and E were euthanized at between 14 and 20 h later, and the skin was peeled back from the head to the tail to reveal s.c. tissues. The mouse in panel D was euthanized 6 days after s.c. infection. Panels B and E reveal large, edematous, hemorrhagic lesions that have lesion scores of 3. Mice in panels C and D had lesion scores of 2.
Phage 153A-5-treated mice kept for observation for 6 days after V. vulnificus infection had no detectable bacteria in their livers (the minimum detectable level is shown in Fig. 2) and had more normal temperatures, yet these were not significantly different from those for control mice at day 1 postinfection (Fig. 2). Three of the five long-term phage 153A-5-treated mice also had no detectable bacteria in their skin lesions, while the other two had various severities of skin infection. The survival rate for the long-term 153A-5-treated mice (five of five mice) was significantly higher than that for the short-term control mice (P = 0.038).
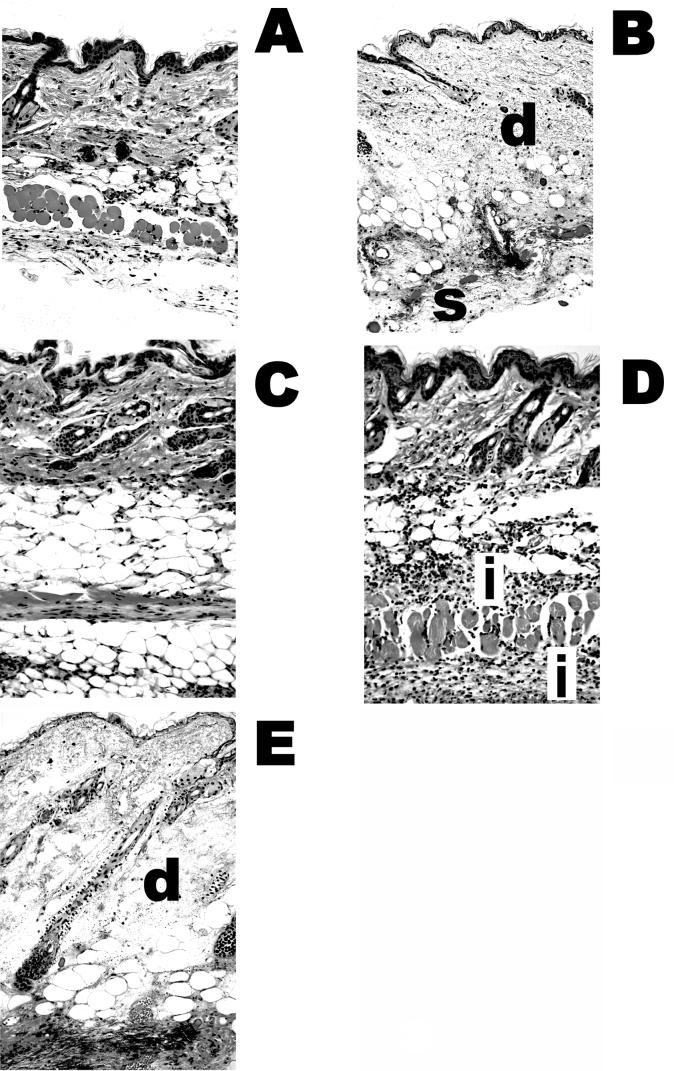
The presence of residual s.c. skin lesions in some of the protected 153A-5-treated mice led us to examine the histopathologic features of the lesions (Fig. 4). We observed that there was very little, if any, damage in the residual lesions in protected phage 153A-5-treated mice (Fig. 4C); however, mild inflammation was observed in treatment failures for 153A-5 (Fig. 4D). Phage 153A-7-treated mice that were not protected exhibited typical histopathologic damage that was previously described for untreated, infected mice (37) (Fig. 4B), namely, severe edema and necrosis of the dermis and s.c. tissues, with extensive infection beneath the s.c. musculature (Fig. 4E). Additionally, we occasionally observed perivascular infection in diseased 153A-7-treated mice (data not shown). Collectively, these data showed that a phage that could lyse V. vulnificus in LB-N could protect mice from a highly virulent strain of V. vulnificus, in both the short and the long terms, whereas a phage that required the presence of sea salts (LB-SS) for lytic activity failed to protect mice.
FIG.4.
Histopathologic features of mice infected with MO6-24/0 and treated with phages 153A-5 and 153A-7. The micrographs depict the histologic features of the mice from the experiment shown in Fig. 3. (A) Healthy mouse that received only an i.p. injection of iron. (B to E) Mice injected s.c. with 417 CFU of MO6-24/0. Tissues were collected from each mouse at death, fixed in buffered formalin, embedded in paraffin, and cut into 5-μm sections. Sections were stained with hematoxylin and eosin and observed at a magnification of ×200. (B) Control mouse (V. vulnificus infected, not phage treated). Necrosis and edema in the dermis (d) and subcutis (s) are indicated. (C) Phage 153A-5-treated mouse with no histologic damage. (D) Failure of phage 153A-5 treatment, with a lesion score of 2. The dermis and subcutis are intact, but inflammation (i) is indicated. (E) Failure of phage 153A-7 treatment. Necrosis in the dermis (d) and subcutis and edema were observed.
Relationship of in vitro host range to phage protection in mice.
To further examine the protective abilities of phage 153A-5 and the lack of protection by phage 153A-7, we performed infections with four additional V. vulnificus strains. V. vulnificus VV1009, like MO6/24-0, is susceptible to phage 153A-5 and resistant to phage 153A-7 in LB-N but susceptible to 153A-7 in LB-SS. V. vulnificus 2400112, NSV-5829, and 99-796DP-E7 are resistant to phage 153A-5 in LB-N or LB-SW. When mice were infected with 398 CFU of V. vulnificus VV1009, simultaneously injected i.v. with 108 PFU of phage, and examined 1 day later, results essentially identical to those for strain MO6/24-0 were obtained (Fig. 5A). Phage 153A-5 significantly protected the mice in terms of all three criteria of disease, whereas phage 153A-7 failed to protect in terms of any of the three criteria. Additionally, the survival rate for 153A-5-treated mice (five of five) was significantly higher than (P = 0.01) while that for 153A-7-treated mice (two of five) was not significantly different from (P = 0.5) that for control mice (one of five).
FIG. 5.
Analysis of protection of mice by phage 153-A5 with additional V. vulnificus strains. VV1009 is phage susceptible and 2400112, NSV-5829, and 99-796DP-E7 are phage resistant in vitro. (A) Iron-dextran-treated mice were inoculated s.c. with 398 CFU of V. vulnificus VV1009. Mice were injected i.v. with BSG (control) (C), phage 153A-5 (A-5), or phage 153A-7 (A-7) immediately after s.c. infection. Samples were harvested at 14 h. Data are means and standard deviations for log CFU per gram of skin lesion (Lesion CFU), log CFU per gram of liver lesion (Liver CFU), and rectal temperature (Temp). For lesion score (Lesion), the mean is shown. All groups contained five animals. Protection was observed for Lesion CFU, Liver CFU, and Temp (A-5) (P < 0.02 [compared to control]). Asterisks indicate significant differences from control. Phage 153A-7-treated mice were not protected in terms of any criteria. (B) Iron-dextran-treated mice were inoculated s.c. with approximately 1,000 CFU of V. vulnificus 2400112 (bars with vertical lines), NSV-5829 (NSV; bars with stippling), or 99-796DP-E7 (99-796; bars with horizontal lines). Mice were injected i.v. with BSG (control) (white background) or phage 153A-5 (grey background) immediately after s.c. infection. Samples were harvested 15 to 20 h later. Data are means and standard deviations for log CFU per gram of skin lesion (Lesion CFU), log CFU per gram of liver lesion (Liver CFU), and rectal temperature (Temp). Data represent two or three combined experiments. Numbers of animals in groups were as follows: for 2400112, without phage, n = 15, and with phage, n = 16; for NSV-5829, with or without phage, n = 15; and for 99-796DP-E7, without phage, n = 10, and with phage, n = 8. No significant differences were observed with phage treatment in the pooled results for any strain; however, some individual experiments for some strains yielded statistically significant differences (see the text).
To examine the specificity of protection by phage 153A-5 against V. vulnificus strains that were phage susceptible in vitro, we infected mice with strain 2400112, NSV-5829, or 99-796DP-E7, each of which is resistant to phage 153A-5, and treated them with 108 PFU of phage 153A-5. For three infections with strain 2400112, we observed sporadic, statistically significant differences in some virulence measures after phage treatment. The combined results of these experiments are shown in Fig. 5B, and the pooled results showed no significant differences in any virulence measure after phage treatment. In one infection, the liver CFU and temperature, measures of systemic disease, were statistically significantly improved by phage treatment (P = 0.0006 and P = 0.0003, respectively); however, a second repetition yielded no differences. The third infection yielded statistically significantly improved skin CFU and liver CFU (P = 0.00014 and P = 0.03, respectively); however the temperatures were not statistically different between the groups in the last two infections (32 and 29°C, respectively). These low temperatures indicate that the mice were moribund. Because we observed occasional improvement in terms of various measures of virulence with phage 153-A5 treatment for strain 2400112, which is not lysed by this phage in vitro, we examined whether the bacteria might become phage susceptible during growth in the mouse host. As a surrogate, we examined the susceptibility of the bacteria to phage 153-A5 during growth in rat serum. As a positive control for phage susceptibility, strain MO6/24-0 was also examined. In both LB-N and rat serum, strain MO6/24-0 was killed by phage 153-A5, while strain 2400112 was not affected by the phage at all (data not shown).
Two infection and protection experiments each were performed with strains NSV-5829 and 99-796DP-E7 and phage 153-A5, and the combined results are shown in Fig. 5B. No statistically significant differences were observed for lesion CFU, liver CFU, or temperature with phage treatment. However, in one experiment, strain NSV-5829 produced marginally lower skin lesion CFU with phage treatment (P = 0.03).
Effects of delaying phage 153A-5 treatment on infection with MO6/24-0.
As was attempted for phage CK-2 and V. vulnificus MLT403, we examined how long phage treatment could be delayed after s.c. administration of a lethal dose of V. vulnificus to iron-dextran-treated mice. Our failure to observe significant protection with delays of 6 and 12 h led us to examine delays of 6 h or less. Mice infected with 1,070 CFU of MO6/24-0 were injected with 108 PFU of phage 153A-5 at 0, 3, and 6 h postinfection and then examined for disease at day 1 postinfection. As shown in Fig. 6, all three times of administering the phage treatment resulted in significant protection in terms of all four criteria in comparison with the results for the control group. However, the numbers of CFU per gram of lesion were significantly higher for the 3- and 6-h treatment delay groups than for the 0-h treatment delay group. The numbers of CFU per gram of liver and temperature were not significantly different among the phage-treated groups. The survival rates for all phage-treated groups (five of five mice) were significantly higher than that for the control group (zero of five mice) (P = 0.0016). Delaying phage treatment for as long as 6 h seemed to provide protection over a short infection period of 1 day for this phage-host pair.
FIG. 6.
Delay of phage 153A-5 treatment of V. vulnificus MO6-24/0-infected mice. Iron-dextran-treated mice were inoculated s.c. with 1,070 CFU of V. vulnificus MO6-24/0. Mice were injected i.v. with BSG (control) (C) immediately or with 108 PFU of phage 153A-5 immediately (0), 3 h later (3), or 6 h later (6). Samples were harvested 14 h after s.c. inoculation. Data are means and standard deviations for log CFU per gram of skin lesion (Lesion CFU), log CFU per gram of liver lesion (Liver CFU), and rectal temperature (Temp). For lesion score (Lesion), the mean is shown. All groups contained five animals. Single asterisks indicate that values for the control group were significantly different from those for all of the phage-treated groups (10−9 < P < 0.013). Double asterisks indicate that the 0-h treatment delay group was significantly protected compared with the 3- and 6-h treatment delay groups (6 × 10−6 < P < 0.0035).
In a follow-up experiment, we examined whether the mice protected over a 1-day period with 6-h delayed phage treatment were destined to recover or whether the delayed phage treatment had only caused a temporary delay in lethal V. vulnificus infection. Seven mice were infected with V. vulnificus MO6/24-0 and treated with 108 PFU of phage 153A-5 6 h postinfection. The experiment had to be terminated at 2 days postinfection because three mice died. One of the remaining mice had 106.2 CFU/g of s.c. lesion, and the other three had undetectable bacteria in skin lesions. Therefore, although some longer-term protection was observed with a 6-h delayed phage treatment, the efficiency of protection was compromised compared with that obtained with simultaneous V. vulnificus infection and phage treatment.
Dose response in mice for phage 153A-5 protection against V. vulnificus MO6/24-0.
Since we had been treating mice with phage 153A-5 doses of up to 106-fold higher than the V. vulnificus inoculum, we titrated the minimum protective dose of the phage for a 1-day V. vulnificus infection (Fig. 7). Iron-dextran-treated mice were injected s.c. with 1,150 CFU of V. vulnificus MO6/24-0 and immediately injected i.v. with 108, 106, or 104 PFU of phage 153A-5. Only the highest dose of phage, 108 PFU, afforded significant protection to the mice in terms of every criterion. The lower doses of phage did not significantly protect the mice over the course of a 1-day infection, except for the CFU per gram of liver in the group treated with 106 PFU. The trends in terms of all four criteria progressed from the control group to the highest-dose phage-treated group, giving the appearance of a dose response; however, the values for the groups treated with 0, 104, and 106 PFU were generally not significantly different from each other. The survival rate for only the group treated with 108 PFU (five of five mice) was significantly higher than that for the control group (one of five mice) (P = 0.01). The survival rate for the group treated with 106 PFU (four of five mice) was nearly significantly higher (P = 0.06). However, when similarly infected mice treated with 106 PFU of phage 153A-5 were monitored for 2 days, three of five mice died and a fourth had nearly 107 CFU/g of skin lesion (data not shown). Therefore, it appeared that very high multiplicities of phage treatment were required for significant protection against s.c. infection by V. vulnificus in iron-dextran-treated mice.
FIG. 7.
Titration of phage 153A-5 in V. vulnificus MO6-24/0-infected mice. Iron-dextran-treated mice were inoculated s.c. with 1,150 CFU of V. vulnificus MO6-24/0. Mice were injected i.v. with BSG (control) (−) or with 108 PFU (8), 106 PFU (6), or 104 PFU (4) of phage 153A-5 immediately after s.c. infection. Samples were harvested 14 to 20 h later. Data are means and standard deviations for log CFU per gram of skin lesion (Lesion CFU), log CFU per gram of liver lesion (Liver CFU), and rectal temperature (Temp). For lesion score (Lesion), the mean is shown. Single asterisks indicated that values for the phage-treated group were significantly different from those for the control group (2.5 × 10−5 < P < 0.02). Double asterisks indicate that values for the 108 PFU-treated group were significantly different from those for the 106 or 104 PFU-treated group (P < 0.02).
Half-life of phage in mouse tissues.
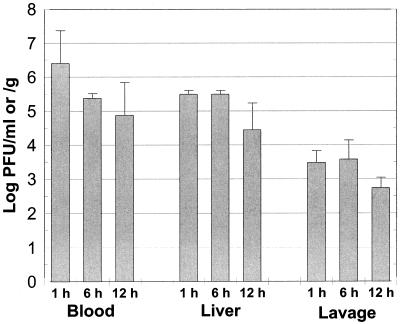
Since delayed treatment with phages resulted in decreased efficacy of protection, we measured the stability of phage 153A-5 in mice after i.v. injection. Mice that were not infected with V. vulnificus were injected i.v. with 108 PFU of phage, and the numbers of phage were measured in the blood, liver, and peritoneal fluid. From 1 h postinjection to 12 h postinjection, the PFU per milliliter of blood dropped over 43-fold from 106.4 to 104.9 PFU/ml, yielding a half-life of 2.2 h (Fig. 8). The PFU per gram of liver was approximately 10% the PFU per milliliter of blood at 1 h postinjection, and liver PFU dropped only 10-fold over the course of the experiment. The ability of the i.v. injected phage to translocate out of the vasculature into the peritoneal cavity was very low. At 1 h postinjection, only about 0.1% the dose of injected phage was recovered from the peritoneal lavage fluid, and the level dropped less than 10-fold over the 12-h period. i.v. injected phage 153A-5 appeared to be relatively stable in blood, yet it was unable to escape the vasculature into the tissues of these animals not infected with V. vulnificus.
FIG. 8.
Half-life of phages in mouse tissues. Mice were treated with iron dextran but not s.c. infected with V. vulnificus. Phage 153A-5 was injected i.v. at 108 PFU per mouse. Samples of the blood, liver, and peritoneal lavage fluid were sampled at 1, 6, and 12 h after injection of the phage, and numbers of PFU per milliliter or PFU per gram of tissue were enumerated. The half-life of the phage in the blood was calculated to be 2.2 h. Data are means and standard deviations.
DISCUSSION
To examine the feasibility, kinetics, and limitations of phage therapy, we used a mouse model of V. vulnificus infection. We show that it is possible to prevent both local and systemic disease caused by V. vulnificus in an iron-dextran-treated mouse model by administering bacteriophages that are lytic for the infecting bacterial strain. Phage CK-2 could prevent infection by V. vulnificus MLT403, and phage 153A-5 could prevent infection by V. vulnificus MO6/24-0 and VV1009. We quantitatively measured disease and protection by using four criteria: s.c. lesion score and CFU per gram of s.c. lesion as measures of local disease and body temperature and CFU per gram of liver as measures of systemic disease. We also measured the survival of mice. In most, but not all, treated mice, these phages could significantly reduce these measures of disease and in many instances resulted in apparently sterile lesions and livers. Achievement of this level of protection required the administration of high doses of phage (108 PFU/mouse) relatively soon after inoculation.
We hypothesize the phage-bacterium interaction in this mouse model for V. vulnificus disease to be follows. After s.c. inoculation into iron-dextran-treated mice, the vibrios begin to replicate at an extremely rapid rate. In the initial characterization of this model, the slowest doubling time of the bacteria in the mice was calculated to be 45 min (37). Although the vibrios and phages are often administered to mice simultaneously, they may not come into contact until some time after injection. Early on, the vibrios may be sequestered in the intercellular fluid in the s.c. tissues, while the phages are present in the bloodstream. Particles as large as phages may not freely pass through the vascular endothelium. The very small amounts of phages obtained from the peritoneal lavage fluid over a 12-h time course are in agreement with this hypothesis. However, as the vibrios reach sufficient numbers in the tissues, they induce vasodilation and vascular permeability (3), thereby enabling the phages access to the bacteria as edema floods the infected tissues. Therefore, the bacteria may actually induce their own demise in this particular model system. The fact that phages administered i.v. can be effective at clearing local infection of the skin tissues suggests that such treatment may be useful in a clinical setting for V. vulnificus disease, where i.v. administration would be most efficient at delivering the phages throughout the body.
With regard to the mechanism whereby phages elicit their protective effect, we have shown that it is, as expected, killing of the bacteria. We generally did not observe protection when the chosen phage was unable to lyse the inoculating V. vulnificus strain in vitro. This relationship was shown in two ways. First, we noted that phage 153A-7 formed plaques on the host bacteria only when bacterial medium containing sea salts or seawater was used. Since these conditions are not present in animals, such phages should not be able to lyse the bacteria in the infected animal host. We demonstrated that phage 153A-7 failed to protect mice from infection with V. vulnificus MO6/24-0 (Fig. 2) and VV1009 (Fig. 5). There are at least two possible reasons for the failure of phage 153A-7 to form plaques on V. vulnificus in the absence of sea salts. Either the receptor on V. vulnificus cells is not expressed in the absence of sea salts or the salts are required for the binding of the phage to the receptor. We have yet to examine these possibilities. Clearly, phages such as phage 153A-7 would not be clinically useful.
A second reason that phages failed to form plaques was the limited host range for the V. vulnificus strains in any media tested. We would not expect these phage-bacterium mixtures to yield protection in our mouse model. As shown in Fig. 5B, phage 153A-5 failed to consistently protect mice from s.c. infection with V. vulnificus 2400112, NSV-5829, and 99-796DP-E7, which were resistant to 153A-5. Figure 5B depicts the combined results of three experiments for strain 2400112 and two experiments for NSV-5829 and 99-796DP-E7 because it was noted that for strains 2400112 and NSV-5829, various measures of virulence were sporadically significantly ameliorated by phage treatment. The occasional statistically significant differences were not nearly on the same order as those seen with the productive phage-host strain combinations, in which the mice were completely protected. These statistically significant differences for the apparently resistant V. vulnificus strains were of questionable biological significance. The reason for these sporadic differences in the absence of in vitro plaque-forming activity of the phage is unknown. One possibility is that the phage is able to infect and kill or debilitate the bacteria without releasing enough progeny phage to form a plaque in vitro. In this scenario, the phage in the bloodstream of the mice would be able to kill vibrios that leak into the vasculature as the disease progresses in the skin. However, we found that strain 2400112 was not killed by phage 153-A5 in either LB-N or rat serum. It is also possible that residual bacterial components in the phage lysates, e.g., lipopolysaccharide, induced an inflammatory response upon i.v. injection and that this inflammatory response slightly boosted the resistance of the mice to infection. However, such a phenomenon was not always apparent, because phage 153-A7, which could lyse its host bacteria only in the presence of seawater, offered no such protection.
To examine the limitations of our phage therapy model, we either delayed treatment with phages after bacterial infection or titrated the minimum numbers of phages required for protection. As shown in Fig. 6, delaying phage treatment for more than 3 h resulted in failure. Even though mice were significantly protected at 1 day postinfection, many became ill within 2 days. The reason for the inability to confer protection after delayed treatment in our model is unknown. The progression of disease in our model is extremely rapid, with death occurring as soon as 12 h after infection with a minimum lethal dose. Since the histopathologic features of the s.c. lesions consist of thrombosis of capillaries (37), it is possible that with delayed phage treatment, the localized vasculature is clotted off, thereby preventing the entry of subsequently administered phages into the infected tissues. In contrast, when the phages are present early during the infection, they may leak into the infected tissues before thrombosis occurs and begin to kill and replicate in the infecting vibrios. Another possibility is that the numbers of bacteria have increased to such a high level that the phages cannot kill them all. Figure 7 shows that a high level of phage, i.e., 108 PFU, is required to protect against a minimum lethal dose of bacteria, and the half-life of phage 153A-5 in mice is approximately 2.2 h. It therefore appears that the phages are relatively stable in mouse fluids, particularly the vasculature. The reason for the ineffectiveness of lower doses of the phages is unknown. However, very high levels of phages do not appear to have harmful side effects.
Phage therapy has been widely used in Eastern Europe for many decades, and animal model studies have been investigated in the West since the early 1980s (10, 39). Almost all of these studies have shown that phage therapy has great potential, but more research needs to be done to understand the parameters that limit the effectiveness of phage therapy in different diseases. Phages have some unique advantageous features that could make them highly efficacious under certain conditions. (i) Phages are capable of replicating in a treated patient by infecting the bacteria, with the subsequent release of 100-fold more phage. This effect is in marked contrast to that of antibiotics, which only decrease in concentration from the time when they are administered. (ii) Phages are specific to bacterial species; hence, the normal microbial flora will be preserved. In contrast, the use of many antibiotics disrupts the normal flora, a process which may lead to secondary symptoms. (iii) Phages can be isolated that recognize virulence factors as receptors so that phage-resistant mutants that have lost their receptors will be attenuated. (iv) Because of their specificity to bacteria, phages have very few, if any, side effects in the treated patient. Many antibiotics exert toxic side effects. (v) Phages can be used topically as well as in the environment to control populations of bacterial pathogens. In spite of these advantages, phage therapy also has its drawbacks. The specificity of phages for host bacteria necessitates the use of broad-host-range phages or pools of phages with broad collective activity or at least the identification of phages with activity against the infecting bacterial strains. Bacteria can become resistant to phages by spontaneous mutation. In theory, the production of antiphage antibodies by phage-treated hosts could interfere with subsequent treatments with the same phage. Finally, in our experimental system, high numbers of phages needed to be administered soon after bacterial infection for treatment to be effective. Optimizing the beneficial attributes of phage therapy while decreasing the potential problems will require continuing analysis of animal models of infection.
In summary, our results demonstrate that treatment of V. vulnificus-infected mice can prevent local and systemic disease as well as death. These results contribute to the growing body of evidence that phage therapy is a viable alternative treatment modality for bacterial infectious disease. Our V. vulnificus phage therapy model will now be used to define the major benefits and limitations of phage therapy.
Acknowledgments
This work was supported by Department of Commerce sea grant R/LR-Q-20 to D.H.D. and P.A.G. and a focused giving award from Johnson and Johnson, Inc., to P.A.G.
We thank Anita C. Wright for review of the manuscript and Thomas J. Doyle, Julio Martin, and Eric Wilkening for expert technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Amako, K., K. Okada, and S. Miake. 1984. Evidence for the presence of a capsule in Vibrio vulnificus. J. Gen. Microbiol. 130:2741-2743. [DOI] [PubMed] [Google Scholar]
- 2.Berchieri, A., M. A. Lovell, and P. A. Barrow. 1991. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 142:541-549. [DOI] [PubMed] [Google Scholar]
- 3.Bowdre, J. H., M. D. Poole, and J. D. Oliver. 1981. Edema and hemoconcentration in mice experimentally infected with Vibrio vulnificus. Infect. Immun. 32:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennt, C. E., A. C. Wright, S. K. Dutta, and J. G. Morris, Jr. 1991. Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J. Infect. Dis. 164:1030-1032. [DOI] [PubMed] [Google Scholar]
- 5.Buchrieser, C., V. V. Gangar, R. L. Murphree, M. L. Tamplin, and C. W. Kaspar. 1995. Multiple Vibrio vulnificus strains in oysters as demonstrated by clamped homogeneous electric field gel electrophoresis. Appl. Environ. Microbiol. 61:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullen, J. J., P. B. Spalding, C. G. Ward, and J. M. Gutteridge. 1991. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch. Intern. Med. 151:1606-1609. [PubMed] [Google Scholar]
- 7.Cook, D. W., P. Oleary, J. C. Hunsucker, E. M. Sloan, J. C. Bowers, R. J. Blodgett, and A. DePaola. 2002. Vibrio vulnificus and Vibrio parahaemolyticus in U.S. retail shell oysters: a national survey from June 1998 to July 1999. J. Food Prot. 65:79-87. [DOI] [PubMed] [Google Scholar]
- 8.DePaola, A., S. McLeroy, and G. McManus. 1997. Distribution of Vibrio vulnificus phage in oyster tissues and other estuarine habitats. Appl. Environ. Microbiol. 63:2464-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePaola, A., M. L. Motes, A. M. Chan, and C. A. Suttle. 1998. Phages infecting Vibrio vulnificus are abundant and diverse in oysters (Crassostrea virginica) collected from the Gulf of Mexico. Appl. Environ. Microbiol. 64:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth, D. H., and P. A. Gulig. 2001. Bacteriophages: potential treatment for bacterial infections. BioDrugs 16:57-62. [DOI] [PubMed] [Google Scholar]
- 11.Fan, J. J., C. P. Shao, Y. C. Ho, C. K. Yu, and L. I. Hor. 2001. Isolation and characterization of a Vibrio vulnificus mutant deficient in both extracellular metalloprotease and cytotysin. Infect. Immun. 69:5943-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaherty, J. E., J. B. Jones, B. K. Harbaugh, G. C. Somodi, and L. E. Jackson. 2000. Control of bacterial spot on tomato in the greenhouse and field with H-mutant bacteriophages. Hortscience 35:882-884. [Google Scholar]
- 13.Gray, L. D., and A. S. Kreger. 1985. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect. Immun. 48:62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hlady, W. G., and K. C. Klontz. 1996. The epidemiology of Vibrio infections in Florida, 1981-1993. J. Infect. Dis. 173:1176-1183. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, J. K., R. L. Murphree, and M. L. Tamplin. 1997. Evidence that mortality from Vibrio vulnificus infection results from single strains among heterogeneous populations in shellfish. J. Clin. Microbiol. 35:2098-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong, K. C., H. S. Jeong, J. H. Rhee, S. E. Lee, S. S. Chung, A. M. Starks, G. M. Escudero, P. A. Gulig, and S. H. Choi. 2000. Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect. Immun. 68:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kook, H., S. E. Lee, Y. H. Baik, S. S. Chung, and J. H. Rhee. 1996. Vibrio vulnificus hemolysin dilates rat thoracic aorta by activating guanylate cyclase. Life Sci. 59:PL41-PL47. [DOI] [PubMed] [Google Scholar]
- 18.Kraffert, C. A., and D. J. Hogan. 1992. Vibrio vulnificus infection and iron overload. J. Am. Acad. Dermatol. 26:140.. [DOI] [PubMed] [Google Scholar]
- 19.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 20.Litwin, C. M., T. W. Rayback, and J. Skinner. 1996. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 64:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merril, C. R., B. Biswas, R. Carlton, N. C. Jensen, G. J. Creed, S. Zullo, and S. Adhya. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyoshi, S., and S. Shinoda. 1988. Role of the protease in the permeability enhancement by Vibrio vulnificus. Microbiol. Immunol. 32:1025-1032. [DOI] [PubMed] [Google Scholar]
- 23.Motes, M. L., A. DePaola, D. W. Cook, J. E. Veazey, J. C. Hunsucker, W. E. Garthright, R. J. Blodgett, and S. J. Chirtel. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 64:1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paranjpye, R. N., J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, J. W., S. N. Ma, E. S. Song, C. H. Song, M. R. Chae, B. H. Park, R. W. Rho, S. D. Park, and H. R. Kim. 1996. Pulmonary damage by Vibrio vulnificus cytolysin. Infect. Immun. 64:2873-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, S. C., I. Shimamura, M. Fukunaga, K. I. Mori, and T. Nakai. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 66:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelon, W., R. J. Siebeling, J. Simonson, and R. B. Luftig. 1995. Isolation of bacteriophage infectious for Vibrio vulnificus. Curr. Microbiol. 30:331-336. [DOI] [PubMed] [Google Scholar]
- 28.Provence, D. L., and R. Curtiss III. 1994. Gene transfer in gram-negative bacteria, p. 317-347. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 29.Shao, C. P., and L. I. Hor. 2000. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect. Immun. 68:3569-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinoda, S., M. Kobayashi, H. Yamada, S. Yoshida, M. Ogawa, and Y. Mizuguchi. 1987. Inhibitory effect of capsular antigen of Vibrio vulnificus on bactericidal activity of human serum. Microbiol. Immunol. 31:393-401. [DOI] [PubMed] [Google Scholar]
- 31.Smith, H. W., and M. B. Huggins. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J. Gen. Microbiol. 128:307-318. [DOI] [PubMed] [Google Scholar]
- 32.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 33.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 133:1127-1135. [DOI] [PubMed] [Google Scholar]
- 34.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 133:1111-1126. [DOI] [PubMed] [Google Scholar]
- 35.Soothill, J. S. 1992. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 37:258-261. [DOI] [PubMed] [Google Scholar]
- 36.Soothill, J. S. 1994. Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns 20:209-211. [DOI] [PubMed] [Google Scholar]
- 37.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. 2000. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 68:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 39.Sulakvelidze, A., Z. Alavidze, and J. G. Morris. 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamplin, M. L., J. K. Jackson, C. Buchrieser, R. L. Murphree, K. M. Portier, V. Gangar, L. G. Miller, and C. W. Kaspar. 1996. Pulsed-field gel electrophoresis and ribotype profiles of clinical and environmental Vibrio vulnificus isolates. Appl. Environ. Microbiol. 62:3572-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamplin, M. L., S. Specter, G. E. Rodrick, and H. Friedman. 1983. Differential complement activation and susceptibility to human serum bactericidal action by Vibrio species. Infect. Immun. 42:1187-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamplin, M. L., S. Specter, G. E. Rodrick, and H. Friedman. 1985. Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect. Immun. 49:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vollberg, C. M., and J. L. Herrera. 1997. Vibrio vulnificus infection: an important cause of septicemia in patients with cirrhosis. South. Med. J. 90:1040-1042. [DOI] [PubMed] [Google Scholar]
- 44.Wright, A. C., and J. G. Morris, Jr. 1991. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect. Immun. 59:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida, S., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]